Abstract
Acanthamoeba species can cause serious, debilitating, and sometimes life-threatening infections. Three groups have been identified using morphological and immunological comparisons. Previous serological studies have utilized a variety of antigen preparations and assay methods and reported disparate (3 to 100%) results. This study was designed to (i) optimize an enzyme-linked immunosorbent assay for detecting serum antibodies to each of the Acanthamoeba serogroups and (ii) test 55 healthy individuals for specific immunoglobulin G reactivity. The highest signal-to-background ratio was found when 3,000 fixed, intact trophozoites per well were used with a 1:10 serum dilution. Sera yielding optical densities of <0.25 against all three Acanthamoeba serogroups were used to define the cutoff for positive results. The highest background reactivity with these sera was seen with Acanthamoeba polyphaga (serogroup 2), followed by Acanthamoeba culbertsoni (serogroup 3) and Acanthamoeba astronyxis (serogroup 1). Of 55 subjects tested, the highest number of positive results was seen with A. polyphaga (81.8%), followed by A. astronyxis (52.8%) and A. culbertsoni (40%). Seven serum samples (12.7%) were negative for all three Acanthamoeba serogroups, 16 (29.1%) were positive for one serogroup only, 16 were positive for two serogroups, and 16 reacted to all three serogroups. Further analysis showed no significant associations between serogroup reactivity and age or gender. However, some ethnic differences were noted, especially with A. polyphaga antigens. In that case, serum samples from Hispanic subjects were 14.5 times less likely to be positive (P = 0.0025) and had lower mean absorbance values (P = 0.047) than those from Caucasian subjects. Overall, these data suggest that Acanthamoeba colonization or infection is more common than previously thought. Mild or asymptomatic infections may contribute to the observed serum reactivities.
Acanthamoebae are free-living protozoans found in the soil worldwide. Infection with Acanthamoeba spp. can cause serious disease with high morbidity and/or mortality (20). Central nervous system (CNS) infection is uniformly fatal within weeks to months. The organism appears to have a relatively low virulence, as evidenced by the rarity of the infection, and it is an opportunist in individuals compromised by human immunodeficiency virus infection, diabetes, immunosuppressive therapy, malignancies, malnutrition, or chronic alcoholism (19). In comparison, Acanthamoeba keratitis does not typically lead to CNS infection but has very significant morbidity, often requiring one or more successive corneal transplants or complete enucleation (16). Contact lens wearers are at higher risk of infection, especially where microabrasions are present (11). Skin infections have also been documented and may serve as the nidus for a hematogenous spread to the CNS (17). Likewise, Acanthamoeba has been found within alveoli of compromised patients with pneumonitis (18) and has been recovered from nasal and pharyngeal swabs from immunocompetent, asymptomatic individuals (1, 3, 15, 28); the latter suggests that transient respiratory infections may occur.
Taxonomic relationships among Acanthamoeba species are currently based on morphological and serological evidence (22, 27) and suggest the existence of three distinct groups. Morphological differences based on the cyst stage have been confirmed by immunological studies. Antibodies specific to trophozoites from various Acanthamoeba species have been generated and cross-tested. These data show high reactivity within a morphological group, but little to no reactivity between groups. Specifically, groups 2 and 3 show minor cross-reactivity, but neither shows cross-reactivity with group 1. These findings suggest that each Acanthamoeba group displays a unique set of antigens and would elicit a group-specific antibody in infected hosts, including humans. The ubiquitousness of the organism in soil and surface waters suggests that all humans are exposed to this potential pathogen. Further, mild or subclinical infections (skin or respiratory infections) may be self-limited and not diagnosed. If such infections occur, immune stimulation, including a serum antibody response, presumably ensues and should be detectable. Therefore, the finding of serum antibodies specific to Acanthamoeba would suggest previous exposure and/or colonization by this organism. Serum antibodies have been found in individuals with systemic Acanthamoeba infections (13) and in some patients with keratitis (7, 26).
Population studies of serum antibodies to Acanthamoeba are few in number (2, 6) and contradictory in their findings. Cursons et al. (6) studied sera from 80 persons from three New Zealand health clinics. Immunoglobulin reactivities in indirect fluorescence antibody assays using Acanthamoeba castellanii (serogroup 2) and Acanthamoeba culbertsoni (serogroup 3) trophozoites were judged to be uniformly positive, with titers of 1:20 or 1:40, respectively, although no definition of a positive reaction was provided. In another study (2), sera from 1,054 individuals were tested against A. culbertsoni using an indirect hemagglutination assay. Titers of 1:40 were considered positive. A positive reaction was found in 3.2 to 3.3% of 282 healthy individuals and 274 psychiatric patients. A higher seroprevalence was seen in 448 hospitalized patients (9.1% positive), especially among 94 diagnosed with liver and gall bladder diseases (17% positive). In response to this observation, 50 individuals from a hepatitis A outbreak were studied, and 52% were positive.
Neither of the seroprevalence studies provided methodological details or information on the definition of a positive result. Also, comparison of these studies is complicated by the fact that two different methods, indirect hemagglutination and indirect fluorescence antibody assays, were employed. The purposes of the present study were (i) to develop a well-characterized enzyme-linked immunosorbent assay (ELISA) for detecting serum antibodies to Acanthamoeba, (ii) to compare the immunoglobulin G (IgG) reactivities among representative species from each of the three Acanthamoeba serogroups, (iii) to define a negative population and cutoff values for a positive reaction, and (iv) to report the range of IgG reactivity in a healthy population.
MATERIALS AND METHODS
Study population.
Fifty-five healthy adults were recruited as part of an ongoing, unrelated study at the University of Texas School of Public Health (4, 8, 24) (Table 1). The study was approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston. These 55 individuals were in excellent general health, with no previous episodes of encephalitis, keratitis, or serious soft tissue infection. Study subjects had a median age of 28.4 years (mean, 30.7 years; range, 19 to 51 years). Females made up 56.4% of the population. Four ethnic groups were represented, with the majority (58.2%) being Caucasian.
TABLE 1.
Study populationa categorized for age, gender, and ethnic origin
| Characteristic | No. (%) with characteristic |
|---|---|
| Age (yr) | |
| <25 | 14 (25.5) |
| 25–29 | 19 (34.5) |
| 30–34 | 10 (18.1) |
| ≥35 | 12 (21.8) |
| Gender | |
| Female | 31 (56.4) |
| Male | 24 (43.6) |
| Ethnic origin | |
| Caucasian | 32 (58.2) |
| African-American | 10 (18.1) |
| Hispanic | 10 (18.1) |
| Asian | 3 (5.5) |
n = 55.
Serum collection.
After informed consent was obtained, blood was collected from volunteers, and serum was separated, aliquoted, and frozen at −86°C prior to use. Specimens were obtained from March 1992 to March 1999.
Antigen for developing hyperimmune antisera was prepared by adjusting amoebae to 2 × 105 cells per ml in 0.15 M phosphate-buffered saline (PBS) pH 7.2, and then subjecting them to four freeze-thaw cycles with liquid nitrogen. New Zealand White rabbits (weight, 2 kg) were immunized via the marginal ear vein weekly with 2 ml of the antigen preparation for a total of 4 weeks. One week after the final immunization, rabbits were bled and sera were collected. Negative-control sera were drawn from rabbits prior to inoculation with amoebae. These experiments were carried out previously by one of the authors (A.L.N.) at the Indiana University School of Medicine (Indianapolis). Sera were sent to Houston on dry ice and stored at −86°C before use.
Growth of Acanthamoeba trophozoites and ELISA antigen preparation.
Acanthamoeba astronyxis cultures were obtained from the American Type Culture Collection (ATCC 30137), and Acanthamoeba polyphaga was transferred to Houston from the laboratory of one of the authors (A.L.N.). The A. culbertsoni culture was a gift from Gene Siders (Indiana University). All of the amoeba species were grown in 15-ml conical tubes containing peptone-yeast extract-glucose medium (PYG) supplemented with 5% heat-inactivated fetal bovine serum plus minimal essential medium (MEM) vitamin mixture (1:2 dilution; Life Technologies-Gibco BRL, Rockville, Md.). Tubes containing A. astronyxis and A. polyphaga were incubated at room temperature, while A. culbertsoni required 37°C for optimal growth. All cultures were grown for 7 to 10 days before being transferred to 75-cm2 tissue culture flasks. Flasks were incubated under the same conditions, and trophozoites were harvested when they grew to 80 to 100% confluency.
To induce rounding and release of the amoebae into the medium, medium in flasks containing adherent trophozoites was replaced with 50 ml of 0.15 M PBS, pH 7.6, and placed in an ice bath for 30 min. The cell suspension was centrifuged at 2,500 × g for 10 min, and the pellet was resuspended in 10 ml of PBS containing 1% formalin. Fixed amoebae were stored at 4°C for as long as 1 week. Prior to use, fixed amoebae were washed three times in PBS as above and then resuspended in 10 ml of PBS, and aliquots were counted on a hemacytometer. Amoebae were then diluted (1:7 to 1:20) in 0.05 M sodium carbonate buffer, pH 9.6, to achieve the desired concentration for coating of microtiter wells.
In one set of experiments, a known number of fixed trophozoites were washed free of formalin and resuspended in carbonate buffer before being disrupted by homogenization (20 s; Tissue Tearor; Biospec Products, Inc., Bartlesville, Okla.) and sonication (60 Sonic Dismembrator; Fisher Scientific, Pittsburgh, Pa.) for 15 s. This resulted in complete disruption of the amoebae as assessed by microscopy. The trophozoite extract was used immediately.
ELISA procedure.
The ELISA method used in these studies was adapted from the work of Sheets et al. (25). A known number of fixed amoebae (or antigens from the same number of disrupted amoebae) were placed in microtiter wells and incubated at 37°C for 1 h, followed by overnight incubation at 4°C. Plates were washed three times with 0.15 M PBS, pH 7.2, containing 0.1% Tween 20, between each incubation step. Wells were blocked for 90 min at 37°C with 200 μl of 5% dry milk-PBS. Human sera (1:10 in 100 μl) were then added to wells and incubated at 37°C for 1 h. This was followed by the addition of horseradish peroxidase (HRP)-conjugated goat anti-human IgG (1:1,000; Zymed Laboratories, Inc., South San Francisco, Calif.). For some experiments where rabbit sera were used, conjugate consisted of HRP-conjugated anti-rabbit IgG (ICN Biomedical, Aurora, Ohio). Reactions were visualized by the addition of peroxidase-activated (final concentration, 0.03%) 2,2′-azino-di-[3-ethylbenzthiazolinesulfonate(6)] (Boehringer Mannheim, Indianapolis, Ind.). Plates were read spectrophotometrically (TiterTek Multiskan MCC/340 ELISA Reader; Flow Laboratories, McLean, Va.) at 414 nm after 3, 5, 10, 15, and 30 min. Each plate included triplicate wells of two to three individual human serum samples, which consistently yielded low absorbance values (≤0.300; negative sera) or high absorbance values (>1.0; positive sera), and other wells, which contained all reagents except primary serum (reagent control). All unknown sera were tested in duplicate. Data were expressed as net absorbance, calculated by subtracting the mean absorbance of the reagent control wells from the mean absorbance of the specimen wells.
Statistical evaluation.
Fifty-five sera were tested against each of the amoeba serogroups, and the absorbance values of all 55, as well as the absorbances of the nonresponder subset (n = 7), were compared using a one-way analysis of variance (ANOVA) and a Tukey-Kramer multiple comparisons test. ANOVA was also used to compare age and ethnic groups. To test for potential cross-reactivity among the three Acanthamoeba serogroups, the data were subjected to McNemar's test for correlated proportions. Ethnic groups tested against A. culbertsoni were compared using the Kruskal-Wallis test because the data were non-Gaussian. Mean absorbances of gender groups were analyzed with Student's t test (unpaired). Other categorical data were compared using a chi-square test or Fisher's exact test. In all analyses, a P value of <0.05 was considered significant.
RESULTS
Selection of antigen concentration.
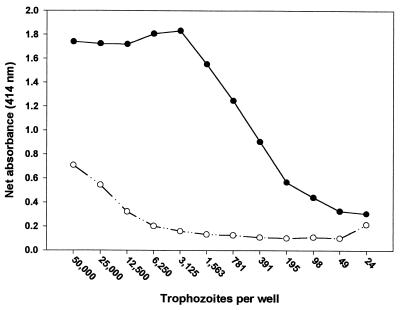
A checkerboard pattern utilizing twofold serial dilutions of fixed A. polyphaga trophozoites (24 to 50,000 trophozoites) and rabbit anti-A. polyphaga serum (1:100 to 1:12,800) was reacted to establish the optimal antigen concentration. Preimmune rabbit serum was tested in the same fashion on a separate plate. All dilutions of the hyperimmune serum showed high reactivity to the trophozoites (data not shown); however, a serum dilution of 1:400 was chosen to establish the optimal antigen concentration (Fig. 1). High reactivity (optical density [OD], >1.5) was seen at concentrations of 1,563 to 50,000 trophozoites, and reactivity decreased in a linear fashion to 195 trophozoites per well. Lower trophozoite concentrations (down to 24/well) yielded OD readings near background levels. In comparison, negative-control serum showed high reactivity (OD, 0.7) in wells containing 50,000 trophozoites, but reactivity decreased linearly with trophozoite concentrations down to approximately 6,250. At lower concentrations, absorbance values plateaued at an OD of approximately 0.1 to 0.2. From these data, an amoeba concentration of approximately 3,000/well was chosen as optimal for subsequent experiments, since that number yielded the highest signal-to-background ratio.
FIG. 1.
Net absorbances (IgG) of rabbit sera in wells containing different numbers of A. polyphaga trophozoites. Sera from a rabbit immunized with A. polyphaga (solid circles) and an unimmunized rabbit (open circles) were tested at a 1:400 dilution.
In a separate experiment, we compared the antibody reactivity to intact versus disrupted A. polyphaga trophozoites. Each well contained the equivalent of 3,000 trophozoites, whether intact or disrupted. Six sera showing low (OD, <0.2) to high (OD, >0.6) reactivity against intact, fixed trophozoites were used in the assays. Each of the sera showed higher absorbance values when incubated with the disrupted compared to the intact trophozoites (data not shown), with an overall mean increase in OD of 2.2-fold. Thus, sera showing low absorbances against intact trophozoites had significantly higher absorbances when an equivalent number of disrupted trophozoites were used, which led to false-positive results. These data indicate that cytosolic antigens may contribute to nonspecific reactivity and a high estimation of seroprevalence. This would be most problematic in Acanthamoeba serogroups which yield a relatively low seroprevalence when intact trophozoites are used. In these cases, cytosolic antigens would likely increase the absorbance values above the cutoff for positivity and cause serious overestimation of the seroprevalence.
Definition of positive results.
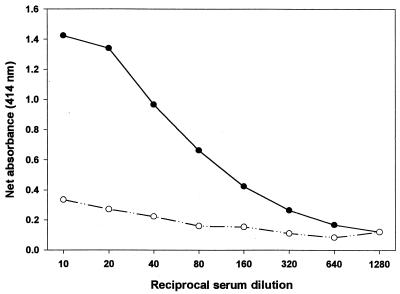
Sera from six individuals were tested at a dilution of 1:10 against A. polyphaga trophozoites in order to examine their relative reactivities (data not shown). Two sera yielding high or low absorbance values were selected for titration (Fig. 2). In this experiment, twofold serial dilutions (1:10 to 1:1,280) of the sera were tested. With the highly reactive serum, dilutions of 1:10 and 1:20 yielded similar absorbance values (ODs of 1.4 or higher). Dilutions between 1:40 and 1:160 were essentially linear in OD, but declined more slowly thereafter until “background” levels were reached at 1:1,280. In comparison, the low-reactivity serum showed only a slight decline in absorbance value from 1:10 to 1:80 and remained relatively stable thereafter. No prozone effect was observed with either serum. Based on the high signal-to-background ratio, a serum dilution of 1:10 was chosen for all subsequent experiments.
FIG. 2.
Net absorbance of IgG from healthy humans to A. polyphaga trophozoites. Sera from a responder (solid circles) and a nonresponder (open circles) were tested against 3,000 trophozoites per well. Each point represents the mean ± SD of triplicate assays.
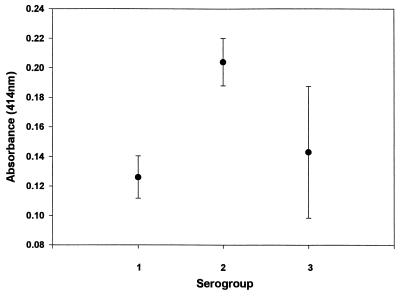
Since a known unexposed population could not be determined, background reactivity (i.e., negative control) had to be empirically defined. All 55 human serum samples were tested against three Acanthamoeba species representing the three serogroups. Persons whose serum samples had low absorbance values (OD, <0.25) with all three serogroups were considered nonreactive; seven individuals fell into this group. The mean absorbance values and standard deviations (SD) for each serogroup are shown (Fig. 3). Serogroups 1 (A. astronyxis) and 3 (A. culbertsoni) yielded mean absorbance values in the same range (0.126 and 0.143, respectively), while serogroup 2 (A. polyphaga) yielded a mean absorbance of 0.204, which was significantly higher (P < 0.02) than those with the other serogroups. These mean absorbance values and SD were used to estimate the antibody reactivities of the remaining 48 serum samples. Positive reactions were defined as any absorbance value that exceeded 3 SD above the mean for each serogroup and cutoff values were as follows: for serogroup 1, >0.288; for serogroup 2, >0.255; for serogroup 3, >0.172.
FIG. 3.
Mean absorbance values of nonresponders to each of the three Acanthamoeba serogroups. Each value is the mean ± SD for seven healthy individuals tested against serogroup 1 (A. astronyxis), serogroup 2 (A. polyphaga), or serogroup 3 (A. culbertsoni) antigens. Serum IgG from each individual was tested in triplicate against each serogroup by ELISA.
Reproducibility of results.
Serum samples yielding low to moderate absorbance values were evaluated for assay variability because these lower values were expected to show a greater coefficient of variation (CV) than higher absorbance values. Each serum sample was tested in duplicate against each of the three serogroups. Two serum samples incubated with A. astronyxis and four serum samples incubated with A. polyphaga showed similar well-to-well variabilities in absorbance, with CVs in the range of 0.11 to 0.14 and 0.06 to 0.14, respectively. One serum sample tested with A. culbertsoni had a well-to-well variation of 29%. In addition, plate-to-plate variation of the same serum samples ranged from 8 to 26% among amoeba species. These data suggest that the results within and between plates were repeatable; however, it is prudent to include negative-control sera on each plate in order to standardize results.
Reactivity to Acanthamoeba serogroups.
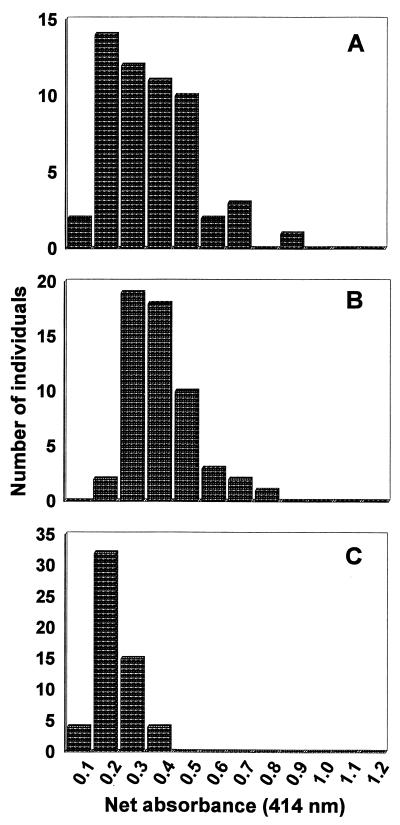
All 55 serum samples were tested for IgG reactivity to each of the Acanthamoeba serogroups, and the frequency distributions of net absorbances were calculated (Fig. 4). Serogroup 1 reactivities ranged from absorbances of 0.068 to 0.806, with a median value of 0.300. In comparison, serogroups 2 and 3 yielded absorbances from 0.184 to 0.707 (median, 0.333) and 0.071 to 0.392 (median, 0.156), respectively. Overall absorbance values with A. culbertsoni were significantly lower than those with the other two Acanthamoeba species (P = 0.0001).
FIG. 4.
Frequency distribution of net absorbances of IgG from 55 healthy subjects in response to Acanthamoeba serogroup antigens. Each serum was tested at a 1:10 dilution in duplicate wells against serogroup 1 (A), serogroup 2 (B), or serogroup 3 (C) trophozoites.
Based on the defined cutoff value (mean + 3 SD) for each serogroup, the percent positive sera was calculated. Percent antibody reactivity was high in each serogroup: for serogroup 1, 52.8%; for serogroup 2, 81.8%; for serogroup 3, 40.0%. These data indicate that colonization or mild infection with Acanthamoeba may be a relatively common occurrence. Analysis of the difference in proportions revealed significant differences between serogroup 2 and serogroup 1 (P = 0.004) or serogroup 3 (P = 0.0001) but no difference between serogroups 1 and 3 (P = 0.130). Thus, A. polyphaga yielded the highest background absorbances (Fig. 3), the highest overall absorbance values (Fig. 4), and the highest percent positive reactivities compared to the other two amoeba species. In contrast, A. culbertsoni yielded low background absorbance values, the lowest overall absorbances, and the lowest percent positive reactivities. These results suggest that among the three Acanthamoeba species studied A. polyphaga is most commonly and A. culbertsoni is the least commonly encountered by the healthy population.
A table of positive or negative reactivity for each of the 55 serum samples tested against each serogroup was prepared (data not shown). All possible combinations of reactivity to each amoeba species were analyzed (Table 2). Forty-eight (87.3%) individuals were positive for one or more serogroups. Of these, equal numbers (i.e., 16 subjects) were positive for one serogroup only, two serogroups, or all three serogroups. Thirteen (81.2%) of the 16 subjects who were positive for one serogroup only had serum samples that reacted against A. polyphaga.
TABLE 2.
IgG reactivities of serum samples of 55 healthy adults to Acanthamoeba species representing each serogroup
| Reactivity to serogroupa:
|
No. of subjects | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| − | − | − | 7 |
| + | − | − | 2 |
| − | + | − | 13 |
| − | − | + | 1 |
| + | + | − | 11 |
| + | − | + | 0 |
| − | + | + | 5 |
| + | + | + | 16 |
Every possible combination of results is given. +, reactive; based on the 3-SD cutoff value for each serogroup. See Materials and Methods. −, nonreactive.
Potential cross-reactivity among serogroups was assessed by examining the number of individuals whose serum samples were reactive to any one of the serogroups alone. Two samples were positive for serogroup 1 (A. astronyxis) alone, 13 for serogroup 2 (A. polyphaga) alone, and 1 for serogroup 3 (A. culbertsoni) alone. If significant cross-reactivity were occurring, one would expect that the A. polyphaga positive serum samples would also be positive for the other serogroups. Since this was not the case, we conclude that the antibody response to each serogroup appears to be independent. We further subjected the data to statistical analysis (McNemar's test for correlated proportions) and found that antibody reactivities against the three serogroup antigens were independent.
Potential risk factors for antibody reactivity to Acanthamoeba species.
Age, gender, and ethnic data were available for all 55 study subjects. Comparisons of percent positive serum samples (for each serogroup) were made among all four age groups (see Table 1). No significant differences or trends were found to suggest an increased (or decreased) antibody reactivity in older age groups (data not shown). Further, comparison of absorbance values confirmed a lack of association between age and reactivity to Acanthamoeba antigens. In a similar manner, gender was assessed as a factor in antibody reactivity to Acanthamoeba serogroups. No significant differences were seen between males and females when the number of positive serum samples or the absorbance values were compared.
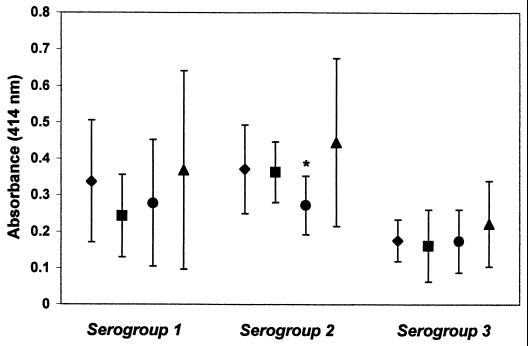
Four ethnic groups were represented in the study population; however, low numbers of Asians prevented a complete analysis. The percent positive serum samples in each ethnic group and the mean absorbance values for each ethnic group were compared among the three Acanthamoeba serogroups. When serum samples were tested against serogroup 1 antigens, the percent positive samples was >65% for Caucasians (n = 32) and Asians (n = 3) versus 40% for Hispanics (n = 10) and 20% for African-Americans (n = 10). In this analysis, the percent positive Caucasians was significantly higher than the percent positive African-Americans (P = 0.026; odds ratio [OR] = 7.6; 95% confidence interval [CI] = 1.4 to 42.4). For serogroup 2 antigens, Caucasians, African-Americans, and Asians were each ≥90% positive compared with 40% positive Hispanics (n = 10); however, the only difference that reached statistical significance was that between Caucasians and Hispanics (P = 0.0025; OR = 14.5; 95% CI = 2.6 to 82.3). The latter difference was also seen when A. polyphaga absorbance values from Caucasians and Hispanics were compared (P = 0.047) (Fig. 5). No other significant associations were seen among the ethnic groups regardless of the Acanthamoeba serogroup which was examined.
FIG. 5.
Mean (and SD) absorbance values for serum samples of various ethnic groups tested against each Acanthamoeba serogroup. Ethnic groups are shown in the following order: Caucasian (⧫), African-American (■), Hispanic (●), and Asian (▴). The asterisk indicates a statistically significant difference (P < 0.05) between Caucasians and Hispanics.
In addition, subjects whose serum samples reacted to no serogroup or to one, two, or all three serogroups were compared with regard to age, gender, and ethnic origin. No significant associations were found between gender or age and reactivity to these serogroups. When ethnicity was analyzed, it was noted that the serum samples of 75% of Caucasians and 100% of Asians were positive for two or three serogroups, compared with 30% for African-Americans and 20% for Hispanics. Since the number of individuals in each ethnic group was limited, the groups with high percentages of positive samples (Caucasians and Asians) were combined, and those with low percentages of positive samples (African-Americans and Hispanics) were combined. Analysis indicated a significant difference (P = 0.0013), with Caucasians and Asians being 7.8 (95% CI, 2.2 to 28) times more likely to be positive for two or three serogroups than African-Americans and Hispanics. The results were similar (P = 0.0051; OR = 5.6; 95% CI = 1.7 to 18.2) when Caucasians were compared to all other ethnic groups combined.
DISCUSSION
An ELISA method has been optimized for detecting anti-Acanthamoeba IgG in human serum samples. The method utilizes whole, fixed trophozoites rather than disrupted trophozoites, which gave higher background reactivity. This suggests that cytosolic antigens from the amoebae can contribute to nonspecific binding and/or cross-reactivity that would obfuscate interpretation of results. The method has been optimized for antigen and serum concentrations; 3,000 trophozoites per well and a serum dilution of 1:10 were found to yield the highest signal-to-background ratios. Since the ubiquitousness of the amoeba precludes an easy definition of an unexposed negative population, an empirical definition was required. For this, serum samples with low absorbance values (OD, ≤0.250) in response to all of the serogroups were chosen to represent nonreactive (negative-control) serum samples. Cutoff values (mean + 3 SD) for each serogroup were then calculated from the mean and SD of these absorbances. Inclusion of negative-control serum samples and a reagent control on each plate ensured standardized results.
Two early studies examining different populations report conflicting results, one with seroprevalences in the 3% range (2) and another with seroprevalences at 100% (6). In each of these studies, A. culbertsoni was used as the antigen in an indirect hemagglutination or indirect fluorescence antibody format. Interestingly, in one of these studies (6) the amoebae used in the antigen preparation were grown on agar seeded with Enterobacter cloacae, which could have contributed to the 100% reactivity reported. Neither report described the antigen preparation in detail or provided a rationale for its definition of positive reactivity. It is also of interest that the antibody titers reported in both studies were all in the range of 1:20 to 1:40; however, differences in what was considered negative led to essentially opposite interpretations. A recent publication examines a leptomyxid amoeba, Balamuthia mandrillaris, a species also known to cause granulomatous amoebic encephalitis (12). Whole, fixed trophozoites were used in an indirect fluorescence antibody assay, and results were confirmed by flow cytometry; however, no negative-control serum samples were described. This paper demonstrated that all 50 children and adults tested had anti-amoeba serum antibody titers (IgM and IgG) in the range of 1:64 to 1:256. This response was shown to be non-cross-reactive with Acanthamoeba or Naegleria.
In comparison to the earlier reports, the present study uses well-characterized parameters in the testing procedure and has taken a detailed approach to defining negative and positive results. A. culbertsoni, also used in the earlier studies, gave the lowest overall absorbances and the lowest percent seropositive results (39.3%) compared to the other two amoeba species. The highest overall absorbances and the highest seropositivity (81.8%) were seen with A. polyphaga.
The relative occurrence of Acanthamoeba species in soil and/or surface water has not been thoroughly studied, but the species associated with human Acanthamoeba infections are thought to be representative of amoebic species in the environment. Additional data on Acanthamoeba species distribution in the environment will be necessary to confirm this notion and to further examine the effects of geographic locations (tropical to temperate zones), seasons, rainfall, and other factors influencing amoeba growth. However, if we are to accept the current belief that group 2 species (including A. polyphaga) are the most common in the environment (27) and group 3 species (including A. culbertsoni) are the least common (21), then our findings are consistent. According to this view, we might then predict from our data that A. culbertsoni and the group 3 species are the least common in the environment. Thus, it appears that the differences seen in the reactivities of serum samples of healthy persons to the various serogroups may reflect the relative exposure of the population to these species in addition to virulence differences. However, additional studies on this point are needed.
When serological studies are undertaken, the potential for cross-reactivity is always a concern. While we found no evidence of cross-reactivity among the three Acanthamoeba serogroups, we did not test for reactivity toward other amoebae that are known to cause human infections. Earlier studies examining cross-reactivity between Acanthamoeba and Naegleria (10) or Balamuthia (11) found none. Nevertheless, the potential for cross-reactivity needs to be further studied but will require antisera produced in animals that are strictly raised so as to prevent any inadvertent exposure to Acanthamoeba in dirt or water.
It is clear from the data presented here that the interpretations of serological studies are heavily influenced by the Acanthamoeba species used as the antigen. Since ≥80% of tested individuals had antibodies to A. polyphaga, it is unlikely that a “high risk” group would show any significant increase in seroprevalence. Thus, for epidemiological studies of healthy versus other populations, serogroup 1 and/or 3 antigens would likely be more useful. The recognition that transient infections with Acanthamoeba are possible could open new avenues for epidemiological studies. Recently, a number of reports have linked human bacterial pathogens, such as Legionella (23), Chlamydia (9), and Mycobacterium (5, 14) spp., to Acanthamoeba. These bacteria are able to survive phagocytosis and replicate in amoebic vacuoles. Further, the Acanthamoeba vector appears to maintain or even enhance the virulence of these bacteria (5). Such observations suggest that Acanthamoeba may play an important role in the survival of bacterial pathogens in the environment and may serve as a mode of transmission, perhaps through aerosols or other mechanisms. If this is indeed the case, then Acanthamoeba antibodies could be detectable in individuals who have had bacterial pathogens transmitted in this way. Selected patient populations are currently being examined in our laboratory for an increased prevalence of Acanthamoeba antibodies.
The number of subjects studied can only provide a preliminary notion of the seroprevalence of Acanthamoeba in the general population. Likewise, attempts to identify potential risk factors were hampered by low numbers in each group. Although no significant associations were seen between Acanthamoeba antibody reactivity and gender or age group, this may not be true of the larger population. The data presented here suggest that the males and females studied were equally exposed. Also, the ages of the study population were relatively narrow and did not include children or those older than 51 years. It is possible that an association may be seen in infants and young children as they are increasingly exposed to soil and surface water. Interestingly, there was some indication that ethnic origin may play a role in the number of seropositive individuals and the degree of reactivity to A. polyphaga antigens. Taken together, the results suggest that Caucasians have a higher percent reactivity to serogroup 1 and 2 antigens than other ethnic groups and that they are more likely to be positive for multiple serogroups. It is not clear whether these findings indicate an increase in exposure, an enhanced antibody response to amoebic antigens, or some other, unknown factor.
In summary, the data reported herein suggest that mucosal colonization and/or transient, mild, or asymptomatic Acanthamoeba infections, perhaps of the skin or respiratory tract, are common. Previous isolation of Acanthamoeba in nasal washings, pharyngeal swabs, and broncheoalveolar lavage support this view (1, 3, 15, 28). Thus, while serious ocular disease and life-threatening CNS infections are rare, mucosal infection may contribute significantly to the large number of undiagnosed sinus infections and pulmonary illnesses suffered each year.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health General Clinical Research Centers grant (M01-RR-02558) and a University of Texas School of Public Health Excellence in Research Award (to C.L.C.).
REFERENCES
- 1.Badenoch P R, Grimmond T R, Cadwgan J, Deayton S E, Essery M S L, Hill B D. Nasal carriage of free-living amoebae. Microb Ecol Health Dis. 1988;1:209–211. [Google Scholar]
- 2.Cerva L. Acanthamoeba culbertsoni and Naegleria fowleri: occurrence of antibodies in man. J Hyg Epidemiol Microbiol Immunol. 1989;33:99–103. [PubMed] [Google Scholar]
- 3.Cerva L, Serbus C, Skocil V. Isolation of limax amoebas from the nasal mucosa of man. Folia Parasitol. 1973;20:97–103. [PubMed] [Google Scholar]
- 4.Chappell C L, Okhuysen P C, Sterling C R, DuPont H L. Cryptosporidium parvum: intensity of infection and oocyst excretion patterns in healthy volunteers. J Infect Dis. 1996;173:232–236. doi: 10.1093/infdis/173.1.232. [DOI] [PubMed] [Google Scholar]
- 5.Cirillo J D, Falkow S, Tompkins L S, Bermudez L E. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect Immun. 1997;65:3759–3767. doi: 10.1128/iai.65.9.3759-3767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cursons R T M, Brown T J, Keys E A, Moriarty K M, Till D. Immunity to pathogenic free-living amoebae: role of humoral antibody. Infect Immun. 1980;29:401–407. doi: 10.1128/iai.29.2.401-407.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driebe W T, Jr, Stern G A, Epstein R J, Visvesvara G S, Adi M, Komadina T. Acanthamoeba keratitis. Potential role for topical clotrimazole in combination chemotherapy. Arch Ophthalmol. 1988;106:1196–1201. doi: 10.1001/archopht.1988.01060140356031. [DOI] [PubMed] [Google Scholar]
- 8.DuPont H L, Chappell C L, Sterling C R, Okhuysen P C, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;30:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 9.Essig A, Heinemann M, Simnacher U, Marre R. Infection of Acanthamoeba castellanii by Chlamydia pneumoniae. Appl Environ Microbiol. 1997;63:1396–1399. doi: 10.1128/aem.63.4.1396-1399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores B M, Garcia C A, Stamm W E, Torian B E. Differentiation of Naegleria fowleri from Acanthamoeba species by using monoclonal antibodies and flow cytometry. J Clin Microbiol. 1990;28:1999–2005. doi: 10.1128/jcm.28.9.1999-2005.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garner A. Pathogenesis of acanthamoebic keratitis: hypothesis based on a histological analysis of 30 cases. Br J Ophthalmol. 1993;77:366–370. doi: 10.1136/bjo.77.6.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z H, Ferrante A, Carter R F. Serum antibodies to Balamuthia mandrillaris, a free-living amoeba recently demonstrated to cause granulomatous amoebic encephalitis. J Infect Dis. 1999;179:1305–1308. doi: 10.1086/314731. [DOI] [PubMed] [Google Scholar]
- 13.Kenney M. The micro-Kolmer complement fixation test in routine screening for soil amoeba infection. Health Lab Sci. 1971;8:5–10. [PubMed] [Google Scholar]
- 14.Krishna-Prashad R N, Gupta S K. Preliminary report on engulfment and retention of mycobacteria by trophozoites of axenically grown Acanthamoeba castellanii Douglas. Curr Sci. 1978;47:245–247. [Google Scholar]
- 15.Lawande R V, Abraham S N, John I, Egler L J. Recovery of soil amoebas from the nasal passages of children during the dusty hartmattan period in Zaria. Am J Clin Pathol. 1979;71:201–203. doi: 10.1093/ajcp/71.2.201. [DOI] [PubMed] [Google Scholar]
- 16.Ma P, Visvesvara G S, Martinez A J, Theodore F H, Daggett P-M, Sawyer T K. Naegleria and Acanthamoeba infections: review. Rev Infect Dis. 1990;1:490–513. doi: 10.1093/clinids/12.3.490. [DOI] [PubMed] [Google Scholar]
- 17.Martinez A J. Is acanthamoebic encephalitis an opportunistic infection? Neurology. 1980;30:567–574. doi: 10.1212/wnl.30.6.567. [DOI] [PubMed] [Google Scholar]
- 18.Martinez A J. Acanthamoebiasis and immunosuppression. Case report. Neuropathol Exp Neurol. 1982;41:548–557. doi: 10.1097/00005072-198209000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Martinez A J, Visvesvara G S. Laboratory diagnosis of pathogenic free-living amoebas: Naegleria, Acanthamoeba, and Leptomyxid. Clin Lab Med. 1991;11:861–872. [PubMed] [Google Scholar]
- 20.Martinez A J, Visvesvara G S. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 1997;7:583–598. doi: 10.1111/j.1750-3639.1997.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mergeryan H. The prevalence of Acanthamoeba in the human environment. Rev Infect Dis. 1991;13(Suppl. 5):S390–S391. doi: 10.1093/clind/13.supplement_5.s390. [DOI] [PubMed] [Google Scholar]
- 22.Moura H, Wallace S, Visvesvara G S. Acanthamoeba healyi n. sp. and the isoenzyme and immunoblot profiles of Acanthamoeba spp., groups 1 and 3. J Protozool. 1992;39:573–583. doi: 10.1111/j.1550-7408.1992.tb04853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newsome A L, Scott T M, Benson R E, Fields B S. Isolation of an amoeba naturally harboring a distinctive Legionella species. Appl Environ Microbiol. 1998;64:1688–1693. doi: 10.1128/aem.64.5.1688-1693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okhuysen P C, Chappell C L, Crabb J H, Sterling C R, DuPont H L. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dist. 1999;180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- 25.Sheets P B, Newsome A L, Allen S D. Detection of human serum antibodies reactive with Acanthamoeba polyphaga by use of an indirect fluorescence antibody (IFA) test, p. D-8.1–D-8.6. In: Lee J J, Soldo A T, editors. Protocols in protozoology. Lawrence, Kans: Allen Press, Inc.; 1992. [Google Scholar]
- 26.Stehr-Green J K, Bailey T M, Visvesvara G S. The epidemiology of Acanthamoeba keratitis in the United States. Am J Ophthalmol. 1989;107:331–336. doi: 10.1016/0002-9394(89)90654-5. [DOI] [PubMed] [Google Scholar]
- 27.Visvesvara G S. Classification of Acanthamoeba. Rev Infect Dis. 1991;13(Suppl. 5):S369–S372. doi: 10.1093/clind/13.supplement_5.s369. [DOI] [PubMed] [Google Scholar]
- 28.Wang S S, Feldman H A. Isolation of Hartmanella species from human throats. N Engl J Med. 1967;277:1174–1179. doi: 10.1056/NEJM196711302772204. [DOI] [PubMed] [Google Scholar]