Abstract
Objective:
Sex, racial, and ethnic disparities in post-operative outcomes following abdominal aortic aneurysm (AAA) repair have been described, but differences in long-term outcomes are poorly understood. Our aim was to identify differences in 5-year outcomes and imaging surveillance after elective endovascular aortic aneurysm repair (EVAR) by sex, race, and ethnicity and to explore potential mechanisms underlying these differences.
Methods:
We identified patients undergoing elective EVAR in the VQI from 2003-2017 with linkage to Medicare claims through 2018 for long-term outcomes. Our primary outcome was 5-year aneurysm rupture. Secondary outcomes were 5-year reintervention and mortality and 2-year loss-to-imaging-follow-up (defined as no aortic imaging from 6-24 months after EVAR). We used Kaplan Meier and Cox regression analyses to evaluate these outcomes by sex/race/ethnicity and constructed multivariable models to explore potential contributing factors.
Results:
Among 16,040 patients, 11,764 (73%) were White males, 2,891 (18%) were White females, 417 (2.6%) were Black males, 175 (1.1%) were Black females, 141 (0.9%) were Asian males, 34 (0.2%) were Asian females, 277 (1.7%) were Hispanic males, and 60 (0.4%) were Hispanic females. At 5 years, rupture rates were highest in Black females at 6.4% and lowest in White males at 2.3%. Compared with White males, rupture rates were higher in White females (hazard ratio [HR] 1.5, 95% confidence interval 1.1-2.0), Black females (HR 2.5, 1.0-6.0), and Asian females (HR 5.2, 1.3-21). White females also had higher mortality (HR 1.2, 1.2-1.3) and loss-to-imaging-follow-up (HR 1.2, 1.1-1.3), whereas Black females had higher mortality (HR 1.4, 1.1-1.8) and reintervention (HR 2.0, 1.4-2.8). Among other groups, Black males had higher reintervention (HR 1.4, 1.0-1.8), and both Black and Hispanic males had higher loss-to-imaging-follow-up (Black: HR 1.4, 1.1-1.7; Hispanic: HR 1.3, 1.0-1.8). In adjusted analyses, White, Black, and Asian females remained at significantly higher risk for 5-year rupture after accounting for procedure year, clinical and anatomic characteristics, surgeon and hospital volume, and loss-to-imaging-follow-up.
Conclusions:
Compared with White male patients, Black females had higher 5-year aneurysm rupture, reintervention, and mortality after elective EVAR, while White females had higher rupture, mortality and loss-to-imaging-follow-up. Asian females also had higher rupture, and Black males had higher reintervention and loss-to-imaging-follow-up. These populations may benefit from improved preoperative counseling and clinical outreach after EVAR. A larger-scale investigation of current practice patterns and their impact on sex, racial, and ethnic disparities in late outcomes after EVAR is needed to identify tangible targets for improvement.
Keywords: Sex, race, ethnicity, late rupture, EVAR
Table of Contents Summary
Compared with White males, Black females had higher 5-year aneurysm rupture, reintervention, and mortality after elective EVAR; White females had higher rupture, mortality, and loss-to-imaging-follow-up; Asian females had higher rupture; and Black males had higher reintervention and loss-to-imaging-follow-up. These subgroups may benefit from improved preoperative counseling and outreach after EVAR.
INTRODUCTION
Sex and racial disparities have been described in abdominal aortic aneurysm (AAA) disease severity and management. Female, Black, and Hispanic patients are more likely to present with symptomatic or ruptured AAA compared with their male and White counterparts.1–6 Female patients are also less likely to undergo endovascular aortic repair (EVAR) compared with males,5,6 and Black and Hispanic patients are less likely to undergo elective AAA repair compared with White patients.7,8 These disparities may be related to differences in prevalence and management of risk factors, AAA screening, and possibly delays in vascular care. For female patients, lower rates of EVAR may also be due to sex-based anatomic differences, as female patients are more likely to have anatomy that is unsuitable for traditional EVAR devices.9
Several studies reported inferior post-operative outcomes in female and Black patients following both open and endovascular AAA repair.10–12 However, these outcomes may not be the best quality metrics for AAA repair. Despite an early survival benefit after EVAR,11,13 accumulating evidence suggests inferior late outcomes with EVAR compared with open repair, which necessitates lifelong imaging surveillance and reintervention if necessary to prevent late rupture.14,15 As such, rates of surveillance, reintervention, and rupture over time may prove to be more useful quality metrics for AAA repair, particularly for EVAR. Importantly, it is unknown whether these outcomes vary by sex, race, and ethnicity. Therefore, we examined differences in 5-year aneurysm rupture, reintervention, and mortality, as well as 2-year loss-to-imaging-follow-up after elective EVAR by sex, race, and ethnicity and explored potential mechanisms underlying these differences.
METHODS
Data source
We performed a retrospective cohort study using the Vascular Implant Surveillance and Interventional Outcomes Network (VISION), which includes Vascular Quality Initiative (VQI) registry data linked with Medicare claims using a previously described method.16 The Society for Vascular Surgery (SVS) VQI contains over 250 patient- and procedure-specific variables as well as in-hospital outcomes from more than 700 centers and 4000 physicians in the United States and Canada (www.vqi.org). Though long-term data are limited in the VQI, Medicare-linkage provides long-term follow-up data, enabling us to study late rupture, reintervention, imaging surveillance, and mortality. Therefore, we utilized data on patient and procedural characteristics from the VQI and data on long-term outcomes from Medicare.
This study adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) standards for observational studies.17 The VQI Research Advisory Committee and Patient Safety Organization as well as the Institutional Review Board at Beth Israel Deaconess Medical Center approved this study and gave permission to use data without the need for informed consent, given the retrospective and deidentified nature of the data.
Patient cohort
We identified all patients who underwent EVAR from 2003-2017 with linked Medicare records (n=28,399). Long-term follow-up data were available through 2018. We excluded patients who were not under Medicare fee-for-service coverage at the time of their index EVAR (n=9242) to allow for complete capture of claims-derived outcomes throughout the study period (i.e., rupture, reinterventions, and imaging studies). To restrict our study to patients undergoing primary elective EVAR, we excluded patients with prior aneurysm repair (n=584), ruptured presentation (n=902), symptomatic presentation (n=1419), missing symptom status (n=49), and those who underwent EVAR on a weekend (n=65).
Patients were divided into 8 groups by sex, race, and ethnicity: non-Hispanic White (White) males, non-Hispanic White (White) females, Black males, Black females, Asian males, Asian females, Hispanic males, and Hispanic females. Though other racial subgroups such as Native American (n=30) and Pacific Islander (n=18) were also represented, the sample sizes were very low and precluded any meaningful analyses. Therefore, these patients were excluded.
Variable definitions
We used pre-specified race and ethnicity variables from the VQI. The VQI classifies race the same as the United States Census Bureau: White race is a person having origins in Europe, the Middle East, or North Africa; Black race is a person having origins in Africa; and Asian race is a person having origins in the Far East, Southeast Asia, or the Indian subcontinent. Hispanic ethnicity is defined as a person of Spanish culture or origin, regardless of race.18 Body mass index (BMI) was calculated according to the standard weight(kg)/height(m)2 formula. Underweight was defined as a BMI <18.5, and obese as a BMI ≥30. Glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation,19 and chronic kidney disease was defined as estimated glomerular filtration rate <30 mL/min/1.73m2 or need for hemodialysis. All other clinical variables were predefined within the VQI.
To account for physician and center experience with EVAR, physician and center volumes were determined by the total number of intact and ruptured EVARs performed within the 12 months prior to the index EVAR. These volumes were separated into quintiles, and the lowest quintile was defined as low volume.20 Physicians and centers with <12 months of data prior to the index EVAR were excluded from the quintile calculations but were assigned to the appropriate quintile based on the total number of cases performed prior to the index EVAR.
Outcomes
The primary outcome was 5-year aneurysm rupture, defined as any Medicare patient encounter with a primary diagnosis of aortic rupture after the index EVAR admission that was associated with a death or reintervention within 90 days. This included diagnosis codes corresponding to rupture of the abdominal aorta, thoracoabdominal aorta, or an unspecified aortic rupture (Supplemental Table I). Prior work using VQI-Medicare-linked data restricted this rupture definition to require death/reintervention within 14 days of diagnosis.21 We broadened this definition to allow for death/reintervention within 90 days to allow for wider variation in the timing of billing claims in Medicare, after exploring the relative timing of deaths and reinterventions surrounding rupture events. As a sensitivity analysis, we also assessed 5-year rupture using the prior definition with a 14-day restriction.
Secondary outcomes were 5-year reintervention and all-cause mortality and 2-year loss-to-imaging-follow-up. Reintervention was determined from procedure codes in Medicare and was defined as any procedure performed after hospital discharge from the index EVAR that was related to the aneurysm or the index aneurysm repair. Mortality data were determined from the Medicare denominator file. Loss-to-imaging-follow-up was determined from imaging procedure codes in Medicare and was defined as having no imaging of the aorta between 6 and 24 months after the index EVAR, based on current SVS surveillance guidelines recommending annual CT or duplex ultrasound imaging after EVAR.22
Statistical analysis
Baseline and operative characteristics were described across the 8 sex/racial/ethnic groups. Categorical variables were presented as frequencies and percentages and continuous variables as the median and interquartile range. Kaplan-Meier estimation and unadjusted Cox regression were used to compare outcomes across sex/racial/ethnic groups, and formal statistical testing was performed to ensure that the proportional hazards assumption was not violated.23,24 As a secondary analysis, we tested for an interaction between sex and race/ethnicity for each outcome. To explore the individual effects of sex or race/ethnicity on each outcome, we also evaluated outcomes by race/ethnicity after stratifying patients by sex, and then by sex after stratifying patients by race/ethnicity. For non-survival outcomes, patients who died were censored at the date of death, and patients who left Medicare-fee-for-service were censored at the date of the switch (n=1510) due to inability to capture data on rupture, reintervention, and loss-to-imaging-follow-up after that time point. Although the majority of patients were Medicare-eligible based on age ≥65 years old, 4% of the cohort was <65 years old and had other indications for Medicare eligibility (e.g., permanent dialysis or disability). To assess whether these younger patients may bias our results, we tested for an interaction between age<65 (as a binary variable) and sex/race/ethnicity for all outcomes. When a significant interaction was present, we included age<65 and the interaction term in our models.
We then performed adjusted Cox regression analyses to explore factors that may be contributing to differences in 5-year aneurysm rupture across groups. We selected the following factors for inclusion a priori based on potential clinical relevance to 5-year aneurysm rupture: procedure year, clinical factors (age, hypertension, smoking history), anatomic factors (AAA diameter, iliac aneurysm), environmental factors (low hospital volume, low surgeon volume, allowing for clustering by center), and loss-to-imaging-follow-up (as a time-varying covariate). We then evaluated whether the association between sex/race/ethnicity and aneurysm rupture changed with the addition of each set of covariates. As a post-hoc analysis, we also created a Cox regression model to assess for an association between loss-to-imaging-follow-up and 5-year mortality, adjusting for the same covariates. All variables had <5% missing data.
All tests were two-sided, and p<.05 was considered statistically significant. All analyses were performed using Stata version 16 software (StataCorp LP, College Station, Texas, USA).
RESULTS
Patients
We identified 16,040 patients who underwent elective EVAR over the study period, of whom 73% were White males, 18% were White females, 2.6% were Black males, 1.1% were Black females, 0.9% were Asian males, 0.2% were Asian females, 1.7% were Hispanic males, and 0.4% were Hispanic females (Table I). Compared with all other groups, White patients were more likely to have a family history of AAA. Meanwhile, Black patients were younger and more likely to be current smokers. Black male patients were more likely to have concurrent iliac aneurysms, and Black female patients were more likely to be treated in a low-volume center or by a low-volume physician. Asian female patients were older and were less likely to have a smoking history or a family history of AAA. Hispanic patients were less likely to be on a statin or aspirin prior to EVAR.
Table I.
Baseline and anatomic characteristics for patients undergoing elective EVAR by sex, race, and ethnicity.
| N (%) or median (IQR) | NH White | Black | Asian | Hispanic | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| M | F | M | F | M | F | M | F | |
|
| ||||||||
| Total patients | 11764 | 2891 | 417 | 175 | 141 | 34 | 277 | 60 |
|
| ||||||||
| Age, years | 75 (70, 81) | 77 (72, 82) | 72 (68, 78) | 76 (69, 82) | 76 (69, 82) | 81 (75, 85) | 76 (70, 82) | 78 (72, 84) |
| Age <65 years | 442 (3.8%) | 96 (3.3%) | 44 (11%) | 13 (7.4%) | <11 (<7.8%) | 0 (0%) | 13 (4.7%) | <11 (<18%) |
| Obese (BMI≥30) | 3569 (30%) | 823 (29%) | 106 (25%) | 69 (40%) | 11 (7.8%) | <11 (<32%) | 65 (24%) | 18 (30%) |
| Underweight (BMI<18.5) | 185 (1.6%) | 143 (5.0%) | 20 (4.8%) | <11 (6.8%) | <11 (<7.8%) | <11 (<32%) | <11 (<4%) | <11 (<18%) |
| Smoker, ever | 10203 (87%) | 2394 (83%) | 371 (89%) | 133 (76%) | 103 (73%) | <11 (<32%) | 217 (78%) | 42 (70%) |
| Smoker, current | 2978 (25%) | 899 (31%) | 150 (36%) | 50 (29%) | 24 (17%) | <11 (<32%) | 63 (23%) | 15 (25%) |
| Comorbidities | ||||||||
| COPD | 3794 (32%) | 1238 (43%) | 114 (27%) | 55 (31%) | 21 (15%) | <11 (<32%) | 57 (21%) | 20 (33%) |
| Hypertension | 9684 (83%) | 2432 (84%) | 375 (90%) | 163 (93%) | 117 (83%) | 32 (94%) | 223 (81%) | 49 (83%) |
| Diabetes | 2401 (20%) | 542 (19%) | 109 (26%) | 51 (29%) | 48 (34%) | <11 (<32%) | 69 (25%) | 15 (25%) |
| Coronary artery disease | 5555 (47%) | 899 (31%) | 171 (41%) | 50 (29%) | 61 (43%) | <11 (<32%) | 115 (42%) | 13 (22%) |
| Congestive heart failure | 1545 (13%) | 314 (11%) | 68 (16%) | 30 (17%) | 10 (7.1%) | <11 (<32%) | 33 (12%) | <11 (<18%) |
| Prior cardiac intervention | 4610 (39%) | 653 (23%) | 137 (33%) | 31 (18%) | 59 (42%) | <11 (<32%) | 97 (35%) | 11 (18%) |
| CKD (eGFR<30) | 328 (2.8%) | 155 (5.4%) | 16 (4.1%) | 11 (6.6%) | <11 (<7.8%) | <11 (<32%) | <11 (<4%) | <11 (<18%) |
| ESRD | 119 (1.0%) | 32 (1.1%) | 25 (6.0%) | <11 (<6.3%) | <11 (<7.8%) | 0 (0%) | <11 (<4%) | <11 (<18%) |
| Family history of AAA | 995 (8.5%) | 317 (11%) | 18 (4.3%) | <11 (<6.3%) | <11 (<7.8%) | <11 (<32%) | 14 (5.1%) | <11 (<18%) |
| Preoperative medication | ||||||||
| Statin | 8477 (72%) | 1888 (65%) | 280 (67%) | 122 (70%) | 111 (79%) | 25 (74%) | 176 (64%) | 37 (62%) |
| Aspirin | 8050 (68%) | 1764 (61%) | 260 (62%) | 112 (64%) | 84 (60%) | 22 (65%) | 155 (56%) | 35 (58%) |
| P2Y12 inhibitor | 1438 (12%) | 332 (12%) | 50 (12%) | 19 (11%) | 26 (18%) | <11 (<32%) | 42 (15%) | <11 (<18%) |
| Low volume center (≤19 EVARs) | 3533 (30%) | 883 (31%) | 127 (31%) | 60 (34%) | 45 (32%) | <11 (<32%) | 83 (30%) | 17 (28%) |
| Low volume physician (≤4 EVARs) | 3865 (33%) | 963 (33%) | 152 (37%) | 77 (44%) | 55 (39%) | 11 (32%) | 117(42%) | 24 (40%) |
| Anatomic factors | ||||||||
| AAA diameter (mm) | 55 (51, 60) | 53 (50, 58) | 54 (50, 60) | 53 (50, 58) | 56 (51, 60) | 52 (47, 56) | 55 (50, 60) | 52 (48, 59) |
| Iliac aneurysm, any | 3045 (27%) | 315 (11%) | 198 (49%) | 47 (27%) | 44 (31%) | <11 (<32%) | 69 (25%) | <11 (<18%) |
NH = non-Hispanic; M = male; F = female; COPD = chronic obstructive pulmonary disease; BMI = body mass index; CKD = chronic kidney disease; ESRD = end stage renal disease; AAA = abdominal aortic aneurysm.
Unadjusted long-term outcomes
Aneurysm rupture.
Median follow up time was >2.3 years across all groups. At 5 years, estimated rates of rupture were highest in Black females at 6.4% and lowest in White males at 2.3% (Figure I). Compared with White males, rupture rates were higher in White females (hazard ratio [HR]:1.5, 95% confidence interval [CI]:[1.1-2.0], p=.004), Black females (HR:2.5, 95%CI:[1.0-6.0], p=047) and Asian females (HR:5.2, 95%CI:[1.3-21], p=020; Table II). When rupture was assessed using the 14-day restriction, there remained a higher risk for 5-year rupture in Black females (HR:3.1, 95%CI:[1.3-7.5]) and Asian females (HR:6.6, 95%CI:[1.6-27]). The higher risk of rupture in White females was attenuated and no longer significant, but a trend toward higher rupture remained (HR:1.3, 95%CI:[0.96-1.9]). There was no significant interaction between sex and race/ethnicity (Supplemental Table II). When patients were stratified by sex, there was a trend toward higher rates of rupture in Asian females compared with White females (HR:3.4, 95%CI:[0.82-14]; Supplemental Table II). When patients were stratified by race/ethnicity, White females had higher rates of rupture compared with White males (HR:1.5, 95%CI:[1.1-2.0]; Supplemental Table II).
Figure I.
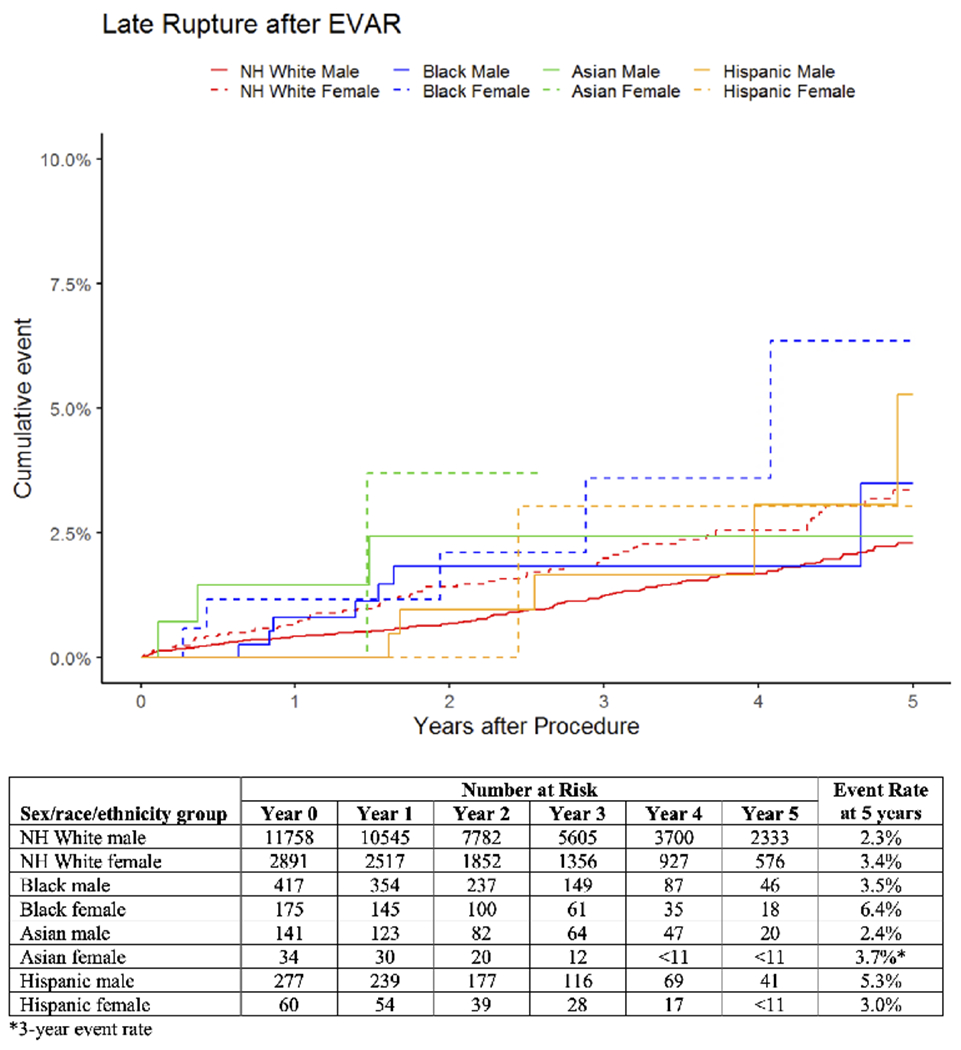
Unadjusted cumulative rates of late aneurysm rupture after elective EVAR by sex, race, and ethnicity. For Asian females, the standard error exceeds 10% at 3 years, so the corresponding curve is truncated at this time, and the 3-year event rate is reported. NH = non-Hispanic.
Table II.
Cox regression for long-term outcomes after elective EVAR by sex, race, and ethnicity. Sex, race, and ethnicity were included in all models, with non-Hispanic White male as the reference group.
| Covariate | Late Rupture | Reintervention* | Mortality | Loss-to-imaging-follow-up | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| NH White male | ref | ref | ref | ref | ref | ref | ref | ref |
| NH White female | 1.5 (1.1-2.0) | .004 | 1.1 (0.95-1.2) | .284 | 1.2 (1.2-1.3) | <.001 | 1.2 (1.1-1.3) | <.001 |
| Black male | 1.4 (0.67-3.0) | .354 | 1.4 (1.0-1.8) | .029 | 1.1 (0.92-1.3) | .311 | 1.4 (1.1-1.7) | <.001 |
| Black female | 2.5 (1.0-6.0) | .047 | 2.0 (1.4-2.8) | <.001 | 1.4 (1.1-1.8) | .007 | 0.97 (0.66-1.4) | .881 |
| Asian male | 1.6 (0.52-5.1) | .402 | 1.2 (0.74-1.9) | .473 | 0.82 (0.58-1.2) | .270 | 0.72 (0.44-1.2) | .185 |
| Asian female | 5.2 (1.3-21) | .020 | 1.6 (0.66-3.8) | .306 | 1.3 (0.70-2.4) | .408 | 0.95 (0.39-2.3) | .907 |
| Hispanic male | 1.7 (0.74-3.8) | .212 | 1.2 (0.87-1.7) | .245 | 0.92 (0.73-1.2) | .481 | 1.3 (1.0-1.8) | .029 |
| Hispanic female | 1.2 (0.17-8.8) | .837 | 1.1 (0.51-2.3) | .844 | 0.77 (0.46-1.3) | .338 | 1.3 (0.76-2.4) | .322 |
HR = hazard ratio; CI = confidence interval; NH = non-Hispanic; ref = reference group.
Indicates a statistically significant interaction between age<65 and sex/race/ethnicity for the specified outcome.
Reintervention.
Estimated rates of reintervention at 5 years were highest in Black females at 34% and lowest in White females at 17% (Figure II). Compared with White males, reintervention rates were higher in Black males (HR:1.4, 95%CI:[1.0-1.8]) and Black females (HR:2.0, 95%CI:[1.4-2.8]; Table II). There was no significant interaction between sex and race/ethnicity (Supplemental Table II). When patients were stratified by sex, reintervention rates were higher in Black males compared with White males (HR:1.4; 95%CI:[1.1-1.8]) and in Black females compared with White females (HR:1.8, 95%CI:[1.3-2.6]; Supplemental Table II). When patients were stratified by race/ethnicity, there were no differences in reintervention by sex across groups (Supplemental Table II).
Figure II.
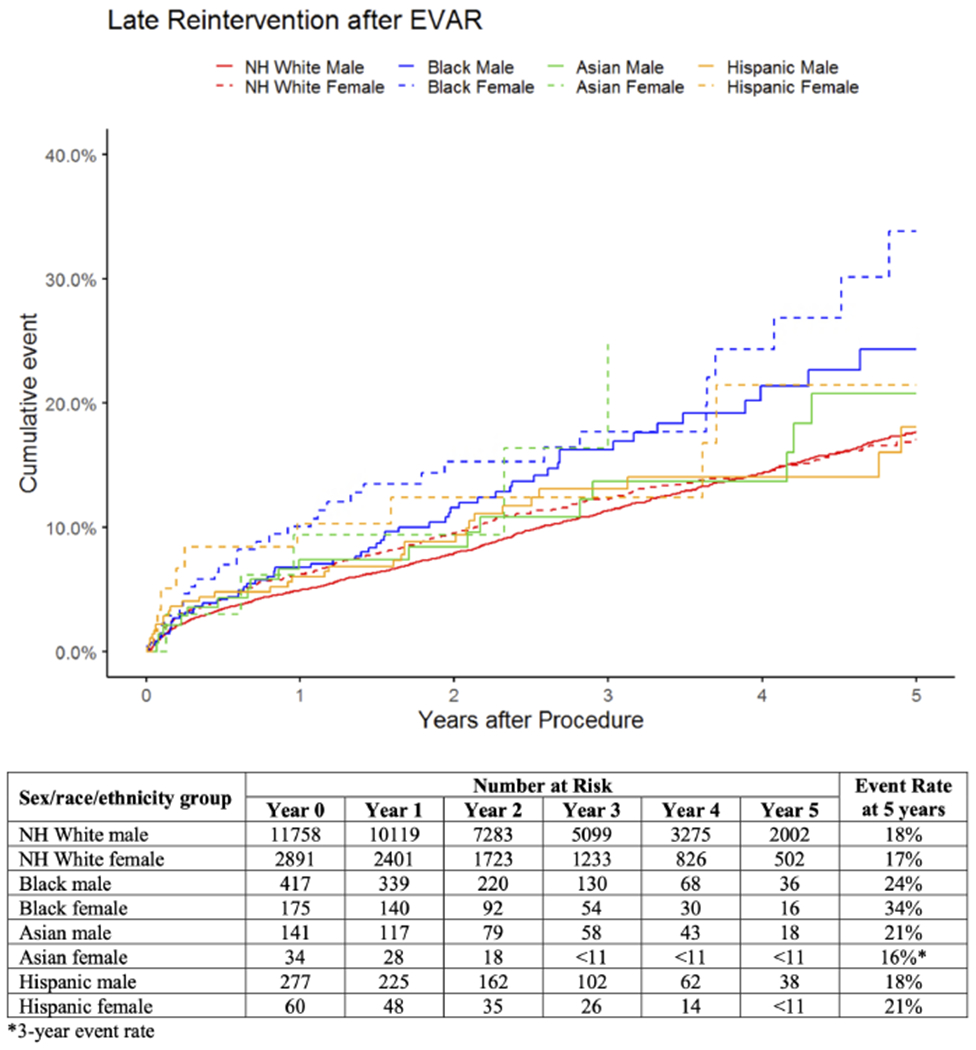
Unadjusted cumulative rates of late reintervention after elective EVAR by sex, race, and ethnicity. For Asian females, the standard error exceeds 10% at 3 years, so the corresponding curve is truncated at this time, and the 3-year event rate is reported. NH = non-Hispanic.
Mortality.
Estimated mortality at 5 years was highest in Black females at 45% and lowest in Hispanic females at 28% (Figure III). Compared with White males, mortality was higher in White females (HR:1.2, 95%CI:[1.2-1.3]) and Black females (HR:1.4, 95%CI:[1.1-1.8]; Table II). There was no significant interaction between sex and race/ethnicity (Supplemental Table II). When patients were stratified by sex, there were no differences in mortality by race/ethnicity (Supplemental Table II). When patients were stratified by race/ethnicity, mortality was higher in White females compared with White males (HR:1.2, 95%CI:[1.1-1.3]; Supplemental Table II).
Figure III.
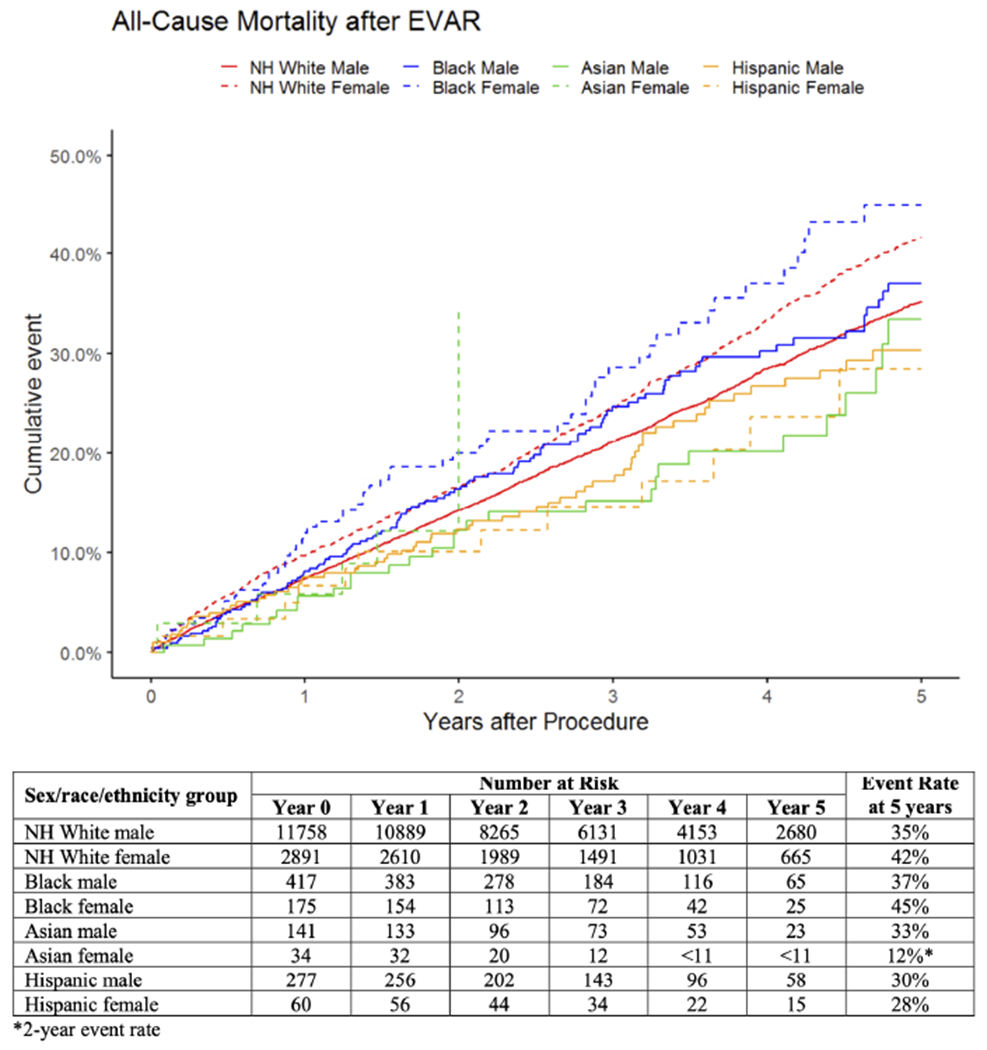
Unadjusted mortality after elective EVAR by sex, race, and ethnicity. For Asian females, the standard error exceeds 10% at 2 years, so the corresponding curve is truncated at this time, and the 2-year event rate is reported. NH = non-Hispanic.
Loss-to-imaging-follow-up.
Estimated loss-to-imaging-follow-up at 2 years was 16% overall, ranging from 21% in Black and Hispanic males to 11% in Asian males (Figure IV). Compared with White males, loss-to-imaging-follow-up was higher in White females (HR:1.2; 95%CI:[1.1-1.3]), Black males (HR:1.4, 95%CI:[1.1-1.7]), and Hispanic males (HR:1.3, 95%CI:[1.0-1.8]; Table II). There was no significant interaction between sex and race/ethnicity (Supplemental Table II). When patients were stratified by sex, loss-to-imaging-follow-up was higher in Black and Hispanic males compared with White males (Black: HR:1.4, 95%CI:[1.1-1.7]; Hispanic: HR:1.3, 95%CI:[1.0-1.8]; Supplemental Table II). When patients were stratified by race/ethnicity, loss-to-imaging-follow-up was higher in White females compared with White males (HR:1.2, 95%CI:[1.1-1.3]; Supplemental Table II).
Figure IV.
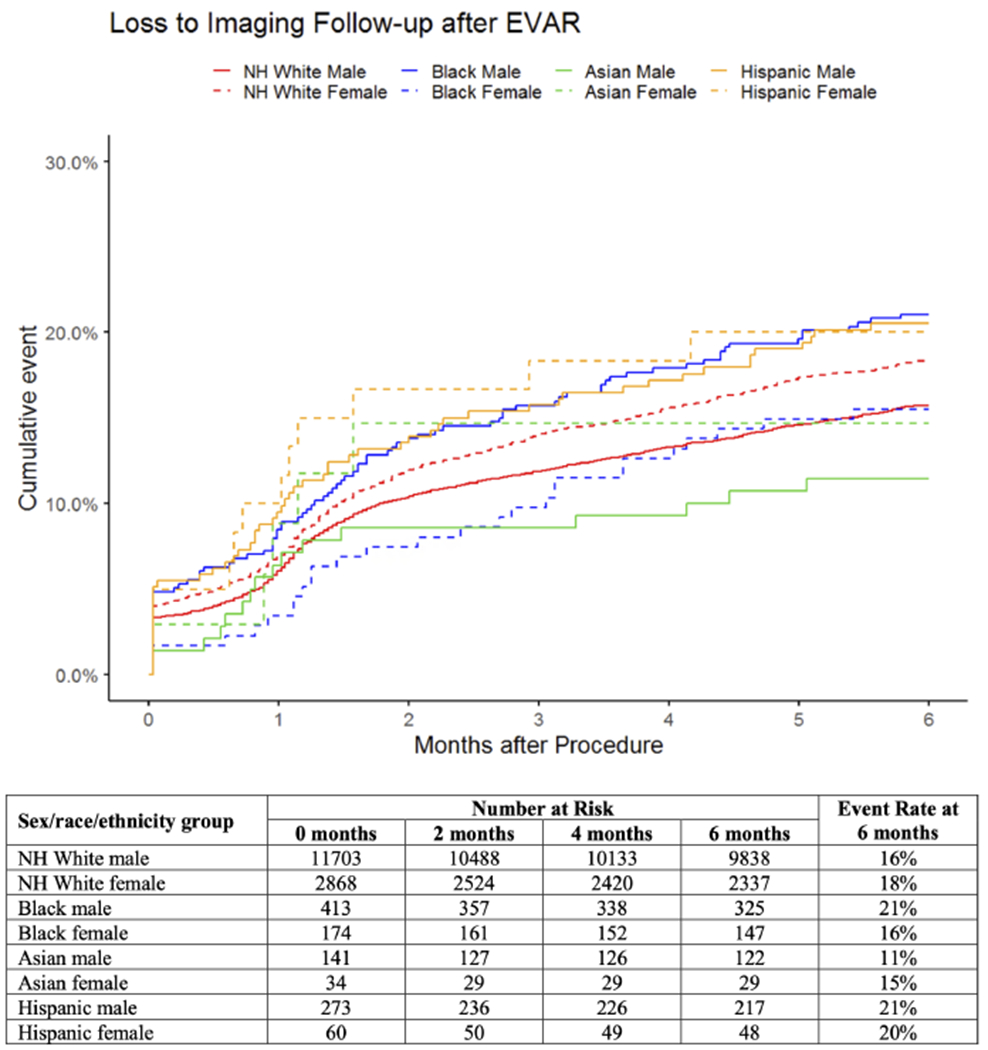
Unadjusted cumulative rates of loss-to-imaging-follow-up after elective EVAR by sex, race, and ethnicity. Patients were designated as lost-to-imaging-follow-up at the time of their last imaging study prior to the 6-to-24-month interval. All standard errors are <10%. NH = non-Hispanic.
Adjusted analyses
Aneurysm rupture.
In adjusted analyses, White and Black females remained at significantly higher risk for 5-year rupture after accounting for procedure year, clinical factors, anatomic factors, environmental factors, and loss-to-imaging-follow-up (Supplemental Table III). Loss-to-imaging-follow-up was not associated with rupture (HR:1.0, 95%CI:[0.66-1.7]).
Mortality.
In a post-hoc analysis, loss-to-imaging-follow-up was independently associated with higher 5-year mortality after elective EVAR after accounting for sex/race/ethnicity, procedure year, clinical factors, anatomic factors, and environmental factors (HR:2.8; 95%CI:[2.7-3.0]; Supplemental Table IV). Mortality remained higher in White females and Black females compared with White males after adjustment.
DISCUSSION
In this observational study of 16,040 VQI-Medicare patients who underwent elective EVAR, we identified significant differences in 5-year outcomes by sex, race, and ethnicity, with White and Black female patients having the worst outcomes. Compared with White males, Black females had higher rates of 5-year aneurysm rupture, reintervention, and mortality, while White females had higher rupture, mortality, and loss-to-imaging-follow-up. Asian females also had higher rupture, Black males had higher reintervention, and both Black and Hispanic males had higher loss-to-imaging-follow-up. These subgroups may benefit from improved preoperative counseling and clinical outreach after EVAR.
Several existing studies provide insight into the higher risk of rupture in White, Black, and Asian females. First, female patients with ruptured AAA are known to rupture at smaller aortic diameters compared with male patients.25 As such, the higher rates of rupture in female patients may result from limited understanding of how to best identify female patients at risk for rupture after EVAR. Additionally, Black patients are more likely to undergo AAA repair at low-volume facilities,7,26,27 which may influence late rupture. In exploratory analyses, we found that risk of rupture and mortality remained higher in White, Black, and Asian females after adjusting for several relevant factors including age, comorbidities, AAA diameter, and surgeon/hospital experience. This suggests that these factors, alone, do not explain the observed outcome disparities in these patients. It is likely that other social determinants of health such as economic stability, neighborhood environment, education, caregiver responsibilities, and systemic sexism and/or racism also contribute.28 Importantly, the higher risk of rupture in White, Black, and Asian females may contribute to higher 5-year mortality in these groups compared with their male counterparts, as Hispanic females had both lower rupture and lower mortality compared with Hispanic males. These differences in sex-based outcomes across racial/ethnic groups warrant further investigation.
The rates of 5-year aneurysm rupture in our EVAR cohort were similar to rates reported in Medicare previously, ranging from 2.3-6.4% across groups (2.7% overall) compared with 3.0% among beneficiaries who underwent EVAR for intact AAA between 2001-2008.15 However, this Medicare-only cohort is not a perfect comparator, as it had fewer patients who were Black (2.8% vs 3.7%) or Hispanic (0.6% vs 2.1%). Rupture rates in our cohort and in the Medicare-only cohort are notably lower compared with recent long-term follow up data from the EVAR-I trial, which reported a rupture rate of 7% beyond 8 years.14 There are a few possible explanations for this discrepancy. First, Medicare only identifies patients who present to a hospital with rupture and fails to capture those who suffer rupture and death at home. Second, it is possible that the cause of death was misclassified as a cardiac event rather than a rupture in some patients who were dead upon arrival to a hospital. These limitations may lead to underestimation of late rupture in Medicare. Alternatively, the lower rates of rupture in Medicare may reflect improvement over time. Patients in the EVAR-I cohort underwent EVAR between 1999-2004,14 compared with 2001-2008 in the Medicare-only cohort15 and 2003-2017 in our VQI-Medicare-linked cohort. Though temporal trends in late rupture have not been assessed, midterm survival after EVAR improved between 2003-2018, likely attributable to advances in clinical experience and endograft design.29,30 Overall, our data may underestimate 5-year rupture after EVAR, but we are confident that the relatively higher rates of rupture in White, Black, and Asian females represent a true disparity.
We observed that Black male and female patients had a >40% higher risk of reintervention at 5-years after elective EVAR compared with White patients. Our findings are comparable to prior work showing 5-year reintervention rates of 31% in Black patients versus 20% in White patients after elective, urgent, or emergent EVAR,21 suggesting that this difference in reintervention is not explained by higher rates of symptomatic and ruptured AAAs in Black patients.1,18 Prior work has also demonstrated that Black patients undergoing AAA repair are more likely to have concomitant iliac artery aneurysms compared with White patients.1,10,18,31 We found that this was only the case for Black males, thus highlighting the importance of examining more granular patient subgroups, and this factor likely contributes to their higher rate of reintervention.31 In Black females, the higher rate of reintervention may reflect the need to intervene to prevent and/or treat higher rates of rupture. It is also possible that Black females had higher rates of major vascular reinterventions in particular, which may contribute to their higher mortality. Importantly, not all reinterventions necessarily reflect EVAR failures and could instead imply better follow-up for interventions before major complications (like rupture) arise; but the higher loss-to-imaging-follow-up in Black males and higher rupture rates in Black females are more suggestive of the former. Although information on reintervention type and indication were not available, these data will be critical to understanding the reasons for higher reintervention rates in Black patients.
We found that 16% of patients did not undergo aortic imaging between 6-24 months after elective EVAR. This is non-compliant with current SVS guidelines recommending annual imaging for graft surveillance to prevent late aneurysm rupture.22 Compared with White male patients, White females had higher loss-to-imaging-follow-up, but this did not contribute to 5-year rupture. This finding is consistent with prior work in Medicare showing that annual EVAR surveillance did not decrease aneurysm-related mortality32 and may be partly due to underestimation of rupture in Medicare, for reasons described above. In line with this possibility, we found that loss-to-imaging-follow-up was associated with higher all-cause mortality (which Medicare captures whether deaths occur in the hospital or at home). This association may reflect undocumented ruptures leading to death or a decision to cease surveillance imaging in patients who are deemed to have poor life expectancy due to other comorbidities. Further work is needed to examine the impact of imaging surveillance on aneurysm-related complications after EVAR, particularly as we were unable to assess aneurysm-related mortality in the context of this data source. Nonetheless, the high loss-to-imaging-follow-up in our study represents an important opportunity for quality improvement, and differences observed across sex/race/ethnicity cohorts highlight subgroups that may benefit from improved outreach after EVAR.
This study has several limitations. First, the VQI is a quality improvement registry, and centers and surgeons opting to join the registry may not be fully generalizable to EVARs performed nationally. Although certain racial and ethnic populations may be underrepresented in the VQI, it is possible that participating hospitals have better outcomes and/or fewer disparities. As such, our findings may actually underrepresent disparities on a national level. Second, Medicare fee-for-service only includes about 60% of the overall Medicare population and is comprised mostly of individuals aged ≥65 years with select individuals aged <65 years,33 so these results may not be generalizable to patients without Medicare fee-for-service or to younger patients. However, our prior work suggests that about 75-80% of EVARs in the United States are performed in Medicare beneficiaries.34 Third, the small number of patients in some sex/race/ethnicity subgroups precluded meaningful analysis of long-term outcomes. Fourth, due to limitations of Medicare data, we could not be certain that late rupture events were related to the index EVAR rather than due to metachronous aneurysms, but in restricting our rupture definition exclude thoracic aortic ruptures, we feel that we have minimized this potential bias. We were also unable to assess for an association between reintervention and late rupture due to our definition of late rupture and challenges determining the relative timing of these events in Medicare. Lastly, we were not able to assess or evaluate the impact of certain key social determinants of health on outcome disparities in the context of this data source.28
CONCLUSION
Among patients who underwent elective EVAR in the VQI-Medicare population, the majority (73%) were White males. Compared with White males, Black females had higher rates of 5-year aneurysm rupture, reintervention, and mortality, while White females had higher rupture, mortality, loss-to-imaging-follow-up. Asian females also had higher rupture, and Black males had higher reintervention and loss-to-imaging-follow-up. These subgroups may benefit from improved preoperative counseling and clinical outreach after EVAR. A larger-scale investigation of current practice patterns and their impact on disparities in late outcomes after EVAR is needed to identify tangible targets for improvement.
Supplementary Material
ARTICLE HIGHLIGHTS:
Type of Research:
Retrospective cohort study of prospectively collected data from the Vascular Quality Initiative registry with Medicare-linkage for long-term outcomes.
Key Findings:
Among 16,040 elective EVAR patients, the majority of patients (73%) were White males. Compared with these patients, Black females had higher rates of 5-year aneurysm rupture, reintervention, and mortality; White females had higher rupture, mortality, and loss-to-imaging-follow-up; Asian females had higher rupture; and Black males had higher reintervention and loss-to-imaging-follow-up.
Take home Message:
There are significant differences in 5-year outcomes after elective EVAR by sex, race, and ethnicity, with White and Black female patients having the worst outcomes.
ACKNOWLEDGEMENTS
This work was conducted with support from Harvard Catalyst | The Harvard Clinical Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic healthcare centers, and the NIH T32 Harvard-Longwood Research Training in Vascular Surgery Grant 5T32HL007734. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic healthcare centers, or the National Institutes of Health.
Data for this project was generated by the Vascular Implant Surveillance and Interventional Outcomes Network (VISION) (http://mdepinet.org/vision-crn/), a partnership between the Vascular Quality Initiative and the Medical Device Epidemiology Network (MDEpiNet), a Food and Drug Administration supported initiative aimed at developing distributed research networks. VISION links VQI data to Medicare claims to enhance long-term follow-up data in the VQI. Use of the VQI-Medicare linked data is governed by CMS Data Use Agreement 52144 and the Society for Vascular Surgery Patient Safety Organization.
FUNDING INFORMATION:
CM is supported by grant number F32HS027285 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. PP & JW are supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant 5T32HL007734. The study was partially supported by The Assistant Secretary for Planning and Evaluation (ASPE) and Patient-Centered Outcomes Research Trust Fund (PCORTF) of the U.S. Department of Health and Human Services under Interagency Agreement #750119PE060048, through the U.S. Food and Drug Administration (FDA) Grant (#U01FD006936). The funder had no influence on design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
PRESENTATION INFORMATION:
This study was presented as a poster at the 2021 Vascular Annual Meeting of the Society for Vascular Surgery, San Diego, California, August 18-21, 2021.
CONFLICTS OF INTEREST:
None
REFERENCES
- 1.Soden PA, Zettervall SL, Deery SE, Hughes K, Stoner MC, Goodney PP, et al. Black patients present with more severe vascular disease and a greater burden of risk factors than white patients at time of major vascular intervention. J Vasc Surg 2018;67(2):549–556.e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrissey NJ, Giacovelli J, Egorova N, Gelijns A, Moskowitz A, Kent KC, et al. Disparities in the treatment and outcomes of vascular disease in Hispanic patients. J Vasc Surg 2007;46(5):971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 2010;52(3):539–548. [DOI] [PubMed] [Google Scholar]
- 4.Tang W, Yao L, Roetker NS, Alonso A, Lutsey PL, Steenson CC, et al. Lifetime Risk and Risk Factors for Abdominal Aortic Aneurysm in a 24-Year Prospective Study: The ARIC Study (Atherosclerosis Risk in Communities). Arterioscler Thromb Vasc Biol 2016;36(12):2468–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo RC, Bensley RP, Hamdan AD, Wyers M, Adams JE, Schermerhorn ML. Gender differences in abdominal aortic aneurysm presentation, repair, and mortality in the Vascular Study Group of New England. J Vasc Surg 2013;57(5):1261–1268, 1268.e1261-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPhee JT, Hill JS, Eslami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg 2007;45(5):891–899. [DOI] [PubMed] [Google Scholar]
- 7.Williams TK, Schneider EB, Black JH, Lum YW, Freischlag JA, Perler BA, et al. Disparities in Outcomes for Hispanic Patients Undergoing Endovascular and Open Abdominal Aortic Aneurysm Repair. Ann Vasc Surg 2013;27(1):29–37. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Lehman EB, Aziz F. African Americans are less likely to have elective endovascular repair of abdominal aortic aneurysms. J Vasc Surg 2019;70(2):462–470. [DOI] [PubMed] [Google Scholar]
- 9.Mwipatayi BP, Anwari T, Wong J, Verhoeven E, Dubenec S, Heyligers JM, et al. Sex-Related Outcomes After Endovascular Aneurysm Repair Within the Global Registry for Endovascular Aortic Treatment. Ann Vasc Surg 2020;67:242–253 e244. [DOI] [PubMed] [Google Scholar]
- 10.Deery SE, O’Donnell TFX, Shean KE, Darling JD, Soden PA, Hughes K, et al. Racial disparities in outcomes after intact abdominal aortic aneurysm repair. J Vasc Surg 2018;67(4): 1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deery SE, Soden PA, Zettervall SL, Shean KE, Bodewes TCF, Pothof AB, et al. Sex differences in mortality and morbidity following repair of intact abdominal aortic aneurysms. J Vasc Surg 2017;65(4): 1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locham S, Shaaban A, Wang L, Bandyk D, Schermerhorn M, Malas MB. Impact of Gender on Outcomes Following Abdominal Aortic Aneurysm Repair. Vasc Endovascular Surg 2019;53(8):636–643. [DOI] [PubMed] [Google Scholar]
- 13.Schermerhorn ML, O’Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. Open Repair of Abdominal Aortic Aneurysms in the Medicare Population. New England Journal of Medicine 2008;358(5):464–474. [DOI] [PubMed] [Google Scholar]
- 14.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM, EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 2016;388(10058):2366–2374. [DOI] [PubMed] [Google Scholar]
- 15.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. New England Journal of Medicine 2015;373(4):328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoel AW, Faerber AE, Moore KO, Ramkumar N, Brooke BS, Scali ST, et al. A pilot study for long-term outcome assessment after aortic aneurysm repair using Vascular Quality Initiative data matched to Medicare claims. J Vasc Surg 2017;66(3):751–759.e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemio. 2008;61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 18.de Guerre L, Rice J, Cheng J, Li C, Dansey KD, Marcaccio C, et al. Racial Differences in Isolated Aortic, Concomitant Aortoiliac, and Isolated Iliac Aneurysms: This is a Retrospective Observational Study. Ann Surg 2020. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zettervall SL, Schermerhorn ML, Soden PA, McCallum JC, Shean KE, Deery SE, et al. The effect of surgeon and hospital volume on mortality after open and endovascular repair of abdominal aortic aneurysms. J Vasc Surg 2017;65(3):626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Columbo JA, Ramkumar N, Martinez-Camblor P, Kang R, Suckow BD, O’Malley AJ, et al. Five-year reintervention after endovascular abdominal aortic aneurysm repair in the Vascular Quality Initiative. J Vasc Surg 2020;71(3):799–805 e791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 2018;67(1):2–77 e72. [DOI] [PubMed] [Google Scholar]
- 23.Schoenfeld D Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–241. [Google Scholar]
- 24.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81(3):515–526. [Google Scholar]
- 25.Patel PB, De Guerre L, Marcaccio CL, Dansey KD, Li C, Lo R, et al. Sex Specific Criteria for Repair Should be Utilized in Patients Undergoing Aortic Aneurysm Repair. J Vasc Surg 2022;75(2):515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemaire A, Cook C, Tackett S, Mendes DM, Shortell CK. The impact of race and insurance type on the outcome of endovascular abdominal aortic aneurysm (AAA) repair. J Vasc Surg 2008;47(6): 1172–1180. [DOI] [PubMed] [Google Scholar]
- 27.Vogel TR, Cantor JC, Dombrovskiy VY, Haser PB, Graham AM. AAA repair: sociodemographic disparities in management and outcomes. Vasc Endovascular Surg 2009;42(6):555–560. [DOI] [PubMed] [Google Scholar]
- 28.Healthy People 2020: Social Determinants of Health. Office of Disease Prevention and Health Promotion. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed January 14, 2022.
- 29.Varkevisser RRB, Swerdlow NJ, de Guerre L, Dansey K, Stangenberg L, Giles KA, et al. Five-year survival following endovascular repair of ruptured abdominal aortic aneurysms is improving. J Vasc Surg 2020;72(1):105–113 e104. [DOI] [PubMed] [Google Scholar]
- 30.Varkevisser RRB, Swerdlow NJ, de Guerre L, Dansey K, Zarkowsky DS, Goodney PP, et al. Midterm survival after endovascular repair of intact abdominal aortic aneurysms is improving over time. J Vasc Surg 2020;72(2):556–565 e556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Columbo JA, Martinez-Camblor P, O’Malley AJ, Suckow BD, Hoel AW, Stone DH, et al. Long-term Reintervention After Endovascular Abdominal Aortic Aneurysm Repair. Ann Surg 2021;274(1):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garg T, Baker LC, Mell MW. Postoperative Surveillance and Long-term Outcomes After Endovascular Aneurysm Repair Among Medicare Beneficiaries. JAMA Surg 2015;150(10):957–963. [DOI] [PubMed] [Google Scholar]
- 33.Murphy-Barron C, Pyenson B, Ferro C, Emery M. Comparing the Demographics of Enrollees in Medicare Advantage and Fee-For-Service Medicare. Better Medicare Alliance; October 2020. 2020. [Google Scholar]
- 34.Dansey KD, Varkevisser RRB, Swerdlow NJ, Li C, de Guerre LEVM, Liang P, et al. Epidemiology of endovascular and open repair for abdominal aortic aneurysms in the United States from 2004 to 2015 and implications for screening. J Vasc Surg 2021;74(2):414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


