Abstract
Chronic kidney disease (CKD) and kidney failure are global health problems associated with morbidity, mortality and healthcare costs, with unequal access to kidney replacement therapy between countries. The diversity of guidelines concerning referral from primary care to a specialist nephrologist determines different outcomes around the world among patients with CKD where several guidelines recommend referral when the glomerular filtration rate (GFR) is <30 mL/min/1.73 m2 regardless of age. Additionally, fixed non-age-adapted diagnostic criteria for CKD that do not distinguish correctly between normal kidney senescence and true kidney disease can lead to overdiagnosis of CKD in the elderly and underdiagnosis of CKD in young patients and contributes to the unfair referral of CKD patients to a kidney specialist. Non-age-adapted recommendations contribute to unnecessary referral in the very elderly with a mild disease where the risk of death consistently exceeds the risk of progression to kidney failure and ignore the possibility of effective interventions of a young patient with long life expectancy. The opportunity of mitigating CKD progression and cardiovascular complications in young patients with early stages of CKD is a task entrusted to primary care providers who are possibly unable to optimally accomplish guideline-directed medical therapy for this purpose. The shortage in the nephrology workforce has classically led to focused referral on advanced CKD stages preparing for kidney replacement, but the need for hasty referral to a nephrologist because of the urgent requirement for kidney replacement therapy in advanced CKD is still observed and changes are required to move toward reducing the kidney failure burden. The Kidney Failure Risk Equation (KFRE) is a novel tool that can guide wiser nephrology referrals and impact patients.
Keywords: age-adapted diagnosis and referral of CKD, chronic kidney disease, Kidney Failure Risk Equation, nephrology referral
INTRODUCTION
The global prevalence of chronic kidney disease (CKD) in the general population is thought to be very high, 697–850 million prevalent cases worldwide, with substantial variation observed between countries and regions of the world [1]. Over diagnosis due to a lack of age-adapted estimated glomerular filtration rate (eGFR) criteria for identification of CKD confounds the veracity of these prevalence estimates [2], nevertheless, CKD signifies an important impact on global health, both as a direct cause of excess mortality and as an important risk factor for cardiovascular disease [1, 3, 4]. The global distribution of CKD and increasing evidence for the effectiveness of measures designed to slow the progression to kidney failure inspires a need for greater attention to CKD in general and specifically to action plans for CKD prevention, detection and management, including timely and appropriate referral for specialized care [5].
The role nephrologists play in preventing kidney disease progression or preparing kidney replacement therapies is highly dependent on the referral by primary care. Prompt referral to nephrology care is associated with improved clinical outcomes among CKD patients [6, 7]. Most referral guidelines for CKD limit the attention to when eGFR is consistently <30 mL/min/1.73 m2 and the likelihood of kidney replacement therapy is proximate [8–14] and when most of kidney is already irretrievably lost and opportunities for slowing progression are more limited. The Kidney Failure Risk Equation (KFRE) may be a useful adjunctive tool for clinical assessment as part of referral decision-making.
In this review, we discuss the issues of the referral event in the care of CKD adults across the continuum of declining kidney function and its pace that embrace the interface between primary care and the nephrologist. We compare the Kidney Disease: Improving Global Outcomes (KDIGO) referral model based on eGFR thresholds with the use of risk-based equations with their pitfalls.
MAIN GUIDELINES OF REFERRAL TO NEPROLOGISTS
KDIGO 2013 referral criteria
The KDIGO guidelines of 2013 [8] recommend that adult patients with CKD [as defined by an eGFR/albuminuria matrix and time (3 months)] be referred to a nephrologist, regardless of age ˃20 years, when any one of the following conditions are present: persistent eGFR <30 mL/min/1.73 m2, a consistent finding of urinary albumin:creatinine ratio (uACR) >300 mg/g (33.9 mg/mmol), abrupt or rapidly progressive deterioration of kidney function, concurrent treatment-resistant hypertension, red blood cell casts in a urinary sediment examination, persistent serum potassium abnormalities, CKD-associated anaemia, when hereditary CKD or polycystic kidney disease is suspected and when recurrent/extensive nephrolithiasis is present.
The advantages of the KDIGO criteria are the wide awareness and usage of these guidelines and also the explicit description of reasons for referral. The disadvantages are that the eGFR thresholds for referral are neither age-adapted nor based on specific risks.
National Institute for Health and Clinical Excellence (NICE) 2021 referral criteria
The 2008–14 NICE guidelines [9] recommendation of referring when eGFR is <30 mL/min/ 1.73 m2 (CKD Stages 4–5) has been substituted in the 2021 guidelines [15] by a 5-year risk of needing renal replacement therapy of ˃5% (measured using the four-variable KFRE); other reasons for referral include: marked proteinuria [uACR ≥70 mg/mmol (≥619 mg/g)] unless known to be caused by diabetes already appropriately treated, uACR ≥30 mg/mmol together with haematuria, a sustained decrease of eGFR of ≥15 mL/min/1.73 m2 per year, hypertension that remains poorly controlled despite the use of at least four antihypertensive drugs at therapeutic doses, people with or suspected of having rare or genetic causes of CKD, and patients with suspected renal artery stenosis.
The advantages of the NICE criteria are that the KFRE is included. The disadvantages are that hyperkalaemia and anaemia are not included and the eGFR thresholds are not age-adapted.
Other guidelines, referral criteria
Guidelines such as the National Kidney Foundation (Kidney Disease Outcomes Quality Initiative) [10], Caring for Australasians with Renal Impairment (CARI) [11], American Diabetes Association of 2022 [12] and European guidelines [13, 14] in general recommend that patients with GFR <30 mL/min/ 1.73 m2 (CKD Stages 4–5), regardless of the age of the patient, should be referred to a nephrologist, among other causes. French guidelines (Haute Autorité de Sante) recommend a higher cut-off value of 45 mL/min/1.73 m2 [16]. The different main guidelines [17] that address referral recommendations from primary care to nephrologists are shown in Table 1.
Table 1.
Recommendation summary of different guidelines comparing criteria for referral to nephrologists
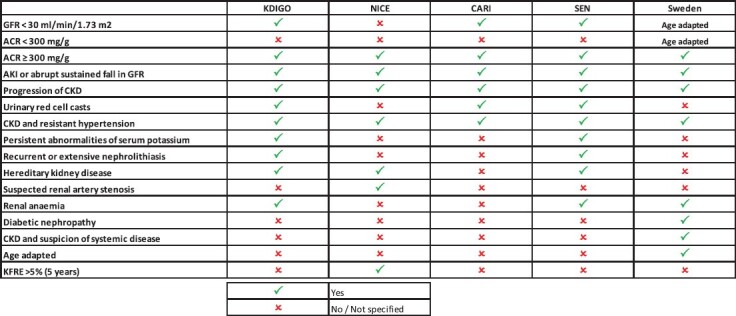
|
KDIGO: Kidney Disease: Improving Global Outcomes 2013; NICE: National Institute for Health and Clinical Excellence 2021; SEN: Sociedad Española Nefrologia 2022 (Spanish Society of Nephrology); CARI: Caring for Australasians & New Zealanders with Kidney Impairment; Sweden: Southern Health Region.
A different approach of referral in CKD adults, which introduces an age adaptation of eGFR thresholds for referral, can be exemplified by the guidelines for the Southern Health Region in Sweden [18]. In patients ˂55 years of age, all patients with uACR >30 mg/mmol are suitable for referral and if uACR is <30 mg/mmol, referral is recommended if eGFR is ˂60 mL/min/1.73 m2. Patients 55–75 years of age with uACR >30 mg/mmol are suitable for referral if eGFR is ˂60 mL/min/1.73 m2 and in patients with uACR <30 mg/mmol if eGFR is ˂45 mL/min/1.73 m2. Finally, in patients >75 years of age, referral is indicated when the uACR is >30 mg/mmol if eGFR is ˂30 mL/min/1.73 m2, with non-referral consultation with nephrologists in case of an eGFR of 30–45 mL/min/1.73 m2, and uACR <30 mg/mmol if eGFR ˂15 mL/min/1.73 m2, and with consultation in if eGFR is 15–30 mL/min/1.73 m2. Interestingly, besides the age-adapted referral, the specifics of the timing of referral, are also established; rapid referral and urgent referral are considered in low eGFR situations, depending on the age of the patient instead of ordinary timing of the referral. High risk for rapid progression of CKD, and therefore a direct referral recommendation, includes increased albuminuria uACR >300 mg/mmol (>2654 mg/g), uncontrolled hypertension, rapid decline in eGFR, renal anaemia, diabetic nephropathy or suspicion of systemic disease.
The advantages of these criteria are that the thresholds of eGFR are age-adapted and the urgency of referral (in weeks) is included and clearly defined. A potential disadvantage is that a diagnosis of diabetic nephropathy may not be practical, but the rapid evolution of reno protective therapies for early stages of diabetic nephropathy may offset this potential impracticability.
Only a few guidelines differentiate between low-threshold consultation (NICE, CARI) or co-management versus long-term referral for management of advanced CKD. Multidisciplinary or co-management is mentioned by several guidelines [17]. The Belgium Centre for Evidence-Based Medicine only explicitly describes the role of general practitioners (GPs) and recommends the GP be responsible for detecting and monitoring CKD, detecting complications and treating cardiovascular risk [17]. Other recent guidelines additionally recommend referral in earlier stages of CKD (30–60 mL/min/1.73 m2) if there is a confirmed progression of CKD and add special recommendations for specific populations, such as patients >80 years old [14]. Thus coexistent guidelines reflect a diversity of recommendations for GPs (Table 1).
KFRE FOR REFERRAL
The KFRE is a widely validated tool for estimating the absolute risk of kidney failure over 2 and 5 years that incorporates age, sex, GFR and uACR [19, 20]. Accurate prediction of progression to kidney failure can help for a more appropriate transition from primary care to secondary care nephrology and avoid referrals in those who are unlikely to progress [21]. Of course, the KFRE focuses only on kidney failure risk and not on other risks that are increased in CKD, such as cardiovascular disease (especially congestive heart failure), all-cause mortality and acute kidney injury.
Validation studies of the KFRE in UK primary care suggest a threshold of >5% over 5 years instead of <30 mL/min/1.73 m2 may reduce nephrology referral [22] or may reduce wait times of referral as evaluated in Canada—using risk >3% instead of 5% over 5 years [23]. Major et al. [22], in one region of the UK, examined discriminatory ability as well as calibration and external validity of the four-variable KFRE using a primary care cohort database. The KFRE over predicted risk in lower-risk groups and underpredicted risk in high-risk groups. The Hingwala et al. study [23], from Manitoba, Canada, examined the practical utility of the four-variable KFRE as risk-based triage in a province-wide cohort. The goal was to compare a KFRE-based triage system with a non-KFRE-based referral system sequentially. A validation and calibration study from the same group was previously published [24]. Using a threshold of >3% over 5 years, they found that referrals increased but wait times decreased. Long-term follow-up of low-risk patients was not available, so discrimination was not tested. More validation and calibration in diverse populations are needed for the KFRE to be fully implemented across many health systems.
A recent retrospective study [25] compared referral volume based on the estimated risk of kidney failure and laboratory criteria. About 18% of CKD patients met laboratory indications for referral (eGFR <30 mL/min/1.73 m2), where most were not truly referred in the following year and the median 2-year risk of kidney failure was 1.5%. If the referral was restricted to patients with a predicted risk >1% in addition to laboratory indications, potential referral volume would be reduced drastically by 42%. If referrals were based only on risk equations (KFRE >1% over 2-year risk), referral volume was ∼20% [25]. Health systems seeking to optimize CKD care delivery will need to assess the capacity of their nephrology workforce and can choose referral criteria that identify the highest-risk subset of patients [7]. Another recent study [26] compared previous criteria that included eGFR <30 mL/min/1.73 m2 with NICE criteria of KFRE >3% risk of kidney failure at 5 years. A similar referral rate (19.2% versus 21.9%) was found. Interestingly, a number of patients were reallocated between primary care and specialist nephrology care [26]. Probably the application of more stringent risk thresholds (>5% over 5 years) would identify progressively fewer patients and would imply fewer referrals to nephrologists.
The lack of albuminuria (or proteinuria) testing in CKD patients cared for in primary care settings [26, 27], particularly in patients without diabetes, is perhaps the largest barrier to widespread implementation of these interesting prognostic tools. Education in primary care to help increase the limited measurements of albuminuria (or proteinuria) in CKD patients is very desirable. Single values of eGFR, as used in equations of kidney failure risk, for referral decision-making may be inappropriate, as they are highly imprecise [28, 29]. The tendency of CKD to progress over time (the eGFR trajectory) has to be taken into consideration. Regression can be as common as progression [30]. Understanding ‘CKD as a movie and not a portrait’ helps to use the KFRE correctly and distinctly. Whether the decline in eGFR represents a sustained true disease progression, a self-limited episode of acute kidney injury, a superimposed complication (such as urinary tract obstruction or interstitial nephritis) or a transient haemodynamic insult that is likely to ultimately regress to its baseline values requires careful interpretation by an experienced clinician. Additional urinalysis, blood, or urine biochemical testing, imaging or even kidney biopsy may be required to aid in this distinction. Serial eGFR values may be informative for timely referral for vascular access preparation. While the KFRE tool is helpful, it was not intended to replace clinical judgment or guide individual patient care. Other limitations of this equation require consideration; for example, the KFRE was developed in individuals with CKD Stages G3A–G5 and therefore should not be used to determine risk in CKD patients with eGFR >60 mL/min/1.73 m2 [19]. Additionally, nephrologists may be required to evaluate patients with other multiple disorders or/and low risk of CKD progression to kidney failure. In particular, in young individuals, a low-risk CKD progression patient such as a 30-year-old female with an eGFR of 25 mL/min/1.73 m2 with no albuminuria (KFRE <5% over 5 years) should, in our opinion, require referral. Risk over 5 years can be a short time frame for young patients because of their long life expectancy and the ever-present possibility to optimize treatment and slow the decline of kidney function in the long term. Patients with a lower risk of progression to end-stage renal disease, especially young adults, may require nephrological evaluation.
Despite these limitations (Table 2), the risk of kidney failure may be a useful adjunct tool to clinical assessment as part of referral decision-making and encourage more albuminuria (or proteinuria) testing by primary care physicians. The use of the KFRE may incentivize the user to introduce albuminuria levels, as with the implementation of smartphone apps [31]. Additional studies to examine the impact of these risk thresholds, either 3% or 5% over 5 years, on the number of generated nephrology referrals and comparisons between new 2021 NICE criteria for referral and previous guidelines are needed [21]. These tools may help prioritize referrals based on the risk of adverse outcomes, especially when long wait times for appointments are present, for which defining times of referrals could also be required (urgent, accelerated, ordinary referral). In the mentioned example, hyperkaliaemia or anaemia—not included in the NICE 2021 guidelines [15]—could change the point of view of an ‘unnecessary’ referral by the risk of kidney failure to one requiring urgent referral. Other reasons for referral, such as complications related to CKD (e.g. cardiovascular disease), can be the predominant problem and are the key.
Table 2.
Possible disadvantages of KFRE and GFR <30 mL/min/1.73 m2 threshold for referral for all ages
|
KFRE
Low albuminuria testing hinges its use Non-developed for G1–2 stages Single measures, needs interpretation Reduces referral volume Optimum cut-off (3% versus 5%) requires future research Low KFRE in young might require referral Needs more validation and calibration in diverse populations |
GFR
Limits early interventions for nephrologists Unnecessary referrals in elderly Non-age adaptation overestimating CKD in elderly and underestimating in young Unfair moment of referral comparing young and elderly Also refers patients with low risk of progression |
POSSIBLE AREAS TO REVISIT IN FUTURE REFERRAL CRITERIA
While little doubt exists that the 2013 KDIGO criteria for referral of CKD patients to nephrologists should be broadly applied, they might be improved by establishing specific roles for the general, non-nephrological physicians in the care of patients with CKD, particularly with respect to the prevention of CKD and its management to delay progression to kidney failure. Health system-related barriers include a perceived lack of urgency for detecting early CKD among primary care clinicians, lack of knowledge of CKD guidelines, lack of incentives for CKD interventions, lack of CKD-specific clinical quality measures and suboptimal communication between specialties [29]. Primary care physicians face substantial but modifiable barriers in providing care to a patient with CKD at the patient, provider and system level [32]. Identifying high-risk individuals as candidates for CKD screening will require that clinicians are educated about CKD risk factors. Effective CKD risk stratification will also require education about CKD staging, particularly the importance of albuminuria (or proteinuria). To bridge the education gap, existing guidelines could be simplified with quick reference guides for primary care clinicians. Approaches for CKD screening, risk stratification and treatment should be integrated with existing health services and processes [29].
In countries with universal coverage and unrestricted access to healthcare systems funded primarily by taxes, established referral criteria identify the moment in the continuum of care when the nephrologist begins to participate actively in the management of CKD. The timing of this event has economic implications. Co-management models for the care of patients with CKD, created by consensus of different national societies, could help to clarify this issue. Guidelines for referral to a nephrologist might need to adapt to the nature of the healthcare system utilized in specific countries (e.g. national health insurance versus the private-public hybrid systems). Insurance coverage and its impact on access to care determine that a substantial number of patients exist in some high-income countries that also have marked limitations on access to timely and high-quality CKD care. The specific details provided by the KDIGO referral recommendations outlined above may not be feasible for all countries. In fact, in some regions of the world, only a minority of patients are referred to a nephrologist at least 12 months before the start of renal replacement therapy [33]. Also, non-nephrology physicians are often lacking in extensive experience with CKD patients and may possess a low level of awareness of complex guidelines for CKD diagnosis/management [32]. This can lead to delayed intervention for treating kidney disease or an untimely referral [34]. An illustration can be the low rates of albuminuria (or proteinuria) testing in patients with CKD [27, 35] in primary care and at the time of referral [31] despite the consistent recommendations and the vital importance of albuminuria (proteinuria) for prognostication in CKD.
Electronic health record reminders may help address low rates of albuminuria testing in patients with CKD. Smartphone apps may also help address these issues and facilitate the improvement in knowledge/awareness of CKD by primary care physicians and help to enable appropriate referral [31, 36]. Automated laboratory reporting and the use of risk equations and clinical decision support tools could be embedded in existing electronic health records to guide the selection of individuals for testing and the frequency of repeat testing [29]. But the imprecision of the commonly used eGFR equation hampers the utility of such laboratory-based prompts in individual patients. Electronic consultations can also minimize the need for travel to centralized referral centres for patients in rural communities that are underserved by nephrology subspecialists [37] or in pandemic (COVID-19) scenarios. E-consult platforms and CKD registries have already been implemented in some healthcare systems [38, 39].
In this new era of widespread information-sharing capability, electronic consultations or discussion of a case by phone or e-mail can be used in any stage of CKD, but especially in milder cases where nephrologists can assist primary care and other specialists in the care of patients with CKD and advise whether to advance or wait for a referral. Kidney specialists can help to determine the cause of CKD, recommend specific therapy or treatment for the slow progression of CKD, identify and deal with complications related to treatment and/or CKD, assess prognostic considerations and help predict the risk of progression to kidney failure, but these interventions are often too late in many patients referred with an eGFR <30 mL/min/1.73 m2, especially those with a fairly rapid decline of eGFR. An exception to this generalization might be rapidly progressive glomerulonephritis due to extensive crescentic diseases or due to obstructive uropathy.
Accurate and consistent measurements of kidney function avoiding racial bias is an evolving challenge [28, 40] leading to a well-timed referral. The recent recommendations of the National Kidney Foundation-American Society of Nephrology task force have moved forward in this direction by establishing a new refitted eGFR equation that avoids a race coefficient [41–44]. Many forms of CKD, especially diabetic kidney disease, are preventable and treatable [45], and slowing CKD progression at early stages can provide economic benefits [46]. What we do know is that a timely and appropriate referral to outpatient nephrology care is associated with slowed kidney disease progression [6], fewer hospital admissions and reduced total treatment costs [47]. In our opinion, a global action plan for the prevention and control of CKD [48] should include universal evidence-based referral criteria to optimize care for CKD, globally adapted to regional healthcare systems.
The referral to the nephrologist could be hindered by the apprehension that once the patient is referred to the specialist, the GP might lose contact with him/her. Studies suggest that continuity of care should be communicated [49]. Interdisciplinary care, co-management models and the role of GPs in the detection and monitoring of CKD, detection of complications and treatment of cardiovascular risk should also be included in the guidelines [17]. Emerging health care policies should strengthen the primary care screening of CKD and improve coordination with nephrologists. This includes the communication of a clear delineation of the roles and responsibilities of the GP and nephrologists [17, 49].
KIDNEY SENESCENCE, AGEING AND REFERRAL TO NEPHROLOGISTS
Since 1990, the age-standardized prevalence rate of CKD has remained relatively stable and the rising prevalence counts of CKD can be largely attributed to population growth and societal ageing. As the definition of CKD hinges on the eGFR and the duration of its reduction, when the thresholds used for identifying CKD are not age-adapted or rely on single eGFR values, marked overestimates of the true values of CKD prevalence can occur [2, 50].
In considering the issues of diagnosis and referral of patients with presumed CKD, it is crucial to understand and appreciate that the current definition of CKD by the KDIGO (2013) is an abnormality of kidney structure or function present for ≥3 months; basically an eGFR <60 mL/min/1.73 m2 (as assessed by any one of several eGFR equations based on serum creatinine or serum cystatin C levels or both) and/or the presence of a uACR in a ‘spot’ or timed urine sample of >30 mg/g (or 3 mg/mmol) [8]. These criteria are not age- or sex-adapted. It has been known for decades that the measured GFR declines steadily with ageing [51]. Studies of healthy living kidney transplant donors have shown that the normal median GFR at age 20–30 years is ∼107 mL/min/1.73 m2 and at age 70–80 years is ∼76 mL/min/1.73 m2 [52]. The lower limit of normal GFR is 78 mL/min/1.73 m2 for a healthy 20- to 30-year-old, whereas the lower limit for GFR in a healthy 70- to 80-year-old is 49 mL/min/1.73 m2 [53]. Thus the fixed, non-age-adapted threshold of <60 mL/min/1.73 m2 for the definition of CKD for all patients, irrespective of age, is questionable [54]. An age-adapted definition of CKD, based on eGFR or measured GFR, is a matter of open debate, but if an age-adapted threshold of eGFR for defining CKD were to be universally adopted, the high prevalence of the diagnosed CKD in the general population mentioned above would likely decrease, and markedly so in some situations [54], affecting referrals. Thus the prevalence of CKD globally is likely to be overstated [55, 56].
Jonsson et al. [57] evaluated 218 437 adult individuals comparing KDIGO eGFR criteria (two or more values >90 days apart, eGFR <60 mL/min/1.73 m2) versus age-adapted eGFR thresholds (two or more values >90 days apart) in patients <40 years of age with an eGFR <75 mL/min/1.73 m2, age 40–60 years with an eGFR <60 mL/min/1.73 m2 and age >65 years with an eGFR <45 mL/min/1.73 m2. The prevalence of CKD was markedly lower when an age-adapted eGFR threshold was adopted (3.64% versus 5.94%). The prevalence is markedly lower in individuals ˃65 years old [58]. The prevalence of CKD might also be overstated due to a single assessment of eGFR (as the KDIGO definition states, it should be confirmed or present >3 months) [55, 59]. Higher threshold values of eGFR for defining CKD (∼<75 mL/min/1.73 m2) for younger subjects and lower threshold values (∼<45 mL/min/1.73 m2) for older subjects may be more appropriate [53, 57]. Understanding normal kidney ageing may imply a nuanced moment of referral, earlier in young and later in elderly CKD patients, thus delaying referral in an 87-year-old woman with a stable serum creatinine of 1.55 mg/dL (G4 eGFR <30 mL/min/1.73 m2) and accelerating it in a 20-year-old woman with a creatinine of 1.9 mg/dL (G3b eGFR >30 mL/min/1.73 m2) that surprisingly would not be referred by the KDIGO classic referral fixed eGFR threshold of <30 mL/min/1.73 m2.
In order to compare referral to nephrologists with the age-adapted CKD versus KDIGO CKD definition, we used the National Health and Nutrition Examination Survey (NHANES) database [60]. All participants from the NHANES 2009–2016 >18 years of age were reviewed, using only those with available age, gender, race, serum creatinine and uACR. ‘Age-adapted’ CKD was defined as eGFR <75 mL/min/1.73 m2 in subjects from 18–39 years of age, <60 mL/min/1.73 m2 for subject's 40–64 years of age, <45 mL/min/1.73 m2 for subjects ˃65 years of age and/or uACR ≥30 mg/g [52], while KDIGO CKD was, for all ages, an eGFR <60 mL/min/1.73 m2 and/or uACR ≥30 mg/g. According to the NHANES, serum creatinine values were standardized using a reference method on isotope dilution mass spectrometry. eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation using age, gender, race and serum creatinine. Descriptive analysis was performed using measures of central tendency, dispersion and position for quantitative variables and frequency distribution for qualitative variables. Among a total of 21 843 participants, 3158 had CKD by age-adapted criteria, a lower prevalence (by 14.3%) compared with 3686 cases when using the 2013 KDIGO criteria. The mean age of the patients was 47.5 years (standard deviation 18.3), 51.4% were women and 20.7% were blacks. The median CKD-EPI creatinine was 98 mL/min/1.73 m2 [interquartile range (IQR) 32.6] and the median uACR was 7.1 mg/g (IQR 8.9). With KDIGO 2013 [8] referral recommendation criteria (eGFR <30 mL/min/1.73 m2 and/or uACR ≥300 mg/g for all ages), 15.3% (564/3686) of patients with CKD would be referred compared with 17.8% with age-adapted CKD criteria. ‘Would referral of young patients with higher eGFR levels overwhelm nephrologists’ offices?’ No. The very low prevalence of CKD in those <40 years of age (˂10%) would imply a mild increase in referrals, as the greater number of cases of patients with CKD, as expected, are >65 years old according to our analysis.
The albuminuria thresholds for the definition of CKD do not need to be age-adapted, as abnormal albuminuria does not develop with healthy ageing [8]. Albuminuria alongside eGFR for older persons helps distinguish senescence from likely intrinsic kidney disease. In general, albuminuria (or proteinuria) measurements remain underutilized despite general recommendations and are vitally important for prognostication [8, 27]. As an example, the Cleveland Clinic reported 36% of patients with CKD had no proteinuria assessed [35] and a British cohort of 12 988 patients showed a similar percentage of uACR testing in CKD patients over a 7-year period and only 17% had uACR testing within the first year of registration of CKD [61, 62]. In a retrospective single-centre study, only 62.5% of patients included uACR measurements at the moment of referral to a nephrologist [31]. This worrying low albuminuria testing rate can especially occur in developing countries, where CKD causes are different (human immunodeficiency virus, malaria) and affect mainly young people.
Finally, optimizing treatment for reducing cardiovascular disease risk in milder cases of CKD may be a reason for referral. These risks are not captured by the KFRE. Cardiovascular risk in younger CKD patients is enormous compared with age-matched healthy individuals, while in older patients with CKD versus matched healthy individuals this risk is also higher but not so disproportionate [63]. Non-age-adapted CKD does not consider kidney senescence in determining the moment of intervention and referral. The nephrological intervention in young CKD patients can have a greater impact in terms of life expectancy and cardiovascular prevention. This might also reaffirm the need for referring younger patients with higher eGFRs [36] and age-adapted referrals to nephrologists. The possible limitations of the eGFR threshold for referral versus KFRE are shown in Table 2. Societal ageing also brings unaddressed issues in guidelines, such as specific referral criteria for very elderly patients. In patients >85 years of age, CKD regression or death is more likely than CKD progression to kidney failure [30].
SPECIAL GLOBAL PERSPECTIVES ON REFERRAL CRITERIA
The global nephrologist density is estimated as 8.83 per million population (pmp); high-income countries report a nephrologist density of 28.52 pmp compared with 0.31 pmp in low-income countries [64]. Optimal care of patients with CKD, and therefore optimal referral, might not be feasible in low-income or low- to middle-income countries. The first step in many low- to middle-income regions (Africa, southern Asia and others) would be to prioritize increasing population-level access to standardized creatinine testing and more widespread implementation of eGFR [65]. This would enable follow-up testing, allow diagnosis and treatment to slow the progression of CKD if possible and enable correct referrals to nephrologists if possible. In low-income countries, only qualitative urine strip tests are available, which, despite their limitations, can be useful. Optimized referral in young patients, especially in countries with lower life expectancy or a low ratio of nephrologists to population, could be considered in global action plans. This optimal referral could be an issue in countries with a low ratio of nephrologists compared with primary care physicians [64].
Referral to the nephrologist is considered late if it is within 1–6 months before the requirement for kidney replacement therapy is reached [66]. This non-appropriate delayed referral has been linked to worse mortality and increased hospitalization and costs in CKD patients [67–75]. Various studies have shown that an important percentage of patients (25–50%) in the USA require chronic kidney replacement therapy within 1 month of their first nephrology visit [76, 77]. This pattern is also common in other parts of the world, such as France [78] and Brazil [79], and is likely to be extremely common in low- to middle-income countries. This frequency of hastily conducted referrals for kidney replacement can have regional variations in large countries like Australia [80]. Referral criteria often reflect the structure of the healthcare system and the availability of resources and services [17]. Disturbingly, we all still see patients in our clinical practices who require dialysis and have received no medical attention at all. This issue can be a common problem in some countries around the world. Referring patients for evaluation at a low GFR threshold, when a possible requirement for kidney replacement therapy is close at hand, may not help to shift this tendency in a more favourable direction since other factors are associated with urgent-start dialysis [81]. Age-adapted referral criteria have been adopted by some countries, such as Sweden, regions and healthcare systems [18, 82], but no prospective studies have compared models and outcomes. In Table 3 we show a hybrid proposal of referral—age-adapted and KFRE, as well as other reasons for a referral—based on the expert opinions of the authors of this article.
Table 3.
Hybrid proposed criteria for referral to nephrologist (age adapted + KFRE) from primary care or other specialities in CKD
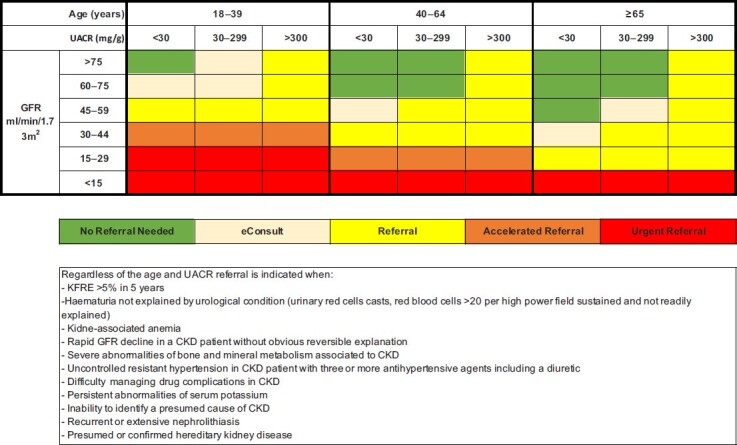
|
These criteria are only applicable to ambulatory CKD patients and not for hospitalized or patients with acute kidney injury. eConsult is defined as a discussion of a case with a nephrologist by phone, e-mail or online platform without a referral. Referral should include a nephrology evaluation in ˂3 months. Accelerated referral should include evaluation in <1 month and urgent referral in <1 week. Common sense should always prevail and cases with suspicious systemic disease, glomerulonephritis, nephritic or nephrotic syndrome do not need confirmation of CKD at 3 months and should be urgently referred. Medical emergency should always be referred to the emergency ward. Depending on the healthcare system, referral can be made for ongoing diagnosis and management, for a one-time consult, for initiating co-management between nephrologists and primary care or for preparation for dialysis or transplantation. This proposal is based on the authors’ expert opinions.
An example of a useful initiative to improve referral and communication between specialists is ‘MONITORED consensus’ [83]. A virtual meeting in the form of a webinar took place between gastroenterologists and nephrologists to define when to refer in inflammatory bowel disease patients with kidney disorders. A previous review of the meeting highlighted the conflicting strategies and different schemes of international societies and the need to homogenize practice. Criteria for referring to the nephrologist were voted on and experts agreed in defining unanimous points such as age-adapted eGFR thresholds for referral.
Different recommendations for referral (KDIGO, NICE, others) do not help clinicians [84], who need standardization of criteria. Consensus is required to generate simple and optimum updated referral criteria for widespread use.
CONCLUSIONS AND SUGGESTIONS
The diversity in guidelines for referrals reflects variations in care in CKD patients worldwide. Nephrology referrals based on GFR thresholds when eGFR is <30 mL/min/1.73 m2 regardless of the age of the patient with CKD may not be appropriate for young patients. For example, a 19-year-old female with serum creatinine 2.1 mg/dL and uACR 280 mg/g would be classified as category G3bA2 and would not meet the criteria to be referred to a nephrologist. Risk equations can help to avoid this gap of several guidelines. Kidney senescence, cardiovascular risk according to age, kidney failure risk and complications associated with CKD have to be considered in an optimum individualized time for referral to a nephrologist. To achieve this, we need to increase uACR testing by primary care practitioners to provide a correct diagnosis and timely intervention that might eventually reduce the burden of kidney failure and the need for dialysis or transplantation. Implementing decision support tools by alerting primary care providers of referral indications may improve the care of CKD patients. Implementing the KFRE is also helpful for referral decision-making and for vascular access planning, but stringent risk thresholds may reduce the volume of referrals and the limitations of these equations at the individual patient level must be recognized. Even individuals at low risk of kidney failure may benefit from secondary care evaluation, especially in young individuals and when CKD-associated complications are present. Standardization of referral criteria to nephrology between guidelines is required.
Contributor Information
Nestor Oliva-Damaso, Department of Medicine, Division of Nephrology, Hospital Costa del Sol, Marbella, Malaga, Spain.
Pierre Delanaye, Department of Nephrology-Dialysis-Transplantation, University of Liege, Centre Hospitalier Universitaire Sart Tilman, ULgCHU, Liege, Belgium; Department of Nephrology-Dialysis-Apheresis, Hôpital Universitaire Carémeau, Nîmes, France.
Elena Oliva-Damaso, Department of Medicine, Division of Nephrology, Hospital Universitario Doctor Negrin, Las Palmas de Gran Canaria, Spain.
Juan Payan, Department of Medicine, Division of Nephrology, Hospital Costa del Sol, Marbella, Malaga, Spain.
Richard J Glassock, Department of Medicine, Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
CONFLICT OF INTEREST STATEMENT
P.D. is a member of the CKJ editorial board.
REFERENCES
- 1. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Hare AM, Rodriguez RA, Rule AD. Overdiagnosis of chronic kidney disease in older adults—an inconvenient truth. JAMA Intern Med 2021; 181: 1366. [DOI] [PubMed] [Google Scholar]
- 3. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1789–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Go AS, Chertow GM, Fan Det al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 5. Carney EF. The impact of chronic kidney disease on global health. Nat Rev Nephrol 2020; 16: 251. [DOI] [PubMed] [Google Scholar]
- 6. Kinchen KS, Sadler J, Fink Net al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137: 479–486 [DOI] [PubMed] [Google Scholar]
- 7. Chu CD, Lamprea-Montealegre JA, Estrella MM. Too many for too few: finding appropriate nephrology referrals for patients with CKD that optimize outcomes. Am J Kidney Dis 2022; 79: 330–332 [DOI] [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 9. National Clinical Guideline Centre . Chronic kidney disease. Early identification and management of chronic kidney disease in adults in primary and secondary care. London: National Institute for Health and care Excellence, 2008 [PubMed]
- 10. National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2 Suppl 1): S1–266 [PubMed] [Google Scholar]
- 11. Caring for Australasians & New Zealanders with Kidney Impairment . Dialysis guidelines. Acceptance onto dialysis. Timing of referral of chronic kidney disease patients to nephrology services (adult). Westmead, NSW: Caring for Australasians & New Zealanders with Kidney Disease, 2010
- 12. American Diabetes Association . 11. Chronic kidney disease and risk management: standards of medical care in diabetes—2022. Diabetes Care 2022. 45(Suppl 1): S175–S184; [DOI] [PubMed]
- 13. Martinez-Castelao A, Gorriz JL, Segura-de la Morena Jet al. Consensus document for the detection and management of chronic kidney disease. Nefrologia 2014; 34: 243–262 [DOI] [PubMed] [Google Scholar]
- 14. García-Maset R, Bover J, Segura de la Morena Jet al. Documento de información y consenso para la detección y manejo de la enfermedad renal crónica. Nefrologia 2022; 10.1016/j.nefro.2021.07.010 [DOI] [Google Scholar]
- 15. National Institute for Health and Care Excellence . Chronic kidney disease: assessment and management. NG203. London: National Institute for Health and Care Exellence, 2021 [PubMed]
- 16. Haute Autorité de Santé. Maladie rénale chronique de l'adulte. Parcours de soins. https://www.has-sante.fr/portail/jcms/r_1506285/fr/maladie-renale-chronique-de-l-adulte-parcours-de-soins.
- 17. Weckmann GFC, Stracke S, Haase Aet al. Diagnosis and management of non-dialysis chronic kidney disease in ambulatory care: a systematic review of clinical practice guidelines. BMC Nephrol 2018; 19: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kidney Society of Southerrn Health Region in Sweden (2013 version). Personal communication from Anders Christensson, Malmo, Sweden.
- 19. Tangri N, Stevens LA, Griffith Jet al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011; 305: 1553–1559 [DOI] [PubMed] [Google Scholar]
- 20. Tangri N, Grams ME, Levey ASet al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA 2016; 315: 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tangri N, Major RW. Risk-based triage for nephrology referrals: the time is now. Kidney Int Rep 2021; 6: 2028–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Major RW, Shepherd D, Medcalf JFet al. The Kidney Failure Risk Equation for prediction of end stage renal disease in UK primary care: an external validation and clinical impact projection cohort study. PLoS Med 2019; 16: e1002955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hingwala J, Wojciechowski P, Hiebert Bet al. Risk-based triage for nephrology referrals using the kidney failure risk equation. Can J Kidney Health Dis 2017; 4: 2054358117722782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whitlock RH, Chartier M, Komenda Pet al. Validation of the kidney failure risk equation in Manitoba. Can J Kidney Health Dis 2017; 4: 2054358117705372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duggal V, Montez-Rath ME, Thomas I-Cet al. Nephrology referral based on laboratory values, kidney failure risk, or both: a study using veterans affairs health system data. Am J Kidney Dis 2022; 79: 347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhachu HK, Cockwell P, Subramanian Aet al. Impact of using risk-based stratification on referral of patients with chronic kidney disease from primary care to specialist care in the United Kingdom. Kidney Int Rep 2021; 6: 2189–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shin JI, Chang AR, Grams MEet al. Albuminuria testing in hypertension and diabetes: an individual-participant data meta-analysis in a global consortium. Hypertension 2021; 78: 1042–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Porrini E, Ruggenenti P, Luis-Lima Set al. Estimated GFR: time for a critical appraisal. Nat Rev Nephrol 2019; 15: 177–190 [DOI] [PubMed] [Google Scholar]
- 29. Shlipak MG, Tummalapalli SL, Boulware LEet al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2021; 99: 34–47 [DOI] [PubMed] [Google Scholar]
- 30. Liu P, Quinn RR, Lam NNet al. Progression and regression of chronic kidney disease by age among adults in a population-based cohort in Alberta, Canada. JAMA Netw Open 2021; 4: e2112828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oliva-Damaso N, Oliva-Damaso E, Rivas-Ruiz Fet al. Impact of a phone app on nephrology referral. Clin Kidney J 2019; 12: 427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sperati CJ, Soman S, Agrawal Vet al. Primary care physicians’ perceptions of barriers and facilitators to management of chronic kidney disease: a mixed methods study. PLoS One 2019; 14: e0221325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh K, Waikar SS, Samal L. Evaluating the feasibility of the KDIGO CKD referral recommendations. BMC Nephrol 2017; 18: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agrawal V, Ghosh AK, Barnes MAet al. Perception of indications for nephrology referral among internal medicine residents: a national online survey. Clin J Am Soc Nephrol 2009; 4: 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jolly SE, Navaneethan SD, Schold JDet al. Chronic kidney disease in an electronic health record problem list: quality of care, ESRD, and mortality. Am J Nephrol 2014; 39: 288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oliva-Damaso N, Oliva-Damaso E, Rodriguez-Perez JCet al. Improved nephrology referral of chronic kidney disease patients: potential role of smartphone apps. Clin Kidney J 2019; 12: 767–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koraishy FM, Rohatgi R. Telenephrology: an emerging platform for delivering renal health care. Am J Kidney Dis 2020; 76: 417–426 [DOI] [PubMed] [Google Scholar]
- 38. Tuot DS, Leeds K, Murphy EJet al. Facilitators and barriers to implementing electronic referral and/or consultation systems: a qualitative study of 16 health organizations. BMC Health Serv Res 2015; 15: 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tuot DS, McCulloch CE, Velasquez Aet al. Impact of a primary care CKD registry in a us public safety-net health care delivery system: a pragmatic randomized trial. Am J Kidney Dis 2018; 72: 168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feldman HI, Briggs JP. Race and the estimation of GFR: getting it right. Am J Kidney Dis 2021; 78: 3–4 [DOI] [PubMed] [Google Scholar]
- 41. Williams WW, Hogan JW, Ingelfinger JR. Time to eliminate health care disparities in the estimation of kidney function. N Engl J Med 2021; 385: 1804–1806 [DOI] [PubMed] [Google Scholar]
- 42. Inker LA, Eneanya ND, Coresh Jet al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med 2021; 385: 1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Delgado C, Baweja M, Crews DCet al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. J Am Soc Nephrol 2021; 32: 2994–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Delgado C, Baweja M, Crews DCet al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis 2022; 79: 268–288 [DOI] [PubMed] [Google Scholar]
- 45. Chen TK, Sperati CJ, Thavarajah Set al. Reducing kidney function decline in patients with CKD: core curriculum 2021. Am J Kidney Dis 2021; 77: 969–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trivedi HS, Pang MMH, Campbell Aet al. Slowing the progression of chronic renal failure: economic benefits and patients’ perspectives. Am J Kidney Dis 2002; 39: 721–729 [DOI] [PubMed] [Google Scholar]
- 47. Lonnemann G, Duttlinger J, Hohmann Det al. Timely referral to outpatient nephrology care slows progression and reduces treatment costs of chronic kidney diseases. Kidney Int Rep 2017; 2: 142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Luyckx VA, Tonelli M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull World Health Org 2018; 96: 414–422D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Raffray M, Vigneau C, Couchoud Cet al. Predialysis care trajectories of patients with ESKD starting dialysis in emergency in France. Kidney Int Rep 2021; 6: 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu P, Quinn RR, Lam NNet al. Accounting for age in the definition of chronic kidney disease. JAMA Intern Med 2021; 181: 1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest 1950; 29: 496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pottel H, Delanaye P, Weekers Let al. Age-dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J 2017; 10: 545–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Delanaye P, Jager KJ, Bökenkamp Aet al. CKD: a call for an age-adapted definition. J Am Soc Nephrol 2019; 30: 1785–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Glassock RJ, Delanaye P, Rule AD. Should the definition of CKD be changed to include age-adapted GFR criteria? YES. Kidney Int 2020; 97: 34–37 [DOI] [PubMed] [Google Scholar]
- 55. Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol 2017; 13: 104–114 [DOI] [PubMed] [Google Scholar]
- 56. Delanaye P. Too much nephrology? The CKD epidemic is real and concerning. A CON view. Nephrol Dial Transplant 2019; 34: 581–584 [DOI] [PubMed] [Google Scholar]
- 57. Jonsson AJ, Lund SH, Eriksen BOet al. The prevalence of chronic kidney disease in Iceland according to KDIGO criteria and age-adapted estimated glomerular filtration rate thresholds. Kidney Int 2020; 98: 1286–1295 [DOI] [PubMed] [Google Scholar]
- 58. Ren Q, Zhou Y, Chen Get al. Age-adapted definition of chronic kidney disease based on Chronic Kidney Disease Epidemiology Collaboration and full age spectrum equation. Kidney Int 2020; 98: 1350–1352 [DOI] [PubMed] [Google Scholar]
- 59. De Broe M, Delanaye P. How to interpret an estimated glomerular filtration rate (eGFR) in 2020? Kidney Int 2020; 98: 1090–1092 [DOI] [PubMed] [Google Scholar]
- 60. National Center for Health Statistics . National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2021 (May 2021, date last accessed) [Google Scholar]
- 61. Naranjo FS, Sang Y, Ballew SHet al. Estimating kidney failure risk using electronic medical records. Kidney 360 2021; 2: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fraser SD, Parkes J, Culliford Det al. Timeliness in chronic kidney disease and albuminuria identification: a retrospective cohort study. BMC Fam Pract 2015; 16: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Foley R, Parfrey P, Sarnak M. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32(5 Suppl 3): S112–S119 [DOI] [PubMed] [Google Scholar]
- 64. Osman MA, Alrukhaimi M, Ashuntantang GEet al. Global nephrology workforce: gaps and opportunities toward a sustainable kidney care system. Kidney Int Suppl (2011) 2018; 8: 52–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jha V, Modi GK. eGFR testing around the world: justice, access, and accuracy. Clin J Am Soc Nephrol 2021; 16: 963–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Eadington DW. Delayed referral for dialysis. Nephrol Dial Transplant 1996; 11: 2124–2126 [DOI] [PubMed] [Google Scholar]
- 67. Smart NA, Dieberg G, Ladhani Met al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev 2014; 18: CD007333. [DOI] [PubMed] [Google Scholar]
- 68. Tseng C-L, Kern EFO, Miller DRet al. Survival benefit of nephrologic care in patients with diabetes mellitus and chronic kidney disease. Arch Intern Med 2008; 168: 55–62 [DOI] [PubMed] [Google Scholar]
- 69. Chan MR, Dall AT, Fletcher KEet al. Outcomes in patients with chronic kidney disease referred late to nephrologists: a meta-analysis. Am J Med 2007; 120: 1063–1070 [DOI] [PubMed] [Google Scholar]
- 70. Patwardhan MB, Samsa GP, Matchar DBet al. Advanced chronic kidney disease practice patterns among nephrologists and non-nephrologists: a database analysis. Clin J Am Soc Nephrol 2007; 2: 277–283 [DOI] [PubMed] [Google Scholar]
- 71. Khan SS, Xue JL, Kazmi WHet al. Does predialysis nephrology care influence patient survival after initiation of dialysis? Kidney Int 2005; 67: 1038–1046 [DOI] [PubMed] [Google Scholar]
- 72. Stack AG. Impact of timing of nephrology referral and pre-ESRD care on mortality risk among new ESRD patients in the United States. Am J Kidney Dis 2003; 41: 310–318 [DOI] [PubMed] [Google Scholar]
- 73. Winkelmayer WC, Owen WFJ, Levin Ret al. A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J Am Soc Nephrol 2003; 14: 486–492 [DOI] [PubMed] [Google Scholar]
- 74. Bradbury BD, Fissell RB, Albert JMet al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2007; 2: 89–99 [DOI] [PubMed] [Google Scholar]
- 75. Astor BC, Eustace JA, Powe NRet al. Timing of nephrologist referral and arteriovenous access use: the CHOICE Study. Am J Kidney Dis 2001; 38: 494–501 [DOI] [PubMed] [Google Scholar]
- 76. US Renal Data System . USRDS 1997 Annual Data Report. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 1997
- 77. Morbidity and mortality of renal dialysis: an NIH consensus conference statement. Consensus Development Conference Panel. Ann Intern Med 1994; 121: 62–70 [DOI] [PubMed] [Google Scholar]
- 78. Jungers P, Zingraff J, Albouze Get al. Late referral to maintenance dialysis: detrimental consequences. Nephrol Dial Transplant 1993; 8: 1089–1093 [PubMed] [Google Scholar]
- 79. Sesso R, Belasco AG. Late diagnosis of chronic renal failure and mortality on maintenance dialysis. Nephrol Dial Transplant 1996; 11: 2417–2420 [DOI] [PubMed] [Google Scholar]
- 80. Cass A, Cunningham J, Snelling Pet al. Urban disadvantage and delayed nephrology referral in Australia. Health Place 2003; 9: 175–182 [DOI] [PubMed] [Google Scholar]
- 81. Fages V, de Pinho NA, Hamroun Aet al. Urgent-start dialysis in patients referred early to a nephrologist—the CKD-REIN prospective cohort study. Nephrol Dial Transplant 2021; 36: 1500–1510 [DOI] [PubMed] [Google Scholar]
- 82. Rutkowski M, Mann W, Derose Set al. Implementing KDIGO definition and staging guidelines in Southerrn California Kaiser Permanente. Am J Kidney Dis 2009; 53(3 Suppl 3): S86–S99 [DOI] [PubMed] [Google Scholar]
- 83. Guillo L, Delanaye P, Flamant Met al. Kidney function monitoring in inflammatory bowel disease: the MONITORED consensus. Dig Liver Dis 2022; 54: 309–315 [DOI] [PubMed] [Google Scholar]
- 84. Schulz C, Messikh Z, Reboul Pet al. Characteristics of outpatients referred for a first consultation with a nephrologist: impact of different guidelines. J Nephrol 2022; doi: 10.1007/s40620-021-01204-w [DOI] [PubMed] [Google Scholar]


