Abstract
An extracellular polysaccharide was purified from culture supernatants of Paenibacillus jamilae CP-7, a gram-positive bacillus that was isolated from compost prepared with olive mill wastewaters. The extracellular polysaccharide was produced under aerobic conditions in a medium containing olive mill wastewaters (80% [vol/vol]). This exopolymer had a low level of acute toxicity when it is administered to BALB/c mice by the intraperitoneal route. Interesting immunomodulatory effects were detected when mice were given 10 mg of exopolysaccharide per kg of body weight; the proliferative responses of splenocytes to B-cell and T-cell mitogens were suppressed, the in vitro levels of production of gamma interferon and granulocyte-macrophage colony-stimulating factor by unstimulated and lipopolysaccharide-stimulated splenocytes were enhanced, and the levels of resistance to the intracellular pathogen Listeria monocytogenes was increased in mice. Also, the exopolysaccharide was able to induce lymphocyte proliferation in vitro. We conclude that P. jamilae produces an exopolysaccharide with interesting immunomodulatory properties.
Microbial exopolysaccharides (EPSs) often show clearly identified properties that form the basis for a wide range of applications in food, pharmaceutical, petroleum, and other industries (32). Thus, several EPSs have been shown to possess immunological activities with potential pharmacological applications as biological response modifiers (BRMs). BRMs are agents that alter the normal immune response and whose mechanisms of action include induction of cytokines (29). Research on pharmacological applications of BRMs has led to development of both immunosuppressive and immunostimulating drugs that are effective in preventing the rejection of transplanted organs, for the treatment of some autoimmune diseases, as cancer immunotherapy, or as adjuvants for vaccine construction (14). Lentinan and other fungal glucans, yeast mannan fractions, and a number of bacterial EPSs have been identified as BRMs and have been found to have the ability to stimulate tumor rejection (for a review, see reference 40). In recent years, research has focused on the mechanisms of action of these compounds (12, 41), as well as on the discovery of new ones (7, 33).
Although polysaccharides are considered to be T-cell-independent antigens, a number of microbial EPSs are immunomodulators, with activities for T cells and macrophages (for a review, see reference 35). Polysaccharide A, a component of the capsular complex of Bacteroides fragilis, possesses mitogenic activity for T lymphocytes (6), and the production of interleukin-2 (IL-2) by CD4+ T cells appears to play an essential role in the in vivo immunomodulation by this EPS (37). A number of fungi and yeasts produce β-(1,3)-glucans with immunomodulatory properties (4, 35). Studies on the mechanisms of immunomodulation by a soluble derivative of β-(1,3)-glucan have shown that it has the ability to prime granulocytes and macrophages for enhanced cytokine release (30), reactive nitrogen intermediate production (11), and bactericidal capacity (39) in response to a secondary stimulus. In addition, this polymer modulates cytokine production by lymphocytes (31). Other levels of the immune response may be also affected by polysaccharides: mannuronan, an EPS from Pseudomonas aeruginosa, enhances natural cytotoxicity by increasing Fas ligand expression in NK cells (17). As a consequence of their BRM properties, a number of EPSs are able to induce resistance to bacterial infections in experimental models (20, 36), and some of them have been evaluated in clinical trials (3).
In the investigation described here, an EPS was purified from culture supernatants of a Paenibacillus jamilae strain growing on olive mill wastewaters under aerobic conditions, and the BRM properties of this EPS were evaluated.
MATERIALS AND METHODS
Production and isolation of EPSs.
Strain CP-7 was isolated from compost prepared with olive mill wastewaters and was identified as P. jamilae on the basis of phenotypic and phylogenetic analyses and DNA-DNA relatedness studies (1). The growth medium for seed cultures contained olive mill wastewaters (80% [vol/vol]), NH4Cl (0.1% [wt/vol]), and yeast extract (0.1% [wt/vol]); and the pH was adjusted to 7.0 before sterilization by autoclaving at 112°C. Bacteria were grown in this medium at 30°C for 48 h, and 10 ml of seed culture was transferred to a 300-ml preculture flask containing 90 ml of the same medium. The flasks were incubated on a shaker (120 rpm) at 30°C for 48 h, and each culture (100 ml) was used as an inoculum for 900 ml of a culture medium containing olive mill wastewaters (80% [vol/vol]) and NH4Cl (0.1% [wt/vol]). EPS was produced by bacteria during incubation at 30°C for 72 h in a 2.0-liter jar-fermentor (Biostat M; Braun-Biotech, Melsungen AG, Germany), with aereation provided by bubbling (850 ml/min) and agitation at 150 rpm. Bacterial cells were separated from the fermented broth by centrifugation, and the EPS present in the supernatant was precipitated by the addition of 2 volumes of cold ethanol. The precipitated material was collected by centrifugation, dissolved in distilled water, and dialyzed against distilled water. The EPS was purified by chromatography on a column of Sepharose CL-2B and elution with 50 mM phosphate (pH 7.0) containing 0.5 M NaCl. Carbohydrates and proteins were determined in the eluted fractions by the methods of Dubois et al. (13) and Bradford (5), respectively. The elution profile showed two EPS fractions with molecular masses of 500 kDa and >2,000 kDa, respectively. The light fraction showed a high carbohydrate/protein ratio and represented about 40% of the total EPS, whereas the heavy fraction contained only sugars and represented about 60% of the total EPS.
Mouse treatment.
Six- to 8-week-old female BALB/c mice were provided by Technical Services of the University of Granada (Granada, Spain). They were maintained under pathogen-free conditions. EPS was dispersed at the desired doses in pyrogen-free water, and each mouse received one injection (200 μl per 20 g of body weight) by the intraperitoneal route.
Mouse toxicity test.
Mice were weighed and injected intraperitoneally with pyrogen-free water (control group) and several EPS doses (treated groups). Following injection, the mice were observed and their body weights were recorded daily for 10 days. The mice were killed on day 10 after injection, and the spleens were removed and weighed. Splenic index was expressed as the spleen weight (in grams) per 20 g of body weight.
Spleen cell proliferation assay.
The spleens were removed aseptically and homogenized in Hanks' balanced salt solution (Sigma Chemical Co, St. Louis, Mo.). Splenocytes were sedimented by centrifugation; resuspended in red blood cell lysing buffer (Sigma) for 10 min; washed; and resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 50 μM 2-mercaptoethanol, penicillin G (100 U/ml), streptomycin (100 μg/ml), amphotericin B (0.25 μg/ml), 1 mM sodium pyruvate, and 2 mM l-glutamine (Sigma). Cell suspensions were distributed (5 × 105 viable cells per well) into 96-well tissue culture clusters with flat-bottom wells (Costar, Cambridge, Mass.). Salmonella enterica serovar Typhi lipopolysaccharide (LPS; Sigma) was used at 2.5 μg/ml as the B-cell mitogen, and concanavalin A (ConA; Sigma) was used at 1 μg/ml as the T-cell mitogen; these mitogen concentrations have been shown to induce optimum splenocyte proliferation under our assay conditions (18). After incubation at 37°C in 5% CO2 for 3 days, proliferation of spleen cells was measured by colorimetric reading of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reduction as described by Mosmann (23).
Cytokine assays.
Spleen cells were cultured with LPS or ConA as described above, and supernatants were removed after 24 h for determination of IL-2 levels and after 72 h for determination of gamma interferon (IFN-γ) and granulocyte-macrophage colony-stimulating factor (GM-CSF) levels. Supernatants were stored at −20°C until they were assayed. Cytokines were quantified by enzyme immunoassays (Endogen, Cambridge, Mass.); the concentrations of IL-2, IFN-γ, and GM-CSF were interpolated from the standard curve for the appropriate recombinant cytokine.
Challenge with Listeria monocytogenes.
A virulent isolate of L. monocytogenes was kindly provided by M. De La Rosa (Hospital Virgen de las Nieves, Granada, Spain). Bacteria were grown on blood agar at 37°C for 24 h, harvested in sterile phosphate-buffered saline (PBS) solution, washed twice, and resuspended in PBS. The bacteria were counted by using a Petroff-Hausser chamber, and the bacterial suspension was adjusted to inject 103 organisms into a mouse tail vein. The mice were observed daily, and deaths were recorded.
Statistical analysis.
The differences between the treated and the control groups were analyzed by using Student's t test. A P value of less than 0.05 was considered significant.
RESULTS
EPS production.
The culture conditions used in the present study were determined in previous work as appropriate to obtain the maximal level of EPS production (26). After 72 h of incubation, the EPS yield was 5.5 g/liter. EPS was obtained as a water-soluble, white powder.
EPS toxicity.
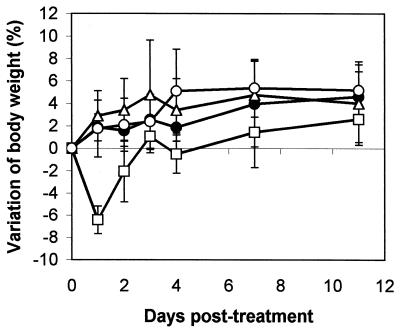
In the acute toxicity tests, no mortality was observed after intraperitoneal administration of 100, 10, and 1 mg of EPS per kg of body weight (data not shown). Mice that received 100 mg/kg showed a significant decrease in body weight at the first day after injection (P < 0.0001), but this effect dissapeared on the second day (Fig. 1). When these animals were killed at 10 days after injection, significant splenomegaly was observed. For EPS dosages of 0, 1, 10, and 100 mg/kg of body weight, splenic indices (measured on day 10 after treatment and expressed as spleen weight [in grams] per 20 g of body weight) were 0.1016 ± 0.01024, 0.1080 ± 0.00707, 0.1072 ± 0.00563, and 0.1254 ± 0.01024 (P < 0.01), respectively (the results represent the means ± standard deviations for five mice). Each mouse received a single injection by the intraperitoneal route. Mice in the control group (0 mg/kg) were given sterile water. The mean values of the splenic index for treated mice represented 123% of the splenic indices for untreated controls (P < 0.01). No variations in body weight or splenomegaly were observed in mice treated with EPS doses of 10 or 1 mg/kg. For in vivo immunomodulation studies, the dosage of 10 mg/kg was selected on the basis of the lack of adverse effects in toxicity tests.
FIG. 1.
Effect of EPS on the variation of body weight of BALB/c mice. Each mouse received a single dose of EPS by the intraperitoneal route on day 0. Data are expressed as the mean ± standard deviation for five mice. ●, no treatment; □, 100 mg of EPS/kg; ▵, 10 mg of EPS/kg; ○, 1 mg of EPS/kg.
Effect of treatment with EPS on mitogen-induced responses of splenocytes.
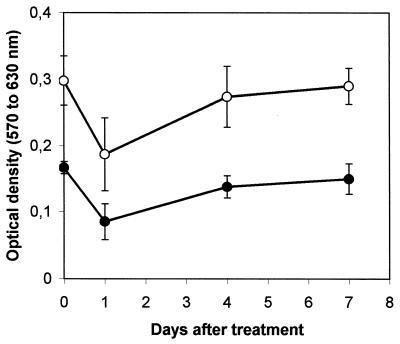
The capacity of splenocytes to proliferate in response to mitogens was assayed at days 1, 4, and 7 after single-dose EPS treatment. Results are shown in Fig. 2. EPS given 1 day before the assay suppressed 49% (P < 0.002) of the proliferation in response to LPS and 37% (P < 0.01) of the proliferation in response to ConA. Treatment of mice with EPS on day 4 before the assay was also suppressive: the response to LPS was decreased by 17% (P < 0.02), although the response to ConA was not significantly suppressed. The administration of EPS on day 7 before the test did not modify spleen cell proliferation in response to either mitogen.
FIG. 2.
Mitogen-induced proliferation of splenocytes from EPS-treated mice. Each mouse received a single intraperitoneal injection of EPS (10 mg per kg of body weight) on day 0. Results are means for five mice and are representative of two separate experiments. Error bars represent standard deviations. ●, LPS; ○, ConA.
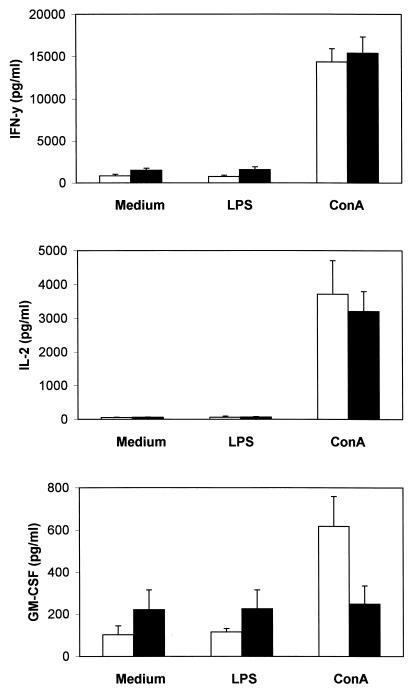
To determine whether the suppression of lymphoproliferative responses observed in cultures of spleen cells from EPS-treated mice was accompanied by changes in the production of cytokines, mice were killed on the first day after EPS administration, and the production of IL-2, IFN-γ, and GM-CSF by unstimulated and LPS- or ConA-stimulated splenocytes was measured in vitro. The results are shown in Fig. 3. In the absence of mitogenic stimuli, the basal levels of IFN-γ and GM-CSF in cultures of splenocytes from EPS-treated mice were higher than those in cultures of splenocytes from untreated controls (mean increases, 1.8- and 2.2-fold, respectively, with P values of <0.05 for both cytokines). LPS did not modify EPS-induced increases from basal levels of IFN-γ and GM-CSF production (mean increases, 2.1- and 2.0-fold, respectively, with P values of <0.05 for both cytokines). In contrast, ConA induced higher levels of GM-CSF secretion in untreated controls than in EPS-treated mice (P < 0.002). The production of IL-2 by unstimulated or mitogen-stimulated splenocytes was not affected by the treatment with EPS.
FIG. 3.
Cytokine production by splenocytes from EPS-treated mice. Each mouse received a single intraperitoneal injection of EPS (10 mg per kg of body weight) 1 day before the assay. Spleen cells from control mice (open bars) and EPS-treated mice (solid bars) were stimulated in vitro with LPS or ConA, and the cytokine levels in the supernatants were measured by specific immunoassays. Results are means for five mice and are representative of two separate experiments. Error bars represent standard deviations. IFN-y, IFN-γ.
Protective effect of EPS against experimental infection with L. monocytogenes.
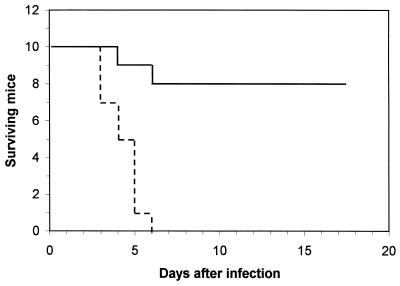
To determine wether EPS-induced modifications in splenocyte responsiveness could affect cell-mediated immunity and resistance against intracellular pathogens, mice were challenged with a lethal inoculum of L. monocytogenes on day 1 after EPS treatment. This bacterium causes a systemic infection in mice, and both activated T cells and macrophages are required to overcome infection (38). As shown in Fig. 4, 80% of the EPS-treated animals survived a challenge by the intravenous route with a dose of L. monocytogenes that was lethal for 100% of the control mice.
FIG. 4.
Resistance of EPS-treated mice to L. monocytogenes. Control mice (broken line) and mice given 10 mg of EPS per kg of body weight (solid line) were challenged by the intravenous route with L. monocytogenes (103 organisms per mouse) on day 0 (1 day after EPS administration). The results presented here are from one of two experiments with similar results.
Lymphocyte-proliferating activity of EPS.
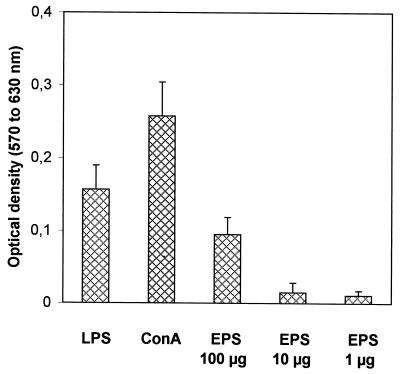
To investigate the in vitro effect of EPS on lymphocytes, spleen cells from untreated mice were incubated with a range of EPS concentrations: 100, 10, and 1 μg/ml. The lymphoproliferation assay was performed as decribed above. Results from experiments that compared the mitogenic abilities of LPS, ConA, and EPS are presented in Fig. 5. An EPS concentration of 100 μg/ml elicited a proliferative response representing 60 and 37% of those elicited by LPS (2.5 μg/ml) and ConA (1 μg/ml), respectively. Little proliferation was observed at low EPS concentrations.
FIG. 5.
Lymphoproliferative activity of EPS. Splenocytes from untreated mice were incubated with control mitogens (LPS and ConA) or EPS (100, 10, and 1 μg/ml). The results are the means for three mice (with eight replicate wells per mitogen and per mouse). Error bars represent standard deviations.
DISCUSSION
EPS can be produced by P. jamilae strain CP-7 in a medium containing olive oil wastewater (80%). Olive oil waste effluents are major pollulants in the Mediterranean area and represent a severe environmental problem because of its content of large amounts of phenolic compounds, which have antimicrobial and phytotoxic activities (9, 10, 15, 22). Ramos-Cormenzana et al. (27) suggested that olive mill wastewater could be used as an alternative, low-cost substrate for EPS production because of its high carbon/nitrogen ratio, which is similar to that of standard media used for polysaccharide production. In the study described here, an EPS with interesting BRM properties was purified from culture supernatants of strain CP-7 grown in an olive oil wastewater-based medium.
EPS had a low level of acute toxicity: no adverse effects were observed in mice given a single dose of 10 mg/kg by the intraperitoneal route. However, this treatment caused a marked immunomodulation: there was an inhibitory effect on the proliferative responses of splenocytes to B-cell and T-cell mitogens; the in vitro level of production of IFN-γ and GM-CSF by unstimulated and LPS-stimulated splenocytes was enhanced, and the ConA-induced level of production of GM-CSF was decreased. The EPS-induced immunomodulation resulted in a remarkable increase in the level of resistance to the intracellular pathogen L. monocytogenes in mice. The ability of EPS to induce lymphocyte proliferation in vitro confirms its immunomodulatory properties.
A global picture can be proposed to describe the BRM activity of EPS. IFN-γ-activated macrophages are central to the restriction of listerial replication (21). IFN-γ stimulates macrophages to produce reactive nitrogen intermediates (16), which, in turn, exert inhibitory effects on lymphocyte proliferation (2, 8). Thus, the increased ability of splenocytes from EPS-treated mice to produce IFN-γ is consistent with both the decrease in the level of splenocyte proliferation in response to mitogens and the increased level of resistance of mice to experimental listeriosis.
Several cell types may be involved in IFN-γ production: NK cells, γ/δ T cells, and Th1 cells (19). The in vitro level of production of IFN-γ by splenocytes from EPS-treated mice was not increased in cultures stimulated with ConA, suggesting that IFN-γ was not produced by T cells. Moreover, production of IL-2, which is a typical Th1 cytokine (24), was not affected by treatment with EPS. Thus, our results indirectly indicate that NK cells may be involved in the immunopotentiation by EPS, although further work is required to confirm this.
Regarding GM-CSF, T cells and activated macrophages are two known sources of this cytokine (28, 34). Since the levels of GM-CSF in supernatants of ConA-stimulated splenocytes were decreased by EPS treatment, it is likely that activated macrophages contributed to increased levels of this cytokine in unstimulated or LPS-stimulated cultures of splenocytes from EPS-treated mice. The central role of GM-CSF in the differentiation of precursor cells into antigen-presenting cells (25) could also be important in the immunomodulation by EPS.
In conclusion, our results demonstrate the production of EPS with BRM properties by P. jamilae. Additional studies on the chemical characterization of this EPS are in progress.
ACKNOWLEDGMENTS
This work was supported by grants OLI96-2189 from Plan Nacional de I + D and PB96-1403 from DGICYT (Ministerio de Educación y Cultura of Spain). V.G. received a grant from the Instituto de Cooperación Iberoamericana (Agencia Española de Cooperación Internacional of Spain).
REFERENCES
- 1.Aguilera, M., M. Monteoliva-Sanchez, A. Suarez, V. Guerra, C. Lizama, A. Bennasar, and A. Ramos-Cormenzana.Paenibacillus jamilae sp. nov., an exopolysaccharide-producing bacterium able to grow in olive-mill wastewater. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 2.Albina J E, Abate J A, Henry W L. Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. J Immunol. 1991;147:144–148. [PubMed] [Google Scholar]
- 3.Babineau T J, Marcello P, Swails W, Kenler A, Bistrian B, Forse R A. Randomized phase I/II trial of a macrophage-specific immunomodulator (PGG-glucan) in high-risk surgical patients. Ann Surg. 1994;220:601–609. doi: 10.1097/00000658-199411000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleicher P, Mackin W. Betafectin PGG-glucan: a novel carbohydrate immunomodulator with anti-infective properties. Annu Rev Pharmacol Toxicol. 1995;37:143–166. [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Brubaker J O, Li Q, Tzianabos A O, Kasper D L, Finberg R W. Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: differential stimulatory effect on mouse and rat lymphocytes in vitro. J Immunol. 1999;162:2235–2242. [PubMed] [Google Scholar]
- 7.Calazans G M T, Lopes C E, Lima R M O C, de França F P. Antitumor activities of levans produced by Zymomonas mobilis strains. Biotechnol Lett. 1997;19:19–21. [Google Scholar]
- 8.Candolfi E, Hunter C A, Remington J S. Roles of gamma interferon and other cytokines in suppression of the spleen cell proliferative response to concanavalin A and toxoplasma antigen during acute toxoplasmosis. Infect Immun. 1995;63:751–756. doi: 10.1128/iai.63.3.751-756.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capasso R. The chemistry, biotechnology, and ecotoxicology of the polyphenols naturally occurring in vegetable wastes. Curr Top Phytochem. 1997;1:145–156. [Google Scholar]
- 10.Capasso R, Evidente A, Shivo L, Orru G, Marcialis M A, Cristinzio G. Antibacterial polyphenols from olive oil mill wastewaters. J Appl Bacteriol. 1995;79:393–398. doi: 10.1111/j.1365-2672.1995.tb03153.x. [DOI] [PubMed] [Google Scholar]
- 11.Cleary J A, Kelly G E, Husband A J. The effect of molecular weight and beta-1,6-linkages on priming of macrophage function in mice by (1,3)-beta-d-glucan. Immunol Cell Biol. 1999;77:395–403. doi: 10.1046/j.1440-1711.1999.00848.x. [DOI] [PubMed] [Google Scholar]
- 12.Donmez C, Groves M J. Activity of mycobacterial antineoplastic glycan against human breast cancer. Anticancer Res. 1997;17:445–450. [PubMed] [Google Scholar]
- 13.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric methods for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 14.Georgiev V S. Immunomodulating drugs: major advances in research and development. Ann N Y Acad Sci. 1993;685:1–10. doi: 10.1111/j.1749-6632.1993.tb35844.x. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez M D, Moreno E, Quevedo-Sarmiento J, Ramos-Cormenzana A. Studies on antibacterial activity of waste waters from olive oil mills (alpechin): inhibitory activity of phenolic and fatty acids. Chemosphere. 1990;20:423–432. [Google Scholar]
- 16.Grazzinelli R T, Oswald I P, James S T, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ-activated macrophages. J Immunol. 1992;148:1792–1796. [PubMed] [Google Scholar]
- 17.Halaas O, Vik R, Espevik T. Induction of Fas ligand in murine bone marrow NK cells by bacterial polysaccharides. J Immunol. 1998;160:4330–4336. [PubMed] [Google Scholar]
- 18.Jimenez-Valera M, Sampedro A, Moreno E, Ruiz-Bravo A. Modification of immune response in mice by ciprofloxacin. Antimicrobi Agents Chemother. 1995;39:150–154. doi: 10.1128/aac.39.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann S H E. Immunity to intracellular bacteria. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Raven Press; 1993. pp. 1251–1286. [Google Scholar]
- 20.Kernodle D S, Gates H, Kaiser A B. Prophylactic anti-infective activity of poly-[1-6]-β-d-glucopyranosyl-[1-3]-β-d-glucopyranose glucan in a guinea pig model of staphylococcal wound infection. Antimicrob Agents Chemother. 1998;42:545–549. doi: 10.1128/aac.42.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladel C H, Blum C, Kaufmann S H E. Control of natural killer cell-mediated innate resistance against the intracellular pathogen Listeria monocytogenes by γ/δ T lymphocytes. Infect Immun. 1996;64:1744–1749. doi: 10.1128/iai.64.5.1744-1749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno E, Quevedo-Sarmiento J, Ramos-Cormenzana A. Antibacterial activity of waste waters of olive oil mills. In: Cheremisinoff P N, editor. Encyclopedia of environmental control technology. Vol. 4. Houston, Tex: Gulf Publishing; 1990. pp. 731–757. [Google Scholar]
- 23.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:56–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T R, Coffman R L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 25.Palucka K A, Taquet N, Sanchez-Chapuis F, Gluckman J C. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587–4595. [PubMed] [Google Scholar]
- 26.Ramos-Cormenzana A, Guerra V, Monteoliva-Sanchez M. Production of microbial polysaccharides in wastewaters from olive oil mills. In: Verachtert H, Verstraete W, editors. International symposium on environmental biotechnology, part II. Ostend, Belgium: Technologisch Instituut; 1997. pp. 259–262. [Google Scholar]
- 27.Ramos-Cormenzana A, Monteoliva-Sanchez M, Lopez M J. Bioremediation of alpechin. Int Biodeterion Biodegr. 1995;35:249–268. [Google Scholar]
- 28.Rao P, Falk L A, Dougherty S F, Sawada T, Pluznik D H. Colchicine down-regulates lipopolysaccharide-induced granulocyte-macrophage colony-stimulating factor production in murine macrophages. J Immunol. 1997;159:3531–3539. [PubMed] [Google Scholar]
- 29.Roth J A. Enhancement of nonspecific resistance to bacterial infection by biologic response modifiers. In: Roth J A, editor. Virulence mechanisms of bacterial pathogens. Washington, D.C.: American Society for Microbioogy; 1988. pp. 329–342. [Google Scholar]
- 30.Sherwood E R, Williams D L, McNamee R B, Jones E L, Browdwe I W, DiLuzio N R. Enhancement of interleukin-1 and interleukin-2 production of soluble glucan. Int J Immunopharmacol. 1987;9:261–267. doi: 10.1016/0192-0561(87)90049-x. [DOI] [PubMed] [Google Scholar]
- 31.Soltys J, Quinn M T. Modulation of endotoxin- and enterotoxin-induced cytokine release by in vivo treatment with β-(1,6)-branched β-(1,3)-glucan. Infect Immun. 1999;67:244–252. doi: 10.1128/iai.67.1.244-252.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutherland I W. Biotechnology of microbial exopolysaccharides. London, United Kingdom: Cambridge University Press; 1990. [Google Scholar]
- 33.Tian X X, Li A F, Zhou W C, Farrugia I V, Groves M J. Isolation and biological activities of an antineoplastic protein-polysaccharide complex (PS4A) obtained from Mycobacterium vaccae. Anticancer Res. 1999;19:237–243. [PubMed] [Google Scholar]
- 34.Tretter T, Aman M J, Bug G, Huber C, Peschel C. Hematopoietic growth factors are differentially regulated in monocytes and CD4(+) T lymphocytes: influence of IFN-alpha and interleukin-4. J Interferon Cytokine Res. 1998;18:95–102. doi: 10.1089/jir.1998.18.95. [DOI] [PubMed] [Google Scholar]
- 35.Tzianabos A O. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin Microbiol Rev. 2000;13:523–533. doi: 10.1128/cmr.13.4.523-533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzianabos A O, Gibson F C, Cisneros R L, Kasper D L. Protection against experimental intraabdominal sepsis by two polysaccharide immunomodulators. J Infect Dis. 1998;178:200–206. doi: 10.1086/515594. [DOI] [PubMed] [Google Scholar]
- 37.Tzianabos A O, Russell P R, Onderdonk A B, Gibson F C, Cywes C, Chan M, Finberg R W, Kasper D L. IL-2 mediates protection against abscess formation in an experimental model of sepsis. J Immunol. 1999;163:893–897. [PubMed] [Google Scholar]
- 38.Unanue E R. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 39.Wakshull E, Brunke-Reese D, Lindermuth J, Fisette L, Nathans R S, Crowley J J, Tufts J C, Zimmerman J, Mackin W, Adams D S. PGG-glucan, a soluble beta-(1,3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-kappa B-like factor in human PMN: evidence for a glycosphingolipid beta-(1,3)-glucan receptor. Immunopharmacology. 1999;41:89–107. doi: 10.1016/s0162-3109(98)00059-9. [DOI] [PubMed] [Google Scholar]
- 40.Whistler R L, Bushway A A, Singh P P, Nakahara W, Tokuzen R. Noncytotoxic, antitumor polysaccharides. Adv Carbohydr Chem Biochem. 1976;32:235–275. doi: 10.1016/s0065-2318(08)60338-8. [DOI] [PubMed] [Google Scholar]
- 41.Yan J, Vetvicka V, Xia Y, Coxon A, Carroll M C, Mayadas T N, Ross G D. Beta-glucan, a “specific” biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18) J Immunol. 1999;163:3045–3052. [PubMed] [Google Scholar]