Abstract
The Helicobacter pylori stool antigen enzyme immunoassay (HpSA) was evaluated during posttreatment follow-up of patients in a country with a very high prevalence of H. pylori infection. From among 273 dyspeptic individuals (18 to 55 years) initially recruited from a shantytown in Lima, Peru, 238 participants who met the inclusion criteria and were suspected to be H. pylori positive based on 14C urea breath test (UBT) results underwent endoscopy. Participants with endoscopy-proven infections received standard eradication therapy and were monitored by UBT and HpSA at 1 month following treatment and at 3-month intervals for 9 months posttreatment. A second endoscopy was performed if UBT results showed evidence of treatment failure or H. pylori recurrence. Biopsy results were considered the “gold standard” in all analyses. Among patients who underwent endoscopy, HpSA had a pretreatment sensitivity of 93%. Two-hundred thirty patients completed the treatment regimen, of whom 201 (93%) were considered to have had successful treatment outcomes based on a negative follow-up UBT. Thirty-two patients with UBT-defined treatment failures or H. pylori recurrences at any point during the 9-month follow-up underwent a second endoscopy. In the posttreatment setting, HpSA had an overall sensitivity of 73% and a specificity of 67%. Agreement between UBT and HpSA diminished throughout the follow-up. Among 14 participants in whom HpSA remained positive at 1 month following treatment despite UBT evidence of treatment success, 12 (86%) became HpSA negative within 3 months posttreatment. Although this study confirmed the validity of the HpSA in the initial assessment of dyspeptic patients, the test demonstrated a reduced overall accuracy in the detection of treatment failures and H. pylori recurrences during 9 months of posttreatment follow-up. Furthermore, in some patients it may take up to 3 months after successful eradication for antigen shedding to diminish to levels within the negative HpSA range.
Helicobacter pylori was first linked with gastritis in 1975 and was subsequently shown to be associated with peptic ulcer in 1985. Since then, the detection of the spiral bacterium in the gastric mucosa has become a principal aspect of the diagnostic investigation of patients with upper gastrointestinal symptoms suggestive of peptic ulcer disease (7). Chronic superficial gastritis due to infection with H. pylori is responsible for up to 95% of duodenal ulcers and 80% of gastric ulcers throughout the world (25). Perhaps the most convincing evidence for the pathogenic role of H. pylori is the dramatic effect of antibiotic therapy. Current treatment regimens combining at least two antibiotics and a proton pump inhibitor can eradicate H. pylori infections in greater than 90% of patients (21), and they significantly reduced ulcer recurrence in long-term follow-up studies (29). The organism has also been classified as a class I carcinogen due to its pathogenic role in intestinal-type gastric adenocarcinoma and has further been linked to diffuse-type gastric neoplasia and B-cell gastric maltoma. The relationship of H. pylori infection to nonulcer dyspepsia, however, remains controversial (7).
Although H. pylori infection affects populations globally, the prevalence varies widely between the developed world and developing regions (20, 23). In Peru, children are infected from a very young age, and over 50% are seropositive by age five (17). Consistent with the suggestion that major transmission risk factors are poor sanitation and overcrowding, infection rates of over 70% were found in even younger age groups in lower socioeconomic classes (10). Over 80% of Peruvian patients undergoing endoscopy to investigate symptoms of gastritis are infected with H. pylori (14, 16).
The diagnosis of H. pylori infection has traditionally relied upon upper endoscopy to obtain gastric biopsies for urease tests, histological preparation, and culture of the organism. These methods all have high sensitivities and specificities, yet the invasiveness and expense of direct observation of the organism have led to the search for valid and reliable noninvasive alternatives (30).
The urea breath test (UBT), based on the detection of exhaled 13C- or 14C-labeled carbon dioxide resulting from H. pylori urease activity, has both a sensitivity and a specificity of between 95 and 100% and has been the most widely used noninvasive test. In addition to being more cost-effective than endoscopy, the advantages of UBT include its ability to detect the current presence of H. pylori in the entire stomach and its usefulness in children (13C UBT) and posteradication patients for whom endoscopy is difficult to justify (30). Serologic tests, such as those based on enzyme-linked immunoassays, are the least expensive methods of detecting H. pylori in untreated patients but are not useful in follow-up of treated patients because antibody titers do not sufficiently decrease until 12 months after successful eradication (5).
The detection of H. pylori in feces raised the possibility of stool-based diagnostic assays (24). Several studies have recently shown that the Premier Platinum H. pylori stool antigen test kit (HpSA) (Meridian Diagnostics, Inc) is comparable to other noninvasive tests for initial diagnosis of H. pylori infection (2, 4, 11, 12, 13, 26, 28). For monitoring the efficacy of eradication treatment, however, the results have been equivocal (11, 13, 26, 27, 28). By comparing HpSA test results to endoscopy and UBT findings, we attempted to confirm the validity of HpSA for diagnosis and to establish the long-term posttreatment follow-up in patients in a developing country where there is a very high prevalence of H. pylori infection.
MATERIALS AND METHODS
Participants.
Individuals with symptoms of dyspepsia, age 18 to 55, were recruited by PRISMA staff field workers in Las Pampas de San Juan de Miraflores, a previously described pueblo joven (shantytown) on the outskirts of Lima, Peru (10). The presence of dyspepsia was defined by the patient's report of two or more specific symptoms as assessed by a dyspepsia questionnaire previously validated in Lima (O. Bisbal, R. León-Barua, R. Berendson, et al., personal communication). Exclusion criteria were the following: breast-feeding; positive pregnancy test at enrollment (Pregnosticon); unwillingness to practice birth control for the period of treatment; physical or mental impairment; serious concomitant illness (including human immunodeficiency virus infection or AIDS); another study participant in the same household; gastric cancer, active peptic ulcer disease, or Zollinger-Ellison syndrome; history of adverse reactions to any of the drugs used in the treatment regimen; or treatment with antibiotics, bismuth preparations, or antacids within the month prior to entering the study.
Participants signed written informed consent forms for all procedures. The protocol was approved by the institutional ethics committees of the Naval Medical Research Center Detachment, Lima, Peru (NAMRCD); Asociación Benéfica PRISMA, Lima, Peru; and The Johns Hopkins School of Hygiene and Public Health, Baltimore, Md.
Procedures.
All suitable volunteers provided a stool sample for HpSA and underwent 14C UBT using the PY test kit and a microCOUNT scintillation counter (Ballard Medical Products, Draper, Utah), conducted at the Department of Pathology, Cayetano Heredia Hospital. Quantification was reported as disintegrations per minute. Ranges used for analysis during all study periods were the following: <50 dpm was a negative or normal result, ≥50 but <200 dpm was an indeterminate result (which was excluded from analysis), and ≥200 dpm was a positive result. For both initial pretreatment evaluation and immediate follow-up assessment at 1 month posttreatment, patients with UBT values equal to or greater than 50 dpm were referred for endoscopy at the Hospital de Apoyo Maria Auxiliadora, the referral hospital for this population. At follow-up points past 1 month posttreatment, results in the predefined indeterminate UBT range generally correlate with negative biopsy findings (R. Gilman, unpublished results); therefore, only patients with a UBT result of ≥200 dpm were referred for a second endoscopy during this period.
Gastroendoscopy was performed to obtain 13 antral and corporeal gastric biopsies for culture (H. pylori-selective agar plates), histology (Giemsa, Warthin-Starry silver, and hematoxylin-eosin staining), and rapid urease testing (6, 17). All laboratory procedures were performed at Cayetano Heredia Hospital Department of Pathology and NAMRCD in Lima.
Patients with positive results for any of the three biopsy-based tests were considered to be infected with H. pylori and were treated with a combination of omeprazole (20 mg twice a day [BID]), clarithromycin (500 mg BID), and amoxicillin (1 g BID) for 14 days. Compliance was ensured by the administration of treatment under the direct supervision of study personnel.
At 1 month following completion of treatment, 14C UBT and HpSA tests were repeated. If the result of this posttreatment UBT was negative, the UBT was repeated 1 week later for confirmation. Participants with two consecutive negative UBT results (<50 dpm) were considered to have had a successful treatment course and were included in further surveillance. Those with equivocal or positive results were referred for a second endoscopy and excluded from further follow-up. Participants who remained H. pylori negative based on UBT were monitored every 3 months for 9 months posttreatment. At each visit, participants provided samples for UBT and HpSA. All patients referred for a second endoscopy based on a UBT suggestive of recurrence (i.e., a UBT result greater than or equal to 200 dpm) were excluded from further follow-up.
Diagnosis with HpSA.
Diluted stool samples were analyzed at NAMRCD using the HpSA enzyme immunoassay kit as per the instructions of the manufacturer (Meridian Diagnostics, Inc.). The kit employs affinity-purified polyclonal anti-H. pylori rabbit antibodies adsorbed to microwell plates. Following addition of peroxidase-coupled antibody and substrate, the color reaction was read using quantitative spectrophotometric determination (450 nm). Positive and negative control wells did not initially reveal spectrophotometric reading within expected ranges; the problem was corrected by rinsing the wells seven times instead of four times between steps, a modification recommended by the manufacturer. Some investigators have deemed HpSA optical density (OD) values above 0.160 to be positive, values between 0.140 and 0.159 to be indeterminate, and values below 0.140 to be negative (27). We considered values equal to or above 0.140 to be positive in accordance with a calculated optimal cutoff point (see below). H. pylori infection as defined by the endoscopic criteria described above was considered the “gold standard” diagnosis against which HpSA was compared.
Statistical analysis.
EpiInfo version 6.01 was used to calculate sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), with Fleiss quadratic 95% confidence intervals (95% CI). SPSS for Windows version 10.0 was used for the calculation of the Mann-Whitney U test, the plotting of the receiver operating characteristic curve, and the kappa statistic.
RESULTS
Initial diagnosis.
Two-hundred seventy-three individuals were initially recruited, of whom 252 (92%) were referred for further investigation based on an initial screening UBT result suggestive of possible infection (>50 dpm). Eight people refused endoscopy and withdrew from the study. Two hundred forty-four participants (72 men and 172 women) underwent initial endoscopic evaluation (mean age, 37 years; standard deviation, 8.7; range, 18 to 55). Six people found to have actively bleeding ulcers were referred for appropriate treatment and excluded from further analysis. Among the 238 included participants, 235 (99%) were found to be H. pylori positive and 3 (1%) were H. pylori negative based on biopsy findings as per the predefined study criteria (Table 1). Results for each gold standard test were the following: 198 (83%) positive by rapid urease test; 235 (99%) positive by Warthin-Starry silver stain; and 221 (93%) positive by culture. All participants classified as H. pylori positive had positive histological specimens using Warthin-Starry silver stain.
TABLE 1.
Comparison of UBT and HpSA results with analysis of biopsies from initial pretreatment endoscopy
| Test (n) and result | No. (%) of patients with the following biopsy resultsa:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive
|
Negative (U−, H−, C−) | ||||||||
| U+, H+, C+ | U+, H+, C− | U−, H+, C+b | U+, H−, C+ | U+, H−, C− | U−, H+, C− | U−, H−, C+ | Total | ||
| UBT (238) | |||||||||
| ≥200 dpm | 184 (98) | 11 (100) | 30 (88) | 0 | 0 | 2 (67) | 0 | 227 (97) | 0 |
| 50–200 dpm | 3 | 0 | 4 | 0 | 0 | 1 | 0 | 8 | 3 (100) |
| HpSA (235c) | |||||||||
| + | 172 (93) | 11 (100) | 31 (94) | 0 | 0 | 3 (100) | 0 | 217 (93) | 0 |
| − | 14 | 0 | 2 | 0 | 0 | 0 | 0 | 16 | 2 (100) |
U, rapid urease test; H, histology (Warthin-Starry silver stain); C, culture.
Includes two patients for whom rapid urease results were unavailable.
A diagnostic HpSA was not performed for three H. pylori-positive patients for whom biopsy results were available.
HpSA results were obtained for 235 (99%) of the 238 participants included in the analysis (Table 2). Median ODs determined by spectrophotometric HpSA readings were 0.342 and 0.120 for H. pylori-positive and -negative patients, respectively (P = 0.026 [Mann-Whitney U test]). By plotting a receiver operating characteristic curve of sensitivity versus (1 − specificity), the optimal OD cutoff point above which colorimetric readings could be considered to be positive was determined to be 0.14. The following measures of the validity of HpSA for initial diagnosis were calculated: sensitivity, 93% (217/233) (95% CI, 89 to 96%); PPV, 100% (217/217) (95% CI, 98 to 100%); and NPV, 11% (2/18) (95% CI, 2 to 36%). Results were identical if HpSA results in the range of 0.140 to 0.159 were deemed indeterminate and were excluded from analysis (data not shown).
TABLE 2.
Validity of HpSA results in patients with suspected treatment failures or recurrences posttreatment using biopsy as the gold standard
| Category | % Sensitivity [95% CI] | % Specificity [95% CI] |
|---|---|---|
| Treatment failures (1 mo posttreatment) | 86 [42–99] (6/7) | 33 [2–88] (1/3) |
| Recurrences (3, 6, or 9 mo posttreatment) | 68 [44–86] (13/19) | 100 [31–100] (3/3) |
| All treatment failures and recurrences (at any follow-up point up to 9 mo posttreatment) | 73 [52–88] (19/26) | 67 [24–94] (4/6) |
Posttreatment follow-up.
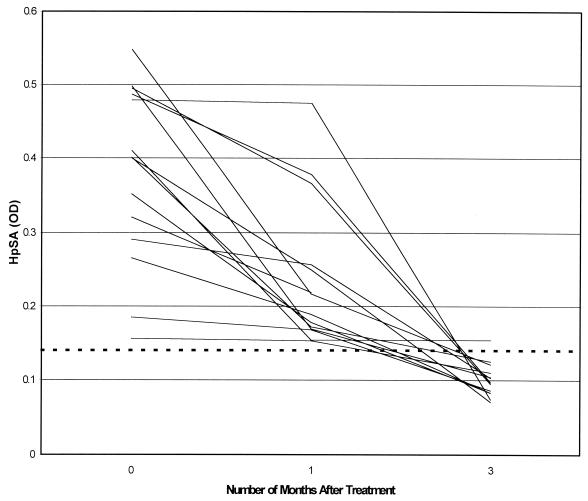
Two-hundred thirty participants completed the treatment regimen, of whom 201 (93%) were considered to have had successful treatment outcomes based on two consecutive negative UBT results (<50 dpm) at 4 and 5 weeks following completion of treatment. Of these 201 UBT-negative patients, 187 (93%) also had concordant negative HpSA results. Among the 14 participants (7%) in whom HpSA remained positive at 1 month following treatment despite UBT evidence of treatment success, 12 (86%) became HpSA negative 2 months later (Fig. 1).
FIG. 1.
Serial HpSA results for 14 patients with a positive HpSA result at 1 month following successful H. pylori eradication (based on UBT values of <50 dpm). The dashed line indicates the threshold above which HpSA results were considered to be positive (OD = 0.14). All patients but one were HpSA negative at 3 months of follow-up.
Participants underwent a second endoscopy only if there was UBT evidence of persistent or recurrent infection posttreatment. Fifteen participants (7%) had UBT results that suggested treatment failure, and 27 additional participants fulfilled the UBT criteria for recurrence at any point during 9 months following treatment completion. Thirty-five of the patients from both groups underwent a second endoscopy, and 32 of these patients also had an HpSA result for the time period when the endoscopy was performed. Using biopsy results as the gold standard, the sensitivity and specificity of HpSA in this posttreatment group were 73 and 67%, respectively (Table 2).
In order to assess the suitability of the criteria by which participants were referred for endoscopy in the pre- and posttreatment settings, we compared the biopsy findings and UBT values among those patients who underwent endoscopy at different stages of the study (Table 3). In the pretreatment setting, 8 of 11 participants (73%) with UBT results in the range from 50 to 200 dpm had positive biopsy findings, and 217 of 217 participants (100%) with UBT results above 200 dpm had positive biopsies. In contrast, 4 of 6 participants (67%) with UBT values in the range of 200 to 500 dpm in the posttreatment setting had negative biopsy results, while 27 of 29 participants (93%) with UBT results of >500 dpm had positive biopsies.
TABLE 3.
Comparison of biopsy results and corresponding UBT values for each study period.
| Study period (n) and biopsy result | No. (%) of patients with the following UBT result:
|
||
|---|---|---|---|
| >50 dpm, <200 dpm | ≥200 dpm, <500 dpm | ≥500 dpm | |
| Pretreatment (238) | |||
| Positive | 8 (73) | 17 (100) | 210 (100) |
| Negative | 3 (27) | 0 | 0 |
| 1 mo posttreatment (11) | |||
| Positive | 0 | 0 | 8 (89) |
| Negative | 0 | 2 (100) | 1 (11) |
| 3–9 mo posttreatment (24) | |||
| Positive | NAa | 2 (50) | 19 (95) |
| Negative | 2 (50) | 1 (5) | |
NA, not applicable
Agreement of HpSA with UBT.
HpSA was compared with 14C UBT when both test results were available from among all participants initially recruited at the beginning of the study, including those included for treatment and follow-up. Comparisons were made from the point of initial diagnosis up to 9 months following completion of treatment (Table 4). Participants for whom UBT results were equivocal (between 50 and 200 dpm) were excluded from analysis, as per the predefined study criteria. Agreement, based on the kappa statistic, decreased throughout the follow-up period.
TABLE 4.
Agreement between 14C UBT and HpSA for initial diagnosis and posttreatment follow-up
| Mo posttreatment (n) | Agreement (κa) between 14C UBT and HpSA | P |
|---|---|---|
| 0 (initial diagnosis) (256) | 0.58 | <0.001 |
| 1 (220) | 0.44 | <0.001 |
| 3 (178) | 0.27 | <0.001 |
| 6 (164) | 0.12 | 0.004 |
| 9 (121) | 0.04 | 0.49 |
κ, kappa statistic.
DISCUSSION
The validation of novel noninvasive and inexpensive techniques for the investigation of upper gastrointestinal symptoms has garnered considerable recent attention. The primary focus of the present study was to evaluate the performance of the HpSA during the long-term follow-up of patients treated for proven H. pylori infection. However, it was important to first confirm the accuracy of the test in the pretreatment setting. Because participants were preselected for initial endoscopic evaluation based on UBT results suggestive of infection, a very high frequency of H. pylori infection (99%) was predictably found among the participants for whom a gold standard diagnosis was available. Even before preselection, however, the prevalence of infection was 92% based on UBT. Employing biopsy results (rapid urease, histology, or culture) as the gold standard, the sensitivity of HpSA in this pre selected population prior to treatment was 93%, a finding within the range of previous studies (89 to 96%) (2, 4, 11, 12, 13, 26, 27, 28). It should be noted that all but 3 of the 235 patients considered to be H. pylori positive by our study criteria are also considered according to published recommendations requiring at least two positive biopsy-based tests or positive culture alone (8). The nature of the preselection process prevented the calculation of the specificity of HpSA, within reasonable confidence intervals, for initial diagnosis.
Although the 100% PPV in our preselected study population implies that patients with a positive HpSA result in combination with a positive UBT result are suitable candidates for empirical eradication therapy, investigators have cautioned against the complete avoidance of endoscopic investigation given the risk of missing important pathological findings (19). Furthermore, because there are widespread antimicrobial resistance, high recurrence rates of H. pylori, and a high incidence of gastric cancer in developing countries such as Peru, there is a stronger argument against the use of empiric antibacterial therapy as the primary means of preventing the long-term sequelae of H. pylori-induced gastritis (15).
Although the present study confirmed the sensitivity of the HpSA in the diagnosis of H. pylori infection, exploring its use in posttreatment follow-up may have greater clinical implications, since it is following eradication therapy that endoscopy becomes less justifiable. Consistent with some previous reports (2, 11, 13), we found the HpSA to perform less well in its ability to detect cases of treatment failure or recurrence at any point during follow-up (overall sensitivity of 73%). However, the test had been as sensitive at detecting the original infection in these particular patients (sensitivity, 92% [results not shown]) as in the group of successfully treated patients. Although there is significant antigenic variability of H. pylori within geographic regions (9), the importance of this observation with regard to this immunoassay is likely negligible. It is more likely that the test failed to detect a large number of recurrences because of a reduced quantity of antigen. Although some investigators have deemed HpSA to be the preferred noninvasive test for posttreatment monitoring (27), the present results demonstrate that the validity of HpSA is substantially reduced after treatment, even among patients monitored for up to 9 months posteradication. There is particular concern that the low sensitivity in this context implies that the test would lead to a considerable number of incorrect diagnoses of eradication.
A limitation of this study arose due to the use of UBT criteria for referring participants for endoscopy that differed between the initial and posttreatment evaluations and long-term follow-up evaluations. In the initial evaluation and immediately following treatment, UBT results of between 50 and 200 dpm prompted referral for endoscopy. In the pretreatment setting, it was found that results in this range were likely to correlate with positive endoscopy findings (Tables 1 and 3). In posttreatment follow-up between 3 and 9 months, endoscopy was performed if the UBT result was at least 200 dpm. We have found that in long-term follow-up, UBT results in the range of 50 to 200 dpm are likely to correlate with negative biopsy findings (data not presented), which was the justification for the criterion for referral. In the present study, participants with UBT results in the range of 200 to 500 dpm during follow-up were at least as likely to have negative results as positive results on endoscopy, suggesting that the original assumption (i.e., that in the lower UBT range of 50 to 200 dpm, most biopsies would be negative) was justified. The disadvantage is that this may have reduced the accuracy of the calculation of specificity of HpSA during follow-up, since it implies that participants who were likely to be biopsy negative were excluded from the posttreatment evaluation of HpSA.
In the follow-up of treated patients, 14C UBT and HpSA showed low to moderate agreement at 1 month following treatment (κ = 0.44; P < 0.001) but poor and diminishing agreement thereafter. Some investigators have suggested that 14C UBT should become a standard for treatment follow-up (1, 18, 22, 30); however, unlike for 13C UBT, definitive cutoff points for 14C UBT have yet to be widely accepted, and we have found the latter test to be unreliable in the context of treatment follow-up (unpublished results). Therefore, it cannot be assumed that the disagreement between the tests is necessarily a result of a diminishing accuracy of HpSA. Further evaluation of these two tests in long-term follow-up is needed to establish the reason for the discrepancies between them. It was especially notable, however, that among the patients deemed treatment successes by UBT but found to be HpSA positive (i.e., apparent false positives) at 1 month posttreatment follow-up, virtually all became HpSA negative by the 3-month follow-up interval. This observation supports the suggestion that in a small minority of successfully treated patients, 1 month following treatment may be too early to detect diminished stool antigen shedding following H. pylori eradication (3, 11). Of note, a higher OD limit for the positive range at 1 month posteradication would likely not reduce the number of false positives, since among the 14 false-positives we observed, the HpSA OD values were spread across a range from 0.156 to 0.548.
The advantages of the HpSA are its noninvasiveness and logistical ease of use. Relative disadvantages of the UBT are that it requires a scintillation counter for the analysis of 14C (or an isotope ratio mass spectrometer for 13C) and that patients may be hesitant to ingest radioactive test material (30).
With these results and those of previous studies (11, 13, 26, 27, 28), the accumulated data concerning the use of HpSA in evaluating treatment outcome remain unconvincing. The present study evaluated HpSA in longer-term surveillance and suggests a diminished validity of the test in follow-up during 9 months after treatment. However, the data have confirmed the validity of the HpSA as a diagnostic tool in the initial assessment of patients from a population with a very high prevalence of H. pylori infection.
ACKNOWLEDGMENTS
This study was partially funded by Oravax Co. as well as through the Fogarty Center at the NIH via the ITREID and FIRCA grants.
We are grateful to Meridian for the donation of the HpSA kits, and we thank Michel Cadoz, Aventis Pasteur, and March l'Etoile France. We also appreciate the assistance of Giselle Soto, J. B. Phu, and D. Sara. We are grateful to the community of the Pampas de San Juan de Miraflores for its participation. We thank Mariano Alarcon and Alberto Zolezzi, Gastroenterology Service, Hospital de Apoyo Maria Auxiliadora.
REFERENCES
- 1.Ahuja V, Bal C S, Sharma M P. Can the C-14 urea breath test replace follow-up endoscopic biopsies in patients treated for Helicobacter pylori infection? Clin Nuclear Med. 1998;23:815–819. doi: 10.1097/00003072-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Archimandritis A, Giontzis A, Smilakou A, Tzivras M, Davaris P. Diagnosis of Helicobacter pylori infection by HpSA test. Lancet. 1999;354:1210–1211. doi: 10.1016/S0140-6736(05)75420-9. [DOI] [PubMed] [Google Scholar]
- 3.Caselli M, Elisa Z, Trevisani L, Sartori S, Vittorio A. Diagnosis of Helicobacter pylori infection by HpSA test. Lancet. 1999;354:1209–1210. doi: 10.1016/S0140-6736(05)75418-0. [DOI] [PubMed] [Google Scholar]
- 4.Chang M C, Wu M S, Wang H H, Wang H P, Lin J T. Helicobacter pylori stool antigen (HpSA) test—a simple, accurate and non-invasive test for detection of Helicobacter pylori infection. Hepatogastroenterology. 1999;46:299–302. [PubMed] [Google Scholar]
- 5.Cutler A F, Prasad V H. Long-term follow-up of Helicobacter pylori serology after successful eradication. Am J Gastroenterol. 1996;31:85–88. [PubMed] [Google Scholar]
- 6.Gastrointestinal Physiology Working Group. Rapid identification of pyloric Campylobacter in Peruvians with gastritis. Dig Dis Sci. 1986;31:1089–1094. [PubMed] [Google Scholar]
- 7.Goodwin S C, Mendall M M, Northfield T C. Helicobacter pylori infection. Lancet. 1997;349:265–269. doi: 10.1016/S0140-6736(96)07023-7. [DOI] [PubMed] [Google Scholar]
- 8.Guidelines for Clinical Trials in Helicobacter pylori infection. Technical annex: tests used to assess Helicobacter pylori infection. Gut. 1997;41(3S):10S–18S. [PubMed] [Google Scholar]
- 9.Hook-Nikanne J, Perez-Perez G, Blaser M J. Antigenic characterization of Helicobacter pylori strains from different parts of the world. Clin Diagn Lab Immunol. 1997;4:592–597. doi: 10.1128/cdli.4.5.592-597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein P D, Gilman R H, Leon-Barua R, Diaz F, O'Brien Smith E, Graham D Y. The epidemiology of Helicobacter pylori in Peruvian children between 6 and 30 months of age. Am J Gastroenterol. 1994;89:2196–2200. [PubMed] [Google Scholar]
- 11.Makristathis A, Pasching E, Schutze K, Wimmer M, Rotter M L, Hirschi A M. Detection of Helicobacter pylori in stool specimens by PCR and antigen enzyme immunoassay. J Clin Microbiol. 1998;36:2772–2774. doi: 10.1128/jcm.36.9.2772-2774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamara D, Whelan H, Hamilton H, Beattie S, O'Morain C. HpSA: assessment of a new non-invasive diagnostic assay for Helicobacter pylori infection in an Irish population. Irish J Med Sci. 1999;168:111–113. doi: 10.1007/BF02946478. [DOI] [PubMed] [Google Scholar]
- 13.Plebani M, Basso D. Diagnosis of Helicobacter pylori infection by HpSA test. Lancet. 1999;354:1210. doi: 10.1016/S0140-6736(05)75419-2. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez-Ramos A, Gilman R H, Watanabe J, Recavarren A S, Spira W, Miyagui J, Rodriguez U C, Ramirez-Icaza C. Helicobacter pylori infection in long-term and short-term Japanese visitors to Peru. Lancet. 1994;244:1017–1018. doi: 10.1016/s0140-6736(94)91673-x. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez-Ramos A, Gilman R H, Leon-Barua R, Recavarren-Arce S, et al. Rapid Recurrence of Helicobacter pylori infection in Peruvian patients after successful eradication. Clin Infect Dis. 1997;25:1027–1031. doi: 10.1086/516083. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez-Ramos A, Gilman R H, Spira W, Recavarren S, Watanabe J, Leon-Barua R, et al. Ecology of Helicobacter pylori in Peru: infection rates in coastal, high-altitude, and jungle communities. Gut. 1992;33:604–605. doi: 10.1136/gut.33.5.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramirez-Ramos A, Leon-Barua R, Gilman R H, Recavarren A S, et al. Helicobacter pylori and gastritis in Peruvian patients: relationship to socioeconomic level, age, and sex. Am J Gastroenterol. 1990;86:819–823. [PubMed] [Google Scholar]
- 18.Rollan A, Giancapsero R, Arrese M, Figueroa C, Vollrath V, Schultz M, Duarte I, Vial P. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection after antibiotic treatment. Am J Gastroenterol. 1997;92:268–274. [PubMed] [Google Scholar]
- 19.Roseveare C D, Van Heel D A, Arthur M J P, Lawrance R J. Helicobacter pylori: beware “blind” eradication! Gut. 1998;42:757. doi: 10.1136/gut.42.5.757a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sack R B, Gyr K. Helicobacter pylori infections in the developing world. J Diarrhoeal Dis Res. 1994;12:144–145. [PubMed] [Google Scholar]
- 21.Salcedo J A, Al-Kawas F. Treatment of Helicobacter pylori infection. Arch Intern Med. 1998;158:842–851. doi: 10.1001/archinte.158.8.842. [DOI] [PubMed] [Google Scholar]
- 22.Sharma B C, Bhasin D K, Pathak C M, Sinha S K, Ray P, Vaiphei K, Singh K. [14C]-urea breath test to confirm eradication of Helicobacter pylori. J Gastroenterol Hepatol. 1999;14:309–312. doi: 10.1046/j.1440-1746.1999.01869.x. [DOI] [PubMed] [Google Scholar]
- 23.Taylor D N, Blaser M J. The epidemiology of Helicobacter pylori infection. Epidemiol Rev. 1991;13:42–59. doi: 10.1093/oxfordjournals.epirev.a036078. [DOI] [PubMed] [Google Scholar]
- 24.Thomas J E, Gibson G R, Darboe M K, Dale A, Weaver L T. Isolation of Helicobacter pylori from human feces. Lancet. 1992;342:1419–1420. doi: 10.1016/0140-6736(92)92894-l. [DOI] [PubMed] [Google Scholar]
- 25.Thomson M. Helicobacter pylori—the story so far. Br J Med. 1999;319:541. [PubMed] [Google Scholar]
- 26.Trevisani L, Sartori S, Galvani F, et al. Evaluation of a new enzyme immunoassay for detecting Helicobacter pylori in feces: a prospective pilot study Am. J Gastroenterol. 1999;94:1830–1833. doi: 10.1111/j.1572-0241.1999.01213.x. [DOI] [PubMed] [Google Scholar]
- 27.Vaira D P, Malfertheiner P, Megraud F, Axon A T R, Deltenre M, Gasbarrini G, O'Morain C, Pajares Garcia J M, Quina M, Tytgat G N J the European Helicobacter HpSA Study group. Noninvasive antigen-based assay for assessing Helicobacter pylori eradication. A European multicentre study. Am J Gastroenterol. 2000;95:925–929. doi: 10.1111/j.1572-0241.2000.01931.x. [DOI] [PubMed] [Google Scholar]
- 28.Vaira D, Malfertheiner P, Megraud F, Axon A T, Deltenre M, Hirschi A M, Gasbarrini G, O'Morain C, Garcia J M, Quina M, Tytgat G N. Diagnosis of Helicobacter pylori infection with a new non-invasive antigen-based assay. HpSA European study group. Lancet. 1999;354:30–33. doi: 10.1016/s0140-6736(98)08103-3. [DOI] [PubMed] [Google Scholar]
- 29.Walsh J H, Peterson W L. Drug therapy: the treatment of Helicobacter pylori infection in the management of peptic ulcer disease. N Engl J Med. 1995;333:948–991. doi: 10.1056/NEJM199510123331508. [DOI] [PubMed] [Google Scholar]
- 30.Westblom T U, Bhatt B D. Diagnosis of Helicobacter pylori infection. Curr Top Microbiol Immunol. 1999;241:215–235. doi: 10.1007/978-3-642-60013-5_11. [DOI] [PubMed] [Google Scholar]