ABSTRACT
Background
There is a lack of information regarding which is the best dialysis technique after kidney transplant (KT) failure. The aim of this study is to compare the effect of kidney replacement therapy modality-peritoneal dialysis (TX-PD-TX), haemodialysis (TX-HD-TX) and preemptive deceased donor retransplantation (TX-TX) on patient survival and second KT outcomes.
Methods
A retrospective observational study from the Catalan Renal Registry was carried out. We included adult patients with failing of their first KT from 2000 to 2018.
Results
Among 2045 patients, 1829 started on HD (89.4%), 168 on PD (8.2%) and 48 (2.4%) received a preemptive KT. Non-inclusion on the KT waiting list and HD were associated with worse patient survival. For patients included on the waiting list, the probability of human leucocyte antigens (HLA) sensitization and to receive a second KT was similar in HD and PD. A total of 776 patients received a second KT (38%), 656 in TX-HD-TX, 72 in TX-PD-TX and 48 in TX-TX groups. Adjusted mortality after second KT was higher in TX-HD-TX patients compared with TX-TX and TX-PD-TX groups, without differences between TX-TX and TX-PD-TX groups. Death-censored second graft survival was similar in all three groups.
Conclusions
Our results suggest that after first KT failure, PD is superior to HD in reducing mortality in candidates for a second KT without options for preemptive retransplantation.
Keywords: allograft failure, dialysis, kidney replacement therapy, kidney transplantation, transitional care
INTRODUCTION
Kidney graft failure is an increasingly common issue because of a greater pool of kidney transplant (KT) recipients. Failed KTs account for 13.1% of the incident dialysis population in Catalonia and represent 35.2% of the patients on the kidney transplant waiting list [1]. This population shows increased morbidity and mortality in comparison with non-previously transplanted end-stage kidney disease (ESKD) patients starting dialysis. However, little attention has been given to this period of kidney replacement therapy (KRT) transition, when patients suffer from higher rates of complications [2]. Therefore, there is a lack of data on most issues related to patient and graft management of the failing transplant.
Regarding the modality of KRT, preemptive kidney retransplantation provides the best outcomes, in view of larger survival and better quality of life [3]. However, this option is nearly exclusive for living donor transplants in most countries, and most patients return to dialysis. However, there are few studies comparing the outcomes depending on the preferred dialysis technique. In a recent review [4], Fiorentino et al. summarized five studies [5–9] comparing outcomes of peritoneal dialysis (PD) and haemodialysis (HD) patients with failed primary KTs and concluded that it is still a matter of debate. Most of the studies were published ˃10 years ago and did not find differences in survival [5, 6] or if found, they were attributed to patient comorbidities. Regarding PD, it seems that there was a trend to better survival during the first year but worse thereafter. Moreover, there are no data about the impact of the dialysis modality on second KT outcomes.
In the present study, we have analyzed data from the Catalan Registry of Renal Patients to determine whether the type of dialysis modality (HD or PD) after first kidney allograft failure, in comparison with those patients receiving a preemptive deceased donor second KT, has an impact on patient survival and second KT outcomes.
MATERIALS AND METHODS
After gaining the approval of the Institutional Review Board, we used data from the Registry of Renal Patients of Catalonia (RMRC). This is a mandatory population-based registry covering 7.5 million people that collects information on all patients with ESKD requiring KRT in Catalonia. At the time of starting KRT and at every change of treatment throughout KRT, a registration form is filled in. Every year an update must be carried out and sent to the RMRC up to the finalization of KRT, death of patient or loss of follow-up.
Study population
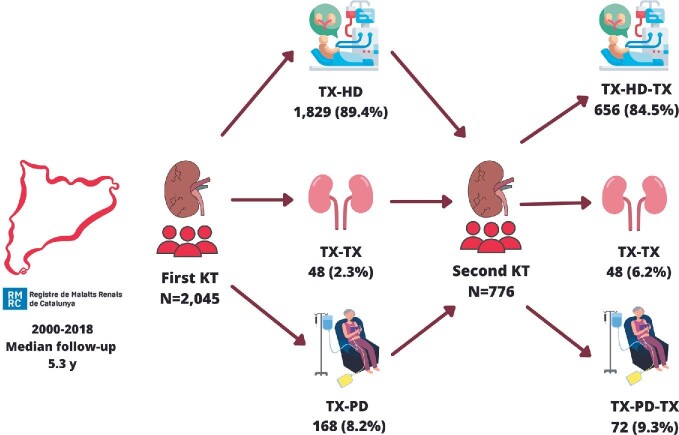
A retrospective observational study has been carried out with the analysis of data from patients with failing of their first kidney allograft in Catalonia from 2000 to 2018. Patients with multiorgan transplantation and patients that received a second living donor KT were excluded. Among the 2045 patients identified and selected for the study, 776 received a second KT from a deceased donor (Fig. 1). All cases were followed-up until December 2018.
FIGURE 1:
Flowchart of the study.
Data analysis
Patients were classified in the following groups depending on the KRT strategy for the second transplant: from failed first KT to HD and then second KT (TX-HD-TX); from failed first KT to PD and then second KT (TX-PD-TX) and from failed KT directly to second KT (TX-TX). Details on group assignments are shown in 9. We also analysed the patients who did not receive a second KT and remained on dialysis (HD or PD) depending on their KT waiting list situation (included or not included). The collected variables were gender, age, diabetes mellitus, cardiovascular disease, panel reactive antibody (PRA) by cellular cytotoxicity (CDC), functional autonomy defined by the Karnofsky scale adapted for dialysis patients [10], inclusion on the waiting list and time in dialysis before second KT. We collected and also calculated PRA (Luminex) in patients who lost their graft between 2014 and 2018.
Comparisons between groups were performed by Chi-squared test for categorical data and analysis of variance or Kruskal–Wallis equality-of-populations rank test for continuous data (P < .05 was considered significant). Baseline characteristics of the study cohort were expressed as a number and a proportion, mean ± standard deviation (SD) or median and interquartile range.
For the multivariable analysis, all models were initially adjusted by gender, age, diabetes mellitus, previous cardiovascular event (ischaemic heart disease, heart failure, peripheral vascular disease and cerebrovascular disease), type of first KT (living or deceased donor), CDC before first KT, functional status and dialysis duration before second KT. Finally, only statically significant variables (P < .05) and gender, age and type of KRT after first graft failure were considered in the chosen models.
We analysed patient survival after the first graft loss and after the second KT. Also, survival of the second graft was determined for each group. Survival analysis was performed by using the Kaplan–Meier in the univariate and the Cox regression in the multivariable analysis. All statistical tests were considered significant if P < .05 for two-tailed tests. Analyses were performed using STATA software version 13.
RESULTS
Failing kidney allograft population outcomes
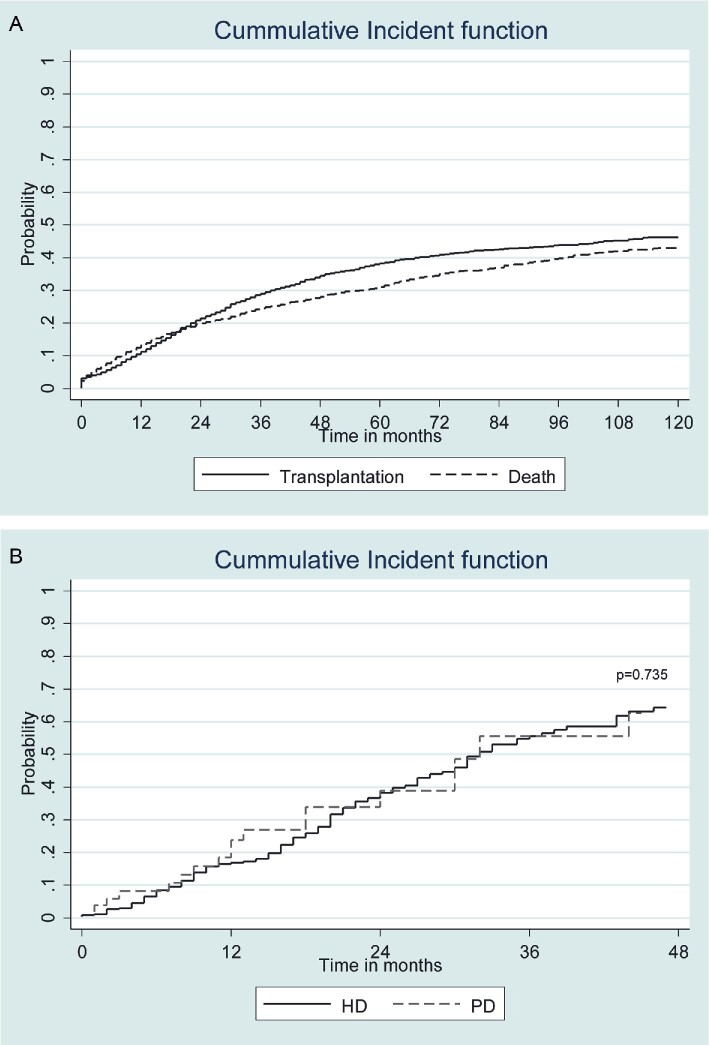
Overall, there were 2045 patients with failing kidney allografts during the study period. Among them, 1829 started on in-centre HD (89.4%), 168 started on PD (8.2%) and 48 (2.4%) received a preemptive deceased donor kidney allograft. Baseline characteristics of this population are shown in Table 1. The mean follow-up was 5.3 years. After the first graft loss, the probability of receiving a second KT was similar to decease (Fig. 2A). Patients returning to HD were older, had a higher prevalence of diabetes and cardiovascular disease and showed lower functional autonomy. Therefore, the proportion of patients reincluded in the KT waiting list was higher in PD (77.9%) than in HD (58.3%), P = .002. However, for patients included on the waiting list, the probability of receiving a second KT was similar in HD and PD (Fig. 2B). For patients included on the waiting list the meantime to receive a second KT is 18.4 ± 11 and 18.1 ± 13 months for TX-HD-TX and TX-PD-TX, respectively. Human leucocyte antigens (HLA) sensitization determined by Luminex was available after first kidney failure in patients who lost their graft between 2014 and 2018 without showing differences between HD and PD (data not shown).
Table 1.
Baseline characteristics of patients after first kidney graft failure depending on kidney replacement therapy
| Variable | Total (n = 2045) | HD (n = 1829) | PD (n = 168) | TX (n = 48) | P-value | Missing (%) |
|---|---|---|---|---|---|---|
| Gender (n, %) | ||||||
| Male | 1244 (60.8) | 1122 (61.3) | 93 (55.4) | 29 (60.4) | ||
| Female | 801 (39.2) | 707 (38.7) | 75 (44.6) | 19 (39.6) | .314 | 0 |
| Age at first TX (n, %) | ||||||
| 0–44 years | 737 (36.0) | 636 (34.8) | 81 (48.2) | 20 (41.7) | ||
| 45–64 years | 932 (45.6) | 837 (45.8) | 74 (44.0) | 21 (43.7) | ||
| >65 years | 376 (18.4) | 356 (19.4) | 13 (7.8) | 7 (14.6) | <.001 | 0 |
| Age at first TX | ||||||
| Years (SD) | 46.0 (16.5) | 49.7 (16.2) | 43.0 (16.5) | 43.7 (19.8) | <.001 | 0 |
| Diabetes | ||||||
| No | 1412 (69.0) | 1241 (67.9) | 137 (81.5) | 34 (70.8) | ||
| Yes | 633 (31.0 | 588 (32.1) | 31 (18.5) | 14 (29.2) | .001 | 0 |
| Cardiovascular disease (n, %) | ||||||
| No | 988 (48.9) | 851 (47.1) | 109 (65.2) | 28 (62.2) | ||
| Yes | 1030 (51.1) | 955 (52.9) | 58 (34.8) | 17 (37.8) | <.001 | 1 |
| First TX type (n, %) | ||||||
| Deceased | 1919 (93.8) | 1719 (94.0) | 158 (94.0) | 42 (87.5) | ||
| Live | 126 (6.2) | 110 (6.0) | 10 (6.0) | 6 (12.5) | .181 | 0 |
| %PRA (n, %) | ||||||
| 0–10% | 1666 (95.4) | 1500 (95.5) | 127 (93.4) | 29 (93.5) | ||
| 11–50% | 66 (3.8) | 57 (3.7) | 7 (5.1) | 2 (6.5) | ||
| >50% | 15 (0.8) | 13 (0.8) | 2 (1.5) | 0 (0) | .548 | 15 |
| Cause of first TX failure (n, %) | ||||||
| PNF | 60 (3.4) | 59 (3.7) | 1 (0.7) | 0 (0) | ||
| Surgical complications | 199 (11.1) | 176 (10.9) | 16 (10.5) | 7 (33.3) | ||
| Chronic damage | 1177 (65.9) | 1062 (65.8) | 102 (67.1) | 13 (61.9) | ||
| Acute rejection | 102 (5.7) | 93 (5.8) | 9 (5.9) | 0 (0) | ||
| Other | 249 (13.9) | 224 (13.8) | 24 (15.8) | 1 (4.8) | .084 | 13 |
| First TX survival | ||||||
| (median month, P25-P75) | 81 (23–148) | 83 (23–147) | 86 (37–155) | 58 (12–159) | .359 | 0 |
| Age at first graft failure | ||||||
| Years (SD) | 56.9 (15.1) | 57.6 (14.9) | 51.5 (15.6) | 51.9 (18.2) | <.001 | 0 |
| Functional autonomy (n, %) | ||||||
| Normal/nearly normal | 1357 (80.9) | 1212 (79.8) | 127 (92.0) | 18 (85.7) | ||
| Limited | 244 (14.6) | 232 (15.3) | 10 (7.2) | 2 (9.5) | ||
| Dependent/hospitalized | 76 (4.5) | 74 (4.9) | 1 (0.8) | 1 (4.8) | .004 | 18 |
HD, haemodialysis; PD, peritoneal dialysis; PNF, primary non function; PRA, panel reactive antibody; SD, standard deviation; TX, transplant.
FIGURE 2:
(A) Death and second kidney transplantation probability after first kidney graft failure. (B) Probability of receiving a second kidney transplant in waitlisted patients, comparing haemodialysis (HD) with peritoneal dialysis (PD). Once included on the waiting list, probability of receiving a second transplant is similar in both HD and PD groups (P = .735).
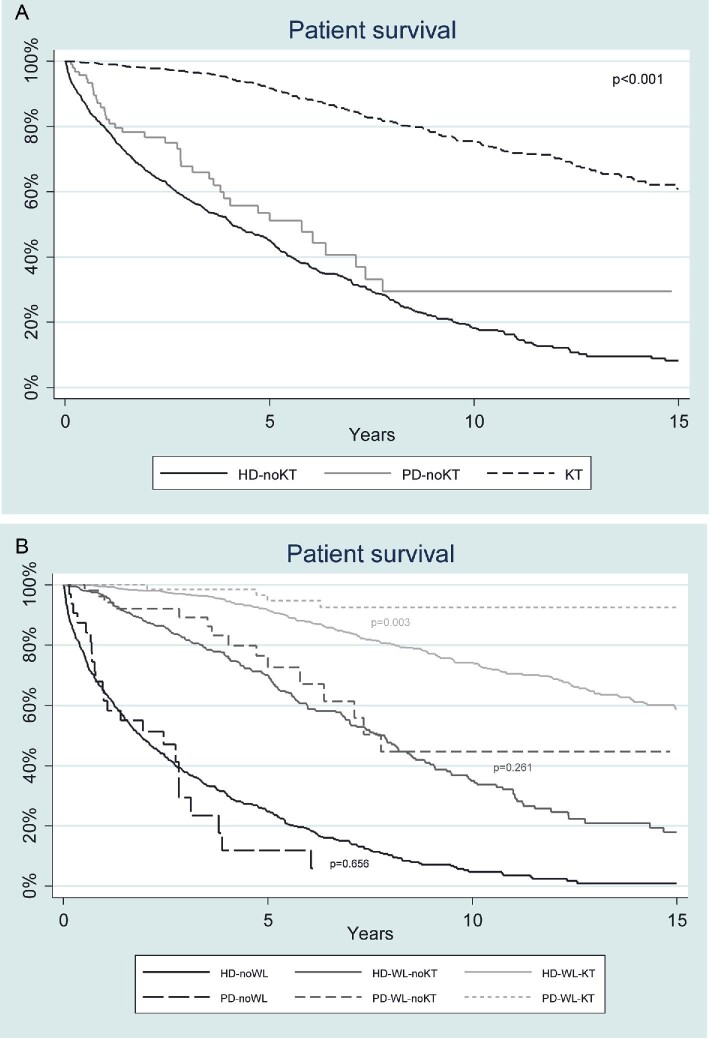
We then compared patient survival between patients remaining on HD, PD and patients receiving a second KT (Fig. 3A). Survival was similar either in patients remaining on PD or HD and both significantly lower than survival in patients that received a second deceased donor KT. Patients on dialysis, waitlisted and not retransplanted had better survival than patients on dialysis not waitlisted, yet lower than those retransplanted (Fig. 3B). Comparing PD versus HD, the beneficial effect of PD on survival was only observed in the cohort of patients that received a second kidney allograft (Fig. 3B). Multivariable adjusted models for patient survival after the first KT showed that variables associated with survival were age, diabetes, cardiovascular disease, non-inclusion on the waiting list, HD as KRT and functional status (Table 2). Interestingly, better survival was associated with TX-TX and TX-PD transition. Major causes of death in HD were cardiovascular (39.3%), infection (24.6%) and cancer (9.2%) and in PD were cardiovascular (46.6%), infection (15.6%) and cancer (2.2%). Causes of death after first kidney allograft failure are detailed in Supplementary data, Table S2.
FIGURE 3:
(A) Patient survival after first kidney graft failure in non-transplanted patients on haemodialysis (HD) and peritoneal dialysis (PD); and in patients receiving a second kidney transplant (KT). Non-transplanted patients exhibit worse survival compared with those retransplanted (P < .001). (B) Patient survival after first kidney graft failure according to: kidney replacement therapy, inclusion on the waiting list for second transplantation and retransplantation. Survival between haemodialysis (HD) and peritoneal dialysis (PD) patients and not included in waiting list (WL) was not statistically different (P = .656), nor between HD and PD groups when included in WL (P = .261). However, survival is superior in the PD group receiving a second transplantation, compared with the also retransplanted HD group (P = .0030).
Table 2.
Multivariable model of patient survival after first kidney transplant
| HR | 95% CI | |
|---|---|---|
| Gender Male Female |
Ref 0.90 |
0.78–1.04 |
| Age 0–44 years 45–64 years >64 years |
Ref 3.70 10.86 |
3.02–4.52 8.38–14.07 |
| Diabetes No Yes |
Ref 1.21 |
1.05–1.41 |
| Cardiovascular disease No Yes |
Ref 1.23 |
1.06–1.43 |
| KRT transition after first graft failure TX-PD-TX TX-TX TX-HD (no WL) TX-HD (WL) TX-HD-TX TX-PD (no WL) TX-PD (WL) |
Ref 2.80 9.28 7.08 3.71 13.32 5.39 |
0.79–9.93 3.45–25.00 2.62–19.08 1.37–10.01 4.56–38.94 1.77–16.41 |
| Functional autonomy Normal/nearly normal Limited Dependent/hospitalized |
Ref 1.48 4.34 |
1.17–1.87 2.89–6.53 |
CI, confidence interval; KRT, kidney replacement therapy; HR, hazard ratio; WL, waiting list.
Recipients of a second KT
Among the 2045 patients included, 776 received a second KT (38%): 656 TX-HD-TX (84.5%), 72 TX-PD-TX (9.3%) and 48 TX-TX (6.2%). Baseline characteristics are shown in Table 3. TX-HD-TX patients were older and had a higher prevalence of diabetes and cardiovascular disease. The period on dialysis was similar in TX-HD-TX and TX-PD-TX. Early graft loss (<90 days after kidney transplantation), acute rejection as the cause of graft failure and renal function during the first year after retransplantation were similar among all the three groups, as shown in Table 4.
Table 3.
Second kidney transplant recipient baseline characteristics depending on previous kidney replacement therapy
| Variable, n (%) | Total (n = 776) | TX-TX (n = 48) | TX-HD-TX (n = 656) | TX-PD-TX (n = 72) | P-value | Missing (%) |
|---|---|---|---|---|---|---|
| Gender Male Female |
471 (60.7) 305 (39.3) |
29 (60.4) 19 (39.6) |
401 (61.1) 255 (38.9) |
41 (56.9) 19 (43.1) |
.788 |
0 |
| Age at first TX 0–44 years 45–64 years >64 years |
254 (32.7) 380 (49.0) 142 (18.3) |
12 (25) 25 (52.1) 11 (22.9) |
208 (31.7) 323 (49.2) 125 (19.1) |
34 (47.2) 32 (44.4) 6 (8.3) |
.029 |
0 |
| Diabetes No Yes |
652 (84.6) 119 (15.4) |
41 (89.1) 5 (10.9) |
541 (82.9) 112 (17.1) |
70 (97.2) 2 (2.8) |
.004 |
1 |
| Cardiovascular disease No Yes |
336 (44.1) 429 (55.9) |
26 (62.2) 17 (37.8) |
267 (41.1) 383 (58.9) |
43 (59.7) 29 (40.3) |
<.001 |
1 |
| First TX type Deceased Live |
714 (92) 62 (8) |
42 (87.5) 6 (12.5) |
606 (92.4) 50 (7.6) |
66 (91.7) 6 (8.3) |
.482 |
0 |
| %PRA 0–10% 11–50% >50% |
637 (95.5) 25 (3.8) 5 (0.8) |
39 (95.1) 2 (4.9) 0 (0) |
541 (95.6) 21 (3.7) 4 (0.7) |
57 (95.0) 2 (3.3) 1 (1.7) |
.741 |
14 |
| Functional autonomy Normal/nearly normal Limited Dependent/hospitalized |
618 (92.1) 47 (7) 6 (0.9) |
18 (85.7) 2 (9.5) 1 (4.8) |
546 (91.8) 44 (7.4) 5 (0.8) |
618 (92.1) 47 (7.0) 6 (0.9) |
.132 |
14 |
| Time until second transplant <1 year >1 year |
158 (21.7) 570 (78.3) |
|
141 (21.5) 515 (78.5) |
17 (23.6) 55 (76.4) |
.679 |
0 |
| Death No Yes |
590 (76) 186 (24) |
40 (83.3) 8 (16.7) |
482 (73.5) 174 (26.5) |
68 (94.4) 4 (5.6) |
<.001 |
0 |
| Second kidney graft failure No Yes |
603 (77.7) 173 (22.3) |
39 (81.2) 9 (18.8) |
504 (76.8) 152 (23.2) |
60 (83.3) 12 (16.7) |
.376 |
0 |
HD, haemodialysis; PD, peritoneal dialysis; PRA, panel reactive antibodies; TX, transplant.
Table 4.
Second kidney graft evolution depending on previous kidney replacement therapy
| Variable (n, %) | Total (n = 776) | TX-TX (n = 48) | TX-HD-TX (n = 656) | TX-PD-TX (n = 72) | P- value | Missing (%) |
|---|---|---|---|---|---|---|
| Early graft failure | 48 (6.2) | 4 (8.3) | 41 (6.3) | 3 (4.2) | .603 | 0 |
| Rejection as cause of graft failure | 14 (1.8) | 0 (0) | 13 (1.98) | 1 (1.39) | 1.000 | 12 |
| First eGFR 0–29 30–59 >59 |
169 (24.5) 327 (47.5) 193 (28) |
13 (29.6) 19 (43.2) 12 (27.3) |
147 (25.4) 268 (46.3) 164 (28.3) |
9 (13.6) 40 (60.6) 17 (25.8) |
.152 |
11 |
| Second eGFR 0–29 30–59 >59 |
92 (16.1) 292 (51.0) 189 (33.0) |
5 (15.2) 13 (39.4) 15 (45.5) |
83 (17.18) 248 (51.35) 152 (31.47) |
4 (7.0) 31 (54.4) 22 (38.6) |
.150 |
26 |
eGFR, estimated glomerular filtration rate (measured in mL/min/1.73 m2); HD, haemodialysis; PD, peritoneal dialysis; TX, transplant.
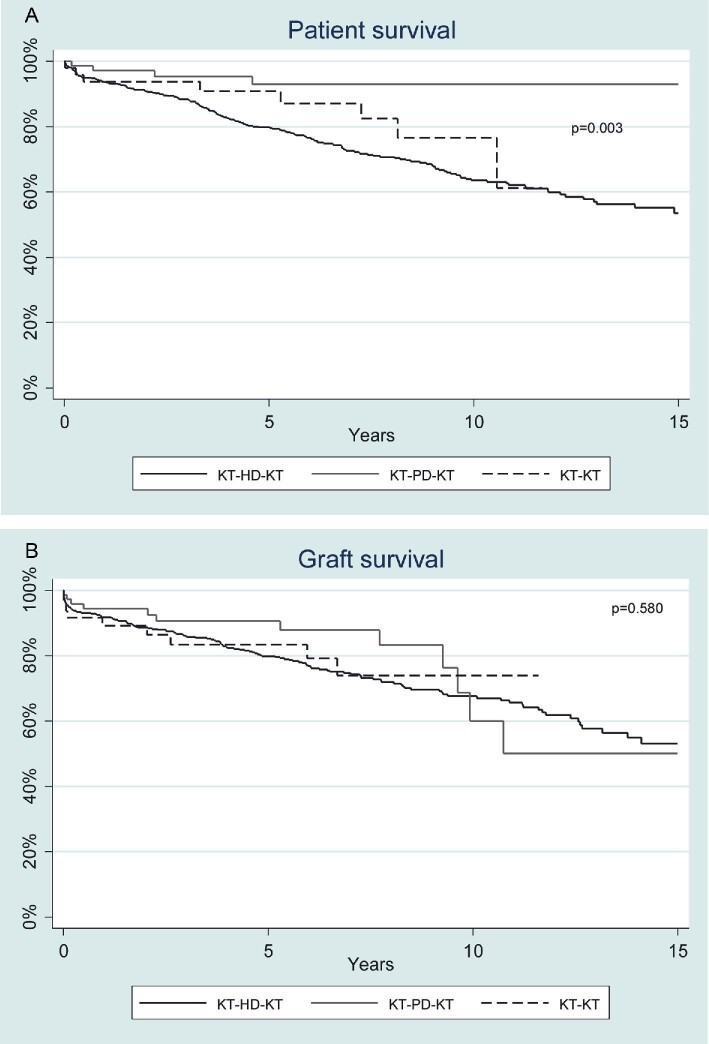
We then compared patient survival (Fig. 4A) and death-censored graft survival (Fig. 4B) between TX-TX, TX-HD-TX and TX-PD-TX groups. Multivariable adjusted models for patient and graft survival after a second KT are shown in Table 5. Variables associated with mortality were age, diabetes, cardiovascular disease, limited functional status and HD-TX transition. Variables associated with death-censored second graft survival were female gender and age. Major causes of death in TX-HD-TX were cardiovascular (31.9%), infection (27.9%), and cancer (11.7%), and in TX-PD-TX were cardiovascular (40%) and infection (20%). All causes of death after the second KT are detailed in Supplementary data, Table S3.
FIGURE 4:
(A) Patient survival after second kidney transplant (KT) according to previous kidney replacement therapy. Survival in TX-PD-TX and TX-TX groups is greater compared with the TX-HD-TX group (P = .003). There was no statistically difference between TX-TX and TX-PD-TX groups. (B) Death-censored second kidney graft survival according to previous kidney replacement therapy. HD, haemodialysis; KT, kidney transplant; PD, peritoneal dialysis. No difference was found among the different groups: HD-KT, PD-KT or KT-KT (P = .580).
Table 5.
Multivariable models of patient and graft survival after second kidney transplant
| Patient survival since first graft failure | Patient survival after second kidney transplant | Death-censored second graft survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Gender Male Female |
Ref 0.85 |
0.62–1.16 |
Ref 0.94 |
0.69–1.29 |
Ref 1.40 |
1.03–1.89 |
| Age 0–44 years 45–64 years >64 years |
Ref 4.19 12.07 |
2.64–6.66 7.28–20.01 |
Ref 3.84 8.75 |
2.40–6.14 5.18–14.79 |
Ref 1.06 1.92 |
0,76–1.49 1.25–2.95 |
| Diabetes No Yes |
|
|
Ref 1.57 |
1.07–2.29 |
|
|
| CV disease No Yes |
|
|
Ref 1.81 |
1.28–2.57 |
|
|
| KRT transition after first graft failure TX-PD-TX TX-TX TX-HD-TX |
Ref 3.93 4.57 |
0.55–26.11 1.13–18.51 |
Ref 1.03 4.84 |
0.09–11.44 1.19–19.61 |
|
|
| Functional autonomy Normal/nearly Limited Dependent/hospitalized |
Ref 1.32 7.00 |
0.76–2.30 2.16–22.72 |
Ref 1.01 5.59 |
0.57–1.79 1.69–18.50 |
|
|
CI, confidence interval; CV, cardiovascular; HD, haemodialysis; KRT, kidney replacement therapy; PD, peritoneal dialysis; HR, hazard ratio; TX, transplant. Statistically significant values are shown in bold.
DISCUSSION
Management of kidney allograft failure is guided by poor evidence and stands as a current clinical issue. Patients with a failed allograft have increased in the last decade, representing ∼ 3% of the incident dialysis population in the USA [11] or even as much as 15% in some European regions like Catalonia [1]. Moreover, this group of patients represents an important proportion of waitlisted people for KT [11].
Following graft failure, living donor repeated transplantation is the best option [12]. However, in Spain this path is uncommon, and most patients are evaluated for a second deceased donor KT. Thus, to minimize transplantation bias, we excluded recipients of second KT from a living donor. The Spanish health system guarantees to chronic kidney disease population universal evaluation for KT feasibility as demonstrated by the fact that almost 60% of patients are relisted for second transplantation in our cohort. Similar [13] or lower [14] overall relisting in patients with allograft failure are depicted in other studies. Despite this fact, after the first kidney graft failure, we observed that the probability to receive a second KT is similar to dying. Patients from our cohort were quite older (∼10 years) compared with other studies [7, 15]. It is well-known that mortality in patients requiring dialysis after kidney graft loss is higher than in transplant-naïve peers [16, 17]. This difference in the evolution between both groups may be caused by the proinflammatory state in which these patients are, causing anaemia and malnutrition and making them more susceptible to infections [18, 19].
When living donor KT is not feasible, preemptive deceased-donor kidney retransplantation represents the optimal therapy for patients who lose kidney allograft, exhibiting greater patient and graft survival, thanks to avoiding the morbidity and mortality associated with dialysis reinitiation [3, 15, 20]. However, this option is not always possible due to patient comorbidities or limited because of HLA sensitization, or by the fact that time on dialysis is a relevant criterion in our allocation score. Thus, only 2.4% of patients from our cohort received a preemptive second KT and therefore, most patients needed to start dialysis. A comparable preemptive retransplantation rate is shown in a US study [13].
In our cohort, most patients were on HD rather than PD. Regarding inclusion on the waiting list for a second KT, patients on PD were more frequently included than patients on HD. This observation is consistent with the fact that HD patients were older, showed more cardiovascular comorbidities and had inferior functional status. However, once included in the waiting list, the probability and the waiting time for receiving a second KT were similar among HD and PD patients. Likewise, in a registry-based study from the US Renal Data System, the median time to retransplantation was ∼2 years, and when comparing time to retransplantation between PD and HD groups in a multivariable and propensity score analysis, no differences were observed [8].
Management of immunosuppression in patients with failing allograft is subjective because of the lack of clinical evidence. Practice for tapering immunosuppression may be critical for preventing HLA sensitization and it could be different between HD and PD. Unfortunately, in our registry, we do not have data on immunosuppression tapering. However, our observation that HLA sensitization determined by Luminex was similar in patients on HD and PD indirectly suggests that the management of immunosuppression was comparable. This similar HLA sensitization after graft failure in patients on HD and PD may explain the similar probability of transplantation in both groups. Comparable HLA sensitization between HD and PD groups after allograft loss is found in other studies [8].
Evidence to determine whether HD or PD after allograft failure is better in terms of survival are scarce and sometimes contradictory. The main studies in the field comparing HD with PD were summarized in a recent review [4] which concluded that the choice of dialysis modality after graft failure should be based on clinical characteristics due to a lack of definitive evidence in the scientific literature. Regarding PD, some studies find a temporary benefit in the first period of the technique (approximately the first year) either in transplant-naïve patients [21] or those suffering from allograft loss [8], which is lost afterward.
Our study underscores the beneficial impact of receiving a second KT in patients on dialysis after first graft failure. Patient survival was higher in both HD and PD patients comparing transplantation versus no transplantation, and even when comparing transplantation versus waitlisted but not transplanted. Beyond the well-known KT benefit, we tried to ascertain whether the dialysis modality may also influence patient survival after the first graft failure. Our results suggest that PD is superior to HD in terms of patient survival, particularly in patients that received a second KT, and provides similar outcomes to preemptive second KT. In spite of this, for patients that are not retransplanted, the increased survival associated with PD seems to have vanished. The different impact of dialysis modality on survival, depending on whether the patient is suitable for a second KT or not, may explain some discrepancies in previous studies, as only patients in good enough health to be considered as candidates for a second KT take advantage of PD after first kidney graft failure. The beneficial effect of residual kidney function in PD patients might be one of the factors improving the survival [22]. Maybe those who maintained it enough until retransplantation are the only ones seeing this effect. However, this is just an unproved hypothesis, given the absence of data on residual kidney function in our study. The lack of differences in survival between HD and PD patients if not retransplanted suggests that the multivariable analysis was properly adjusted for the key comorbidities. The higher mortality due to infection in HD versus PD may be related to the use of catheter as vascular access, given that the lack of fistula has been established as a risk factor for mortality in this population [23]. However, this finding deserves further investigation. In addition to dialysis modality and transplantation, we found that other determinants of patient survival after first KT are age, diabetes mellitus, cardiovascular disease and functional status. Previous publications also report a detrimental effect of these comorbidities [7, 24].
Our study also analysed the outcome after receiving a second KT. As far as we know, this is the first study in the literature reporting the influence of dialysis modality in this setting. Our results show that early and late immunological and non-immunological graft outcomes were comparable between HD and PD and similar to those with preemptive second KT. Thus, it seems that neither the dialysis modality nor preemptive KT may have a significant impact on death-censored second graft survival. In agreement with these results, several studies have shown that the impact of pretransplant dialysis time on second graft survival is related to an increase in the risk of mortality, rather than an independent impact on the graft [12, 25]. Related to the preemptive effect, a registry-based study from the Austrian cohort observed that superior graft survival of preemptive versus non-preemptive primary KT recipients was lost when excluding living donors and KTs performed before the year 2000, yet only when dialysis time was ˂1.5 years [26]. An unexpected observation was that the female gender appeared to be a risk factor for death-censored second allograft survival, as it has not been identified as so in other studies [25, 27, 28]. However, in a study specifically aiming at sex as a possible risk factor for primary allograft failure, female KT recipients were at higher risk of death-censored allograft survival in case of receiving a male donor, and only in the 15–24 years range when receiving a female donor [29].
Despite the absence of any effect on graft survival, the dialysis modality was relevant to patient survival. Again, we found that patients on HD after first graft failure and then transplanted (TX-HD-TX transition) exhibited lower patient survival than patients on PD and then transplanted (TX-PD-TX transition). The decrease in cancer as a death cause in patients on pretransplant PD deserves further investigation. Retrospective studies from different cohorts have shown no impact of dialysis modality on cancer incidence either during dialysis [30] or after primary transplantation [31]. However, no data on mortality are specified in these studies. Other risk factors associated with mortality were similar to those after first KT: age, diabetes, cardiovascular disease and functional autonomy.
Our study has some limitations. Like other registry-based studies, our work is limited by the retrospective nature of the data and, therefore, no causal relationship can be established. As previously mentioned, we have no information regarding the tapering of immunosuppression, graft intolerance, embolization or transplantectomy. Analysis of patient survival could be better based on replacement modality included as time-dependent covariates and not as baseline covariates representing ‘future exposure’ to kidney replacement therapy/ waiting list over the follow-up. Finally, regarding the impact of KRT on second KT outcomes, there could be other unmeasured clinical factors related to the choice of the technique that affected patient selection. But it has also some strengths. This is a national registry including all patients transplanted in the six Catalan kidney transplant centres, as well as a national registry of KRT, so it gathers all the follow-up in dialysis and transplantation. Last but not the least, we have data on HLA sensitization after the first KT failure.
In conclusion, our study suggests that after the first KT failure, PD is superior to HD in reducing mortality in candidates for a second KT without options for preemptive retransplantation.
Supplementary Material
ACKNOWLEDGEMENTS
We thank CERCA Program/Generalitat de Catalunya, ISCIII RETICS RedinRen RD16/0009/0003 for institutional support. This research was supported by Spanish Government ISCIII grant PI18/00910.
Contributor Information
Carlos Couceiro, Nephrology Department, Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Barcelona, Spain.
Inés Rama, Nephrology Department, Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Barcelona, Spain.
Jordi Comas, Department of Health, Catalan Renal Registry, Catalan Transplant Organization, Barcelona, Spain.
Núria Montero, Nephrology Department, Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Barcelona, Spain; Biomedical Research Institute (IDIBELL), Hospital Duran i Reynals, L'Hospitalet de Llobregat, Barcelona, Spain; Facultat de Medicina i Ciències de la Salut, Universitat de Barcelona, L'Hospitalet de Llobregat, Barcelona, Spain.
Anna Manonelles, Nephrology Department, Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Barcelona, Spain; Biomedical Research Institute (IDIBELL), Hospital Duran i Reynals, L'Hospitalet de Llobregat, Barcelona, Spain; Facultat de Medicina i Ciències de la Salut, Universitat de Barcelona, L'Hospitalet de Llobregat, Barcelona, Spain.
Sergi Codina, Nephrology Department, Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Barcelona, Spain; Biomedical Research Institute (IDIBELL), Hospital Duran i Reynals, L'Hospitalet de Llobregat, Barcelona, Spain.
Alexandre Favà, Nephrology Department, Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Barcelona, Spain; Biomedical Research Institute (IDIBELL), Hospital Duran i Reynals, L'Hospitalet de Llobregat, Barcelona, Spain.
Edoardo Melilli, Nephrology Department, Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Barcelona, Spain; Biomedical Research Institute (IDIBELL), Hospital Duran i Reynals, L'Hospitalet de Llobregat, Barcelona, Spain.
Ana Coloma, Nephrology Department, Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Barcelona, Spain; Biomedical Research Institute (IDIBELL), Hospital Duran i Reynals, L'Hospitalet de Llobregat, Barcelona, Spain.
Maria Quero, Nephrology Department, Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Barcelona, Spain; Biomedical Research Institute (IDIBELL), Hospital Duran i Reynals, L'Hospitalet de Llobregat, Barcelona, Spain; Facultat de Medicina i Ciències de la Salut, Universitat de Barcelona, L'Hospitalet de Llobregat, Barcelona, Spain.
Jaume Tort, Department of Health, Catalan Renal Registry, Catalan Transplant Organization, Barcelona, Spain.
Josep M Cruzado, Nephrology Department, Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Barcelona, Spain; Biomedical Research Institute (IDIBELL), Hospital Duran i Reynals, L'Hospitalet de Llobregat, Barcelona, Spain; Facultat de Medicina i Ciències de la Salut, Universitat de Barcelona, L'Hospitalet de Llobregat, Barcelona, Spain.
CONFLICT OF INTEREST STATEMENT
J.M.C. is member of the CKJ editorial board. The other authors of this manuscript have no conflicts of interest to disclose.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
AUTHORS’ CONTRIBUTIONS
C.C., I.R., J.C., M.Q. and J.M.C. helped conceive the study. J.C. performed the statistical analysis. C.C. and J.M.C. prepared the draft manuscript. N.M., A.M., S.C., A.F., E.M., A.C. and J.T. revised critically the article.
REFERENCES
- 1. Organització Catalana de Trasplantaments (OCATT) . Registre de Malalts Renals de Catalunya, Informe Estadístic2019; Barcelona [Google Scholar]
- 2. Perl J, Zhang J, Gillespie Bet al. Reduced survival and quality of life following return to dialysis after transplant failure: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 2012; 27: 4464–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark S, Kadatz M, Gill Jet al. Access to kidney transplantation after a failed first kidney transplant and associations with patient and allograft survival: an analysis of national data to inform allocation policy. Clin J Am Soc Nephrol 2019; 14: 1228–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fiorentino M, Gallo P, Giliberti Met al. Management of patients with a failed kidney transplant: what should we do? Clin Kidney J 2021; 14: 98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies SJ. Peritoneal dialysis in the patient with a failing renal allograft. Perit Dial Int 2001; 21: 280–284 [PubMed] [Google Scholar]
- 6. De Jonge H, Bammens B, Lemahieu Wet al. Comparison of peritoneal dialysis and haemodialysis after renal transplant failure. Nephrol Dial Transplant 2006; 21: 1669–1674 [DOI] [PubMed] [Google Scholar]
- 7. Perl J, Hasan O, Bargman JMet al. Impact of dialysis modality on survival after kidney transplant failure. Clin J Am Soc Nephrol 2011; 6: 582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perl J, Dong J, Rose Cet al. Is dialysis modality a factor in the survival of patients initiating dialysis after kidney transplant failure? Perit Dial Int 2013; 33: 618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salazar AM, Chávez CO, Álvarez TGet al. Returning to dialysis after kidney transplant failure. Does the dialysis treatment modality influence the survival prognosis? Transplantation 2018; 102: S537 [Google Scholar]
- 10. Gutman RA, Stead WW, Robinson RR. Physical activity and employment status of patients on maintenance dialysis. N Engl J Med 1981; 304: 309–313 [DOI] [PubMed] [Google Scholar]
- 11. United States Renal Data System . 2020 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gill JS, Johnston O, Rose CLet al. Risks and benefits of preemptive second kidney transplantation. Transplantation 2013; 95: 705–710 [DOI] [PubMed] [Google Scholar]
- 13. Schold JD, Augustine JJ, Huml AMet al. Modest rates and wide variation in timely access to repeat kidney transplantation in the United States. Am J Transplant 2020; 20: 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schrezenmeier E, Lehner L, Merkel Met al. What happens after graft loss? A large, long-term, single-center observation. Transpl Int 2021; 34: 732–742 [DOI] [PubMed] [Google Scholar]
- 15. Kainz A, Kammer M, Reindl-Schwaighofer Ret al. Waiting time for second kidney transplantation and mortality. Clin J Am Soc Nephrol 2022; 17: 90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rao P, Schaubel D, Jia Xet al. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis 2007; 49: 294–300 [DOI] [PubMed] [Google Scholar]
- 17. Heaphy ELG, Poggio ED, Flechner SMet al. Risk factors for retransplant kidney recipients: relisting and outcomes from patients’ primary transplant. Am J Transplant 2014; 14: 1356–1367 [DOI] [PubMed] [Google Scholar]
- 18. López-Gómez J, Pérez-Flores I, Jofré Ret al. Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol 2004; 15: 2494–2501 [DOI] [PubMed] [Google Scholar]
- 19. Knoll G, Muirhead N, Trpeski Let al. Patient survival following renal transplant failure in Canada. Am J Transplant 2005; 5: 1719–1724 [DOI] [PubMed] [Google Scholar]
- 20. Florit EA, Bennis S, Rodriguez Eet al. Pre-emptive retransplantation in patients with chronic kidney graft failure. Transplant Proc 2015; 47: 2351–2353 [DOI] [PubMed] [Google Scholar]
- 21. McDonald SP, Marshall MR, Johnson DWet al. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009; 20: 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jassal SV, Lok CE, Walele Aet al. Continued transplant immunosuppression may prolong survival after return to peritoneal dialysis: results of a decision analysis. Am J Kidney Dis 2002; 40: 178–183 [DOI] [PubMed] [Google Scholar]
- 23. Brar A, Markell M, Stefanov DGet al. Mortality after renal allograft failure and return to dialysis. Am J Nephrol 2017; 45: 180–186 [DOI] [PubMed] [Google Scholar]
- 24. Stack AG, Martin DR. Association of patient autonomy with increased transplantation and survival among new dialysis patients in the United States. Am J Kidney Dis 2005; 45: 730–742 [DOI] [PubMed] [Google Scholar]
- 25. Wong G, Chua S, Chadban SJet al. Waiting time between failure of first graft and second kidney transplant and graft and patient survival. Transplantation 2016; 100: 1767–1775 [DOI] [PubMed] [Google Scholar]
- 26. Haller MC, Kainz A, Baer Het al. Article dialysis vintage and outcomes after kidney transplantation: a retrospective cohort study. Clin J Am Soc Nephrol 2017; 12: 122–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coupel S, Giral-Classe M, Karam Get al. Ten-year survival of second kidney transplants: impact of immunologic factors and renal function at 12 months. Kidney Int 2003; 64: 674–680 [DOI] [PubMed] [Google Scholar]
- 28. Arnol M, Prather J, Mittalhenkle Aet al. Long-term kidney regraft survival from deceased donors: risk factors and outcomes in a single center. Transplantation 2008; 86: 1084–1089 [DOI] [PubMed] [Google Scholar]
- 29. Lepeytre F, Dahhou M, Zhang Xet al. Association of sex with risk of kidney graft failure differs by age. J Am Soc Nephrol 2017; 28: 3014–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee Y, Hung S, Wang Het al. Is there different risk of cancer among end-stage renal disease patients undergoing hemodialysis and peritoneal dialysis? Cancer Med 2018; 7: 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong G, Turner R, Chapman Jet al. Time on dialysis and cancer risk after kidney transplantation. Transplantation 2013; 95: 114–121 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.