Abstract
We investigated whether certain strains of lactic acid bacteria (LAB) could antagonize specific T-helper functions in vitro and thus have the potential to prevent inflammatory intestinal immunopathologies. All strains tested induced various levels of both interleukin-12 (IL-12) and IL-10 in murine splenocytes. In particular, Lactobacillus paracasei (strain NCC2461) induced the highest levels of these cytokines. Since IL-12 and IL-10 have the potential to induce and suppress Th1 functions, respectively, we addressed the impact of this bacterium on the outcome of CD4+ T-cell differentiation. For this purpose, bacteria were added to mixed lymphocyte cultures where CD4+ T-cells from naive BALB/c mice were stimulated weekly in the presence of irradiated allogeneic splenocytes. In these cultures, L. paracasei NCC2461 strongly inhibited the proliferative activity of CD4+ T cells in a dose-dependent fashion. This was accompanied by a marked decrease of both Th1 and Th2 effector cytokines, including gamma interferon, IL-4, and IL-5. In contrast, IL-10 was maintained and transforming growth factor β (TGF-β) was markedly induced in a dose-dependent manner. The bacteria were not cytotoxic, because cell viability was not affected after two rounds of stimulation. Thus, unidentified bacterial components from L. paracasei NCC2461 induced the development of a population of CD4+ T cells with low proliferative capacity that produced TGF-β and IL-10, reminiscent of previously described subsets of regulatory cells implicated in oral tolerance and gut homeostasis.
The immunological properties of lactic acid bacteria (LAB) have raised a lot of interest in recent years due to their immune-stimulating properties (32). Several strains of LAB were reported to display stimulatory properties on cells of the innate immune system in vitro, including macrophages (24) and NK cells (14, 25). Immune stimulation in vitro was characterized by the induction of proinflammatory cytokines, such as interleukin-12 (IL-12) (20, 23, 33) and tumor necrosis factor alpha (15, 16). This increase of innate immune functions was mirrored in vivo using animal models (39–42) and in humans given fermented milk products containing probiotics (46, 47). It has therefore been proposed that LAB could be used as nonspecific adjuvants of innate immune responses to increase early defense mechanisms in response to gastrointestinal infections.
Innate immune responses not only serve as an early line of defense against invading pathogens but also are crucial for the development of subsequent acquired immune responses that are orchestrated by CD4+ T cells (31). Murine CD4+ T lymphocytes can be classified into several subsets depending on the type of cytokines they produce. Originally, two major subsets of effector CD4+ T cells were described as the Th1 and Th2 subsets. Th1 and Th2 cells produce mainly high levels of gamma interferon (IFN-γ) and IL-4/IL-5 respectively and carry out distinct key regulatory functions in an immune response (35). Th1-derived cytokines mediate principally the cell-mediated immune functions, such as trigger killing of intracellular parasites by macrophages, whereas Th2 cytokines mostly favor the generation of humoral responses dominated by immunoglobulin E that are required for elimination of helminth infections (reviewed in reference 36). In the mouse, when these two types of responses are strongly polarized, they are by and large mutually exclusive and regulate each other through feedback loops mediated by regulatory cytokines, such as IL-12 and IL-10 (5, 8, 29). The balance between the two types of responses is considered important in maintaining homeostasis of the host, since a number of diseases that have been associated with an exaggerated Th1 or Th2 response are linked to abnormal production of these cytokines. This balance is thought to be maintained by specialized subsets of regulatory cells that produce suppressive cytokines such as IL-10 and transforming growth factor β (TGF-β) (2, 12). The signals that drive the differentiation of naive CD4+ T cells toward distinct effector or regulatory phenotypes have been extensively studied. Murine naive CD4+ T cells that are primed by antigen-presenting cells and antigen in the presence of IL-12 preferentially develop toward the Th1 phenotype (26), whereas the presence of IL-4 favors Th2 differentiation (38). While the early sources of IL-4 remain somewhat controversial (4), it appears that early IL-12 production stems from components of the innate immune system, such as macrophages stimulated by pathogenic gram-positive bacteria (22). Interestingly, the genetic background of the responding CD4+ T cells determines the default pathway toward either phenotype if no exogenous factors are added during the priming phase (21).
Most of the studies on mechanisms of in vitro CD4+ T-cell differentiation mentioned above have made use of peptide-specific T-cell receptor transgenic naive CD4+ T cells. A simpler method to study the mechanisms of CD4+ T cell differentiation has been the mixed lymphocyte reaction (MLR), where purified CD4+ T-cell responders from naive mice are mixed with allogeneic irradiated whole splenocytes as accessory cells (17). These MLR studies have allowed study of the proliferative capacity and cytokine production profiles of distinct murine CD4+ T-cell subsets distinguished on the basis of cell surface markers and to address the importance of different types of accessory cells in this process (17, 18). Because LAB strongly induce innate IL-12 in accessory cells (20, 23, 33), we have used this MLR system to evaluate the impact of LAB on the subsequent generation of Th1 and Th2 effector functions.
MATERIALS AND METHODS
Mice and bacteria.
Female 6-week-old BALB/c and C57BL/6 mice were purchased at Iffa-Crédo (L'Abresle, France) and were maintained under specific-pathogen-free conditions in our animal facilities at the Nestlé Research Center, Lausanne, Switzerland, and in accordance with the ethical regulations of the Veterinary Service of the Canton de Vaud, Switzerland. Mice were sacrificed before 8 weeks of age by cervical dislocation under 3% Isoflurane anesthesia (Abbot SA) for sampling of spleens.
All strains of lactobacilli were cultured in MRS broth without acetate at 37°C under anaerobic conditions. L. johnsonii NCC533, L. gasseri NCC2493, and L. paracasei NCC2461 were originally isolated from human feces. L. acidophilus NCC90 was originally provided by The University of Piacenza. L. casei strain Shirota was isolated from a commercial product (Yakult, Japan). L. casei strain GG was obtained from Valio (Finland). All bacteria were harvested by centrifugation (3,000 × g for 15 min) at stationary growth phase. Pelleted bacteria were then washed three times in phosphate-buffered saline (PBS) and diluted to a final working concentration of 109 CFU/ml in complete medium, i.e., RPMI 1640 medium containing 10% inactivated fetal calf serum (FCS), 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, 100 U of penicillin per ml, and 100 mM Streptomycin (all reagents from Life Technologies AG). This stock suspension was aliquoted and stored at −80°C. One fresh aliquot was thawed for every new experiment to avoid variability in the cultures between experiments.
Stimulation of splenocytes with LAB.
Whole splenocytes were obtained from BALB/c or C57BL/6 mice by homogenizing spleens with a tissue grinder. After elimination of erythrocytes, spleen cells were washed three times in ice-cold Hanks' balanced salt solution (Life Technologies AG) containing 5% FCS and were resuspended at 107 cells/ml in complete medium. Cells (106/well) were cultured in 96-well plates in the presence or absence of various concentrations of bacteria (concentrations are indicated in the figure legends). Lipopolysaccharide (LPS) (Escherichia coli serotype O55:B5; Sigma) was added at 1 μg/ml as a positive control culture for IL-10 production. After 24 h of culture, the supernatants were subjected to IL-12p40 and IL-10 quantification by enzyme-linked immunosorbent assay (ELISA).
MLR.
CD4+ T cells were purified from erythrocyte-depleted spleens of BALB/c mice using an anti-CD4 monoclonal antibody coupled to MACS microbeads (Myltenyi Biotec, Bergisch Gladbach, Germany) as specified by the manufacturer. Cell purity was verified by flow cytometric analysis and exceeded 90%. Purified CD4+ T cells (105 cells/well) were mixed with irradiated (3,000 rads) allogeneic splenocytes (106 cells/well) from C57BL/6 mice in 200 μl of complete medium in round-bottom 96-well plates. Cultures were incubated for 7 days at 37°C in a 5% CO2 incubator under 80% humidity. After this primary culture, CD4+ T cells were washed, purified again using the MACS system, and restimulated for another 7 days with freshly isolated irradiated C57BL/6 splenocytes in a secondary culture under conditions identical to these in the primary culture. During the primary and secondary weekly stimulations, the cultures contained either medium alone, LPS (1 μg/ml), blocking monoclonal antibody (MAb) to IL-4 (clone 1D11; Pharmingen), or bacteria added at concentrations of 107 or 106 CFU/ml. After the secondary culture, a fraction of the live CD4+ T cells were analyzed by flow cytometry for memory markers as characterized by low expression of MEL-14 (CD62L) and high expression of CD44 (Pharmingen). The remainder of the cells were washed, purified one last time by MACS, and stimulated for 48 h in medium alone in the presence of fresh irradiated C57BL/6 splenocytes. After this time, supernatants were collected and frozen at −20°C until analyzed by ELISA. To measure cell proliferation, cells were pulsed for a further 16 h with 1 μCi of [methyl-3H] thymidine (Amersham, Zürich, Switzerland). The cells were then harvested on nitrocellulose filters (Packard, Zürich, Switzerland), and bound [methyl-3H]thymidine was measured by scintillation counting (TopCount; Packard).
ELISA.
Cytokines were detected in culture supernatants by sandwich ELISA. IFN-γ was detected using R4-6A2 and biotinylated XMG1.2 as coating and detecting MAbs, respectively. For IL-4 detection, 1D11 and biotinylated 24G2 were used; for IL-5, TRFK4 and biotinylated TRFK5 were used; for IL-10, 16E3 and biotinylated 2A5 were used (all MAbs from Endogen, Woburn, Mass.). Briefly, coating antibodies were incubated on Maxisorp ELISA plates (Life Technologies AG, Basel, Switzerland) at 5 μg/ml in PBS overnight at room temperature. After four washes in PBS containing 0.05% Tween 20, the wells were blocked for 1 h at room temperature with PBS containing 20% FCS and 0.05% Tween 20. After further washing, supernatants were added at a dilution of 1:2 and the samples were serially diluted in culture medium and incubated in the presence of negative (normal medium) and positive (recombinant standard; Pharmingen) controls for 4 h at room temperature. After further washing, secondary biotinylated MAbs were added at a concentration of 0.5 to 2 μg/ml and the samples were incubated for 1 h at room temperature. The wells were then washed, 1 μg of horseradish peroxidase-labeled streptavidin (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was added, and the wells were incubated for 30 min. Plates were washed one last time and incubated with TMB microwell substrate (Kirkegaard & Perry). The reactions were stopped with 1 M phosphoric acid. Optical densities were read at 450 nm. TGF-β and IL-12p40 were quantified using the Quantikine anti-human and Quantikine M ELISA kits, respectively from R&D Systems (Abingdon, United Kingdom). Cytokine levels were extrapolated from the standard curve calculated from dilutions of the recombinant cytokines.
Statistics.
All experiments were carried out at least three times, and cultures were performed in triplicate wells. Values and error bars in the graphics represent the mean ± standard error of the mean (SEM). In Table 1, the SEM is not shown and did not exceed 12% of the means. When necessary, Student's paired t test was performed on the data to assess significant differences (P < 0.05).
TABLE 1.
CD4+ T-cell viability after 2 weekly stimulations of MLR cocultured with L. paracasei NCC2461a
| Culture conditionsc | 4 days after restimulationb
|
7 days after restimulationb
|
||||
|---|---|---|---|---|---|---|
| Total no. of cells (106) | % of CD4+ cells | No. of CD4+ cells (105) | Total no. of cells (106) | % of CD4+ cells | No. of CD4+ cells (105) | |
| Medium | 1.2 | 14.87 | 1.78 | 1.1 | 38.88 | 4.28 |
| NCC2461 (107 CFU/ml) | 1.2 | 11.88 | 1.43 | 1.0 | 19.9 | 1.99 |
| NCC2461 (106 CFU/ml) | 1.2 | 8.94 | 1.07 | 1.1 | 24.09 | 2.65 |
| LPS (1 μg/ml) | 1.5 | 12.26 | 1.84 | 1.2 | 21.81 | 2.62 |
| aIL-4 (10 μg/ml) | 1.5 | 11.03 | 1.65 | 0.9 | 31.84 | 2.87 |
Values are mean of triplicate cultures from one representative experiment. The SEM (not shown) did not exceed 12% of the means.
CD4+ T cells were harvested, and viability was assessed by trypan blue exclusion 4 and 7 days after the second weekly restimulation.
Culture conditions during the first 2 weeks of culture.
RESULTS
Distinct strains of lactobacilli rapidly induce different levels of IL-12 and IL-10 protein synthesis in murine spleen cells.
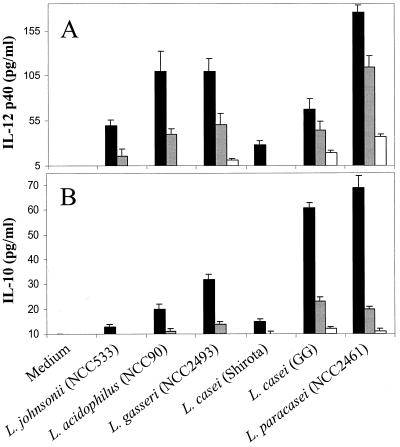
There is evidence that nonpathogenic gram-positive bacteria may be strong IL-12 inducers in mononuclear adherent cells while gram-negative bacteria are more efficient IL-10 inducers (19). Several strains of lactobacilli (LAB) were tested for their capacity to induce the secretion of IL-12 and IL-10 after 24 h of culture with BALB/c splenocytes. All tested strains induced IL-12p40, although at very different levels (Fig. 1A). It appeared that L. paracasei NCC2461 was the strongest IL-12p40 inducer (>160 pg/ml at 107 CFU/ml). Surprisingly, we observed that all LAB strains also induced substantial IL-10 synthesis (Fig. 1B), albeit somewhat lower levels than those of IL-12p40. L. paracasei strain NCC2461 and L. casei strain GG induced the largest amounts of IL-10 (>60 pg/ml at 107 CFU/ml). With these bacteria, detectable amounts of IL-10 were still measured at 106 and 105 CFU/ml whereas no or low levels of IL-10 were measured at these doses with the other strains. Cultures containing 1 μg of LPS per ml produced on average 520 ± 100 pg of IL-10 per ml in their supernatants, while the IL-12p40 level remained low to undetectable (<5 pg/ml).
FIG. 1.
Induction of IL-12p40 (A) and IL-10 (B) by various strains of lactobacilli. Among the bacterial strains used were L. johnsonii NCC533, L. acidophilus NCC90, L. gasseri NCC2493, L. paracasei NCC2461, and two strains of L. casei, Shirota and GG. Bacteria were added to spleen cultures at concentrations of 107 CFU/ml (black boxes), 106 CFU/ml (grey boxes), or 105 CFU/ml (white boxes). Error bars indicate the SEM of triplicate cultures.
Because IL-12 and IL-10 have mutually antagonistic functions (5, 8, 29), we were interested in determining whether, among the LAB strains tested, those inducing high levels of IL-10 would be poor IL-12 inducers and vice versa. This appeared not to be the case, since L. paracasei NCC2461 triggered the highest production of both IL-12 and IL-10 whereas L. casei Shirota, a weak IL-10 inducer, was also a poor IL-12 inducer. Therefore, there did not appear to be a pattern of reciprocal IL-12 and IL-10 induction among the LAB strains tested but, rather, a parallel induction of both cytokines.
A similar pattern of IL-12 and IL-10 synthesis in response to these various LAB strains was found in spleen cells from C57BL/6 mice (data not shown), suggesting that induction of IL-12 and IL-10 by LAB was not dependent on the genetic background of the accessory cells.
L. paracasei NCC2461 inhibits CD4+ T-cell proliferation.
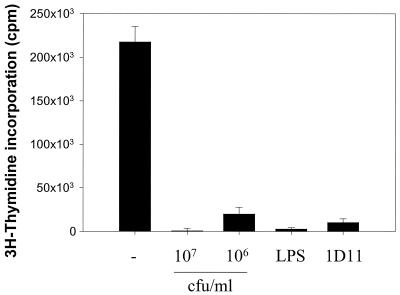
Because L. paracasei NCC2461 was the most effective inducer of IL-12 and IL-10, this strain was used throughout the following experiments. MLR of purified CD4+ T cells from naïve BALB/c mice were maintained by weekly restimulation with irradiated allogeneic splenocytes from C57BL/6 mice. During the 2 first weeks of the cultures, the cells were primed in medium alone or in the presence of various concentrations of L. paracasei NCC2461, 1 μg of LPS per ml, or 10 μg of blocking MAb against IL-4 per ml. LPS was used as a positive control for rapid induction of elevated amounts of IL-10 in these cultures. The anti-IL-4 MAb was added to block endogenous production of IL-4, which has been shown in another system to prevent the default differentiation of BALB/c CD4+ T cells toward the Th2 phenotype and to induce a switch to the Th1 phenotype (38). After the 2-weekly priming cultures, CD4+ T cells were restimulated one last time with irradiated allogeneic splenocytes in medium alone and proliferation was measured. In contrast to cells primed in the presence of medium alone, which proliferated vigorously, we observed a marked (P < 0.05) inhibition of CD4+ T-cell proliferation when L. paracasei NCC2461 was added at 107 CFU/ml to the priming cultures (Fig. 2). This inhibition was still observed (P < 0.05) when the bacteria were added at 106 CFU/ml, but to a lesser degree. When LPS and anti IL-4 MAb were added to the priming cultures, cell proliferation was also strongly decreased (Fig. 2).
FIG. 2.
Inhibition of CD4+ T-cell proliferation by L. paracasei NCC2461. CD4+ T cells were primed in medium alone (−) or in the presence of L. paracasei NCC2461 (107 or 106 CFU/ml), LPS (1 μg/ml), or a blocking MAb against IL-4 (1D11; 10 μg/ml). Proliferation was measured 48 h after the third stimulation. Error bars indicate the SEM of triplicate cultures.
Since proliferation was low in wells containing L. paracasei NCC2461, LPS, and anti-IL-4 MAb, we examined the viability of the cells grown under these conditions. After 4 and 7 days following the second stimulation, the cells were stained with trypan blue to assess viability and with a CD4-specific MAb to quantify the proportion of CD4+ T cells by fluorescence-activated cell sorting. When cells were cultured in medium alone, the total number of cells decreased and the proportion of CD4+ T cells increased (Table 1). This showed that there was an expansion of CD4+ T cells concomitantly with a loss of viable irradiated splenocytes in these cultures. In wells containing L. paracasei NCC2461, LPS, and anti-IL-4 MAb, there was a minor but measurable increase in the relative proportion of CD4+ T cell in the wells. However, because the total number of cells tended to decrease, the actual number of CD4+ T cells increased only a little in these wells. This demonstrated that CD4+ T cells did expand somewhat after 2 weeks of culture in the presence of L. paracasei NCC2461 and that they were viable at this stage. Furthermore, 7 days after the second stimulation, virtually all (>95%) live CD4+ T cells in the different culture conditions displayed a memory phenotype (MEL-14lo CD44hi), suggesting that the bacteria did not prevent T-cell priming (data not shown).
Lastly, when L. paracasei NCC2461 was added to purified CD4+ T cells grown on plastic-bound anti-CD3 (i.e., in the absence of accessory cells) as described previously (9), there was no significant inhibition of cell proliferation (data not shown). This further shows that the bacteria were not toxic to growing CD4+ T cells and also implies that the suppressive effects of L. paracasei NCC2461 required the presence of accessory cells.
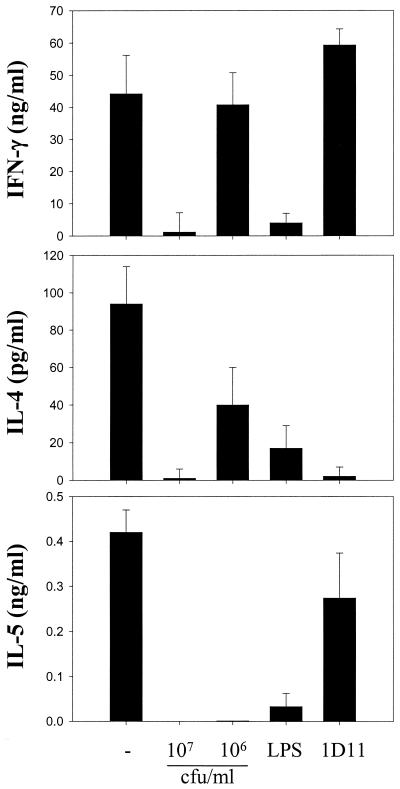
L. paracasei NCC2461 inhibits the secretion of Th1 and Th2 cytokines by CD4+ T cells.
Since L. paracasei NCC2461 inhibited CD4+ T cell proliferation, we wanted to investigate whether production of effector cytokines was also affected. Cytokine levels were measured 48 h after the third MLR restimulation. When cells were differentiated in medium alone, they secreted large amounts of effector cytokines, including IFN-γ, IL-4, and IL-5 (Fig. 3). When they were differentiated in the presence of 107 CFU of L. paracasei NCC2461 per ml, there was a sharp decrease in the amounts of these three cytokines. However, IFN-γ was produced at normal levels in the presence of 106 CFU of L. paracasei NCC2461 per ml, while IL-4 was partially suppressed (P < 0.05) and IL-5 remained undetectable. Thus, the presence of L. paracasei NCC2461 during the differentiation of CD4+ T cells had a negative impact on both Th1 and Th2 effector cytokines, but there appeared to be a dose threshold for the suppression of IFN-γ and IL-4 in this system. Addition of LPS during differentiation suppressed all three cytokines quite effectively (P < 0.05). Addition of the blocking anti-IL-4 MAb increased (P > 0.05) IFN-γ levels but strongly inhibited (P < 0.05) IL-4 and slightly suppressed (P > 0.05) IL-5 secretion in the restimulation cultures. Hence, LPS had an impact similar to the high concentrations of L. paracasei NCC2461 in these cultures whereas anti IL-4 antibodies exclusively decreased the secretion of Th2 cytokines.
FIG. 3.
Inhibition of Th1 and Th2 effector cytokines by L. paracasei NCC2461 in MLR. CD4+ T cells were primed in medium alone (−) or in the presence of L. paracasei NCC2461 (107 or 106 CFU/ml), LPS (1 μg/ml), or a blocking anti-IL-4 MAb (1D11; 10 μg/ml). Cytokine levels were measured 48 h after the third stimulation. Error bars indicate the SEM of triplicate cultures.
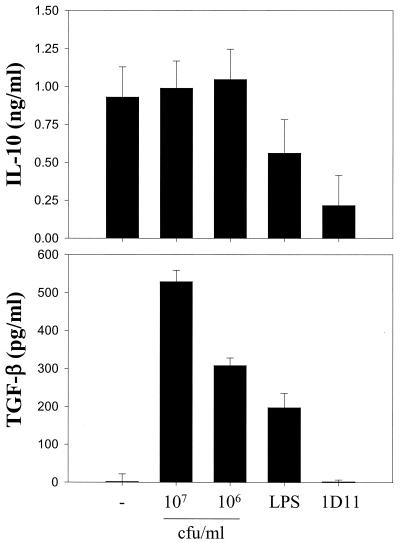
L. paracasei NCC2461 maintains IL-10 and induces TGF-β secretion by CD4+ T cells.
To determine whether the inhibitory properties of L. paracasei NCC2461 could be due to the concomitant induction of a suppressive phenotype, we measured the production of IL-10 and TGF-β in these cultures. When CD4+ T cells were differentiated in medium alone, they secreted high levels of IL-10 but no or low levels of TGF-β (Fig. 4). Unlike all other cytokines tested (Fig. 3), the addition of 107 CFU of L. paracasei NCC2461 per ml had no measurable inhibitory impact on IL-10 secretion (Fig. 4). In addition, there was a dose-dependent induction of TGF-β. Addition of LPS partially (P > 0.05) suppressed IL-10 secretion and induced intermediate levels of TGF-β in these cultures. Anti-IL-4 MAbs markedly inhibited IL-10 secretion (P < 0.05) but had no impact on TGF-β production. Hence, these data show that after 2 weeks of allogeneic splenocyte stimulation in the presence of L. paracasei NCC2461, effector CD4+ T cells produced predominantly IL-10 and TGF-β while cells stimulated in medium alone produced IFN-γ, IL-4, IL-5, and IL-10.
FIG. 4.
L. paracasei NCC2461 does not suppress IL-10 and induces TGF-β production by CD4+ T cells in MLR. CD4+ T cells were primed in medium alone (−) or in the presence of L. paracasei NCC2461 (107 or 106 CFU/ml), LPS (1 μg/ml), or a blocking anti-IL-4 MAb (1D11; 10 μg/ml). Cytokine levels were measured 48 h after the third stimulation. Error bars indicate the SEM of triplicate cultures.
DISCUSSION
Most reported studies on cytokine modulation by LAB in vitro have focused on the induction of modulatory cytokines by innate components of the immune system (15, 16, 20, 23–25, 33). It is well known that innate immunity plays a crucial role in determining the type of adaptive immune response that is generated consequently (reviewed in reference 31). We therefore asked how the induction of those innate cytokines by LAB could impact on the subsequent development of CD4+ T-cell functions in vitro. To study the impact of LAB antigens on CD4+ T-cell priming and differentiation into effector cells, we have used a previously described system of MLR (17, 18). In these cultures, CD4+ T cells from naive BALB/c mice were stimulated repeatedly on a weekly basis with allogeneic splenocytes. In a different system that used naive TCR transgenic CD4+ T cells primed and restimulated with the specific antigen over long periods, BALB/c CD4+ T cells were reported to default toward a dominant Th2 phenotype if no exogenous factor was provided in the priming cultures (21). Because several LAB species were shown to induce innate IL-12 in macrophages (20, 23, 33), our original aim was to see whether certain strains of LAB could thereby antagonize the differentiation of BALB/c CD4+ T cells toward their default Th2 phenotype and, instead, induce a switch toward a dominant Th1 response.
In our hands, both Th1 and Th2 cytokines were produced at high levels by CD4+ T cells from BALB/c mice stimulated by allogeneic splenocytes under neutral conditions. This could have been because splenic CD4+ T cells from naive mice contained a minor (<5%) population that displayed a memory (MEL-14lo CD44hi) phenotype. In previous studies showing a default toward Th2 in the BALB/c background, this subpopulation had been sorted out (21). Surprisingly, L. paracasei (strain NCC2461) inhibited the production not only of Th1 cytokines but also of Th2 cytokines. This suppression correlated with a rapid (probably innate) induction of the suppressive cytokine IL-10 in murine splenic cells and with the emergence of a CD4+ T-cell phenotype characterized by the production of IL-10 and TGF-β and low proliferative capacity. Because we have seen that LPS induced high levels of innate IL-10 in murine splenocytes, we have used LPS in the MLR cultures as a positive control for high levels of endogenous IL-10 and suppression of Th proliferation. Indeed, addition of LPS to the priming cultures had effects similar to those elicited by L. paracasei NCC2461 given at high concentrations (107 CFU/ml), i.e., a strong suppression of effector cytokines and of proliferation. Addition of a blocking anti-IL-4 MAb also inhibited CD4+ T-cell proliferation and Th2 cytokines (including IL-10) but did not impair the Th1 response and did not induce TGF-β in the MLR cultures. Thus, as expected from other systems showing that IL-4 is a key cytokine for driving Th2 differentiation (38), blocking endogenous IL-4 favored the switch to a dominant Th1 response in these cultures. Altogether, the data showed that L. paracasei NCC2461 inhibited the function of BALB/c CD4+ T cells and that this inhibition was not due to a switch to a Th1 phenotype but, rather, to a general suppressed state resembling anergy that coincided with a decreased proliferative capacity and the production of suppressive cytokines.
Previous studies have suggested that gram-positive bacteria tend to preferentially induce IL-12 whereas gram-negative bacteria predominantly induce IL-10 in macrophages (19, 20). We were surprised to detect substantial production of IL-10 in splenocytes cultured with LAB. IL-10 is a suppressive cytokine that inhibits IL-12 production by macrophages and Th1 functions induced by IL-12 and IFN-γ (5, 8). Therefore, it appears that LAB, in particular L. paracasei NCC2461, induced immunoregulatory cytokines which may have opposite effects on Th1 responses. Although the innate induction by L. paracasei NCC2461 of both IL-12 and IL-10 seems difficult to reconcile, it is possible that the residual dominant effect of either cytokine on CD4+ T-cell differentiation may depend on the genetic background of the responding T cells. Indeed, earlier studies have shown that Th1 responsiveness to IL-12 depends on the expression of its receptor β2 chain, which is switched off upon Th2 differentiation (50). Accordingly, the IL-12 receptor β2 chain is regulated differently in various genetic backgrounds (13). The experiments described in this paper were performed with CD4+ T cells from BALB/c mice, a mouse strain that rapidly loses surface expression of IL-12 receptor β2 chain after priming (13). Interestingly, the attenuation of IL-12 receptor expression has been reported to require the presence of TGF-β (10), a cytokine that was markedly induced in our cultures containing L. paracasei NCC2461. Therefore, our current hypothesis is that the early induction of IL-12 by L. paracasei NCC2461 in accessory cells prevented the genetically programmed Th2 differentiation of naive BALB/c T cells and, instead, induced an early differentiation toward a Th1 phenotype. However, this early Th1 response was subsequently downregulated by endogenous IL-10 (which blocked further IL-12 secretion) and TGF-β (which downregulated IL-12 responsiveness) in a synergistic manner.
In line with this hypothesis, we observed that the suppression of IFN-γ and IL-4 was completely and partially lost, respectively, when lower concentrations (106 CFU/ml) of bacteria were added to the cultures, suggesting a threshold in the suppressive effects of the bacteria. This was perhaps because less TGF-β was produced under these conditions and further strengthens the idea that TGF-β may play a key role in the suppressive effects of L. paracasei NCC2461. It should be noted, however, that LPS also strongly suppressed proliferation and IFN-γ production, although it induced less TGF-β than did 106 CFU/ml of bacteria. However, LPS induced far stronger production of IL-10 in primary cultures. It therefore remains likely that LPS and L. paracasei NCC2461 elicited their suppressive effects by different mechanisms. Addition of blocking antibodies to IL-10 and/or TGF-β in the priming cultures may resolve the relative importance of these two cytokines in the suppressive effects elicited by the bacteria or by LPS.
To our knowledge, there have been no previous in vitro data demonstrating inhibition of Th1 cytokines by LAB in developing CD4+ T cells. Instead, LAB have been shown to promote Th1 functions (3, 19, 20, 23) and decrease Th2 responses (30, 37, 49) in numerous studies. A few studies have nevertheless reported induction by LAB of IL-10 (16, 34) and suppression of Th1-related immunopathologies, such as inflammatory bowel disease (27, 48). Inflammatory bowel disease had been associated with elevated levels of Th1 cytokines in the colon (44) and by a dysregulation in the maintenance of homeostasis by IL-10 (1) and TGF-β (43). Experiments in animal models have shown that the endogenous bacterial flora may contribute to the disease (28, 45), but its interaction with pathogenic T cells is not understood. It has nonetheless been suggested that the disease was a consequence of a loss of T-cell tolerance to resident bacteria (6) and that the balance between IL-12 and IL-10 played a key role in this process (7). Recently, it was shown that activation of CD4+ T cells in the presence of IL-10 contributed to their differentiation toward a novel phenotype of cells, named Tr1, that produced high levels of IL-10 and TGF-β (12). The same study showed that Tr1 cells could prevent the onset of colitis in SCID mice containing CD45RBhi cells (12), probably by the induction of an anergic state in pathogenic Th1 cells (11). The existence and specificity of these Tr1 regulatory cells in vivo is not known, but it is conceivable that their activity may be regulated by components of the enteric bacterial flora. This paper reports that components from a LAB strain induce a Tr1-like population that produces substantial levels of TGF-β and IL-10 and provides evidence for the mechanism by which probiotic bacteriotherapy may prevent intestinal inflammation. This question deserves to be addressed in vivo using mouse models of colitis.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Kim-Yen Saudan and the critical reading of the manuscript by Nabila Ibnou-Zekri, Dirk Haller, and Pierre Guesry.
REFERENCES
- 1.Asseman C, Mauze S, Leach M W, Coffman R L, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Kuchroo V K, Inobe J, Hafler D A, Weiner H L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 3.Cleveland M G, Gorham J D, Murphy T L, Tuomanen E, Murphy K M. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffman R L, von der Weid T. Multiple pathways for the initiation of T helper 2 (Th2) responses. J Exp Med. 1997;185:373–375. doi: 10.1084/jem.185.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer Z B K. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duchmann R, Schmitt E, Knolle P, Meyer Z B K, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 8.Fiorentino D F, Zlotnik A, Vieira P, Mosmann T R, Howard M, Moore K W, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 9.Gollob K J, Coffman R L. A minority subpopulation of CD4+ T cells directs the development of naive CD4+ T cells into IL-4-secreting cells. J Immunol. 1994;152:5180–5188. [PubMed] [Google Scholar]
- 10.Gorham J D, Guler M L, Fenoglio D, Gubler U, Murphy K M. Low dose TGF-beta attenuates IL-12 responsiveness in murine Th cells. J Immunol. 1998;161:1664–1670. [PubMed] [Google Scholar]
- 11.Groux H, Bigler M, de Vries J E, Roncarolo M G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries J E, Roncarolo M G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 13.Guler M L, Jacobson N G, Gubler U, Murphy K M. T cell genetic background determines maintenance of IL-12 signaling: effects on BALB/c and B10.D2 T helper cell type 1 phenotype development. J Immunol. 1997;159:1767–1774. [PubMed] [Google Scholar]
- 14.Haller D, Blum S, Bode C, Hammes W P, Schiffrin E J. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro: evidence of NK cells as primary targets. Infect Immun. 2000;68:752–759. doi: 10.1128/iai.68.2.752-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haller D, Bode C, Hammes W P. Cytokine secretion by stimulated monocytes depends on the growth phase and heat treatment of bacteria: a comparative study between lactic acid bacteria and invasive pathogens. Microbiol Immunol. 1999;43:925–935. doi: 10.1111/j.1348-0421.1999.tb03353.x. [DOI] [PubMed] [Google Scholar]
- 16.Haller D, Bode C, Hammes W P, Pfeifer A M, Schiffrin E J, Blum S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut. 2000;47:79–87. doi: 10.1136/gut.47.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayakawa K, Hardy R R. Murine CD4+ T cell subsets defined. J Exp Med. 1988;168:1825–1838. doi: 10.1084/jem.168.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayakawa K, Hardy R R. Phenotypic and functional alteration of CD4+ T cells after antigen stimulation. Resolution of two populations of memory T cells that both secrete interleukin 4. J Exp Med. 1989b;169:2245–2250. doi: 10.1084/jem.169.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hessle C, Andersson B, Wold A E. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect Immun. 2000;68:3581–3586. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hessle C, Hanson L A, Wold A E. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin Exp Immunol. 1999;116:276–282. doi: 10.1046/j.1365-2249.1999.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh C S, Macatonia S E, O'Garra A, Murphy K M. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 23.Kato I, Tanaka K, Yokokura T. Lactic acid bacterium potently induces the production of interleukin-12 and interferon-gamma by mouse splenocytes. Int J Immunopharmacol. 1999;21:121–131. doi: 10.1016/s0192-0561(98)00072-1. [DOI] [PubMed] [Google Scholar]
- 24.Kato I, Yokokura T, Mutai M. Macrophage activation by Lactobacillus casei in mice. Microbiol Immunol. 1983;27:611–618. doi: 10.1111/j.1348-0421.1983.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 25.Kato I, Yokokura T, Mutai M. Augmentation of mouse natural killer cell activity by Lactobacillus casei and its surface antigens. Microbiol Immunol. 1984;28:209–217. doi: 10.1111/j.1348-0421.1984.tb00672.x. [DOI] [PubMed] [Google Scholar]
- 26.Macatonia S E, Hosken N A, Litton M, Vieira P, Hsieh C S, Culpepper J A, Wysocka M, Trinchieri G, Murphy K M, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 27.Madsen K L, Doyle J S, Jewell L D, Tavernini M M, Fedorak R N. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 28.Madsen K L, Doyle J S, Tavernini M M, Jewell L D, Rennie R P, Fedorak R N. Antibiotic therapy attenuates colitis in interleukin 10 gene-deficient mice. Gastroenterology. 2000;118:1094–1105. doi: 10.1016/s0016-5085(00)70362-3. [DOI] [PubMed] [Google Scholar]
- 29.Marshall J D, Secrist H, DeKruyff R H, Wolf S F, Umetsu D T. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+ T lymphocytes. J Immunol. 1995;155:111–117. [PubMed] [Google Scholar]
- 30.Matsuzaki T, Yamazaki R, Hashimoto S, Yokokura T. The effect of oral feeding of Lactobacillus casei strain Shirota on immunoglobulin E production in mice. J Dairy Sci. 1998;81:48–53. doi: 10.3168/jds.S0022-0302(98)75549-3. [DOI] [PubMed] [Google Scholar]
- 31.Medzhitov R, Janeway C A J. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 32.Meydani S N, Ha W K. Immunologic effects of yogurt. Am J Clin Nutr. 2000;71:861–872. doi: 10.1093/ajcn/71.4.861. [DOI] [PubMed] [Google Scholar]
- 33.Miettinen M, Matikainen S, Vuopio-Varkila J, Pirhonen J, Varkila K, Kurimoto M, Julkunen I. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058–6062. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miettinen M, Vuopio-Varkila J, Varkila K. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect Immun. 1996;64:5403–5405. doi: 10.1128/iai.64.12.5403-5405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 36.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 37.Murosaki S, Yamamoto Y, Ito K, Inokuchi T, Kusaka H, Ikeda H, Yoshikai Y. Heat-killed Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice. J Allergy Clin Immunol. 1998;102:57–64. doi: 10.1016/s0091-6749(98)70055-7. [DOI] [PubMed] [Google Scholar]
- 38.O'Garra A, Macatonia S E, Hsieh C S, Murphy K M. Regulatory role of IL4 and other cytokines in T helper cell development in an alpha beta TCR transgenic mouse system. Res Immunol. 1993;144:620–625. doi: 10.1016/s0923-2494(05)80014-8. [DOI] [PubMed] [Google Scholar]
- 39.Perdigon G, Alvarez S, Pesce D R H. Immunoadjuvant activity of oral Lactobacillus casei: influence of dose on the secretory immune response and protective capacity in intestinal infections. J Dairy Res. 1991;58:485–496. doi: 10.1017/s0022029900030090. [DOI] [PubMed] [Google Scholar]
- 40.Perdigon G, Alvarez S, Rachid M, Aguero G, Gobbato N. Immune system stimulation by probiotics. J Dairy Sci. 1995;78:1597–1606. doi: 10.3168/jds.S0022-0302(95)76784-4. [DOI] [PubMed] [Google Scholar]
- 41.Perdigon G, de Macias M E, Alvarez S, Oliver G, de Ruiz H. Effect of perorally administered lactobacilli on macrophage activation in mice. Infect Immun. 1986;53:404–410. doi: 10.1128/iai.53.2.404-410.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perdigon G, de Macias M E, Alvarez S, Oliver G, de Ruiz H. Systemic augmentation of the immune response in mice by feeding fermented milks with Lactobacillus casei and Lactobacillus acidophilus. Immunology. 1988;63:17–23. [PMC free article] [PubMed] [Google Scholar]
- 43.Powrie F, Carlino J, Leach M W, Mauze S, Coffman R L. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powrie F, Leach M W, Mauze S, Menon S, Caddle L B, Coffman R L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 45.Sartor R B. The influence of normal microbial flora on the development of chronic mucosal inflammation. Res Immunol. 1997;148:567–576. doi: 10.1016/s0923-2494(98)80151-x. [DOI] [PubMed] [Google Scholar]
- 46.Schiffrin E J, Brassart D, Servin A L, Rochat F, Donnet-Hughes A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: criteria for strain selection. Am J Clin Nutr. 1997;66:515S–520S. doi: 10.1093/ajcn/66.2.515S. [DOI] [PubMed] [Google Scholar]
- 47.Schiffrin E J, Rochat F, Link-Amster H, Aeschlimann J M, Donnet-Hughes A. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J Dairy Sci. 1995;78:491–497. doi: 10.3168/jds.S0022-0302(95)76659-0. [DOI] [PubMed] [Google Scholar]
- 48.Schultz M, Sartor R B. Probiotics and inflammatory bowel diseases. Am J Gastroenterol. 2000;95:S19–S21. doi: 10.1016/s0002-9270(99)00812-6. [DOI] [PubMed] [Google Scholar]
- 49.Shida K, Makino K, Morishita A, Takamizawa K, Hachimura S, Ametani A, Sato T, Kumagai Y, Habu S, Kaminogawa S. Lactobacillus casei inhibits antigen-induced IgE secretion through regulation of cytokine production in murine splenocyte cultures. Int Arch Allergy Immunol. 1998;115:278–287. doi: 10.1159/000069458. [DOI] [PubMed] [Google Scholar]
- 50.Szabo S J, Dighe A S, Gubler U, Murphy K M. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]