Abstract
Robotic surgical systems were created in part to solve several constraints of laparoscopic surgery and offer technical advantages. With a substantial body of evidence that demonstrates its efficacy in the treatment of rectal cancer, robotic surgery will soon become another conventional treatment. However, further investigations and randomized trials focusing on primary endpoints are needed to establish some advantages for robot-assisted colon surgery. Da Vinci Single-SiteⓇ and SPⓇ platforms were developed to overcome the shortcomings of single-port laparoscopic surgery. Despite the currently insufficient evidence, it appears that the SP platform addresses many of the limitations of single-port transabdominal or transanal surgery. Robotic transanal minimally invasive surgery and total mesorectal excision were developed to overcome some of the limitations of conventional platforms, using wristed instrumentation to enhance dexterity and ergonomics. Studies on the effectiveness and viability of this novel approach are ongoing. The near-infrared fluorescence technique, real-time stereotactic navigation technology, and other surgical data platforms based on artificial intelligence incorporated into the robotic system will play an important role in improving outcomes. Robotic systems for advanced colorectal cancer offer technical advantages for complex and precise surgeries. If the cost of robotic surgery is reduced by expanding its indications and enhancing competition among different robotic platforms, it will provide clinical benefits to more patients and reduce social healthcare costs.
Keywords: robotic surgical procedures, colorectal neoplasm, treatment outcomes, review, forecasting
Introduction
Surgical resection continues to be the most effective treatment option for colorectal cancer with respect to curative resection, staging, prognosis, and other therapeutic considerations[1,2]. The primary objective of cancer surgery is to enhance survival and quality of life while minimizing side effects[3]. To improve colorectal cancer treatment with respect to functional, oncological, surgical, patient-reported, and financial outcomes, advancements in surgical procedures are essential[3-7].
Several randomized trials have proven that laparoscopic surgery is preferable to open surgery because it is associated with less blood loss, an earlier recovery of bowel motility, and a shorter hospital stay without sacrificing oncologic results. Consequently, laparoscopic surgery has become the conventional method for the treatment of colon cancer[8,9]. However, the laparoscopic approach has many intrinsic technical limitations. Particularly for low rectal resection, there is a limited range of motion for long straight instruments in the confined pelvic cavity, a two-dimensional vision, a loss of tactile sensitivity, and a reduction in dexterity.
The use of three-dimensional (3D) magnified imaging, a surgeon-controlled stable camera system, and more maneuverable instruments (e.g., EndoWristⓇ; Intuitive Surgical, Inc., Mountain View, CA, USA) with 7° of freedom, 180° articulation, 540° rotation, filtering of physiologic tremor, motion scaling functions, stronger retraction, and fixed third-arm retraction are some of the technical advantages of these emerging technologies over conventional laparoscopic surgery. In addition, it appears that robotic colorectal surgery has a shorter learning curve than laparoscopic surgery for doctors attempting to master minimally invasive treatments[10].
Robotic surgery, utilizing cutting-edge technologies, has revolutionized the surgical management of colorectal cancer, and this technique is gaining popularity in the field of colorectal surgery. However, the role of robotics in colorectal cancer surgery remains largely uncertain, and it is unknown if robotic surgery provides significant clinical advantages over laparoscopic surgery for the treatment of colorectal cancer. In this review article, we aimed to assess the outcomes of robotic surgery currently performed for specific indications and to project its future direction.
Robotic Surgery for Rectal Cancer
Since large multicenter randomized trials demonstrated its long-term oncological safety, laparoscopic resection has been the treatment of choice for colon cancer[11]. Nevertheless, laparoscopic total mesorectal excision (TME) for rectal cancer is a technically challenging treatment because laparoscopic instruments are straight and rigid, requiring surgeons to conduct a series of camera-dependent intricate movements within the restricted bony pelvis. Several randomized controlled trials investigating the oncological outcomes of laparoscopic TME for rectal cancer have failed to demonstrate that this technique is non-inferior to open surgery[4,12].
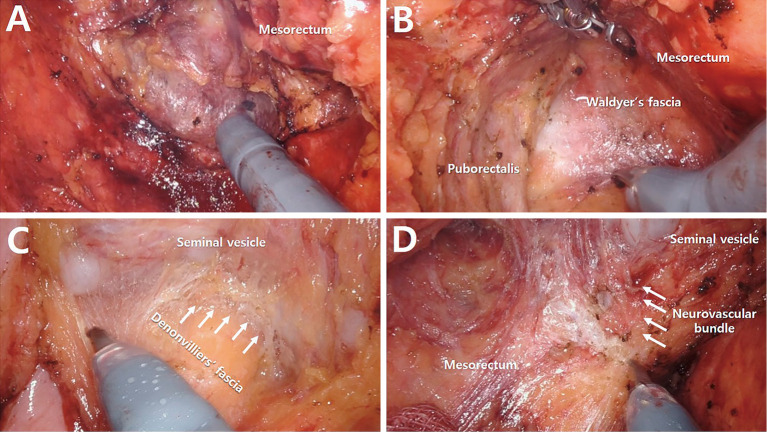
To overcome the constraints of laparoscopic surgery in difficult procedures, such as operating in limited places to treat rectal and prostate malignancies, robotic surgery has been created (Figure 1). No significant difference in short- and long-term outcomes exists between the robotic and laparoscopic approaches for rectal cancer[13,14]. Kim et al.[15] conducted an open-label randomized controlled research and found no significant difference between laparoscopic and robotic TME in the rate of conversion to open surgery. The robotic versus laparoscopic resection for rectal cancer (ROLARR) trial also failed to detect a significant difference in the rate of conversion to open laparotomy after laparoscopic or robotic procedures (12.2% vs. 8.1%, P = 0.16); however, subgroup analyses revealed potential benefits for robotic procedures in men, obese patients, and patients with low-lying rectal cancer[16]. Milone et al. performed a meta-analysis on the completeness of robotic versus laparoscopic TME in rectal cancer and showed a significant difference in favor of robotic surgery (odds ratio 1.83, 95% confidence interval 1.08-3.10, P = 0.03). In a meta-analysis by Kowalewski et al.[17], patients who underwent robotic surgery had a better quality of life and decreased urinary symptoms, ileus, and urinary retention, but no discernible variations were observed in sexual function. Previous studies reported comparable long-term oncological outcomes between laparoscopic and robotic TME in the treatment of rectal cancer, whereas Kim et al. found that robotic surgery is a significant prognostic factor for overall and cancer-specific survival in their multivariate analysis[18,19]. Many studies investigating various endpoints to demonstrate the efficacy of robotic surgery in the treatment of rectal cancer are ongoing, and a substantial body of evidence is growing. Robotic surgery may become another conventional treatment for prostate cancer in the near future.
Figure 1.
Robotic total mesorectal excision using the Xi® platform. (A) Posterior dissection (B) Deep posterior dissection (C) Anterior dissection (white arrows indicate Denonvilliers’ fascia) (D) Right anterolateral dissection (white arrows indicate the neurovascular bundle).
Robotic Surgery for Colon Cancer
For the treatment of colon cancer, a technique incorporating complete mesocolic excision (CME) and central vascular ligation (CVL) was recently introduced, and several trials comparing standard laparoscopic CME to open CME for right-sided colon cancer have demonstrated the feasibility and safety of the laparoscopic technique with satisfactory oncologic results[20]. Nevertheless, minimally invasive CME with CVL for right-sided colon cancer is a challenging procedure. Intracorporeal anastomosis is an additional technical issue for many surgeons, particularly when doing a right-sided colectomy. Colectomy performed robotically for right-sided colon cancer may offer the following benefits for colon cancer treatment. First, it allows safe and precise lymph dissection along the superior mesentery trunk. Second, the approach allows suitable mesocolic resection with enhanced visibility and uniform traction. Lastly, the robotic method makes intracorporeal anastomosis practical and safe. However, there is still insufficient evidence that robotic CME and CVL are superior to laparoscopic surgery in clinical and oncologic outcomes. Park et al.[21] performed a randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy with CME and CVL and reported that clinical and long-term outcomes are similar between the two groups. However, robotic colectomy was associated with longer operation times and higher cost, and they concluded that the benefit was insufficient to outweigh the higher expense. A recent meta-analysis conducted by Tschann et al.[22] indicated that the robotic group had a much lower conversion rate, shorter length of hospital stay, and less blood loss; nonetheless, long-term oncological results were comparable between the two groups. Ferri et al.[23] performed a cost-effectiveness analysis in their propensity score-matched prospective nonrandomized study and found that there is no significant difference with respect to total costs between the robotic and laparoscopic groups. Further investigations and randomized trials focusing on specific primary endpoints, such as CME with CVL, intracorporeal anastomosis, and cost-effectiveness, are needed to establish the advantages of robot-assisted colon surgery.
Robotic Surgery for Single-port Surgery
Single-SiteⓇ platform
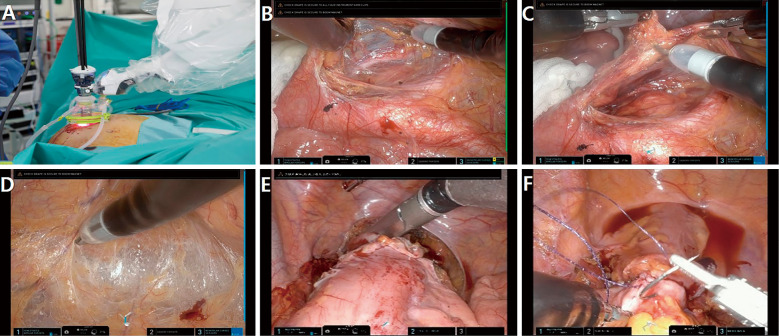
The recently announced da Vinci Single-SiteⓇ platform, which is compatible with the da Vinci Si and Xi systems, enables surgeons to overcome the limitations of single-port laparoscopic surgery. Using the innovative Single-SiteⓇ platform, the software repositions the two curved instruments such that they fit the surgeon's hand on the console. Due to many limitations, including the lack of wristed instrumentation, which is one of the key advantages of the conventional robotic system, this platform has not been widely employed for colorectal surgery. Other limitations include the semi-rigid robotic instrument's limited range of motion, limited tissue retraction (which is required for colorectal surgery), and the Single-SiteⓇ system's limited availability of instruments and accessories. We developed the Single-SiteⓇ plus one-port robotic surgery for right- and left-sided colon cancer and reported the safety and feasibility of this technique (Figure 2)[24,25]. At the 12-week follow-up visit, the evaluation of body image perception and cosmetic satisfaction revealed statistically improved outcomes with the da Vinci Single-Site surgery compared to the multiport laparoscopic colectomy[25]. The advantages of this surgical technique include reduced collisions between robotic instruments and the camera, ease of creating triangulation, cosmetic benefits, sharp dissection with the EndoWristⓇ, and ergonomic comfort, even for the inexperienced surgeon performing single-port laparoscopic surgery. We believe that Single-SiteⓇ plus the one-port technique is worthy of consideration for selected patients because this surgical technique is available in most institutions where da Vinci Si or Xi is installed and some of the shortcomings of SP mentioned below can be addressed.
Figure 2.
Single plus one-port right colectomy with intracorporeal anastomosis using the Single-Site® platform. (A) Access port setup (B) Exposure of the third portion of the duodenum and head of the pancreas (C) Ligation of the ileocolic vessels using the wristed robotic advanced bipolar energy device (D) Side-to-side intracorporeal isoperistaltic anastomosis using a wristed robotic stapler (E) Closure of the stapler insertion site with robotic-assisted continuous stitches (F) Postoperative scar view.
SPⓇ platform
A new robotic approach, the SPⓇ da Vinci robot platform (Intuitive Surgical, Inc., Sunnyvale, CA, USA), has been specifically developed for single-port surgery. The SP system was originally designed for access to tight, difficult-to-reach anatomical areas, such as the prostate, throat, and anorectum; however, it has been utilized in a variety of surgical fields, including visceral surgery. The advantages of the SP platform include instrument positioning display by a hologram, the use of wristed articulation and flexible elbows, a console-controlled camera, and 3D optics. Additionally, the platform's boom can rotate 360° inside and outside the port's remote center, making the performance of multiquadrant surgeries such as colorectal surgery (without having to dock the platform again) possible. Piozzi et al.[26] reported the perioperative short-term outcomes of seven intersphincteric resections, five right colectomies, and one transverse colectomy to evaluate the feasibility and safety of the SP system. They described their SP indications as including right-sided colon cancer, ultralow-lying rectal cancer requiring intersphincteric resection, and small or down-sized/down-staged tumors after preoperative chemoradiation. Kim et al.[27] reported their initial experience with five patients with rectal cancer and demonstrated that the SP platform is safe and feasible with satisfactory perioperative outcomes. Marks et al.[28] documented their initial experiences with 26 patients who underwent SP transanal minimally invasive surgery (TAMIS) of rectal lesions and 2 patients who underwent transanal total mesorectal excision (TaTME) and showed the safety and feasibility of this new platform with excellent optics and dexterity in the anorectal space. In the present study, we had 11 anterior resections for sigmoid colon cancer, 2 low anterior resections for rectal cancer, and 1 TAMIS for a neuroendocrine tumor (not published) (Figure 3). It is clear that the SP platform addresses many of the limitations of single-port transabdominal and transanal surgeries. However, it has a few drawbacks, and additional improvement is required. Notably, except from monopolar curved scissors, there are no staplers, suction irrigators, or surgical energy devices, and the near-infrared fluorescence (NIF) approach cannot be used in SP systems. Additionally, the third arm is at the top, and the camera is at the bottom of the SP system, which is different from the usual surgical view and may be uncomfortable. If the instruments that can be used in the SP system are further developed and the robotic movement is improved, it is expected that the indications for SP will be expanded and it will become an ideal platform for single-port surgery.
Figure 3.
Robotic anterior resection for sigmoid colon cancer using the SP® platform. (A) Access port setup (B) Medial to lateral dissection of the sigmoid colon (C) Lymph node dissection around the inferior mesenteric vessel (D) Posterior dissection of the rectum (E) Endostapling through the single-port (F) Robotic-assisted reinforcing continuous stitches.
Robotic Surgery for Transanal Surgery
TAMIS has been widely used in the treatment of benign lesions and low-risk T1 adenocarcinomas of the rectum. This surgical technique uses a multichannel single-port device implanted in the anal canal as an access system, with a standard laparoscopic scope and straight rigid instruments. While previous studies have established the benefits of the TAMIS technique, it has become clear over time that this approach has its challenges and a steep learning curve[29]. Although TAMIS has many advantages, there are still obstacles, such as the lack of a stable platform, the difficulty of positioning the surgeon and assistant between the device's legs, and the difficulty of suturing and tying using straight instruments in the confined rectal lumen, resulting in instrument collisions and poor visualization during excision.
Robotic TAMIS was developed to overcome the limitations of conventional TAMIS, using wristed instrumentation to enhance dexterity and ergonomics (Figure 4). Several authors have reported on the feasibility and safety of robotic TAMIS, and this approach has been adopted for more complex procedures such as TaTME[30]. TaTME is a novel technique based on natural orifice transluminal endoscopic surgery with a down-to-up approach that has been established to overcome certain technical restrictions, particularly in obese or masculine patients with low-lying rectal cancer in the narrow pelvis. It is also anticipated that this surgical method will enable improved control of the distal margin at the onset of the procedure. However, TaTME is a difficult procedure with a steep learning curve because it is based on a single-port laparoscopic technique, employing straight laparoscopic instruments[31]. Compared to laparoscopic TaTME, improved ambidexterity during lateral dissection and more stable surgical areas with preservation of the pelvic nerves and their autonomic function are additional advantages of robotic TaTME. Since the publication of the first robotic TaTME, other studies have demonstrated the effectiveness and viability of this novel approach[30,32].
Figure 4.
Robotic transanal minimally invasive surgery for rectal neuroendocrine tumor using the SP® platform. (A) Access port setup (B) Radial demarcation of the tumor (C) Full-thickness excision of a rectal lesion (D) Endoluminal suturing with the single-port robot.
Robotic Surgery Using NIF
NIF with indocyanine green (ICG) is beneficial for sentinel lymph node biopsy, lymph node road mapping, identification of the vascular system surrounding the major vessels, detection of the ureters to decrease the risk of iatrogenic ureteral lesions in colorectal surgery, and visual assessment of blood vessels, blood flow, and tissue perfusion (Figure 5)[33]. Recently, an intraoperative NIF imaging system (Firefly™; Intuitive Surgical, Inc., Sunnyvale, CA, USA) installed on a robotic system has made it possible for surgeons to identify intravascular NIF signals in real-time. Many investigations on the use of NIF and ICG in detecting metastatic lymph nodes have been undertaken for the identification of the sentinel lymph node and mapping of additional lymph nodes outside the prescribed resection margins to perform radical lymphadenectomy for curative surgery[34]. Kim et al.[35] demonstrated a unique use of NIF employing ICG during robotic TME with lateral pelvic lymph node dissection (LPND) to locate suspected lateral pelvic lymph nodes and avoid incomplete dissection. Zhou et al.[36] compared patients who underwent TME and LPND with the NIF technique to those who underwent conventional TME and LPND without NIF-guided imaging and found that the NIF group has significantly lower intraoperative blood loss and a significantly larger number of harvested lateral pelvic nodes, and that the lateral pelvic lymph nodes of two NIF patients remain during LPND. Additionally, Park et al.[37] and Bae et al.[38] used the NIF technique for CME with D3 lymphadenectomy in colon cancer surgery. Park et al.[37] injected ICG around the tumor to visualize lymphatic flow and lymph nodes and found that the number of apical lymph nodes and total number of retrieved lymph nodes are considerably more in the NIF group than in the conventional group (Figure 3). During surgery, when blood vessels are exposed, ICG can be simply injected into the bloodstream to aid direct visual inspection. The surgeon doing the NIF imaging found the ideal location of division, which permitted the identification of the left colic branch of the inferior mesenteric artery in 11 patients who underwent robotic TME with preservation of the left colic artery for rectal cancer (IMA)[39]. In addition, NIF imaging was used to identify collateral vessels (Arc of Riolan) around the inferior mesenteric vein[40]. The left colic artery branches mainly at the Griffith point (watershed), which is in the splenic bend, vulnerable to injury and ischemia during surgery. Using NIF to identify collateral vessels in real-time can help ensure safe ligation of the IMA and prevent injury. There are still many unanswered problems, but we hope incorporating the NIF approach into robotic technology will improve clinical and oncologic outcomes.
Figure 5.
Near-infrared fluorescence imaging-guided surgery in colorectal surgery. (A) Dissection around the root of the inferior mesenteric artery (white light image) (B) A near-infrared fluorescence image visualizing the left colic artery using excited fluorescence (C) A white light image before visualizing the ischemic zone of the sigmoid colon using excited fluorescence (D) An intraoperative near-infrared fluorescence image after visualizing the ischemic zone of the sigmoid colon using excited fluorescence (E) A white light image after D3 lymphadenectomy around the superior mesenteric vessels (F) A near-infrared fluorescence image after visualizing the remaining lymph nodes after lymphadenectomy using excited fluorescence.
Robotic Surgery Using Other Advanced and Incorporated Technology
Real-time stereotactic navigation may be useful in enhancing precision and the full understanding of the complex anatomies, during robotic TME[41]. Atallah et al. described real-time stereotactic navigation using the da VinciⓇ Xi platform with the TileProⓇ interface, as well as a blueprint for robotic navigation in TaTME with real-time navigation combining the robotic and Stryker Navigation Systems[42]. We expect that robotic systems and surgical data platforms based on artificial intelligence will complement each other to help surgeons provide more optimized patient care.
Robotic Surgery for Extensive and Selective Procedures
Patients with local invasion of adjacent structures, bowel obstruction, perforation of the colon, and/or significant hemorrhage were traditionally treated with open surgery and considered ineligible for laparoscopic surgery. Robotic colorectal surgery for locally invasive colorectal cancer offers technical advantages for complex surgeries such as combined resection for direct invasion to adjacent organs or extensive lymphadenectomy with optimal wrist movements, 3D visualization, and tremor stabilization, permitting adequate exposure and dissection in selected cases. Surgical radicality is important in the treatment of colorectal cancer, and recent studies showed robotic surgeries for extensive and complex procedures such as para-aortic or LPND or pelvic exenteration[43-45]. In contrast, the benefits of sphincter-preserving surgeries can be maximized when combined with robotic surgery, which enables more precise and selective surgeries such as intersphincteric resection, partial excision of the levator ani muscle for low-lying rectal cancer invading the ipsilateral levator ani muscle (Figure 6), and partial Denonvilliers' fascia excision (Figure 7) for anteriorly-located rectal cancer, especially in the setting of neoadjuvant chemoradiotherapy[1,2,46].
Figure 6.
Tailored Excision of the levators for very low rectal cancer. (A) Schematic representation of transection lines of robotic TME with transabdominal division of the levators for very low rectal cancer before chemoradiotherapy (B) After chemoradiotherapy (C) Intraoperative views during a robotic TME with transabdominal division of the levators for very low rectal cancer (D) Specimen with a resected levator ani muscle (E) Postoperative view.
Figure 7.
Surgical plane of customized excision of Denonvilliers’ fascia according to the location and clinical stage of the tumor located in the anterior rectum. (A) A cT3-4 rectal tumor located at the seminal vesicle level without invasion to the adjacent organ (B) A cT3-4 rectal tumor located at the prostate level (C) A c3-4 rectal tumor that involves half of the rectal wall at the seminal vesicle level.
Competing with Other Robotic Platforms
The da Vinci robotic system has been the sole leader in its field for years; however, new platforms are now being developed, with some focusing on single-port and natural orifice surgery. In October 2017, the Food and Drug Administration (FDA) approved the SenhanceⓇ Surgical Robotic System (TransEnterix, Morrisville, NC, USA), and several studies on this robotic platform for colorectal diseases were published. Samalavicius et al.[47] performed a study on 57 patients who underwent robotic surgery using the Senhance system for colorectal diseases, including malignancy, and confirmed the safety and feasibility of this platform. The FlexⓇ Robotic System (Medrobotics Corp., Raynham, MA, USA) is a miniaturized flexible endoscopic robot optimized for transanal endoscopic microsurgery; it gained FDA approval in 2017. Morino et al.[48] performed a full-thickness excision in 14 patients and a submucosal dissection in 12 patients with rectal tumors and reported a distal margin positivity of 15.4%; there was also a 23.1% rate of conversion to conventional transanal endoscopic operation using the TEOⓇ (Karl Storz, Tuttlingen, Germany) system. Single Port Orifice Robotic Technology-SPORTⓇ (Titan Medical Company, Toronto, ON, Canada), VersiusⓇ (Cambridge Medical Robotics, Cambridge, UK), Revo-IⓇ (model MSR-5000; Meerecompany Inc., Seongnam, Republic of Korea), MiroSurgeⓇ (Medtronic, Minneapolis, MN, USA), and Medicaroid (Kobe, Japan) are under investigation for clinical application. Beyond this era of monopoly, it is expected that the cost of robotic surgery will reduce, and that further development will occur in this field as various robotic platforms will compete with each other.
Cost
Although robot-assisted surgery is widely utilized in a variety of surgical procedures, its exorbitant price has diminished its appeal. According to cost-analysis studies, robotic surgery for colorectal cancer was more expensive than open and laparoscopic procedures. The ROLARR study revealed that the robotic group had greater expenses (£11,853 or US$13,668) than the laparoscopic group (£10,874 or US$12,556)[16]. Kim et al.[49] showed comparable short-term clinical results comparing robotic and laparoscopic surgery groups, but robotic surgery was associated with greater expenditures, including total hospital charges, patients' payment, operation fees, anesthetic fees, and postoperative management expenses. If the cost of robotic surgery is reduced by the expansion of indications and increased competition among different robotic platforms, it will benefit more patients and reduce social health costs.
Wristed Laparoscopic Instruments
Several laparoscopic joint instruments have been introduced as alternatives to robotic systems for minimally invasive surgeries[50]. These tools have several properties that allow them to be articulated or bent into a curved arc. The instrument's handle control allows the handle to control joint movement, whereas the surgeon can control the wrist via a joystick or ball operated with the user's thumb. The ArtiSential (LIVSMED Inc., Republic of Korea) is an 8-mm-diameter pistol-handle instrument with complete articulating function similar to the human wrist and intuitive controllability that was registered with the United States FDA as a Class I medical device in 2019. Surgeons can use two articulating devices at once and get force feedback, all at a significantly reduced cost. Additionally, it allows surgery to be performed directly at the patient's side. We encountered some cases of single-port laparoscopic appendectomy and multiport laparoscopic low anterior resection for rectal cancer (not published) and believe that this instrument will provide us the advantages of robotic surgery (wristed instrumentation) at an affordable price. With the development of robotic surgery, laparoscopic surgery is also gaining the advantages of robotic surgery such as the 3D camera view and NIF using laparoscopic system, wristed instrumentation, and a camera holder system.
Conclusion
Currently, there is a simultaneous and complementary development of robotic systems and surgical techniques for the treatment of colorectal cancer. In general, laparoscopic surgery is the treatment of choice for colorectal cancer; however, robots are increasingly being used for difficult procedures where the limitations of conventional laparoscopic surgery are exposed. If the cost of robotic surgery is reduced via the expansion of indications and increase in competition among different robotic platforms, it will become a platform that complements laparoscopic surgery and benefits more patients.
This article is based on a study first reported in the J Korean Med Assoc 2022; volume (65): pages (577-585)[51], Current Status and Future of Robotic Surgery for Colorectal Cancer.
The original version is available at https://jkma.org/journal/view.php?number=3365
The Editors-in-Chief of Journal of the Korean Medical Association and JMA Journal and the publisher of the original version have permitted the publication of this manuscript.
Conflicts of Interest
There are no conflicts of interest.
Source of Funding
This research was supported by the Bisa Research Grant of Keimyung University in 2022 (20220360).
Author Contributions
Sung Uk Bae: conception and design of the study, drafting and revising the study, and final approval of the version to be submitted for publication
Study conception and design: Bae; Acquisition of data: Bae; Analysis and interpretation of data: Bae; Drafting of the article: Bae; Critical revision: Bae; Final approval of the version to be published: Bae.
References
- 1.Varela C, Kim NK. Surgical treatment of low-lying rectal cancer: updates. Ann Coloproctol. 2021 Dec;37(6):395-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piozzi GN, Kim SH. Robotic intersphincteric resection for low rectal cancer: technical controversies and a systematic review on the perioperative, oncological, and functional outcomes. Ann Coloproctol. 2021 Dec;37(6):351-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh CK, Huh JW, Lee YJ, et al. Long-term oncologic outcome of postoperative complications after colorectal cancer surgery. Ann Coloproctol. 2020 Aug;36(4):273-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevenson ARL, Solomon MJ, Lumley JW, et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015 Oct;314(13):1356-63. [DOI] [PubMed] [Google Scholar]
- 5.De Robles MS, Young CJ. Triple-staple technique effectively reduces operating time for rectal anastomosis. Ann Coloproctol. 2021 Jan;37(1):16-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eldamshety O, Kotb S, Khater A, et al. Early and late functional outcomes of anal sphincter-sparing procedures with total mesorectal excision for anorectal adenocarcinoma. Ann Coloproctol. 2020 Jun;36(3):148-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaloun HE, Lee IK, Kim MK, et al. Influence of the enhanced recovery after surgery protocol on postoperative inflammation and short-term postoperative surgical outcomes after colorectal cancer surgery. Ann Coloproctol. 2020 Aug;36(4):264-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004 May;350(20):2050-9. [DOI] [PubMed] [Google Scholar]
- 9.Komenaka IK, Giffard K, Miller J, et al. COLOR: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Dig Surg. 2000;17(6):617-22. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Choi GS, Park JS, et al. Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: lessons from a single surgeon's experience. Dis Colon Rectum. 2014 Sep;57(9):1066-74. [DOI] [PubMed] [Google Scholar]
- 11.Fleshman J, Sargent DJ, Green E, et al. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007 Oct;246(4):655-62; discussion 62-4. [DOI] [PubMed] [Google Scholar]
- 12.Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014 Jun;15(7):767-74. [DOI] [PubMed] [Google Scholar]
- 13.Cho MS, Baek SJ, Hur H, et al. Short and long-term outcomes of robotic versus laparoscopic total mesorectal excision for rectal cancer: a case-matched retrospective study. Medicine (Baltimore). 2015 Mar;94(11):e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park CH, Bae SU, Jeong WK, et al. Early and late clinico-pathologic outcomes of minimally invasive total mesorectal excision for rectal cancer: a propensity score-matched comparison of robotic and laparoscopic approaches. Int J Med Robot. 2021 Dec;17(6):e2324. [DOI] [PubMed] [Google Scholar]
- 15.Lee D, Kim SK, Kim K, et al. Advantages of single-port laparoscopic myomectomy compared with conventional laparoscopic myomectomy: a randomized controlled study. J Minim Invasive Gynecol. 2018 Jan;25(1):124-32. [DOI] [PubMed] [Google Scholar]
- 16.Jayne D, Pigazzi A, Marshall H, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA. 2017 Oct;318(16):1569-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowalewski KF, Seifert L, Ali S, et al. Functional outcomes after laparoscopic versus robotic-assisted rectal resection: a systematic review and meta-analysis. Surg Endosc. 2021 Jan;35(1):81-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim DR, Bae SU, Hur H, et al. Long-term oncological outcomes of robotic versus laparoscopic total mesorectal excision of mid-low rectal cancer following neoadjuvant chemoradiation therapy. Surg Endosc. 2017 Apr;31(4):1728-37. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Baek SJ, Kang DW, et al. Robotic resection is a good prognostic factor in rectal cancer compared with laparoscopic resection: long-term survival analysis using propensity score matching. Dis Colon Rectum. 2017 Mar;60(3):266-73. [DOI] [PubMed] [Google Scholar]
- 20.Bae SU, Saklani AP, Lim DR, et al. Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol. 2014 Jul;21(7):2288-94. [DOI] [PubMed] [Google Scholar]
- 21.Park JS, Kang H, Park SY, et al. Long-term oncologic after robotic versus laparoscopic right colectomy: a prospective randomized study. Surg Endosc. 2019 Sep;33(9):2975-81. [DOI] [PubMed] [Google Scholar]
- 22.Tschann P, Szeverinski P, Weigl MP, et al. Short- and long-term outcome of laparoscopic- versus robotic-assisted right colectomy: a systematic review and meta-analysis. J Clin Med. 2022 Apr;11(9):2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferri V, Quijano Y, Nunez J, et al. Robotic-assisted right colectomy versus laparoscopic approach: case-matched study and cost-effectiveness analysis. J Robot Surg. 2021 Feb;15(1):115-23. [DOI] [PubMed] [Google Scholar]
- 24.Bae SU, Jeong WK, Baek SK. Reduced-port robotic right colectomy with intracorporeal anastomosis for right-sided colon cancer using the da Vinci Single-SiteⓇ Platform: a pilot case series study. Ann Robot Innov Surg. 2021 Nov;2(2):31-9. [Google Scholar]
- 25.Bae SU, Jegon WK, Baek SK. Single plus one-port robotic surgery using the da Vinci Single-Site Platform versus conventional multi-port laparoscopic surgery for left-sided colon cancer. Wideochir Inne Tech Maloinwazyjne. 2022 Mar;17(1):179-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piozzi GN, Kim JS, Choo JM, et al. Da Vinci SP robotic approach to colorectal surgery: two specific indications and short-term results. Tech Coloproctol. 2022 Jun;26(6):461-70. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ, Choi GS, Song SH, et al. An initial experience with a novel technique of single-port robotic resection for rectal cancer. Tech Coloproctol. 2021 Jul;25(7):857-64. [DOI] [PubMed] [Google Scholar]
- 28.Marks JH, Kunkel E, Salem JF, et al. First clinical experience with single-port robotic transanal minimally invasive surgery: phase II trial of the initial 26 cases. Dis Colon Rectum. 2021 Aug;64(8):1003-13. [DOI] [PubMed] [Google Scholar]
- 29.Lee L, Kelly J, Nassif GJ, et al. Establishing the learning curve of transanal minimally invasive surgery for local excision of rectal neoplasms. Surg Endosc. 2018 Mar;32(3):1368-76. [DOI] [PubMed] [Google Scholar]
- 30.Atallah S, Nassif G, Polavarapu H, et al. Robotic-assisted transanal surgery for total mesorectal excision (RATS-TME): a description of a novel surgical approach with video demonstration. Tech Coloproctol. 2013 Aug;17(4):441-7. [DOI] [PubMed] [Google Scholar]
- 31.Persiani R, Agnes A, Belia F, et al. The learning curve of TaTME for mid-low rectal cancer: a comprehensive analysis from a five-year institutional experience. Surg Endosc. 2021 Nov;35(11):6190-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez Ruiz M, Parra IM, Palazuelos CM, et al. Robotic-assisted laparoscopic transanal total mesorectal excision for rectal cancer: a prospective pilot study. Dis Colon Rectum. 2015 Jan;58(1):145-53. [DOI] [PubMed] [Google Scholar]
- 33.Son GM, Ahn HM, Lee IY, et al. Multifunctional indocyanine green applications for fluorescence-guided laparoscopic colorectal surgery. Ann Coloproctol. 2021 Jun;37(3):133-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kusano M, Tajima Y, Yamazaki K, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging: a new method for sentinel node navigation surgery in gastrointestinal cancer. Dig Surg. 2008;25(2):103-8. [DOI] [PubMed] [Google Scholar]
- 35.Kim HJ, Park JS, Choi GS, et al. Fluorescence-guided robotic total mesorectal excision with lateral pelvic lymph node dissection in locally advanced rectal cancer: a video presentation. Dis Colon Rectum. 2017 Dec;60(12):1332-3. [DOI] [PubMed] [Google Scholar]
- 36.Zhou SC, Tian YT, Wang XW, et al. Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer. World J Gastroenterol. 2019 Aug;25(31):4502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SY, Park JS, Kim HJ, et al. Indocyanine green fluorescence imaging-guided laparoscopic surgery could achieve radical D3 dissection in patients with advanced right-sided colon cancer. Dis Colon Rectum. 2020 Apr;63(4):441-9. [DOI] [PubMed] [Google Scholar]
- 38.Bae SU, Jeong WK, Baek SK. Intra-operative near-infrared fluorescence imaging for robotic complete mesocolic excision and central vascular ligation in right-sided colon cancer - a video vignette. Colorectal Dis. 2019 Dec;21(12):1459. [DOI] [PubMed] [Google Scholar]
- 39.Bae SU, Saklani AP, Hur H, et al. Robotic interface for transabdominal division of the levators and pelvic floor reconstruction in abdominoperineal resection: a case report and technical description. Int J Med Robot. 2015 Sep;11(3):296-301. [DOI] [PubMed] [Google Scholar]
- 40.Bae SU, Min BS, Kim NK. Near infrared fluorescence imaging for real-time assessment of blood flow during totally robotic total mesorectal excision for rectal cancer--a video vignette. Colorectal Dis. 2016 Mar;18(3):313. [DOI] [PubMed] [Google Scholar]
- 41.Atallah S, Parra-Davila E, Melani AGF, et al. Robotic-assisted stereotactic real-time navigation: initial clinical experience and feasibility for rectal cancer surgery. Tech Coloproctol. 2019 Jan;23(1):53-63. [DOI] [PubMed] [Google Scholar]
- 42.Atallah S, Zenoni S, Kelly J, et al. A blueprint for robotic navigation: pre-clinical simulation for transanal total mesorectal excision (taTME). Tech Coloproctol. 2016 Sep;20(9):653-4. [DOI] [PubMed] [Google Scholar]
- 43.Bae SU, Jeong WK, Baek SK, et al. Robotic complete mesocolic excision and para-aortic lymph node dissection for cecal cancer and paraganglioma. Dis Colon Rectum. 2018 Oct;61(10):1235-6. [DOI] [PubMed] [Google Scholar]
- 44.Kim MC, Oh JH. Lateral pelvic lymph node dissection after neoadjuvant chemoradiotherapy in patients with rectal cancer: a single-center experience and literature review. Ann Coloproctol. 2021 Dec;37(6):382-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin JW, Kim J, Kwak JM, et al. First report: robotic pelvic exenteration for locally advanced rectal cancer. Colorectal Dis. 2014 Jan;16(1):O9-14. [DOI] [PubMed] [Google Scholar]
- 46.Yang SY, Kim NK. Robotic partial excision of levator-ani muscle for locally advanced low rectal cancer invading ipsilateral pelvic floor. Ann Coloproctol. 2020 Dec;36(6):415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samalavicius NE, Dulskas A, Janusonis V, et al. Robotic colorectal surgery using the Senhance((R)) robotic system: a single center experience. Tech Coloproctol. 2022 Apr;26(6):437-42. [DOI] [PubMed] [Google Scholar]
- 48.Morino M, Forcignano E, Arezzo A. Initial clinical experience with a novel flexible endoscopic robot for transanal surgery. Tech Coloproctol. 2022 Apr;26(4):301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim CW, Baik SH, Roh YH, et al. Cost-effectiveness of robotic surgery for rectal cancer focusing on short-term outcomes: a propensity score-matching analysis. Medicine (Baltimore). 2015 Jun;94(22):e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson PL, Lathrop RA, Webster RJ, III. Robot-like dexterity without computers and motors: a review of hand-held laparoscopic instruments with wrist-like tip articulation. Expert Rev Med Devices. 2016 Jul;13(7):661-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bae SU. Robotic surgery for colorectal cancer. JKMA. 2022 Sep; 65(9): 577-85. [Google Scholar]