ABSTRACT
Background
Data on the referral rate of chronic kidney disease (CKD) patients to specialists are sparse. Investigating referral rates and characterizing patients with kidney disease not followed by a nephrologist are relevant for future measures in order to optimize public health and guideline implementation.
Methods
Data were extracted from the Kidney Disease Cohort of Southern Denmark (KidDiCo). Referral rates for all incident CKD patients below 60 mL/min/1.73 m² and referral rates according to the KDIGO guidelines based on glomerular filtration rates below 30 mL/min/1.73 m² were calculated. Information on contact with one of the nephrologist outpatient clinics in the Region of Southern Denmark was collected from the Danish National Patient Registry. The individual follow-up time for nephrology contact was 12 months. Additional data were accessed via the respective national databases. CKD patients on dialysis and kidney transplanted patients were excluded.
Results
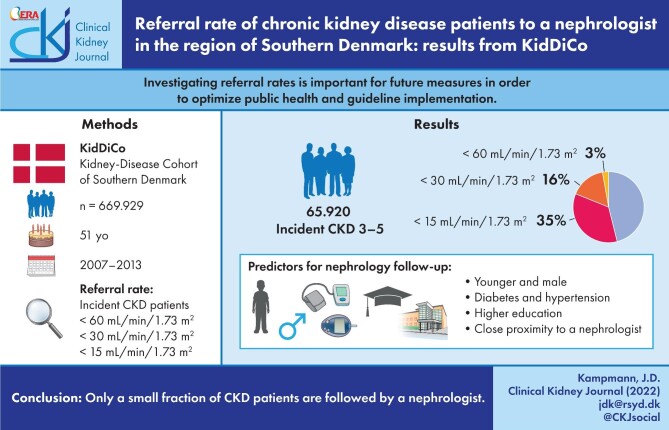
A total of 3% of patients with an eGFR <60 mL/min/1.73 m²–16% of patients with an eGFR <30 mL/min/1.73 m² and 35% of patients with an eGFR <15 mL/min/1.73 m² were in contact with a nephrologist in the outpatient settings. Younger age, male sex, diabetes, hypertension, higher education and proximity to a nephrology outpatient clinic increased the chance of nephrology follow-up.
Conclusion
Only a small fraction of CKD patients are followed by a nephrologist. More studies should be performed in order to find out which patients will profit the most from renal referral and how to optimize the collaboration between nephrologists and general practitioners.
Keywords: chronic kidney disease, guidelines, referral
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Chronic kidney disease (CKD) is a global health challenge affecting 10% of the world population [1]. However, only 20% of patients are aware of having CKD, even at the more advanced stages [2]. Physicians are often not conscious of their patients suffering from decreased renal function [3, 4]. Representable referral rates from health systems with universal healthcare are sparse. The referral rate has been difficult to measure and results have been heterogeneous.
CKD is divided into stages from 1 to 5 according to glomerular filtration rate (GFR) [5]. CKD stages 1 and 2 require either albuminuria or a structural disease. Stages 3 and 4 refer to moderate or clinically significant CKD with a GFR of between 60 and 15 mL/min/1.73 m². A GFR of 60 mL/min/1.73 m² can be seen as a cut-off since it represents a loss of 50% of the normal renal function [6, 7]. In stage 5, GFR is <15 mL/min/1.73 m² and is referred to as end-stage renal disease (ESRD), requiring renal replacement therapy such as dialysis or renal transplantation. Absent or late pre-dialysis nephrology care is associated with poorer blood pressure control, worse biochemical parameters and a lower rate of permanent vascular access among patients at dialysis initiation [8, 9]. Late referral 3–4 months before initiation of dialysis has been shown to have higher mortality in the first [8, 10] or second year [11] after initiation of dialysis when compared with timely referral. Both primary care physician recognition of CKD and nephrology co-management are associated with improved quality of care [12].
The Danish guidelines and the KDIGO workgroup guidelines regarding when to refer CKD patients to a specialist nephrologist were launched in 2015 and 2013, respectively. Both guidelines recommend referring CKD patients with a GFR <30 mL/min/1.73 m² to a nephrologist. Interestingly, KDIGO guidelines for lipids recommend treatment with statins in patients 50 years and older with a GFR 60 mL/min/1.73 m2. Patients with a GFR of between 60 and 30 mL/min/1.73 m² are reliant on their general practitioner’s (GP) awareness of those guidelines. Settings with universal health coverage and aggregated data for the entire population provide an ideal situation for comparing current care to guideline recommendations [13, 14]. Denmark offers universal healthcare and, therefore, referral rates are not influenced by the patients’ financial situation.
The aim of this study is to identify the referral rate to a nephrologist of CKD stage 3–5 patients in general and those fulfiling KDIGO criteria for referral to a nephrologist in terms of GFR being in CKD stage 4–5. Secondly, the study aims to characterize CKD stage 4–5 patients who were followed in a nephrology outpatient clinic.
MATERIALS AND METHODS
All CKD patients followed and treated in special care clinics for nephrology in Denmark are registered according to the relevant International Classification of Diseases (ICD-10) codes. The Danish National Patient Register is highly valid for renal disease, including ESRD [15]. In addition, CKD patients in Denmark are treated exclusively in hospital settings as outpatients. Only physicians working in hospitals, and not GPs, use ICD codes. These codes allow us to identify the proportion of both incident and prevalent cases with CKD referred to and followed in a hospital unit for their kidney disease. CKD patients were identified by the KidDiCo, which has proven to be representative and to have favourable coverage of the Region of Southern Denmark [16]. KidDiCo is a cohort describing patients with creatinine levels measured in participating laboratories in Southern Denmark between 2006 and 2013. The creatinine measurements from 2006 were used as a wash-out phase. Therefore, patients fulfiling CKD 3–5 criteria in 2006 were excluded from this study. All patients fulfiling CKD criteria between 2007 and 2013 registered in the geographical area of the KidDiCo were included in this study. An eGFR of 60 mL/min/1.73 m² or above prior to fulfiling CKD 3–5 criteria was not mandatory. However, we assume that patients with an eGFR <60 mL/min/1.73 m2 would have been identified and excluded during the 1-year washout period.
A previous article has described how the KidDiCo cohort compares with the general population in the time period between 2007 and 2013 [16].
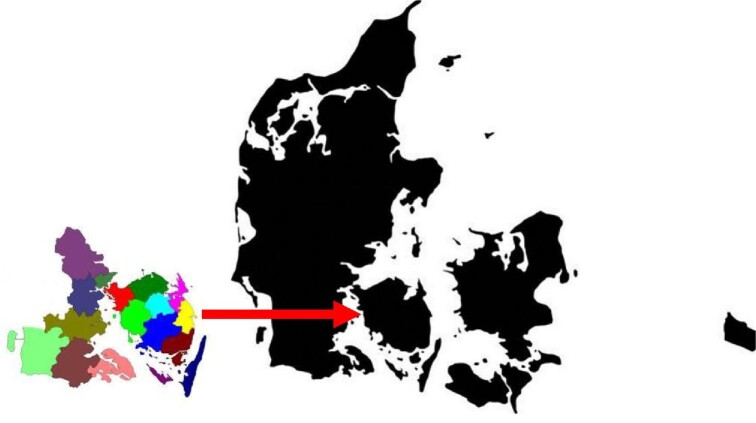
A total of N = 669 929 individuals registered at one point in time in the geographical area of the KidDiCo between 2007 and 2013 had at least one creatinine measured and were, thereby, included in the KidDiCo. Based on data from Statistics Denmark, N = 857 854 individuals lived in the region covered by the KidDiCo between 2007 and 2013. The median age of the general population in the geographical area was 45 years, of whom 51% were male. In comparison, the median age of individuals in the KidDiCo was 51 years old, and 46% were male [16]. Of the 17 municipalities in KidDiCo, n = 3 (18%) municipalities (Kolding, Sønderborg and Odense) have a nephrology department with an outpatient clinic. A map with the included municipalities can be seen in Fig. 1.
FIGURE 1:
A geographical presentation of the municipalities covered by KidDiCo. Maps of municipalities with courtesy from the Department of Regional Development, Global Goals and Analysis in the Region of Southern Denmark (edited by the author). Denmark map GettyImages-1144785834 (edited by the author).
We strictly followed the KDIGO guidelines according to the identification of CKD stages 3–5. Two eGFR measurements at least 3 months apart, but no longer than 1 year, with no normal eGFR measurement in between, had to be present. The incident CKD stages were defined by the eGFR value at the time-point when CKD criteria were fulfiled. Patients on dialysis or kidney-transplanted patients were excluded since we assume that these patients, per definition, are routinely followed by a nephrologist.
We estimated the ratio of nephrologist referrals for two groups. First, patients with an eGFR <60 mL/min/1.73 m², and second, the ratio of patients with an eGFR <30 mL/min/1.73 m², according to KDIGO guidelines should be referred to a nephrologist.
Referral to a nephrologist was defined by a kidney-related primary or secondary ICD-10 diagnosis code within 1 year of CKD incidence. The diagnoses were hand-picked by the authors J.D.K. and J.G.H. (Table 1). Identifying patients directly as ‘being listed as outpatient in nephrology clinics’ was not possible since the codes in the register are referring to medical outpatient contact and cannot be subdivided into the different specialties.
Table 1.
Overview of the ICD-10 codes and respective diagnoses used to identify patients as nephrology outpatients
| ICD-10 code | Description |
|---|---|
| DE102 |
Type 1 DM with kidney complications |
| DE107 | Type 1 diabetes with multiple complications |
| DE112 | Type 2 DM with kidney complications |
| DE117 | Type 2 diabetes with multiple complications |
| DI12 |
Hypertensive CKD |
| DI13 | Hypertensive heart and CKD |
| DM30.0 |
Polyarteritis nodosa and related conditions |
| DM31 |
Other necrotizing vasculopathies |
| DM32 | Systemic lupus erythematosus |
| DM34 | Systemic sclerosis (scleroderma) |
| DN00-N08.9 | Glomerular diseases |
| DN15 | Other renal tubulointerstitial diseases |
| DN16 | Renal tubulointerstitial disorders in diseases classified elsewhere |
| DN17-19.9 | Acute kidney failure and CKD |
| DN26 | Unspecified contracted kidney |
| DN27 | Small kidney of unknown cause |
| DN28 | Other disorders of kidney and ureter, not elsewhere classified |
| DQ61 | Cystic kidney disease |
Age, Charlson comorbidity score (CS) and socioeconomic data were based on the individual CKD stage defining point. Renal diagnoses were removed from CS. Hypertension (HT), diabetes mellitus (DM) and cardiovascular disease (CVD) were enriched with ATC codes for reimbursed prescriptions, as described in a previous study [17]. CVD was defined as ICD-10 codes for myocardial infarction, congestive heart failure, peripheral vascular disease or cerebrovascular disease. HT and DM diagnoses were enriched using redeemed drug prescriptions ±3 months from the time point of CKD incidence. For HT, the following ATC codes were used: C03 ‘diuretics’, C07 ‘beta-blocking agents’, C08 ‘calcium-channel blockers’ and C09 ‘agents on the renin–angiotensin system’. For DM, the ATC code A10 ‘drugs-used-in-diabetes’ was used.
We divided the age category as follows: category 1 = 18–59 years, category 2 = 60–79 years and category 3 = 80 years and over.
In order to define the proximity to a nephrology outpatient department, we used community codes. Patients who lived in a community with a nephrology outpatient clinic at one stage from the CKD incidence date and up to 12 months after were defined as living in a community with a nephrology outpatient clinic. For example, patients living in the municipality of Haderslev are associated with the nephrology department in Sønderborg and would have to travel 71 km to reach the nephrology outpatient clinic.
Stata version 16 was used for the statistical analysis [17]. The manuscript was written in accordance with the STROBE statement [18].
RESULTS
A total of n = 65 920 incident patients with CKD stages 3–5 not transplanted or on dialysis were identified. A total of n = 1975 (3.0%) were followed by a nephrologist according to the predefined outpatient ICD-10 codes 12 months after the CKD incidence date. Since Danish National guidelines and KDIGO guidelines recommend referral at GFR <30 mL/min/1.73 m2, we focused on patients with CKD stages 4–5 not transplanted or in dialysis. A total of N = 5535 patients were identified. Of those, N = 878 (16%) were followed by a nephrologist. Further subdivision resulted in a referral rate for CKD 4 patients of 11% and 35% for CKD 5 patients, not on dialysis and/or not transplanted.
We further examined the characteristics of patients with CKD stages 4 and 5 not on dialysis or transplanted, divided into a referred and a non-referred group (see Table 2). Characteristics of CKD 4 patients can be seen in Supplementary data, Table S1, and characteristics of CKD 5 patients can be seen in Supplementary data, Table S2.
Table 2.
Characteristics of not-referred and referred CKD stages 4–5 patients excluding dialysis patients and kidney transplanted patients
| Not referred | Referred to nephrologist | P-value | ||
|---|---|---|---|---|
| N = 4657 | N = 878 | |||
| Sex | Male | 2007 (43.1%) | 543 (61.8%) | <.001 |
| Female | 2650 (56.9%) | 335 (38.2%) | ||
| Age group category (years) | 18–839 | 49 (1.1%) | 63 (7.2%) | <.001 |
| 40–59 | 269 (5.8%) | 184 (21.0%) | ||
| 60–79 | 1737 (37.3%) | 467 (53.2%) | ||
| 80+ | 2602 (55.9%) | 164 (18.7%) | ||
| Age group (years) | 18–29 | 10 (0.2%) | 22 (2.5%) | <.001 |
| 30–39 | 39 (0.8%) | 41 (4.7%) | ||
| 40–49 | 86 (1.8%) | 72 (8.2%) | ||
| 50–59 | 183 (3.9%) | 112 (12.8%) | ||
| 60–69 | 546 (11.7%) | 225 (25.6%) | ||
| 70–79 | 1191 (25.6%) | 242 (27.6%) | ||
| 80–89 | 1896 (40.7%) | 158 (18.0%) | ||
| 90 and over | 706 (15.2%) | 6 (0.7%) | ||
| Diabetes | No | 3756 (80.7%) | 603 (68.7%) | <.001 |
| Yes | 901 (19.3%) | 275 (31.3%) | ||
| Hypertension | No | 633 (13.6%) | 96 (10.9%) | .033 |
| Yes | 4024 (86.4%) | 782 (89.1%) | ||
| Cardiovascular diseases | No | 2982 (64.0%) | 603 (68.7%) | .008 |
| Yes | 1675 (36.0%) | 275 (31.3%) | ||
| Charlson score | 0 | 2593 (55.7%) | 479 (54.6%) | <.001 |
| 1 | 510 (11.0%) | 147 (16.7%) | ||
| 2 | 1082 (23.2%) | 174 (19.8%) | ||
| 3 | 233 (5.0%) | 46 (5.2%) | ||
| 4+ | 239 (5.1%) | 32 (3.6%) | ||
| Education level | Short | 3112 (66.8%) | 726 (82.7%) | <.001 |
| Middle | 299 (6.4%) | 89 (10.1%) | ||
| Long | 16 (0.3%) | 15 (1.7%) | ||
| Missing | 1230 (26.4%) | 48 (5.5%) | ||
| Occupational status | Active | 243 (5.2%) | 117 (13.3%) | <.001 |
| Temporarily not active | 18 (0.4%) | 12 (1.4%) | ||
| Not active | 4379 (94.0%) | 740 (84.3%) | ||
| Missing/others | 17 (0.4%) | 9 (1.0%) | ||
| Nephrology outpatient clinic in municipality | No | 2864 (61.5%) | 486 (55.4%) | <.001 |
| Yes | 1793 (38.5%) | 392 (44.6%) | ||
| CKD stage | 4 | 3890 (83.5%) | 469 (53.4%) | <.001 |
| 5 | 767 (16.5%) | 409 (46.6%) |
In the referred group, 38.2% were women compared with 56.9% in the non-referred group (P < .001). The biggest share of patients in the referred group was the 60–79 year olds at 53.2%, and in the non-referred group, the ≥80 years patients at 55.9% (P < .001).
The ratio of patients with DM and HT was higher in the referred group. A total of 31.3% had DM in the referred group as opposed to 19.3% (P < .001) in the non-referred group. The number of patients with HT was much higher, in general, in both groups, with 89.1% in the referred group and 86.4% in the non-referred group (P = .033). The ratio of CVD, however, was lower in the referred group at 31.3%, than in the non-referred group at 36.0% (P = .008).
The ratio of patients on the job market was more than twice as high in the referred group when compared with the non-referred group; 13.3% versus 5.2% (P < .001).
Most of the patients in the non-referred group had a lower CKD stage. In the referred group, 46.6% of the patients had CKD stage 5 at incidence, and in the non-referred group, this number was much lower at 16.5% (P < .001).
A multivariate logistic regression analysis was performed to show the odds ratios for being followed by a nephrologist. Missing data from the educational level and occupational status were excluded from the linear regression (see Table 3).
Table 3.
Multivariate logistic regression on referred CKD 4–5 patients
| Referral to a nephrologist | Odds ratio | Standard error | Z | P>|Z| | 95% confidence interval | ||
|---|---|---|---|---|---|---|---|
| Age group category | |||||||
| Age 18–59 | 5.94 | 0.87 | 12.16 | 0 | 4.46 | 7.91 | |
| Age 60–79 | 2.33 | 0.25 | 7.88 | 0 | 1.89 | 2.87 | |
| Age 80< | 1 | (base) | |||||
| Sex | |||||||
| Male | 1.46 | 0.13 | 4.33 | 0 | 1.23 | 1.73 | |
| Female | 1 | (base) | |||||
| Diabetes | |||||||
| No | 1 | (base) | |||||
| Yes | 1.84 | 0.2 | 5.69 | 0 | 1.49 | 2.27 | |
| Hypertension | |||||||
| No | 1 | (base) | |||||
| Yes | 1.75 | 0.24 | 4.17 | 0 | 1.35 | 2.28 | |
| CVD | |||||||
| No | 1 | (base) | |||||
| Yes | 0.9 | 0.09 | –1.09 | 0.277 | 0.74 | 1.09 | |
| Charlson score | |||||||
| 0 | 1 | (base) | |||||
| 1 | 0.83 | 0.11 | –1.37 | 0.17 | 0.63 | 1.08 | |
| 2 | 0.94 | 0.11 | –0.55 | 0.583 | 0.76 | 1.17 | |
| 3 | 0.72 | 0.14 | –1.7 | 0.089 | 0.49 | 1.05 | |
| 4 | 0.64 | 0.14 | –2.07 | 0.038 | 0.42 | 0.98 | |
| Education level | |||||||
| Short | 1 | (base) | |||||
| Middle | 1.07 | 0.15 | 0.46 | 0.646 | 0.81 | 1.41 | |
| Long | 3.09 | 1.21 | 2.86 | 0.004 | 1.43 | 6.67 | |
| Occupational status | |||||||
| Not active | 1 | (base) | |||||
| Active | 0.86 | 0.13 | –0.98 | 0.325 | 0.64 | 1.16 | |
| Temporarily not active | 1.57 | 0.63 | 1.12 | 0.262 | 0.71 | 3.45 | |
| Nephrology outpatient clinic in municipality | |||||||
| No | 1 | (base) | |||||
| Yes | 1.28 | 0.11 | 2.91 | 0.004 | 1.08 | 1.52 | |
| CKD stage | |||||||
| 4 | 1 | (base) | |||||
| 5 | 3.58 | 0.32 | 14.35 | 0 | 3.01 | 4.26 | |
| _CONS | 0.03 | 0.01 | –19.87 | 0 | 0.02 | 0.05 |
Males had a one and a half times higher odds ratio (OR, 1.5; P < .001) for being followed by a nephrologist.
According to age category, 18–59-year-old patients had an almost six times higher OR (OR, 5.9; P < .001) for being followed by a nephrologist when compared with patients ≥80 years. For patients in the age category of 60–79 years of age, the risk was more than twice as high (OR, 2.3; P < .001) when compared with the ≥80 year group.
Patients with DM (OR, 1.8; P < .001) and hypertension (OR, 1.8; P < .001) had a higher OR for being followed by a nephrologist. CVD was not a significant risk factor (OR, 0.9; P = .311). The only statistically significant result regarding CS was patients with a score of four or more. Those patients were less likely to be followed by a nephrologist (OR, 0.6; P = .031).
The ratio of patients followed by a nephrologist increased with educational level, yet this only became significant in patients with high educational levels (OR 3.1; P < .004).
Employment status did not seem to be significant in terms of nephrologist referral.
The odds ratio for being followed by a nephrologist was higher in patients living in a community with a nephrology department (OR, 1.3; P = .002).
CKD patients with CKD stage 5 at incidence were more likely to be followed by a nephrologist (OR, 3.6; P < .001).
DISCUSSION
This study showed that only a few patients suffering from CKD are treated/followed by a nephrologist. The most prominent characteristics of patients followed by nephrologists are males, younger age, comorbidity in the form of HT and/or DM, high educational level, proximity to a nephrology outpatient clinic and higher CKD stage at incidence.
The ratio of women in the referred group in our study was low. This is in accordance with a Swedish study, where women were more likely to be diagnosed with CKD, but 27% were less likely to contact a nephrologist [19].
In this study, the ratio of patients with DM and HT was higher in the referred group. This is in accordance with a study that showed that the awareness rates among those with CKD were higher if comorbid diagnoses of diabetes and hypertension were present [2].
The influence of socioeconomic status (SES) on nephrology referral has, to our knowledge, not been described before. A recent Danish study found that patients with a lower SES had a significantly lower willingness to participate in cardiac rehabilitation (CR) after acute coronary syndrome [20]. In our study, only long educational levels, and not employment status, were significantly associated with referral to a nephrologist. In the CR study low educational level and not being economically active was associated with lower willingness for CR [20].
When becoming CKD incident in stage 5, the OR of being followed by a nephrologist was higher according to our data. CKD, especially in the early stages, is a silent disease, which explains the low awareness in patients with CKD. The low referral rate from doctors may be due to insufficient awareness of guidelines and recommendations regarding the referral of CKD patients [21, 22].
We found that only 16% of patients were referred according to nephrology referral criteria. In Sweden, 36% of individuals with eGFR stages 4–5 not yet on dialysis were referred to nephrologist care [19]. This discrepancy may be explained by the differences in population density, with a lower density of 100/km2 in the Region of Southern Denmark and a higher density of 367.2/km2 in Stockholm, according to www.statista.com/statistics. As our results show, the proximity to a nephrology outpatient clinic was significantly associated with being followed by a nephrologist. Lower population density may be linked to longer distances to access a nephrology outpatient clinic. The higher median age of the non-referred patients could be due to the assumption that older people are less likely to travel longer distances. In the future, telehealth solutions could become an important tool to overcome distance challenges.
In reality, however, one cannot expect that all CKD stage 3–5 patients, and not even all CKD stage 4–5 patients, for that matter, can be followed by a nephrologist. A Swedish study calculated that when strictly following KDIGO recommendations, a >300% increase in nephrology consultation rates should be expected [19]. In order to provide excellent outpatient care, GPs and nephrologists should work closely together. Better access to specialist advice could be the key [23]. According to the study, GPs found it hard to develop working partnerships with nephrologists, including matters regarding a timely exchange, unclear roles and responsibilities, and limited access to nephrologists. In order to provide excellent care for CKD patients, GPs should be familiar with current guidelines and recommendations. Many nephrology guidelines include recommendations for patients with GFR <60 mL/min/1.73 m². In our study, only 3% of this group was followed by a nephrologist. Therefore, distribution strategies for nephrology guidelines should be reconsidered and should reach nephrologists and GPs alike.
Strength
Our study is the first to comprehensively investigate risk factors, including socioeconomic and geographical factors, for nephrology referral. Data are retrieved from a free public health system with solid register data.
Limitations
Using ICD coding instead of actual ambulatory contact information is not ideal. However, it is difficult to imagine having contact at the nephrology outpatient clinic without one of these handpicked diagnoses. The Danish and KDIGO guidelines were first published after the cohort inclusion period ended. Yet, we do not think that the introduction of the guidelines would have a great effect on the numbers. We have no information on whether the patients were referred and then turned down by a nephrologist. In our study, the exact distances from home to the nephrologist were not taken into account. However, we think that using the community codes is a good approximation.
CONCLUSION
Only a small fraction of CKD patients are followed by a nephrologist. The main factors associated with being followed by a nephrologist are; age group 18–59 years, males, having hypertension and/or diabetes, higher education levels and proximity to a department of nephrology. Telehealth could improve nephrology care for patients living further away from nephrology outpatient clinics. Renal guidelines should be distributed to GPs as well. More studies should be performed on who profits most from renal referral and how to optimize the collaboration between nephrologists and GPs.
Supplementary Material
Contributor Information
Jan Dominik Kampmann, Department of Internal Medicine, Hospital of Southern Jutland, Sønderborg, Sønderborg, Denmark; Institute of Regional Health Research, University of Southern Denmark, Odense, Denmark.
James Goya Heaf, Department of Medicine, Zealand University Hospital, Roskilde, Roskilde, Denmark.
Christian Backer Mogensen, Institute of Regional Health Research, University of Southern Denmark, Odense, Denmark; Department of Emergency Medicine, Hospital of Southern Jutland, Aabenraa, Denmark.
Hans Mickley, Department of Cardiology, Odense University Hospital, Odense, Denmark.
Donna Lykke Wolff, Department of Internal Medicine, Hospital of Southern Jutland, Sønderborg, Sønderborg, Denmark; Institute of Regional Health Research, University of Southern Denmark, Odense, Denmark.
Frans Brandt, Department of Internal Medicine, Hospital of Southern Jutland, Sønderborg, Sønderborg, Denmark; Institute of Regional Health Research, University of Southern Denmark, Odense, Denmark.
DATA AVAILABILITY STATEMENT
The data underlying this article were provided by Denmark Statistics by permission. Data will be shared on request to the corresponding author with permission of Denmark Statistics.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Bello AK, Levin A, Tonelli Met al. Assessment of global kidney health care status. JAMA 2017;317:1864–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vassalotti JA, Li S, McCullough PAet al. Kidney early evaluation program: a community-based screening approach to address disparities in chronic kidney disease. Semin Nephrol 2010;30:66–73. [DOI] [PubMed] [Google Scholar]
- 3. Li C, Wen XJ, Pavkov MEet al. Awareness of kidney disease among US adults: findings from the 2011 behavioral risk factor surveillance system. Am J Nephrol 2014;39:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Couser WG, Remuzzi G, Mendis Set al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011;80:1258–70. [DOI] [PubMed] [Google Scholar]
- 5. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 6. Hallan SI, Ritz E, Lydersen Set al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol 2009;20:1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Go AS, Chertow GM, Fan Det al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- 8. Smart NA, Titus TT. Outcomes of early versus late nephrology referral in chronic kidney disease: a systematic review. Am J Med 2011;124:1073–1080.e2. [DOI] [PubMed] [Google Scholar]
- 9. Smart NA, Dieberg G, Ladhani Met al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev 2014;6:CD007333. [DOI] [PubMed] [Google Scholar]
- 10. Chan MR, Dall AT, Fletcher KEet al. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med 2007;120:1063–1070.e2. [DOI] [PubMed] [Google Scholar]
- 11. Stack AG. Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am J Kidney Dis 2003;41:310–8. [DOI] [PubMed] [Google Scholar]
- 12. Allen AS, Forman JP, Orav EJet al. Primary care management of chronic kidney disease. J Gen Intern Med 2011;26:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vart P, Gansevoort RT, Joosten MMet al. Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. Am J Prev Med 2015;48:580–92. [DOI] [PubMed] [Google Scholar]
- 14. Bello A, Hemmelgarn B, Manns Bet al. , Alberta Kidney Disease Network . Use of administrative databases for health-care planning in CKD. Nephrol Dial Transplant 2012;27: 3: iii12–8. [DOI] [PubMed] [Google Scholar]
- 15. Hommel K, Rasmussen S, Madsen Met al. The Danish registry on regular dialysis and transplantation: completeness and validity of incident patient registration. Nephrol Dial Transplant 2010;25:947–51. [DOI] [PubMed] [Google Scholar]
- 16. Kampmann JD, Goya Heaf J, Mogensen CBet al. Kidney Disease Cohort (KidDiCo) of southern Denmark: design, coverage, generalizability and implications for use. Clin Epidemiol 2021;13:971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. StataCorp . Stata Statistical Software: Release 16. College Station, TX: Statacorp LLC, 2019. [Google Scholar]
- 18. von Elm E, Altman DG, Egger Met al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9. [DOI] [PubMed] [Google Scholar]
- 19. Gasparini A, Evans M, Coresh Jet al. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 2016;31:2086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Graversen CB, Johansen MB, Eichhorst Ret al. Influence of socioeconomic status on the referral process to cardiac rehabilitation following acute coronary syndrome: a cross-sectional study. BMJ Open 2020;10:e036088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Navaneethan SD, Kandula P, Jeevanantham Vet al. Referral patterns of primary care physicians for chronic kidney disease in general population and geriatric patients. Clin Nephrol 2010;73:260–7. [DOI] [PubMed] [Google Scholar]
- 22. Boulware LE, Troll MU, Jaar BGet al. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis 2006;48:192–204. [DOI] [PubMed] [Google Scholar]
- 23. Greer RC, Liu Y, Cavanaugh Ket al. Primary care physicians' perceived barriers to nephrology referral and co-management of patients with CKD: a qualitative study. J Gen Intern Med 2019;34:1228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by Denmark Statistics by permission. Data will be shared on request to the corresponding author with permission of Denmark Statistics.