Abstract
Background
Temporary isolation wards have been introduced to meet demands for airborne-infection-isolation-rooms (AIIRs) during the COVID-19 pandemic. Environmental sampling and outbreak investigation was conducted in temporary isolation wards converted from general wards and/or prefabricated containers, in order to evaluate the ability of such temporary isolation wards to safely manage COVID-19 cases over a period of sustained use.
Methods
Environmental sampling for SARS-CoV-2 RNA was conducted in temporary isolation ward rooms constructed from pre-fabricated containers (N = 20) or converted from normal-pressure general wards (N = 47). Whole genome sequencing (WGS) was utilized to ascertain health care-associated transmission when clusters were reported amongst HCWs working in isolation areas from July 2020 to December 2021.
Results
A total of 355 environmental swabs were collected; 22.4% (15/67) of patients had at least one positive environmental sample. Patients housed in temporary isolation ward rooms constructed from pre-fabricated containers (adjusted-odds-ratio, aOR = 10.46, 95% CI = 3.89-58.91, P = .008) had greater odds of detectable environmental contamination, with positive environmental samples obtained from the toilet area (60.0%, 12/20) and patient equipment, including electronic devices used for patient communication (8/20, 40.0%). A single HCW cluster was reported amongst staff working in the temporary isolation ward constructed from pre-fabricated containers; however, health care-associated transmission was deemed unlikely based on WGS and/or epidemiological investigations.
Conclusion
Environmental contamination with SARS-CoV-2 RNA was observed in temporary isolation wards, particularly from the toilet area and smartphones used for patient communication. However, despite intensive surveillance, no healthcare-associated transmission was detected in temporary isolation wards over 18 months of prolonged usage, demonstrating their capacity for sustained use during succeeding pandemic waves.
Key Words: SARS-CoV-2, COVID-19, Environmental contamination, Hospitals, Fomites, Health care-associated
During the ongoing coronavirus disease 2019 (COVID-19) pandemic, requirements for airborne infection isolation rooms (AIIRs) to safely manage patients with COVID-19 has often resulted in demand outstripping existing capacity. Given the significant cost and time required to construct permanent structures with AIIR capabilities, various workarounds have been proposed to create temporary isolation wards, including conversion of pre-existing hospital wards, erection of temporary structures, or conversion of non-medical facilities.1, 2, 3
However, evaluation of the capability of such temporary isolation wards to safely manage COVID-19 cases is significantly lacking; largely because these temporary isolation wards were often introduced at the pandemic peak to provide surge-capacity and resources for detailed evaluation were unavailable. Reports of healthcare-associated SARS-CoV-2 transmission in such temporary isolation wards,4 as well as outbreaks of varicella in COVID-19 isolation facilities,5 highlight the potential for breaches in infection-prevention at such temporary isolation wards, particularly over a sustained duration of usage.
At our institution, a large tertiary hospital in Singapore, pre-pandemic a purpose-built 50-bedded isolation ward (IW) was available, comprising single-occupancy AIIRs with ≥twelve air changes-per-hour, controlled direction of air flow with negative differential pressure of −2.5 Pascal or greater, and anterooms designed to provide an “air-lock” between the adjacent area and the AIIR. However, the substantial increase in demand for AIIRs during the COVID-19 pandemic rapidly outstripped the number of AIIRs available, and COVID-19 patients were housed in temporary isolation wards converted from general ward rooms.6 Our institution opened an additional 50-bedded temporary isolation ward in July 14, 2020, comprising prefabricated containers, each of which were redesigned as a single-occupancy room and met design standards for AIIRs.2 These temporary isolation wards, saw sustained usage throughout 2021, during a surge in community transmission driven by the SARS-CoV-2 delta-variant. We therefore sought to assess SARS-CoV-2 environmental contamination and/or transmission risk associated with such temporary isolation wards over a prolonged period of usage. Notably, our institution conducted contact-tracing for ascertainment of epidemiological exposure, together with active surveillance (including rostered-routine-testing, RRT) and whole genome sequencing (WGS) for all COVID-19 cases amongst HCWs.7 , 8 This allowed us to evaluate if clusters of COVID-19 cases amongst HCWs working in isolation-areas, including temporary isolation wards, could potentially be attributed to healthcare-associated SARS-CoV-2 transmission. By assessing SARS-CoV-2 environmental contamination and potential transmission risk in such temporary isolation wards, we sought to ascertain the safety of these temporary isolation wards over a period of sustained use. Such information could potentially inform hospital practice during subsequent pandemics caused by novel respiratory pathogens.
METHODOLOGY
Institutional setting and study period
Our campus handled COVID-19 and non-COVID-19 admissions and hosts a 1785-bed acute hospital, a 545-bed community hospital, and 4 specialist centers. Environmental sampling to assess the degree of contamination within patient rooms in temporary isolation wards was conducted in. August 2021. Environmental sampling was conducted in the temporary isolation ward constructed from pre-fabricated containers as well as temporary isolation wards converted from normal-pressure general wards These converted general wards contained a mixture of normal-pressure single rooms and cohorted 5-bedded cubicles with en-suite toilets, which opened onto common corridors.6 In the conversion of these general wards, temporary full-height partitions were erected to close off cohorted cubicles from the common corridor.
Each cohorted cubicle and single room had its own ventilation system, and air was not recirculated between cubicles or rooms. Prior to conversion, these general wards met the minimum of ≥6 air-exchanges and/or hour; in line with conversion into temporary isolation wards, air exchange rates were increased to meet the standard of ≥12 air-exchanges and/or hour. The average size of a normal-pressure single room was 4 meters (m) by 10m, while cohorted cubicles on average measured 8 m by 10 m. In contrast, in the temporary isolation ward constructed from pre-fabricated containers, single-occupancy patient rooms (with en-suite toilets) measured 2.4 m x 6 m.2 Each container met design standards for AIIRs, with ≥12 air exchanges and/or hour and laminar (unidirectional) air flow with negative differential pressure of −2.5 Pascal or greater, and had its own ventilation system; air was not recirculated between containers or into the temporary structure containing the 50 prefabricated containers. All temporary isolation wards were air-conditioned and not open to outdoor air, given high heat and humidity in tropical Singapore.
Additionally, to assess potential healthcare-associated transmission from patients to health care workers (HCWs) working in isolation areas, cases of COVID-19 infections amongst HCWs working in isolation areas were collated over an 18 month period from July 2020 to December 2021. Surveillance was conducted for all HCWs working in isolation areas (including temporary isolation wards, purpose-built isolation ward, and isolation areas in the emergency department), given ongoing contact with COVID-19 inpatient cases as well as interactions between staff during patient transfers and handovers. When clusters of COVID-19 infection were reported amongst HCWs working in isolation areas, we utilized epidemiological investigations and whole genome sequencing (WGS) to ascertain the possibility of health care-associated transmission.
COVID-19 infection prevention measures
All HCWs in the institution donned N95 respirators as a mandatory minimum. HCWs in isolation wards (both purpose-built and temporary isolation wards) donned N95 respirators in patient rooms and common areas (eg, common corridors); during entry into patient rooms, single-use disposable gloves, gowns and face shields were additionally utilized as personal protective equipment (PPE). COVID-19 vaccination uptake amongst HCWs was high, with 89.6% fully-vaccinated with mRNA vaccines by end-April 2021.8 Pre-pandemic, all inpatient isolation areas were cleaned with 1000 ppm hypochlorite-based disinfectant 3x-a-day; this was maintained in all temporary isolation wards during the pandemic period, with cleaners required to wear N95 respirators, eye-protection, and disposable gown and/or gloves. In the temporary isolation ward constructed from pre-fabricated containers, in-room smart-phones were also provided for remote communication with the patient via video-conferencing.2 For disinfection of the smart-phone after patient usage, upon patient discharge, HCWs used wipes with quaternary-ammonium-compounds (Schülke mikrozid sensitive) to wipe the screen while ethanol wipes (Schülke mikrozid AF) were used to clean the remaining parts of the smart-phone and its casing. Subsequently, cleaning staff would remove the smart-phone from its casing for decontamination with hydrogen peroxide vaporization prior to re-use.
Surveillance for COVID-19 amongst HCWs
More than 13,000 HCWs worked on-campus. All symptomatic HCWs could access free PCR-testing at our institution's Staff Clinic. Routine-rostered-testing (RRT) was conducted for all asymptomatic HCWs from April 2021, initially with fortnightly PCR. Given surging community transmission, HCW surveillance was stepped up to twice-weekly rapid-antigen-detection (RAD) testing from September 29, 2021. HCWs working in inpatient areas (high-risk) were required to obtain confirmatory PCR-testing if they tested positive on RAD.
Definition of HCW COVID-19 clusters
Epidemiological HCW clusters were defined as ≥2 COVID-19 cases amongst HCWs in the same setting (ward/workplace), with overlap during their infective periods (defined as 2 day prior to symptom-onset if symptomatic or 7 day prior to positive-PCR if asymptomatic);8 ending when no cases were diagnosed for 14 days. Genomic clusters were detected based on whole-genome-similarity analysis (when sequences are≤3 SNPs different and fall in the same branch of the genome-similarity-tree).8 , 9
Epidemiologic and genetic analysis
Contact-tracing was performed for all HCWs at work during their infective periods; cases with a cycle-threshold (CT)-value of <31 on PCR were sent for WGS using the ARTIC protocol on Oxford Nanopore minION sequencers. When clusters of COVID-19 were reported amongst HCWs working in inpatient isolation areas, available isolates from all inpatient cases admitted to that isolation area in the preceding 14 day were also sequenced, in order to ascertain if there was potential patient-to-HCW transmission.
Environmental sampling
Environmental sampling was done in patient rooms within temporary isolation wards to test for SARS-CoV-2 RNA. Sampling was conducted at a single timepoint per room at the point of patient discharge and/or transfer-out, prior to terminal cleaning with sodium hypochlorite 1,000 ppm, and again after terminal cleaning was completed. Per-protocol,6 areas that were sampled routinely included: near-patient environment (bedside, bedside table, which was replaced by a ledge in the prefabricated containers as space constraints could not accommodate a full-sized table), patient equipment (call-bell), toilet area (seat and/or flush-handle), and shower area. The phone screen of the in-room smartphones used for remote communication in the temporary isolation ward constructed from pre-fabricated containers was swabbed as well. HCWs wearing full PPE used sterile premoistened polyester-tipped swab sticks to swab high-touch areas for 2-3 minutes per surface. Air sampling for SARS-CoV-2 RNA was also conducted in the temporary isolation ward converted from normal-pressure general wards, concurrent with environmental sampling. Separately, aerosol samples were collected with the patient present, using NIOSH BC 251, 2 stage cyclone aerosol samplers connected to air sampling pumps set at a flow-rate of 3.5 L/min and run for 4 hour, which were positioned at a distance from the patient's bed of 1 meter (m), 2 meter and in the common corridor outside patient rooms. There were no concurrent aerosol-generating-procedures captured during the period of air sampling. Aerosol sample components (sample tubes and filter cassettes) were vortexed with buffer solution and samples pooled for RNA extraction. Air sampling was not performed in the temporary isolation ward constructed from pre-fabricated containers as their compact nature made it challenging to accommodate the necessary sampling equipment. Investigation for SARS-CoV-2 RNA was done by qualitative real-time reverse transcription PCR (rRT-PCR) using the Cepheid-GeneXpert-Xpert-Xpress-SARS-CoV-2 test-kit.
Statistical analysis
Chi-square-test (univariate analysis) and multivariate logistic regression was used to compare factors associated with detectable SARS-CoV-2 environmental contamination amongst inpatient cases for which environmental sampling was conducted. SPSS (Version 20.0. Armonk, NY, USA: IBM Corp) was used for statistical analysis and a cutoff of P < .05 was set for statistical significance.
Ethics statement
As this study was conducted as part of outbreak investigation, ethics approval was not required under our institutional review board guidelines.
RESULTS
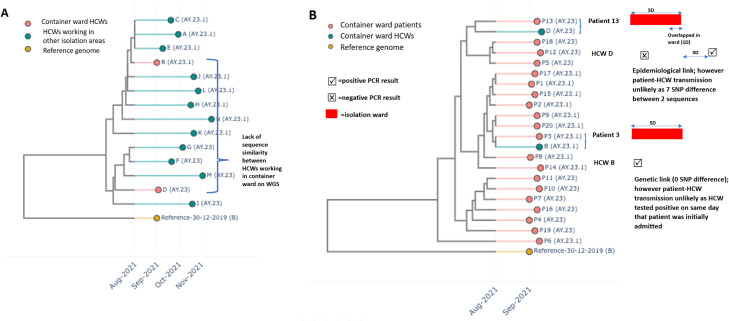
Over the 18 month study period, a total of 4,247 inpatient COVID-19 cases were admitted, of which 35.6% (1510/4247) were initially admitted to the temporary isolation ward constructed from pre-fabricated containers, 26.4% (1125/4247) to the temporary isolation ward converted from normal-pressure general wards and the remainder to the purpose-built isolation ward. The average length-of-stay in temporary isolation wards was 3.89 days (SD = 1.32). A total of 441 COVID-19 infections were reported amongst HCWs over the corresponding period; of which 5.7% (25/441) occurred amongst HCWs working in COVID-19 isolation-areas. The majority of infections amongst HCWs working in isolation areas (60.0%, 15/25) occurred in HCWs working in converted areas of the emergency department (ED) where confirmed/suspected COVID-19 cases were managed.10 Three cases occurred amongst HCWs working in the purpose-built IW, and the remainder occurred amongst HCWs working in temporary isolation wards(converted from general wards, N = 5; prefabricated containers, N = 2). Around half (56.0%, 14/25) of COVID-19 cases amongst HCWs working in isolation-areas were successfully sequenced. A total of 104 HCWs were identified as having had close-contact with the index HCW-cases and placed on furlough. The majority of COVID-19 cases amongst HCWs working in isolation areas were sporadic, with the exception of a single epidemiological cluster of infections reported amongst 2 HCWs working in the temporary isolation ward constructed from pre-fabricated containers, who were diagnosed with COVID-19, 2 days apart. On epidemiological investigation, both HCWs had overlapped at work; however on WGS analysis, the two HCW sequences clustered on separate branches of the genome-similarity tree, suggesting that healthcare-associated HCW-to-HCW transmission in the temporary isolation ward constructed from pre-fabricated containers was less likely (Fig 1 A). On WGS and epidemiological analysis, there was also no evidence of patient-to-HCW transmission in the temporary isolation ward constructed from pre-fabricated containers, based on dissimilarity with sequences of inpatients admitted to the temporary isolation ward constructed from pre-fabricated containers over the preceding 2 weeks and/or absence of an epidemiological link between genetically-linked sequences (Fig 1B).
Fig 1.
Results of epidemiological investigations and whole-genome-sequencing for cluster of COVID-19 infections detected amongst healthcare workers in container isolation ward.
Environmental sampling for SARS-CoV-2 RNA was conducted prior to terminal cleaning amongst representative inpatient cases admitted to temporary isolation wards constructed from pre-fabricated containers (N = 20) or converted from normal-pressure general wards (N = 47). A total of 355 swabs were taken prior to terminal cleaning; 12.4% (44/355) of swabs tested positive, with 22.4% (15/67) of sampled cases having had a positive environmental sample. No swabs tested positive after terminal cleaning. Comparing clinical and demographic characteristics of sampled cases in the temporary isolation ward rooms constructed from pre-fabricated containers versus cases in the converted general-ward (Supplementary Table 1), inpatient cases in the temporary isolation ward rooms constructed from pre-fabricated containers were younger (age<60 years, aOR = 12.74, 95% CI = 2.82-57.46, P = .001) and had lower odds of pneumonia (aOR = 0.04, 95% CI = 0.006-0.26); this was reflective of triage criteria utilized to assign COVID-19 cases to the container-ward. On univariate (Table 1 ) and multivariate analysis, being housed in temporary isolation ward rooms constructed from pre-fabricated containers (adjusted-odds-ratio, aOR = 10.46, 95% CI = 3.89-58.91, P = .008), having had an aerosol-generating procedure at any point (aOR = 6.84, 95% CI = 1.01-49.42, P = .049), having ongoing diarrhea (aOR = 30.20, 95% CI = 1.66-548.05, P = .021), as well as a cycle-threshold value of<20 on SARS-CoV-2 PCR of nasopharyngeal swab specimens (aOR = 8.38, 95% CI = 1.03-67.96, P = .047) were independently associated with greater odds of detectable SARS-CoV-2 environmental contamination. Within temporary isolation ward rooms constructed from pre-fabricated containers, the majority of sampled patients had positive environmental samples from the toilet area (60.0%, 12/20); a substantial proportion (8/20, 40.0%) also had positive samples taken from patient equipment (call-bell and/or phone) (Table 2 ). For patients in temporary isolation wards converted from normal-pressure general wards only 4.3% (2/47) had positive samples from patient equipment (call-bell), and similarly environmental contamination was most frequently detected in the toilet area (17.0%, 8/47) (Table 2). Air samples from representative inpatient COVID-19 cases housed in the temporary isolation ward converted from normal-pressure general wards areas (N = 40) were also tested for SARS-CoV-2 RNA. Only 2 patients had detectable SARS-CoV-2 RNA in air samples taken at a distance of 1 meter from the patient bed, from 1 normal-pressure single room and 1 cohorted 5 bedded cubicle, respectively. No air samples at 2 meter distance or from the common corridor tested positive for SARS-CoV-2 RNA.
Table 1.
Factors associated with PCR detection of SARS-CoV-2 in near-patient environments within temporary isolation ward rooms (N = 67)
| Covariates (index cases) | Detection of SARS-CoV-2 in near-patient environment prior to terminal cleaning, N (%) | Odds ratio, 95% CI* | P-value |
|---|---|---|---|
| Clinical characteristics | |||
| Age | |||
| Age≥60 years | 5/38 (13.2) | 1.00 | |
| Age<60 years | 10/29 (34.5) | 3.47 (1.03-11.68) | 0.074 |
| Gender | |||
| Female | 4/22 (18.2) | 1.00 | |
| Male | 11/45 (24.4) | 1.46 (0.41-5.23) | 0.757 |
| Clinical presentation | |||
| Upper respiratory symptoms alone | 10/44 (22.7) | 1.00 | |
| Pneumonia | 5/23 (21.7) | 0.94 (0.28-3.19) | 1.00 |
| Symptomatic | 12/46 (26.1) | 1.00 | |
| Asymptomatic | 3/21 (14.3) | 0.47 (0.12-1.89) | 0.356 |
| SARS-CoV-2 variant | |||
| SARS-CoV-2 Delta variant | 10/39 (25.6) | 1.00 | |
| Other SARS-CoV-2 variants‡ | 5/28 (17.9) | 0.84 (0.54-1.29) | 0.558 |
| Room characteristics | |||
| Type of temporary isolation ward | |||
| Converted general ward room | 5/47 (10.6) | 1.00 | |
| Prefabricated container-ward room | 10/20 (50.0) | 8.40 (2.35-30.09) | 0.001* |
| Room-occupancy (no. of beds) | |||
| Single-occupancy room | 10/35 (28.6) | 1.00 | |
| Cohort room (5-bedded) | 5/32 (15.6) | 2.16 (0.65-7.20) | 0.250 |
| Admission events | |||
| Aerosol-generating procedure | |||
| No aerosol-generating procedure† | 9/56 (16.1) | 1.00 | |
| Aerosol-generating procedure at any point during isolation ward admission | 6/11 (54.5) | 6.27 (1.57-25.02) | 0.012‖ |
| Presence of diarrhea | |||
| No diarrhea | 12/63 (19.0) | 1.00 | |
| Ongoing diarrhea | 3/4 (75.0) | 12.75 (1.22-133.55) | 0.033‖ |
| SARS-CoV-2 PCR within 48h of environmental sampling§ | |||
| Cycle-threshold>20 | 2/28 (7.1) | 1.00 | |
| Cycle-threshold<20 | 13/32 (40.6) | 1.79 (8.90-44.14) | 0.003‖ |
Chi-square test.
Aerosol-generating procedures defined as: supplemental oxygen, nebulizers, high flow nasal cannula, noninvasive positive pressure ventilation, intubation; at any point during isolation ward admission.
SARS-CoV-2 strains circulating in Singapore prior to emergence of the SARS-CoV-2 Delta variant in April 2021 included Alpha, Beta and Gamma variants. All patients involved in the study had received 2 doses of mRNA vaccinations prior to infection.
If SARS-CoV-2 PCR on nasopharyngeal swab specimens had not been done within 48h of environmental sampling, it was counted as missing data.
P-value < .05 on multivariate logistic regression, including the following variables: room type, aerosol-generating-procedure, diarrhea, and result of SARS-CoV-2 PCR on nasopharyngeal swab samples conducted within 48h of environmental sampling.
Table 2.
Layout, ventilation, and sites of PCR detection of SARS-CoV-2 in temporary isolation wards
| Temporary isolation ward constructed from pre-fabricated containers |
Temporary isolation ward converted from normal-pressure general wards |
||
|---|---|---|---|
 |
 |
||
 |
 |
||
| Site of sampling | Percentage of sampled patients having detectable environmental contamination with SARS-CoV-2 (N%) | Site of sampling | Percentage of sampled patients having detectable environmental contamination with SARS-CoV-2 (N%) |
| Patient equipment (call-bell, phone) | 8/20 (40.0)* | Patient equipment (call-bell) | 2/47 (4.3) |
| Near-patient environment (bedside ledge, bedside) | 0/20 (0.0) | Near-patient environment (bedside table, bedside) | 5/47 (10.6)† |
| Toilet area (seat and flush handle) | 12/20 (60.0) | Toilet area (seat and flush handle) | 8/47 (17.0) |
| Shower area | 1/20 (5.0) | Shower area | 2/47 (4.2) |
Of the 8 patients who had positive environmental swabs from patient equipment (call-bell, phone), 2 had positive swabs from both the phone screen and call-bell, 5 had positive swabs from the phone screen alone, and 1 had positive swabs from the call-bell alone.
Of the 5 patients who had positive environmental swabs from the near-patient environment, 4 had positive swabs from both the table and bedside, and 1 had positive swabs from the table alone.
DISCUSSION
Environmental contamination was detected more frequently in the temporary isolation ward rooms constructed from pre-fabricated containers compared to those converted from normal-pressure general wards. This might be attributable to the more compact size of patient rooms modified from pre-fabricated containers.2 There were also some differences in the observed pattern of contamination. In isolation wards converted from general wards, environmental contamination was detected at the bedside and/or bedside table in 10% of cases sampled, whereas no SARS-CoV-2 RNA was detected on the bedside ledge that replaced a full-sized bedside table in prefabricated containers due to a lack of space. This could potentially reflect different patterns of usage; perhaps in isolation rooms converted from general wards, with more space at the bedside and a proper-sized bedside table for use, patients utilized this more often, resulting in more detectable environmental contamination around the bedside. Contamination of the toilet area was reported in both types of temporary isolation wards. Significant environmental contamination of the toilet area with SARS-CoV-2 RNA in contrast to other patient areas has been reported previously, given that the toilet is usually a smaller confined area compared to the relatively more commodious isolation-room, with a large number of high-touch areas.11 This may have been amplified in temporary isolation ward rooms constructed from pre-fabricated containers due to their more compact nature. The majority of positive swabs from patient equipment in the temporary isolation ward rooms constructed from pre-fabricated containers came from the in-room smart-phones used for remote communication and/or monitoring of the patient. Remote-communication and/or monitoring via in-room smart-phones was a unique feature of the isolation container-ward, as observation was limited due to each container having only a single window.2 SARS-CoV-2 RNA contamination of nursing call-bells has been attributed to high intensity of patient-contact with the device, as patients consider the call-bell a direct conduit to requesting attention from medical and nursing staff.12 The in-room smart-phone may have supplanted the call-bell as a preferred mode of communication due to its more interactive nature with availability of video-communication and two-way feedback. The COVID-19 pandemic has seen the introduction of electronic devices into isolation rooms to allow for patient monitoring via telemedicine, minimizing SARS-CoV-2 exposure for HCWs.13 However, data on the potential persistence of SARS-CoV-2 RNA on such devices remains limited and disinfection of electronic devices remains challenging. SARS-CoV-2 RNA contamination has been detected on the mobile phones of HCWs14 and on communication devices in the “clean-area” of a COVID-19 isolation-ward;15 UV-C disinfecting devices have been mooted as a potential solution for the disinfection of electronic devices.16 While technology has proved useful in improving patients’ experience of care in isolation-wards given the unmet psychosocial needs of patients in isolation during a novel disease outbreak,17 a standardized infection-prevention approach to the disinfection of such electronic devices may be valuable in preventing health care-associated infections.
Despite environmental contamination observed in temporary isolation wards, no evidence of healthcare-associated transmission to HCWs working in isolation areas was observed over an 18 month period of sustained usage, in which more than 2,500 COVID-19 cases were managed in temporary isolation wards. Indeed, the vast majority of COVID-19 infections amongst HCWs at our center were associated with community exposure, rather than in-hospital exposure.7 This observation highlights the importance of adherence to infection-prevention guidelines and donning of appropriate PPE when working in high-risk isolation areas. During the pandemic, rates of compliance with hand hygiene were close to 90% at our institution; HCWs utilized designated PPE (N95 respirators, disposable gowns, gloves and eye protection) during ∼80% of contact episodes with high-risk suspected COVID-19 cases.18 However, despite high PPE compliance and widespread N95 usage, breakthrough infections were still reported amongst vaccinated HCWs caring for patients with unsuspected COVID-19 in non-isolation general ward areas during an outbreak attributed to the SARS-CoV-2 delta strain, highlighting the potential for transmission of SARS-CoV-2 variants with greater infectivity.19 Redesign of temporary isolation wards to incorporate better engineering-controls and allow more effective cleaning and other infection-prevention measures, however, remains critical, as PPE-compliance may not be absolute given unfamiliarity and fatigue, resulting in potential health care-associated transmission in areas with significant soilage from contaminated fomites.4 This is crucial given the potential for sustained usage of temporary isolation wards during succeeding pandemic waves, and the potential for environmental contamination with more transmissible variants-of-concern.20
The limitations of our study are as follows. Detection of viral RNA does not necessarily translate to viable virus; viral culture could not be performed at our institution due to biosafety restrictions. Air sampling was not performed in the temporary isolation ward constructed from pre-fabricated containers as its compact nature made setting up of necessary instrumentation challenging and there were concerns that air sampling equipment could have impeded the entry of resuscitative equipment in the event of a medical emergency within the confined space. Additionally, this was a single-center study; multiple factors including the method of sampling, frequency of disinfection and compliance with infection-prevention measures may impact detection of contamination and limit generalizability. However, to the best of our knowledge, there are no published data on environmental contamination in temporary isolation wards modified from prefabricated containers. Finally, this study was conducted prior to emergence of the SARS-CoV-2 Omicron variant in Singapore; the results may not be reflective of more transmissible SARS-CoV-2 variants. Understanding transmission dynamics of SARS-CoV-2 within temporary isolation wards is crucial, given that these facilities may become a more regular feature to provide isolation surge capacity during successive and future pandemic waves.
Footnotes
Funding: This work was funded by the SingHealth Duke-NUS Academic Medicine COVID-19 Research Grant (AM/COV003/2020)
Conflicts of interest: None to report.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2022.09.004.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Lee JK, Jeong HW. Rapid expansion of temporary, reliable airborne-infection isolation rooms with negative air machines for critical COVID-19 patients. Am J Infect Control. 2020;48:822–824. doi: 10.1016/j.ajic.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wee LE, Fan EMP, Heng R, et al. Construction of a container isolation ward: A rapidly scalable modular approach to expand isolation capacity during the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2021;42:1162–1164. doi: 10.1017/ice.2020.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang D, Pan S, Li Z, et al. Large-scale public venues as medical emergency sites in disasters: lessons from COVID-19 and the use of Fangcang shelter hospitals in Wuhan, China. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Mayahi ZK, Al Kindi N, Al Shaqsi N, et al. Non-respiratory droplet transmission of COVID-19 in the isolation ward of a secondary hospital in Oman: a return to isolation basics. Infect Dis Clin Pract (Baltim Md) 2021;29:e371–e375. doi: 10.1097/IPC.0000000000001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim SYD, Tey HL. Large-scale isolation facilities and potential for secondary infectious disease outbreak. Emerg Infect Dis. 2021;27:334–335. doi: 10.3201/eid2701.203127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wee LEI, Sim XYJ, Conceicao EP, et al. Containing COVID-19 outside the isolation ward: The impact of an infection control bundle on environmental contamination and transmission in a cohorted general ward. Am J Infect Control. 2020;48:1056–1061. doi: 10.1016/j.ajic.2020.06.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wee LE, Sim XYJ, Conceicao EP, et al. Containment of COVID-19 cases among healthcare workers: The role of surveillance, early detection, and outbreak management. Infect Control Hosp Epidemiol. 2020;41:765–771. doi: 10.1017/ice.2020.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wee LE, Ko KK, Conceicao EP, et al. Linking sporadic hospital clusters during a community surge of the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) B.1.617.2 delta variant: The utility of whole-genome sequencing. Infect Control Hosp Epidemiol. 2022:1–5. doi: 10.1017/ice.2022.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yingtaweesittikul H, Ko K, Abdul Rahman N, Tan SYL, Nagarajan N, Suphavilai C. CalmBelt: rapid SARS-CoV-2 genome characterization for outbreak tracking. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.790662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Teo TL, Lim MJ, et al. Dynamic emergency department response to the evolving COVID-19 pandemic: the experience of a tertiary hospital in Singapore. J Am Coll Emerg Physicians Open. 2020;1:1395–1403. doi: 10.1002/emp2.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding Z, Qian H, Xu B, et al. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.141710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore G, Rickard H, Stevenson D, et al. Detection of SARS-CoV-2 within the healthcare environment: a multi-centre study conducted during the first wave of the COVID-19 outbreak in England. J Hosp Infect. 2021;108:189–196. doi: 10.1016/j.jhin.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sagy I, Rosenberg E, Barski L. Converting a standard internal medicine ward into an isolation unit during the COVID-19 outbreak. Int J Clin Pract. 2021;75:e13979. doi: 10.1111/ijcp.13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinoza EPS, Cortes MF, Noguera SV, et al. Are mobile phones part of the chain of transmission of SARS-CoV-2 in hospital settings? Rev Inst Med Trop Sao Paulo. 2021;63:e74. doi: 10.1590/S1678-9946202163074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Mo P, Li G, et al. Environmental virus surveillance in the isolation ward of COVID-19. J Hosp Infect. 2020;105:373–374. doi: 10.1016/j.jhin.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malhotra S, Wlodarczyk J, Kuo C, et al. Shining a light on the pathogenicity of health care providers’ mobile phones: Use of a novel ultraviolet-C wave disinfection device. Am J Infect Control. 2020;48:1370–1374. doi: 10.1016/j.ajic.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woong NL, Ekstrom VSM, Xin X, et al. Empower to connect and connect to empower: experience in using a humanistic approach to improve patients' access to, and experience of, care in isolation wards during the COVID-19 outbreak in Singapore. BMJ Open Qual. 2021;10 doi: 10.1136/bmjoq-2020-000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wee LE, Sim JXY, Conceicao EP, Aung MK, Ng IM, Ling ML. Re: ’Personal protective equipment protecting healthcare workers in the Chinese epicenter of COVID-19′ by Zhao et al. Clin Microbiol Infect. 2020;26:1719–1721. doi: 10.1016/j.cmi.2020.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wee LE, Conceicao EP, Sim JX, et al. Sporadic outbreaks of healthcare-associated COVID-19 infection in a highly-vaccinated inpatient population during a community outbreak of the B.1.617.2 variant: The role of enhanced infection-prevention measures. Am J Infect Control. 2022;50:465–468. doi: 10.1016/j.ajic.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maltezou HC, Tseroni M, Daflos C, et al. Environmental testing for SARS-CoV-2 in three tertiary-care hospitals during the peak of the third COVID-19 wave. Am J Infect Control. 2021;49:1435–1437. doi: 10.1016/j.ajic.2021.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.