Abstract
Background
Chronic kidney disease (CKD) is widely reported to decrease quality of life, increase morbidity and mortality and cause increased healthcare resource utilisation (HCRU) as the disease progresses. However, there is a relative paucity of accurate and recent estimates of HCRU in this patient population. Our aim was to address this evidence gap by reporting HCRU and related costs in patients with CKD from the UK primary and secondary care settings.
Methods
HCRU and cost estimates of CKD were derived for UK patients included in the DISCOVER CKD cohort study using clinical records from the Clinical Practice Research Datalink linked to external databases. Patients with a history of transplant or undergoing dialysis were not included. HCRU and costs were stratified by CKD severity using the urinary albumin:creatinine ratio (UACR) and estimated glomerular filtration rate.
Results
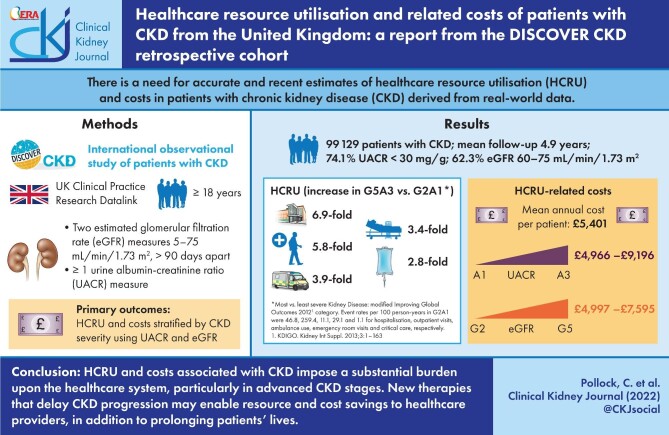
Hospitalisation rates more than tripled between low (A1) and high (A3) UACR categories and the mean annual per-patient costs ranged from £4966 (A1) to £9196 (A3) and from £4997 (G2) to £7595 (G5), demonstrating that a large healthcare burden can be attributed to a relatively small number of patients with later stage CKD, including those with kidney failure and/or albuminuria.
Conclusions
HCRU and costs associated with CKD impose a substantial burden on the healthcare system, particularly in the more advanced stages of CKD. New interventions that can delay the progression of CKD to kidney failure may not only prolong the patient’s life, but would also provide significant resource and cost savings to healthcare providers.
Keywords: chronic kidney disease, Clinical Practice Research Datalink, cost analysis, healthcare resource use, hospitalisations
Graphical Abstract
Graphical Abstract.
INTRODUCTION
In the UK, like elsewhere, the prevalence of chronic kidney disease (CKD) is increasing [1, 2], driven by an ageing population and an increasing incidence of type 2 diabetes mellitus (T2D) [3–5]. Contemporary modelling projections from the UK predict that by 2025, CKD prevalence will increase a further 1%, to a staggering 14% of the adult population. In addition, projections also predict a changing profile of the prevalent CKD population, with an increase in the more advanced stages (3b–5) of ∼7% relative to the total CKD population by 2025 [6].
This increasing prevalence and changing patient profile are of concern to healthcare providers as patient outcomes worsen and costs increase as the disease progresses toward kidney failure. This increase in costs is driven by more frequent and expensive interactions with healthcare services, where costly and invasive procedures, such as dialysis, ultimately become a requirement for survival [7–9]. This is further exacerbated by the costs associated with the management of comorbidities and complications that are relatively frequent in this patient population, such as diabetes, anaemia and cardiovascular complications including heart failure (HF) [4, 10–14].
Despite a high unmet need in this patient population, the treatment landscape for CKD has not changed substantially until quite recently [8]. Sodium–glucose co-transporter-2 inhibitors (SGLT2is) and selective mineralocorticoid receptor antagonists (MRAs) have emerged as new classes of interventions in the management of CKD [15, 16]. While these are well-established in the management of T2D, recent phase 3 trials have also shown them to provide significant and sustained improvements in kidney- and cardiovascular-related outcomes for patients both with and without T2D [17–23]. In addition to the importance of efficacious therapies, categorisation of patients by estimated glomerular filtration rate (eGFR) and urinary albumin:creatinine ratio (UACR) is crucial for accurate prognosis and to guide patient management.
Economic tools underpinned by robust data sources are required to support the formal evaluation of such new and effective interventions and their subsequent integration into routine clinical practice. It is here that an accurate and thorough understanding of healthcare resource utilisation (HCRU) can be of great value, as economic evaluations in the CKD population are often generated using older HCRU estimates or data not derived from real-world evidence [8, 24–28]. Therefore this study sought to describe the HCRU of patients with CKD in the UK, stratified by both eGFR and UACR, to provide a valuable source of contemporary HCRU data to support accurate, evidence-driven health economic evaluations of next-generation therapies for CKD and to better inform the healthcare sector with resourcing requirements for more efficient service delivery.
MATERIALS AND METHODS
Study population
DISCOVER CKD is a hybrid, multinational, observational cohort study in patients with CKD. The patients included in the analysis reported here are a subset derived from DISCOVER CKD based on the UK retrospective patient cohort, which corresponds to patients recorded in the Clinical Practice Research Datalink (CPRD GOLD) electronic health records (EHR) database. The full DISCOVER CKD study was comprised of a retrospective patient cohort capturing secondary data from established anonymised datasets and a prospective cohort collecting primary and secondary data in patients individually recruited from participating centres in Italy, Japan, Sweden, Spain, the UK and the USA (ClinicalTrials.gov identifier: NCT04034992; ISAC protocol number: 19_226A4).
The DISCOVER CKD eligibility criteria have been previously described [29], but in brief the eligible patient cohort included adult patients with CKD after 1 January 2008 and ≥1 year of medical history available prior to the index date. Diagnosis was defined as documented diagnostic code (e.g. International Classification of Diseases, Tenth Revision) for CKD stages 3A through to kidney failure or two consecutive eGFR measures of <75 mL/min/1.73 m2 recorded >90 days apart (maximum 730 days).
The following additional inclusion criteria were applied for this analysis: two consecutive eGFR measures of 5–75 ml/min/1.73 m2 recorded >90 days apart (maximum 730 days) and one or more UACR measurement within 1 year before or any time up to 5 years after the index date. The UACR measure closest to the index was used to categorise patients.
The following additional exclusion criteria were applied: patients without two measures of eGFR <75 mL/min/1.73 m2 recorded at least 90 days apart on or after 1 January 2008, death within 30 days of the index date (where available in the data source), a history of type 1 diabetes mellitus or a history of renal transplant or dialysis at the study index.
Baseline patient demographics and laboratory parameters were defined as the most recent variable prior to the index within a 1-year lookback period (1 year prior to the index). Medication usage at baseline was defined as any treatment received at the index or within the 1-year lookback period. Comorbidity history at baseline was defined as any history prior to the index spanning the patient's entire available medical history. For any repeated measures, the non-missing data closest to the index date were used.
The modified Kidney Disease: Improving Global Outcomes (KDIGO) 2012 classification system was used to assess CKD severity. The KDIGO classification system is an internationally recognized framework for appraising the severity and prognosis of CKD incorporating both eGFR and UACR measurements (Fig S1), which has been previously used to estimate disease-related risk and provide clinical guidance for the management of CKD in the UK [2, 8].
Study period
The study period covered in this analysis spanned January 2008–January 2020. Patients were followed up until the end of data collection, database end, loss to follow-up or death, whichever occurred first. The index date corresponded with the patient's baseline and was defined as the date of the second eGFR measurement recorded >90 days after the first measurement (maximum 730 days).
Data sources
This study used EHR data from CPRD GOLD, representing the primary care population with records coming directly from general practitioners (GPs), and linked Hospital Episode Statistics (HES) databases, representing the collection of secondary care data across the patient cohort: Admitted Patient Care (APC), Outpatient (OP), Accident and Emergency (A&E) and Office of National Statistics (ONS) death data.
This study is based in part on data from the CPRD obtained under licence from the UK Medicines and Healthcare products Regulatory agency. The data are provided by patients and collected by the National Health Service (NHS) as part of their routine care and support. The interpretation and conclusions contained in this study are those of the authors alone. Hospital Episode Statistics (HES)/Office of National Statistics (ONS) data are reused with the permission of the Health and Social Care Information Centre. All rights reserved.
Study endpoints
Study endpoints included in this analysis were patient follow-up (exposure time, patient-years), total length of hospital admission across all episodes, HCRU and associated costs. Number and incidence rates of HCRU included outpatient visits, hospitalisations, emergency room (ER) visits, intensive care unit stays (critical care), required use of an ambulance and GP visits. Total and annualised costs over the observation period were calculated from each HCRU category. HCRU and associated costs correspond to the overall resource utilisation of patients with CKD included in this study, not limited to those relating specifically to nephrological treatment.
Statistical analysis
Descriptive analysis for annualised HCRU/costs were undertaken for the aforementioned categories. The cost perspective was that of the NHS. Costs associated with HCRU were generated by applying the relevant Personal Social Services Research Unit (GP visits) and 2017/2018 NHS reference costs (outpatient visits, hospitalisations, ER visits, critical care and ambulance use); costs were inflated to 2019 values [30, 31]. Healthcare Resource Group (HRG) codes were mapped to costs on a per event basis and grouped into the corresponding HCRU categories. Patients were not censored for transplant or dialysis initiation during the study period, but the specific costs for such procedures were not included in this analysis. The costs predominantly reflect HCRU incurred by patients aside from the costs of treatment in specialist dialysis centres. Costs incurred during the follow-up period were summed per patient. From these, total and annual costs were calculated across the cohort. Mean costs were omitted from this analysis for subgroups where data were available for <11 patients; this included critical care costs for patients in the eGFR G5 category. Annualised event rates were expressed as the incidence of the outcome per 100 person-years. eGFR measures <5 mL/min/1.73 m2 were excluded from the analyses.
RESULTS
Baseline characteristics
DISCOVER CKD captured a large patient cohort representative of the broad spectrum of CKD in UK clinical practice, [32] including 99 129 patients with CKD (Table 1). At baseline, the most common comorbidities were hypertension (58.7%) and T2D (37.3%), with a history of HF reported less frequently (5.1%). The majority of patients (90.8%) had an eGFR of 45–75 mL/min/1.73 m2 and had a UACR <30 mg/g (74.1%). A relatively high proportion of patients had an elevated UACR (A2–A3) but near-normal eGFR (G2; 13.8%), this was particularly prominent in patients with comorbid CKD and HF (15.2%) (Supplementary data, Table S1).
Table 1.
Baseline characteristics
| Variable | Overall CKD cohort (n = 99 129) | Comorbid CKD and T2D (n = 36 960) | Comorbid CKD and HF (n = 5033) |
|---|---|---|---|
| Follow-up (years), mean (SD) | 4.9 (2.9) | 4.5 (2.9) | 4.2 (2.7) |
| Demographics | |||
| Female, n (%) | 50 309 (50.8) | 15 875 (43.0) | 1934 (38.4) |
| Age (years), mean (SD) | 68.5 (11.3) | 68.0 (10.0) | 72.5 (10.3) |
| KDIGO eGFR category (mL/min/1.73 m2), n (%) | |||
| G2a (60–75) | 61 797 (62.3) | 28 487 (77.1) | 2473 (49.1) |
| G3a (45–<60) | 28 248 (28.5) | 6664 (18.0) | 1650 (32.8) |
| G3b (30–<45) | 7514 (7.6) | 1473 (4.0) | 720 (14.3) |
| G4 (15–<30) | 1408 (1.4) | 298 (0.8) | 170 (3.4) |
| G5 (<15) | 162 (0.2) | 38 (0.1) | 20 (0.4) |
| KDIGO UACR category (mg/g), n (%) | |||
| A1 (<30) | 73 440 (74.1) | 27 605 (37.6) | 3332 (66.2) |
| A2 (30–<300) | 21 958 (22.2) | 7728 (35.2) | 1429 (28.4) |
| A3 (≥300) | 3731 (3.8) | 1627 (43.6) | 272 (5.4) |
| Laboratory values, median (IQR) | |||
| eGFR (mL/min/1.73 m2) | 64.0 (55.1–70.2) | 67.8 (60.9–71.8) | 59.8 (48.6–68.1) |
| UACR (mg/g) | 10.4 (4.5–30.1) | 10.6 (5.1–28.3) | 17.7 (6.2–54.0) |
| Hb (g/dl) | 13.7 (12.7–14.7) | 13.6 (12.6–14.7) | 13.3 (12.1–14.4) |
| Medical history/comorbidities at baseline, n (%) | |||
| History of HF | 5033 (5.1) | 2193 (5.9) | 5033 (100.0) |
| History of coronary heart disease | 17 294 (17.4) | 7549 (20.4) | 2923 (58.1) |
| History of myocardial infarction | 7380 (7.4) | 3187 (8.6) | 1690 (33.6) |
| History of hypertension | 58 217 (58.7) | 23 605 (63.9) | 3214 (63.9) |
| History of T2D | 36 960 (37.3) | 36 960 (100.0) | 2193 (43.6) |
| History of stroke | 5046 (5.1) | 2083 (5.6) | 505 (10.0) |
| History of hyperkalaemia | 449 (0.5) | 230 (0.6) | 81 (1.6) |
| Baseline medication use, n (%) | |||
| RAAS inhibitors (ACE inhibitors and ARBs) | 50 769 (51.2) | 23 929 (64.7) | 4308 (85.6) |
| Diuretics (MRAs, loop diuretics and thiazide diuretics) | 17 426 (17.6) | 13 896 (37.6) | 3952 (78.5) |
| Anticoagulants | 6149 (6.2) | 2842 (7.7) | 1726 (34.3) |
| Antiplatelets (aspirin, clopidogrel and other agents) | 33 086 (33.4) | 14 518 (39.3) | 3113 (61.9) |
| Anti-hypertensive therapies [calcium channel blockers (DHP and non-DHP), beta blockers and alpha blockers] | 42 823 (43.2) | 20 325 (55.0) | 4117 (81.8) |
Restricted G2 KDIGO category based on eGFR cut-off from study inclusion criteria.
CKD: chronic kidney disease; CPRD: clinical practice research datalink; eGFR: estimated glomerular filtration rate; HF: heart failure; KDIGO: Kidney Disease Improving Global Outcomes; T2D: type 2 diabetes; UACR: urine albumin-creatinine ratio.
HCRU
Patients in the most severe KDIGO category (G5A3) had elevated HCRU compared with those in the least severe category (G2A1), driven by the rates of hospitalisation and outpatient visits, which were associated with a 6.9- and 5.8-fold increase, respectively. Similarly, patients in the G5A3 category displayed a 3.9- and 3.4-fold increase in ambulance use and ER visits, respectively, compared with the least severe category (Supplementary data, Table S2). For GP visits, there was a ≤1.4-fold increase across any KDIGO category. The mean length of hospital stay was also greater at worsened severities, increasing from 14.7 (G2A1) to 54.3 (G5A3) days.
Healthcare costs
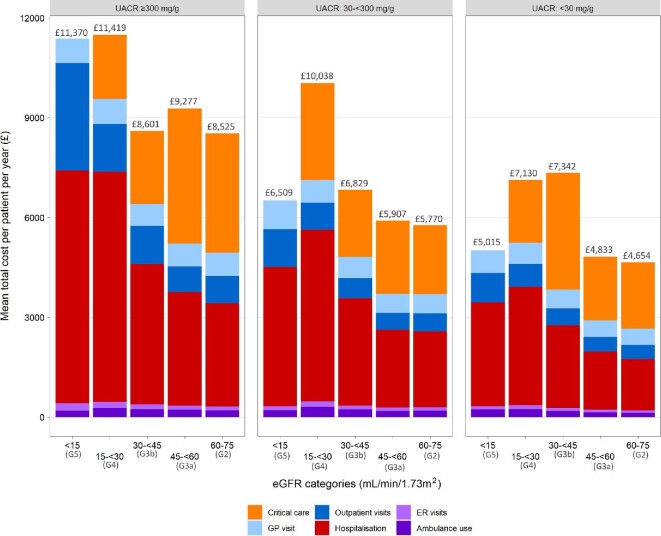
Overall mean healthcare costs regardless of CKD stage were £5401 per patient per year (PPPY) and ranged from £4654 (G2A1) to £11 419 (G4A3; Fig. 1). Critical care costs for patients with G5A3 CKD were excluded due to the small number of events in this small subgroup of patients; nevertheless, costs were high in this most severe population. Hospitalisation and critical care accounted for the majority of healthcare costs across the cohort (Table 2, Table 3 and Fig. 2). Costs for each healthcare resource generally rose with increasing CKD severity, including hospitalisations, outpatient visits, critical care, GP visits, ER visits and ambulance use (Table 3).
Figure 1:
Mean annual per patient healthcare costs for the overall CKD cohort, stratified by KDIGO classification groups. eGFR <15 critical care costs excluded because of the small number of events (<11 patients with event).
Table 2.
Overall HCRU and costs
| Variable | Mean (SD) | Median (IQR) | Range |
|---|---|---|---|
| Follow-up (years) | 4.9 (2.9) | 4.7 (2.6–6.9) | 0.0–11.7 |
| Outpatient visits | |||
| Patients with outpatient visits, n (%) | 37 790 (38.1) | ||
| Visit days per patient from index to end of follow-up | 16.1 (20.6) | 9.0 (4.0–20.0) | 1.0–706.0 |
| Visit days PPPY | 3.6 (4.6) | 2.3 (1.0–4.6) | 0.1–121.8 |
| Total cost per patient from index to end of follow-up | £2121 (2992) | £1190 (474–2633) | £0–107 429 |
| Total cost PPPY | £481 (695) | £285 (125–596) | £0–37 005 |
| Hospitalisations | |||
| Patients with hospitalizations, n (%) | 28 391 (28.6) | ||
| Admissions per patient from index to end of follow-up | 4.3 (9.6) | 2.0 (1.0–5.0) | 1.0–557.0 |
| Admissions PPPY | 1.1 (2.3) | 0.6 (0.3–1.1) | 0.1–107.8 |
| Total cost per patient from index to end of follow-up | £7217 (9085) | £4096 (1433–9609) | £0–168 852 |
| Total cost PPPY | £1958 (3811) | £876 (304–2224) | £0.0–187 406 |
| Length of stay across all hospital admissions (days) | 19.0 (36.4) | 6.0 (2.0–20.0) | 0.0–1532.0 |
| Emergency room visits | |||
| Patients with emergency room visits, n (%) | 24 930 (25.1) | ||
| Visit days per patient from index to end of follow-up | 2.9 (3.4) | 2.0 (1.0–3.0) | 1.0–108.0 |
| Visit days PPPY | 0.7 (1.1) | 0.4 (0.2–0.8) | 0.1–48.5 |
| Total cost per patient from index to end of follow-up | £342 (438) | £216 (133–422) | £0–13 498 |
| Total cost PPPY | £84 (139) | £47 (22–96) | £0.0–5675 |
| GP visits | |||
| Patients with GP visits, n (%) | 98 312 (99.2%) | ||
| Visit days per patient from index to end of follow-up | 56.4 (51.8) | 42.0 (21.0–75.0) | 1.0–997.0 |
| Visit days PPPY | 12.1 (9.3) | 9.8 (6.3–15.2) | 0.1–182.6 |
| Total cost per patient from index to end of follow-up | £2420 (2328) | £1785 (888–3198) | £30–42 705 |
| Total cost PPPY | £524 (444) | £410 (259–648) | £4–11 266 |
| Ambulance usage | |||
| Patients with ambulance usage, n (%) | 14 260 (14.4) | ||
| Use per patient from index to end of follow-up | 2.3 (2.8) | 1.0 (1.0–3.0) | 1.0–92.0 |
| Use PPPY | 0.6 (1.0) | 0.3 (0.2–0.7) | 0.1–27.6 |
| Total cost from index to end of follow-up | £588 (707) | £252 (252–756) | £252–24 185 |
| Total cost per year | £162 (250) | £88 (49–179) | £22–7282 |
| Critical care | |||
| Patients with critical care visits, n (%) | 2167 (2.2) | ||
| Visit days per patient from index to end of follow-up | 1.3 (0.8) | 1.0 (1.0–1.0) | 1.0–10.0 |
| Visit days PPPY | 0.4 (0.6) | 0.2 (0.2–0.4) | 0.1–8.5 |
| Total cost per patient from index to end of follow-up | £8097 (8392) | £6339 (3222–9763) | £0–130 865 |
| Total cost PPPY | £2193 (4120) | £1280 (601–2386) | £0–88 032 |
PPPY: per patient per year.
Table 3.
Healthcare costs per resource category, stratified by UACR and eGFR
| Total cost PPPY, mean (SD) | ||||
|---|---|---|---|---|
| Category | UACR 0–<30 mg/g | UACR 30–<300 mg/g | UACR ≥300 mg/g | Overall (per eGFR category) |
| Hospitalisation | ||||
| eGFR 60–75 | £1534 (2657) | £2284 (4461) | £3101 (4797) | £1712 (3154) |
| eGFR 45–<60 | £1750 (2910) | £2340 (4035) | £3413 (5989) | £1937 (3357) |
| eGFR 30–<45 | £2493 (4600) | £3229 (4789) | £4212 (8074) | £2826 (4994) |
| eGFR 15–<30 | £3559 (5445) | £5161 (10 946) | £6918 (20 265) | £4801 (11 520) |
| eGFR <15 | £3119 (3347) | £4188 (4700) | £7000 (10 560) | £4748 (6770) |
| Overall (per UACR category) | £1702 (2998) | £2553 (4833) | £3804 (8401) | – |
| Outpatient visit | ||||
| eGFR 60–75 | £431 (564) | £537 (724) | £826 (1138) | £458 (617) |
| eGFR 45–<60 | £445 (602) | £508 (637) | £772 (875) | £469 (624) |
| eGFR 30–<45 | £507 (738) | £601 (849) | £1148 (2800) | £579 (1066) |
| eGFR 15–<30 | £688 (866) | £813 (1146) | £1432 (1488) | £873 (1145) |
| eGFR <15 | £881 (604) | £1134 (1093) | £3226 (4319) | £1716 (2680) |
| Overall (per UACR category) | £443 (593) | £547 (737) | £959 (1671) | – |
| Ambulance usage | ||||
| eGFR 60–75 | £133 (196) | £191 (318) | £201 (251) | £147 (230) |
| eGFR 45–<60 | £146 (232) | £187 (289) | £226 (310) | £160 (252) |
| eGFR 30–<45 | £184 (247) | £232 (300) | £237 (308) | £202 (269) |
| eGFR 15–<30 | £243 (367) | £305 (484) | £271 (270) | £274 (411) |
| eGFR <15 | £229 (214) | £211 (190) | £191 (224) | £209 (201) |
| Overall (per UACR category) | £145 (219) | £201 (316) | £222 (282) | – |
| ER visit | ||||
| eGFR 60–75 | £73 (114) | £103 (187) | £119 (182) | £80 (133) |
| eGFR 45–<60 | £74 (110) | £99 (167) | £119 (164) | £81 (127) |
| eGFR 30–<45 | £90 (139) | £118 (199) | £152 (260) | £102 (170) |
| eGFR 15–<30 | £116 (198) | £168 (290) | £188 (306) | £150 (260) |
| eGFR <15 | £102 (93) | £117 (107) | £225 (236) | £147 (163) |
| Overall (per UACR category) | £75 (116) | £106 (187) | £133 (207) | – |
| GP visit | ||||
| eGFR 60–75 | £490 (405) | £581 (472) | £696 (623) | £514 (429) |
| eGFR 45–<60 | £494 (420) | £576 (482) | £687 (629) | £522 (449) |
| eGFR 30–<45 | £558 (458) | £630 (514) | £655 (521) | £589 (483) |
| eGFR 15–<30 | £633 (547) | £683 (573) | £757 (794) | £676 (611) |
| eGFR <15 | £686 (637) | £859 (927) | £728 (595) | £774 (767) |
| Overall (per UACR category) | £497 (415) | £588 (486) | £691 (623) | – |
| Critical care | ||||
| eGFR 60–75 | £1993 (3001) | £2075 (2984) | £3582 (11 980) | £2085 (3895) |
| eGFR 45–<60 | £1913 (2434) | £2196 (4214) | £4059 (11 161) | £2105 (3948) |
| eGFR 30–<45 | £3510 (6953) | £2019 (2154) | £2198 (2671) | £2862 (5427) |
| eGFR 15–<30 | £1890 (1550) | £2908 (3920) | £1925 (2089) | £2279 (2797) |
| eGFR <15a | – | – | – | – |
| Overall (per UACR category) | £2104 (3427) | £2132 (3352) | £3388 (9790) | – |
eGFR: estimated glomerular filtration rate; ER: emergency room; GP: general practitioner; PPPY: per patient per year; UACR: urine albumin creatinine ratio.
aeGFR <15 critical care costs are excluded from the individual subgroups because of the small number of events (<11).
Figure 2:
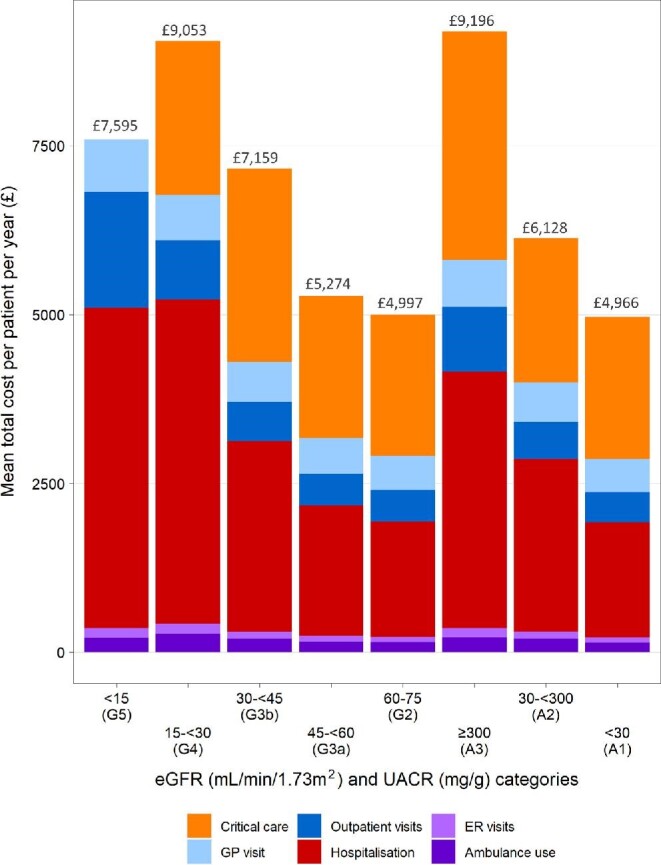
Mean annual per patient healthcare costs for overall CKD cohort, stratified by eGFR and UACR. eGFR <15 critical care costs excluded because of the small number of events (<11 patients with event).
HCRU in people with CKD and T2D
Rates of HCRU were higher in those with comorbid CKD and T2D compared with those with CKD without T2D: 62.1 versus 54.7 hospitalisations and 308.5 versus 274.5 outpatient visits per 100 patient-years, respectively. This general trend was observed across KDIGO categories and was particularly prominent in G4A3 patients, who had a 2.1-fold increase in hospitalisation rates and a 3.8-fold increase in the number of critical care visits between G4A3 patients with comorbid CKD and T2D compared with those with CKD without T2D (Supplementary data, Table S3 and S4).
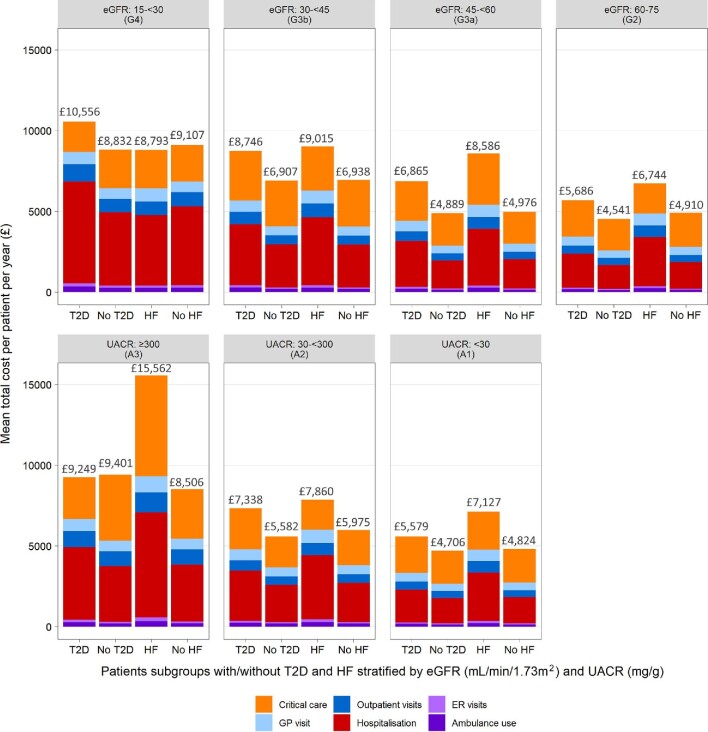
Overall mean healthcare costs regardless of CKD stage were £6149 and £5076 PPPY for those with comorbid CKD and T2D compared with CKD without T2D, respectively. For the overall T2D cohort, cost burden was consistently elevated across all resource categories when compared with patients without T2D (Supplementary data, Table S5 and S6). Hospitalisation and critical care incurred the most significant costs for those with CKD and T2D (mean annual cost £2385 and £2357, respectively). Healthcare costs for patients with and without T2D stratified by eGFR and UACR categories are presented in Fig. 3.
Figure 3:
Mean annual per patient healthcare costs for patient subgroups with/without T2D and HF, stratified by eGFR and UACR. eGFR <15 subgroup excluded because of the small number of events across resource categories and subgroups (<11 patients with event)
HCRU in people with CKD and HF
For patients with comorbid CKD and HF, HCRU rates were greater across each category compared with those with CKD without HF. Rates of ambulance use, critical care and hospitalisation were particularly elevated in patients with HF compared with those without (2.6-, 2.2- and 1.7-fold increase, respectively). The increase in HCRU event rates as CKD worsened was generally greater for patients without HF compared with those with HF; particularly prominent was the difference in rates of hospitalisation and outpatient visits between lower and higher CKD severities (G2A1–G4A3): hospitalisation (2.0- versus 11.6-fold difference) and outpatient visits (1.5- versus 2.9-fold difference) for patients with and without HF, respectively. This indicates that HCRU was already elevated at lower CKD severities for patients with HF (Supplementary data, Table S7 and S8).
Overall mean healthcare costs regardless of CKD stage were £7825 and £5223 PPPY for CKD patients with and without HF, respectively. For the overall HF cohort, the cost burden was consistently elevated across all resource categories (Supplementary data, Table S9 and S10). When stratified by eGFR, HCRU and associated costs were similar in the lower eGFR categories for patients with HF [mean total cost PPPY: £8586 (G3a)–£8793 (G4)] and were considerably less in patients with G2 CKD (£6744), driven by lower hospitalisation costs. When stratified by UACR, total healthcare costs were considerably higher in the most severe patients [mean total cost PPPY: £7127 (A1)–£15 562 (A3)], driven primarily by critical care and hospitalisation costs (Fig. 3).
DISCUSSION
This study shows that CKD represents a significant system-wide healthcare burden beyond the impact of dialysis, transplant and other treatment and management costs usually provided through specialist nephrology care, with a particularly high burden in the advanced stages of CKD. For example, mean rates of hospitalisation were particularly elevated in this CKD cohort and increased in line with CKD severity, with 3.2 hospitalisations PPPY and an average length of stay of 54 days for patients with G5A3 CKD. Paired with critical care, hospitalisation imposed the greatest cost and resource burden. In addition, while individuals with relatively normal kidney function (G2A1) used ambulances at a rate comparable to that of the general population in England, this increased to a 2-fold greater usage at a CKD severity of G3aA2 and to a 4-fold greater usage in G5A3 patients [33], and all KDIGO groups visited GPs at a >2-fold higher rate than the average individual [34]. While higher than the general population, rates of GP visits were generally lower in the more severe KDIGO categories and were independent of comorbid T2D or HF, which may indicate a shift in treatment setting from primary to secondary care, as treatment and follow-up likely transitioned to the secondary setting as CKD worsened. Additionally, when compared with the suggested frequency of eGFR/UACR monitoring visits in contemporary UK guidance, we observed a greater number of outpatient visits across all KDIGO categories in this CKD cohort [35].
As may be expected from previous studies, our data does demonstrate that hospitalisations are the biggest driver behind the cost burden of CKD, adding validity to the findings [25]. However, one of the strengths of this study is its reporting of several aspects of HCRU that are often overlooked because they are considered acute, short-term or lower impact, such as emergency admissions, critical care and ambulance use. These are services that are consistently overstretched and represent areas in which a reduction in CKD-related admissions could be of great benefit to local healthcare providers [36–39]. For example, shortages of beds, staff and ambulances can result in prolonged waiting times for emergency care [39], an issue driven by an ageing and ever-growing population [40]. It is through the detailed reporting of HCRU that this study may help local healthcare systems understand the true burden imposed by CKD.
With increasing prevalence, CKD is expected to comprise a significant and growing impact on both service delivery and costs to the NHS in the future [3–6]. Therefore, any interventions found to significantly delay CKD progression are expected to represent a significant offset to costs and resource use to the NHS. Dialysis imposes a substantial cost burden on the NHS above and beyond the healthcare costs highlighted in our study, with recent estimates of £32 259 PPPY for dialysis [41] and £27 033 for the initial cost of a kidney transplant [42]. The costs of dialysis and transplant alone are more than six times greater than the overall healthcare costs reported here for patients with earlier stage CKD (G2–3a). These high costs emphasise the benefits to the healthcare system of early CKD detection and proactive management to delay patients’ progression to the later stages of CKD, and further signifies the critical need for novel treatment options and effective implementation of new therapies with proven efficacy.
The smaller group of patients with the most advanced disease are responsible for a particularly large cost and resource utilisation burden when compared with the rest of the CKD cohort. This is evident when stratifying patients based on CKD severity, with mean annual per-patient costs increasing substantially to £11 419 at a severity of G4A3, an increase that was also observed when assessing UACR and eGFR separately. Thus increasing HCRU and costs align with all measured parameters of CKD severity. Patients with comorbid HF or T2D were also associated with greater costs than those with CKD without T2D/HF. Given the prevalence of T2D and cardiovascular disease is projected to increase, it can be expected that comorbid CKD will also increase. Patients with comorbidities associated with greater cost, such as T2D/HF and CKD, will place an increasing burden upon the healthcare system.
Some of the major strengths of this study include the extensive follow-up period and the granularity of HCRU and cost reporting. However, this study does not come without limitations. The lack of specific medication, dialysis and transplant costs resulted in an underestimate of the overall costs associated with CKD for patients with kidney failure. In addition, the exclusion of patients undergoing dialysis or transplant at the index resulted in a slight underrepresentation of patients with kidney failure compared with the overall real-world CKD population.
While the data captured from the CPRD were considered robust, the data reflect routine care and were not collected for research purposes. Consequently, data are prone to missingness, which was compounded by the limitations of the linked datasets used to categorise HCRU. For example, the HES was limited by a lack of prescription history and laboratory values, and details of specific outpatient departments utilised by patients during visits were not available, thus limiting the conclusions that can be drawn based on the exact morbidity responsible for healthcare utilisation. In addition, the CPRD does not collect data relating to the socio-economic status of individuals, which may not precisely match a modelled population and can influence HCRU [43]. Reflecting the inclusion criteria of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease trial population, an eGFR range of 60–75 mL/min/1.73 m2 was applied to define G2. Therefore HCRU and costs may be an overestimate if modelled using an eGFR range of 60–89 mL/min/1.73 m2, which typically defines the G2 group [44, 45]. The long-term implications of preventing or delaying CKD progression are not directly addressed in this analysis, only postulated based on HCRU trends. Any conclusions based on the causal mechanisms of CKD in relation to HCRU outcomes should be treated with caution. In a cohort greatly affected by comorbidities and other underlying conditions, it cannot be concluded with certainty that all captured HCRU relates to CKD. However, due to the interrelated nature of CKD and its various sequalae, it was deemed important to capture all HCRU/costs of the cohort. There was no matching made between severity groups, therefore the differences in HCRU and costs as UACR and eGFR worsen may be influenced by confounding factors such as age. Finally, results from this analysis are only reflective of the UK and may have limited generalisability to other settings; in addition, regional/hospital-level practice differences are not accounted for across such a large cohort.
One additional consideration is that the UACR requirements applied to this study were beyond those of the broader DISCOVER CKD inclusion criteria. This greatly limited the number of eligible patients and resulted in ∼75% of the otherwise eligible DISCOVER CKD cohort being excluded from this study. The lack of available UACR data further highlights the need for more thorough recording and capture of UACR across EHRs to aid in the prognosis and management of CKD. This is particularly relevant for patients with comorbid CKD and HF, for which a high proportion (15.2%) had elevated UACR (A2–A3) but near-normal eGFR (G2). Thus a diagnosis of CKD may be missed when screening for eGFR only.
CONCLUSIONS
This study provides contemporary evidence of HCRU in patients with CKD where costs and HCRU were found to be substantial and increased, in line with worsened CKD severity. When factoring in the additional costs of dialysis, CKD can be expected to cost upwards of six times more between the early stages (G2–3a) and kidney failure. The evidence provided by this study will be highly valuable in supporting the evaluation of novel therapies for patients with CKD and to better inform the health sector with resourcing requirements to support more efficient healthcare delivery. The adoption of new and effective therapies that have been associated with delaying or halting disease progression into routine care for patients with CKD, paired with improved early screening and monitoring, particularly of UACR, could potentially play a major role in reducing the HCRU burden while also improving patient outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Alyshah Abdul Sultan for input into the study design, interpretation of results and manuscript outline review, and Ben Wilding of Health Economics and Outcomes Research Ltd for providing medical writing support/editorial support, which was funded by AstraZeneca in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Contributor Information
Carol Pollock, Kolling Institute, Royal North Shore Hospital University of Sydney, Sydney, NSW, Australia.
Glen James, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK.
Juan Jose Garcia Sanchez, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK.
Juan Jesus Carrero, Karolinska Institutet, Department of Medical Epidemiology and Biostatistics, Stockholm, Sweden.
Matthew Arnold, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK.
Carolyn S P Lam, National Heart Centre, Department of Cardiology, Singapore, Singapore; Duke-NUS Medical School, Singapore, Singapore.
Hungta (Tony) Chen, AstraZeneca, Gaithersburg, MD, USA.
Stephen Nolan, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK.
Roberto Pecoits-Filho, School of Medicine, Pontifical Catholic University of Parana, Curitiba, Brazil; Arbor Research Collaborative for Health, Ann Arbor, MI, USA.
David C Wheeler, Department of renal medicine, University College London, London, UK.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study were derived for UK patients included in the DISCOVER CKD study using clinical records from the Clinical Practice Research Datalink (CPRD GOLD) linked to external databases. Data underlying the findings described in this article may be requested in accordance with AstraZeneca's data sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home through www.vivli.org.
FUNDING
This work was supported by AstraZeneca, which provided support for the analysis and medical writing for this study.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the study design, interpretation of results and review/approval of this manuscript.
CONFLICT OF INTEREST STATEMENT
J.J.G.S., M.A., T.C. and S.N. are employees and stockholders of AstraZeneca. C.P. has received consulting fees from AstraZeneca, Eli Lilly/Boehringer Ingelheim, Merck Sharp and Dohme, Novartis, Vifor, Amgen, Otsuka, Sanofi and Janssen and has received speaker fees from Novartis, Janssen Cilag, Otsuka and Vifor. J.J.C. has received institutional grants from AstraZeneca, Vifor and Astellas, speaker fees from AstraZeneca, Abbott and Nutricia and consultancy fees from AstraZeneca, Baxter Healthcare and Bayer. R.P.F. is an employee of Arbor Research Collaborative for Health, which receives global support for the ongoing DOPPS Programs (provided without restriction on publications by a variety of funders; for details see https://www.dopps.org/AboutUs/Support.aspx) and has received research grants from Fresenius Medical Care; consulting fees from AstraZeneca, Akebia, Novo Nordisk and Fresenius; non-financial support from AstraZeneca, Bayer, Boehringer, Novo Nordisk and Akebia and personal fees from Retrophin outside the submitted work. C.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore, has received research support from Bayer and Roche Diagnostics; has served as a consultant or on the advisory board/steering committee/executive committee for Abbott, Actelion, Amgen, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma, EchoNous, Impulse Dynamics, Ionis Pharmaceutical, Janssen Research & Development, Medscape/WebMD Global, Merck, Novartis, Novo Nordisk, Prosciento, Radcliffe Group, Roche Diagnostics, Sanofi and Us2.ai and serves as co-founder and non-executive director of Us2.ai. D.C.W. has received consulting fees from Akebia, Bayer, Baxter, GlaxoSmithKline, Gilead, Janssen and Zydus and reports personal fees and non-financial support from AstraZeneca; personal fees from Bayer, Boehringer Ingelheim, Astellas, GlaxoSmithKline, Janssen, Napp, Mundipharma, Reata, Vifor Fresenius and Tricida and participates in data safety and monitoring boards for Gilead and Zydus. G.J. has nothing to declare. This study was funded by AstraZeneca. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Jha V, Garcia-Garcia G, Iseki Ket al. . Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–72. [DOI] [PubMed] [Google Scholar]
- 2. Forbes A, Gallagher H.. Chronic kidney disease in adults: assessment and management. Clin Med 2020;20:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Midtvedt K, Heldal K.. Chronic kidney disease and the aging population. Transplantation 2014;97:e64. [DOI] [PubMed] [Google Scholar]
- 4. Winocour PH. Diabetes and chronic kidney disease: an increasingly common multi-morbid disease in need of a paradigm shift in care. Diabet Med 2018;35:300–5. [DOI] [PubMed] [Google Scholar]
- 5. Thomas MC, Cooper ME, Zimmet P.. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol 2016;12:73–81. [DOI] [PubMed] [Google Scholar]
- 6. Sanchez JG, Power A, Sultan AAet al. . POS-323 Inside CKD: projecting the future burden of chronic kidney disease in Europe using microsimulation modelling. Kidney Int Rep 2021;6:S139–S140. [Google Scholar]
- 7. Landray MJ, Emberson JR, Blackwell Let al. . Prediction of ESRD and death among people with CKD: the Chronic Renal Impairment in Birmingham (CRIB) prospective cohort study. Am J Kidney Dis 2010;56:1082–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute of Health and Care Excellence . Chronic kidney disease in adults: assessment and management. CG182. London: National Institute of Health and Care Excellence, 2014. [Google Scholar]
- 9. Nguyen NTQ, Cockwell P, Maxwell APet al. . Chronic kidney disease, health-related quality of life and their associated economic burden among a nationally representative sample of community dwelling adults in England. PLoS One 2018;13:e0207960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Kidney Foundation . KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 2006;47(5 Suppl 3):S1–S146. [DOI] [PubMed] [Google Scholar]
- 11. Stauffer ME, Fan T.. Prevalence of anemia in chronic kidney disease in the United States. PLoS One 2014;9:e84943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas R, Kanso A, Sedor JR.. Chronic kidney disease and its complications. Prim Care 2008;35:329–44, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vanholder R, Annemans L, Brown Eet al. . Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol 2017;13: 393–409. [DOI] [PubMed] [Google Scholar]
- 14. Wang V, Vilme H, Maciejewski MLet al. . The economic burden of chronic kidney disease and end-stage renal disease. Semin Nephrol 2016;36:319–30. [DOI] [PubMed] [Google Scholar]
- 15. Toyama T, Neuen BL, Jun Met al. . Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab 2019;21:1237–50. [DOI] [PubMed] [Google Scholar]
- 16. Pecoits-Filho R, Perkovic V.. Are SGLT2 inhibitors ready for prime time for CKD? Clin J Am Soc Nephrol 2018;13:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bakris GL, Agarwal R, Anker SDet al. . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–29. [DOI] [PubMed] [Google Scholar]
- 18. Bayer . Bayer's finerenone meets primary endpoint in Phase III FIGARO-DKD cardiovascular outcomes study in patients with chronic kidney disease and type 2 diabetes [press release]. 2021. https://media.bayer.com/baynews/baynews.nsf/id/Bayers-finerenone-meets-primary-endpoint-Phase-III-FIGARO-DKD-cardiovascular-outcomes-study-patients?OpenDocument&sessionID=1624971510 (June 2021, date last accessed). [Google Scholar]
- 19. Heerspink HJ, Stefánsson BV, Correa-Rotter Ret al. . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. [DOI] [PubMed] [Google Scholar]
- 20. Neal B, Perkovic V, Mahaffey KWet al. . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–57. [DOI] [PubMed] [Google Scholar]
- 21. Perkovic V, Jardine MJ, Neal Bet al. . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 22. Wanner C, Inzucchi SE, Lachin JMet al. . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–34. [DOI] [PubMed] [Google Scholar]
- 23. Wiviott SD, Raz I, Bonaca MPet al. . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–57. [DOI] [PubMed] [Google Scholar]
- 24. Kent S, Schlackow I, Lozano-Kühne Jet al. . What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease? BMC Nephrol 2015;16:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kerr M, Bray B, Medcalf Jet al. . Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012;27(Suppl 3): iii73–iii80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Institute of Health and Care Excellence . Patiromer for treating hyperkalaemia. TA623. London: National Institute of Health and Care Excellence, 2020. [Google Scholar]
- 27. National Institute of Health and Care Excellence . Renal replacement therapy and conservative management. NG107.London: National Institute of Health and Care Excellence, 2018. [PubMed] [Google Scholar]
- 28. National Institute of Health and Care Excellence . Sodium zirconium cyclosilicate for treating hyperkalaemia. TA599. London: National Institute of Health and Care Excellence, 2019. [Google Scholar]
- 29. Pecoits-Filho R, James G, Carrero JJet al. . Methods and rationale of the DISCOVER CKD global observational study. Clin Kidney J 2021;14:1570–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. NHS England . National cost collection for the NHS. https://www.england.nhs.uk/national-cost-collection/ (12 February 2021, date last accessed). [Google Scholar]
- 31. Personal Social Services Research Unit . Unit costs of health and social care. https://www.pssru.ac.uk/project-pages/unit-costs/ (June 2021, date last accessed). [Google Scholar]
- 32. Herrett E, Gallagher AM, Bhaskaran Ket al. . Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Audit Office. NHS Ambulance Services. HC 972. Session 2016–17. London: National Audit Office, 2017. [Google Scholar]
- 34. NHS Digital . Appointments in General Practice December 2019. https://digital.nhs.uk/data-and-information/publications/statistical/appointments-in-general-practice/december-2019 (28 January 2021, date last accessed). [Google Scholar]
- 35. National Institute of Health and Care Excellence . Chronic kidney disease assessment and management. NG203. https://www.nice.org.uk/guidance/ng203/resources/chronic-kidney-disease-assessment-and-management-pdf-66143713055173 (May 2022, date last accessed). [Google Scholar]
- 36. Care Quality Commission. The state of health care and adult social care in England 2019/20. https://www.cqc.org.uk/sites/default/files/20201016_stateofcare1920_fullreport.pdf (June 2021, date last accessed). [Google Scholar]
- 37. NHS England . Clinically-led Review of NHS Access Standards: Interim Report from the NHS National Medical Director. 2019. https://www.england.nhs.uk/wp-content/uploads/2019/03/CRS-Interim-Report.pdf (June 2021, date last accessed). [Google Scholar]
- 38. Nuffield Trust . A&E waiting times. 2021. https://www.nuffieldtrust.org.uk/resource/a-e-waiting-times (June 2021, date last accessed). [Google Scholar]
- 39. Health Foundation . NHS performance and waiting times: priorities for the next government. https://www.health.org.uk/sites/default/files/2019-11/nhs-performance-and-waiting-times-priorities-for-the-next-government-ge02-.pdf (June 2021, date last accessed). [Google Scholar]
- 40. Office for National Statistics . Overview of the UK population: January 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/january2021 (June 2021, date last accessed). [Google Scholar]
- 41. National Institute of Health and Care Excellence . RRT and conservative management. Economic analysis report: Cost-effectiveness analysis of HDF versus high flux HD. NG107. https://www.nice.org.uk/guidance/ng107/documents/supporting-documentation-2 (October 2021, date last accessed). [Google Scholar]
- 42. Department of Health . NHS reference costs 2018/2019. https://www.england.nhs.uk/publication/2018-19-national-cost-collection-data-publication/ (October 2021, date last accessed). [Google Scholar]
- 43. Laires PA, Perelman J.. The association between multimorbidity, socioeconomic factors, and multimorbidity-related excess healthcare use - Results From the 2014 national health interview survey. Value Health 2017;20:A684. [Google Scholar]
- 44. Levey AS, de Jong PE, Coresh Jet al. . The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17–28. [DOI] [PubMed] [Google Scholar]
- 45. Levey AS, Eckardt K-U, Tsukamoto Yet al. . Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–2100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study were derived for UK patients included in the DISCOVER CKD study using clinical records from the Clinical Practice Research Datalink (CPRD GOLD) linked to external databases. Data underlying the findings described in this article may be requested in accordance with AstraZeneca's data sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home through www.vivli.org.