Abstract
Biopsy specimens of the antrum and corpus were obtained from four Helicobacter pylori-infected members of a family and from the same boy (son 1) in whom the infection reappeared after simultaneous successful eradication treatment of three family members, excluding the mother. A total of 18 to 60 H. pylori isolates were obtained from each specimen and subjected to rRNA gene restriction pattern analysis. The father's isolates and the initial isolates from son 1 showed the same HindIII type, which was divided into three HaeIII subtypes. Isolates from the mother and a brother (son 2) and posttreatment isolates from son 1 showed a distinct HindIII type (with one minor subtype), which was divided into six HaeIII subtypes. All subtypes of the initial isolates from son 1 were present in the father's isolates, and all subtypes of the posttreatment isolates from son 1 were present in the mother's isolates but not in son 2's. Electron microscopic analysis of the biopsy specimens demonstrated extremely high levels of H. pylori colonization in the father's gastric mucosa. H. pylori adherence with a ruffle formation was also demonstrated. The findings suggest that son 1 was infected initially with the H. pylori strain of the father and son 2 was infected with the H. pylori strain of the mother and that after eradication therapy son 1 was reinfected with the H. pylori strain of the mother, who did not undergo eradication therapy.
Helicobacter pylori colonizes the gastric mucosa via an oral route, and around 60% of individuals worldwide are infected with H. pylori (5). Children are at high risk for H. pylori infections (21, 34). H. pylori infection may persist for years or be lifelong (30, 35), although spontaneous clearance is also common in childhood (15, 19). H. pylori infection is closely associated with gastritis and peptic ulcers (14, 25), and it is also a bacterial risk factor for gastric cancer (16) and mucosa-associated lymphoid tissue lymphoma (2).
The precise mechanisms of H. pylori transmission are not yet known. Previous investigations by Drumm et al. in 1990 (8), Malaty et al. in 1991 (18), and Oderda et al. in 1991 (27) suggested intrafamilial clustering of H. pylori infections. Molecular DNA analyses of familial strains of H. pylori were then performed by Nwokolo et al. in 1992 (26) and by Bamford et al. in 1993 (1), demonstrating intrafamilial infections due to a single H. pylori strain (or a common source of infection within the family). Now, transmission among family members is considered to constitute the main route of H. pylori infection (6).
In intrafamilial H. pylori infection, the infected parents, particularly infected mothers, have been considered likely to play a key role in the transmission of H. pylori (4, 28). In contrast, in developing countries, environmental factors may be more important than intrafamilial transmission (29, 32).
Those epidemiological studies, however, are not based on molecular biological analysis of H. pylori strains. In this study, we investigated the molecular basis of H. pylori transmission by rRNA gene restriction pattern analysis (ribotyping) for the four members of a Japanese family, including one member (son 1) who showed a recurrence of infection after simultaneous, successful eradication treatment of three family members, excluding the mother. We also characterized the ultrastructure of H. pylori colonization in biopsy specimens.
MATERIALS AND METHODS
Gastric biopsy specimens.
Biopsy specimens were taken from the antrum and corpus of the four H. pylori-infected members of one family at Juntendo University Hospital (Tokyo, Japan) in 1998. They included a 9-year-old boy (son 1), his 7-year-old brother (son 2), his 36-year-old father, and his 36-year-old mother (Fig. 1).
FIG. 1.

Pedigree of H. pylori strains in a family. Shaded symbols indicate H. pylori infection. Numbers in the symbols represent single colonies obtained from the primary H. pylori culture of the biopsy specimens (antrum and corpus) that were examined by ribotyping.
Son 1 was admitted to the hospital because of upper abdominal pain. He was positive by a [13C]-urea breath test and was endoscopically diagnosed with a duodenal ulcer. The father, mother, and son 2 were also positive by a [13C]urea breath test, while the 4-year-old brother (son 3) was negative. The father and son 2 were endoscopically diagnosed with duodenal ulcers, and the mother displayed a normal gastric mucosa.
Son 1, son 2, and the father were simultaneously treated with a combination of anti-H. pylori agents (clarithromycin and amoxicillin) and a proton pump inhibitor (lansoprazole). Seven weeks after treatment, all three had negative results by [13C]urea breath tests and by histologic and culture examinations. However, 36 weeks after treatment, recurrence of the infection was observed only in son 1 by a [13C]urea breath test, and biopsy specimens were again taken from the antrum and corpus of son 1.
Media and bacterial growth.
H. pylori was isolated from biopsy specimens by culturing at 37°C in a microaerophilic atmosphere (10% O2 and 10% CO2) on 5% sheep blood agar (Becton Dickinson, Tokyo, Japan) or on H. pylori-selective plates containing trimethoprim, polymyxin B, vancomycin, and amphotericin B to inhibit the growth of microbes other than H. pylori (Nissui Pharmaceuticals, Tokyo, Japan). Single colonies were subcultured on 5% sheep blood agar and then stored at −80°C in 3% skim milk (Difco Laboratories, Detroit, Mich.) supplemented with 5% glucose (Difco). For liquid culture, H. pylori colonies on 5% sheep blood agar were suspended in brain heart infusion broth (Difco) containing 10% fetal bovine serum (Gibco, Gaithersburg, Md.), followed by incubation for 18 h at 37°C in a microaerophilic atmosphere.
Scanning electron microscopy.
Biopsy specimens were washed in saline, fixed with 2.5% (vol/vol) glutaraldehyde in phosphate-buffered saline (pH 7.4) for 2 h at room temperature, and subsequently postfixed in 1% (wt/vol) osmium tetroxide for 1 h at 4°C. The samples were then dehydrated in acetone, critical-point dried, and coated with gold-palladium. The samples were finally analyzed by scanning electron microscopy (38, 39).
Transmission electron microscopy.
The fixed samples were dehydrated in acetone and embedded in EPOK 812 (Oukenn Inc., Tokyo, Japan). The embedded block was cut with an ultramicrotome (MT-5000) diamond knife and stained with uranyl acetate and lead citrate. The stained samples were analyzed by transmission electron microscopy (38).
Bacterial DNA isolation.
H. pylori DNA was prepared essentially as previously described (23). H. pylori cells grown in 10 ml of brain heart infusion broth containing 10% fetal bovine serum were suspended in 10 mM Tris-HCl (pH 8.0) containing 1 mM EDTA and then lysed by the addition of sodium dodecyl sulfate (0.5%) and proteinase K (100 μg/ml). After treatment with cetyltrimethylammonium bromide (1% in the presence of 0.7 M NaCl) and subsequently with chloroform-isoamyl alcohol (24:1) and phenol-chloroform-isoamyl alcohol (25:24:1), DNA in the aqueous solution was precipitated with 0.6 volume of isopropanol. DNA was then rinsed with 70% ethanol and redissolved in 50 μl of 10 mM Tris-HCl (pH 8.0) containing 1 mM EDTA.
rRNA gene restriction pattern analysis (ribotyping).
Bacterial DNA was digested with HindIII or HaeIII, and the digests were electrophoresed in a 0.8% agarose gel with a 1-kb DNA ladder (Life Technologies, Gaithersburg, Md.) which was used as the molecular size standard. Digoxigenin (DIG)-labeled cDNA probes were prepared by reverse transcription of 16S and 23S rRNA (Roche Diagnostics GmbH, Mannheim, Germany) using a DIG DNA labeling mixture (Roche) and a cDNA synthesis kit (Roche) (36). Southern hybridization was performed as described previously (9), using a nylon membrane (Amersham International, Amersham, United Kingdom). DNA hybrids on the membrane were treated with anti-DIG alkaline phosphatase-conjugated Fab fragments (Roche) and then visualized with color development by adding nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate 4-toluidine salt (substrate) (Roche).
Computer analysis.
Computer-assisted analysis of the ribotype patterns was performed by using a program called Molecular Analyst Fingerprinting Plus (Bio-Rad, Tokyo, Japan) according to the UPGMA clustering algorithm (24, 33). In this analysis, the Dice coefficient [SD = 2nAB/(nA + nB), where nA is the number of bands in lane A, nB is the number of bands in lane B, and nAB is the number of bands found in both lanes A and B] and the matching tolerance of 0.8% were employed.
RESULTS
Ribotyping of H. pylori isolates from the family members.
A total of 18 to 60 single colonies of H. pylori were obtained from each primary culture of the antrum and corpus biopsy specimens of the family members (Fig. 1). Bacterial DNA was then obtained from each H. pylori isolate, digested with HindIII (a six-base cutter) or HaeIII (a four-base cutter, which will produce many more fragments), and subjected to ribotype analysis (Fig. 2).
FIG. 2.
Ribotyping analysis of H. pylori strains from the members of a family. H. pylori chromosomal DNA was digested by HindIII (A) and HaeIII (B). a, before treatment; b, after treatment. Lanes M, 1-kb DNA ladder. In panel B, H. pylori was from the antrum of the father (lanes 1 to 3), the mother (lanes 4 to 7), son 2 (lanes 8 and 9), and son 1 (lanes 10 to 13). The same ribotypes were also observed in H. pylori from the corpus.
When DNAs were digested with HindIII, most of the samples produced only three bands (Fig. 2A). All of the initial isolates from son 1 and the father's isolates gave the same single ribotyping pattern, designated Fhin1 (Fig. 2A, lanes 1 to 4; Table 1). In contrast, most of the mother's isolates, all of son 2's isolates, plus all of the posttreatment isolates from son 1 gave a distinct single pattern, designated Mhin1 (Fig. 2A, lanes 5 to 10; Table 1). The mother's minor isolates gave a related, similar pattern, designated Mhin2 (Fig. 2A, lane 12; Table 1).
TABLE 1.
Ribotyping patterns of H. pylori isolates from family members
| Family member | Source of biopsy specimen (no. of isolates) | % of ribotyping patterns belonging to subtypea:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fhin1 | Mhin1 | Mhin2 | Fhae1 | Fhae2 | Fhae3 | Mhae1 | Mhae2 | Mhae3 | Mhae4 | Bhae1 | Bhae2 | ||
| Son 1b | Antrum (18) | 100 | —c | — | 39 | 61 | 0 | — | — | — | — | — | — |
| Corpus (24) | 100 | — | — | 79 | 21 | 0 | — | — | — | — | — | — | |
| Father | Antrum (24) | 100 | — | — | 21 | 58 | 21 | — | — | — | — | — | — |
| Corpus (24) | 100 | — | — | 21 | 42 | 38 | — | — | — | — | — | — | |
| Mother | Antrum (26) | — | 96 | 4 | — | — | — | 15 | 65 | 15 | 4 | 0 | 0 |
| Corpus (24) | — | 96 | 4 | — | — | — | 33 | 17 | 46 | 4 | 0 | 0 | |
| Son 2 | Antrum (28) | — | 100 | 0 | — | — | — | 0 | 50 | 0 | 0 | 14 | 36 |
| Corpus (26) | — | 100 | 0 | — | — | — | 0 | 69 | 0 | 0 | 19 | 12 | |
| Son 1d | Antrum (60) | — | 100 | 0 | — | — | — | 0 | 60 | 40 | 0 | 0 | 0 |
| Corpus (60) | — | 100 | 0 | — | — | — | 0 | 17 | 83 | 0 | 0 | 0 | |
Subtypes containing “hin” were the result of HindIII digestion. Subtypes containing “hae” were the result of HaeIII digestion.
Before eradication treatment.
—, not detected.
After eradication treatment.
When DNAs were digested with HaeIII, eight or more bands were observed (Fig. 2B). HaeIII digestion divided the Fhin1 type (found in the initial isolates from son 1 and the father's isolates) into three subtypes, designated Fhae1, Fhae2, and Fhae3 (Fig. 2B, lanes 1 to 3). The Fhae1, Fhae2, and Fhae3 subtypes showed a high degree of similarity (more than 92%). The Fhae1 and Fhae2 subtypes were present in both the initial isolates from son 1 and the father's isolates (Table 1; Fig. 2B, lanes 10 and 11), whereas the Fhae3 subtype was present only in the father's isolates (Table 1).
HaeIII digestion also divided the Mhin1 type (found in the isolates from the mother and son 2) into five subtypes, designated Mhae1, Mhae2, Mhae3, and Bhae1, and Bhae2 (Fig. 2B, lanes 4 to 6, 8, and 9). HaeIII digestion of the Mhin2 type (found in the mother's minor isolates) gave a distinct, sixth subtype, designated Mhae4 (Fig. 2B, lane 7). The Mhae1, Mhae2, Mhae3, and Mhae4 subtypes were close to each other, with a similarity of more than 88%. In contrast, the Bhae1 and Bhae2 subtypes were slightly distant from the Mhae1, Mhae2, Mhae3, and Mhae4 subtypes (similarity, 80 to 82%).
The mother's isolates showed the four subtypes Mhae1, Mhae2, Mhae3, and Mhae4; of those, Mhae1 and Mhae4 were unique to the mother's isolates (Table 1). Son 2's isolates had the mother's subtype, Mhae2, plus the unique subtypes Bhae1 and Bhae2 (Table 1). The posttreatment isolates from son 1's had only two (Mhae2 and Mhae3) of the four subtypes found in the mother and no unique subtypes (Table 1; Fig. 2B, lanes 12 and 13). The Mhae2 subtype was commonly found among isolates from the mother and son 2 and posttreatment isolates from son 1.
Characterization of H. pylori colonization by scanning electron microscopy.
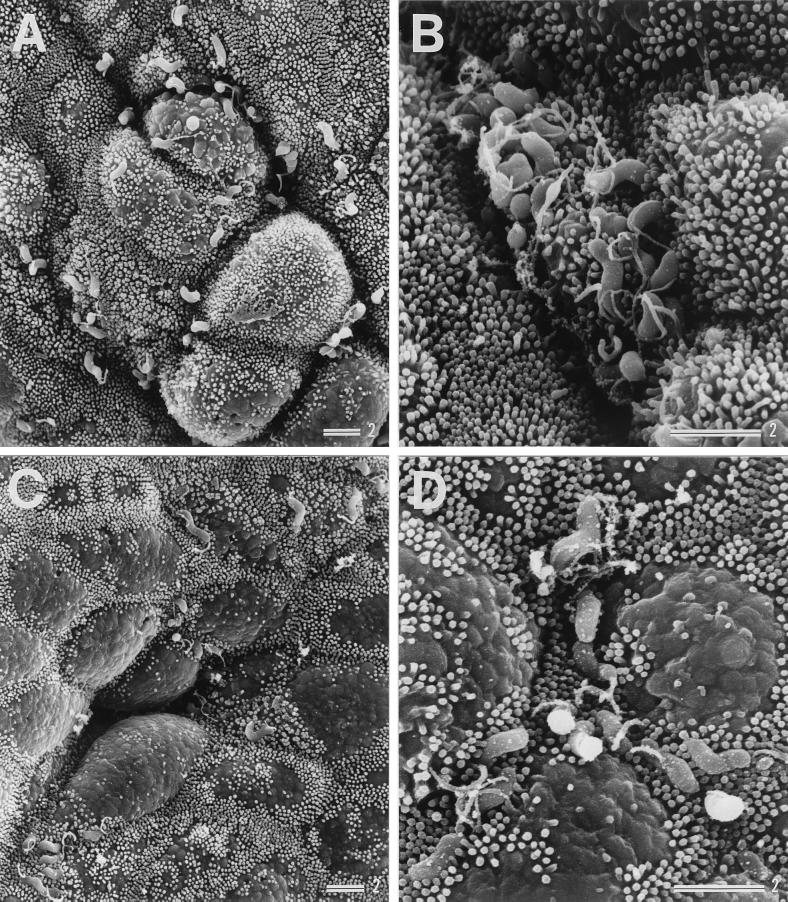
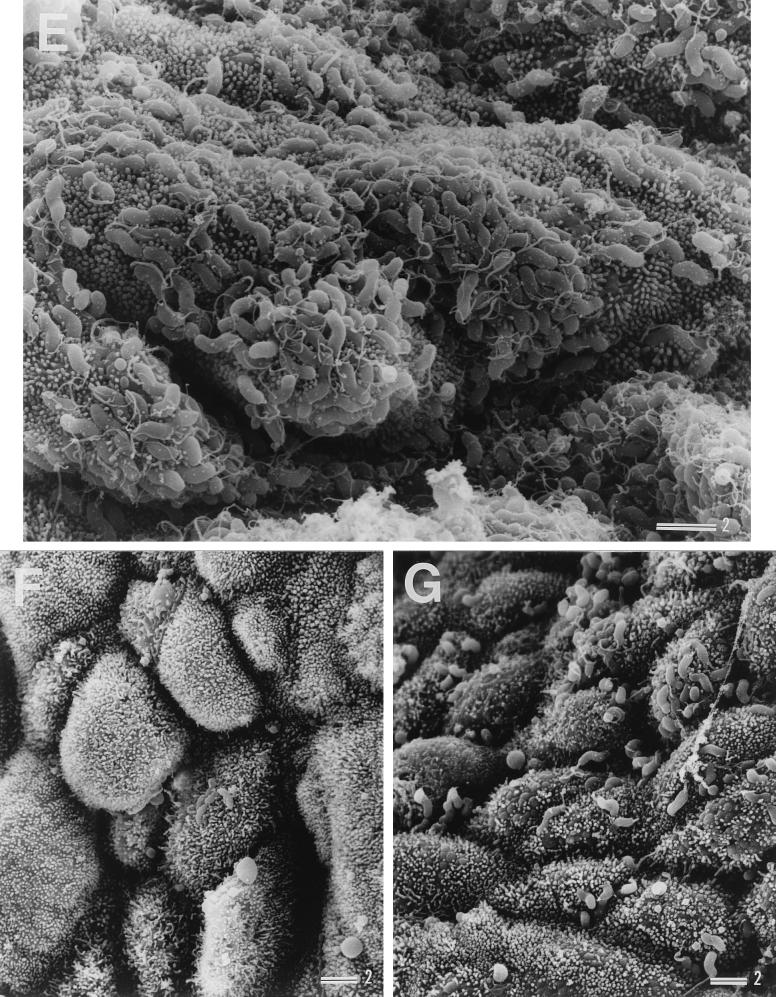
Examination of son 1's biopsy specimens showed that H. pylori had colonized the gastric mucosa of both the antrum and corpus, as shown in Fig. 3A to D. In the father's biopsy specimens, extremely high levels of H. pylori colonization were observed, and examination revealed that the gastric epithelium was almost completely covered by the adherent H. pylori (Fig. 3E). In the mother's gastric mucosa, H. pylori colonization was observed to a lesser extent (Fig. 3F), than in the father's specimens. Although son 1's mucosa had no detectable adherent H. pylori 7 weeks after eradication treatment (as expected from the negative results in culture examinations), 36 weeks after treatment H. pylori colonization was observed at a level similar to or even greater than that before treatment (Fig. 3G).
FIG. 3.
Scanning electron micrographs showing colonization of H. pylori in gastric biopsy specimens from son 1 before treatment (A to D), the father (E), the mother (F), and son 1 after reinfection (G). Panels C and D show specimens from the corpus; all others show specimens from the antrum. Bars = 2 μm.
Unique features of H. pylori adherence to the mucosa.
Transmission electron microscopic analysis of the biopsy specimens from son 1 before and after treatment showed that the H. pylori cells adhered to the gastric epithelial cells via filament-like structures in most cases (Fig. 4A and C). The H. pylori cells also occasionally bound to a pedestal-like structure (Fig. 4B). H. pylori adherence via filament-like structures was also observed in all other biopsy specimens examined, as shown for the father's mucosa (Fig. 4D).
FIG. 4.
Transmission electron micrographs showing H. pylori adherence to epithelial cells in gastric biopsy specimens (antrum) from son 1 before treatment (A and B) and after reinfection (C) and from the father (D). Arrowheads in panels A, C, and D point to filament-like structures between the adherent bacterium and the epithelial cells. An arrowhead in panel B points to a pedestal-like structure. Bars = 1 μm.
An intimate adherence characterized by ruffle formation was found in a sample from the father's mucosa by scanning electron microscopy (Fig. 5).
FIG. 5.
Scanning electron micrographs showing ruffle formation of H. pylori in a gastric biopsy specimen (antrum) from the father. An arrowhead points to a ruffle formation on the epithelial cell surface. Bar = 1 μm.
DISCUSSION
Previous investigations using serological or histological diagnosis and/or the [13C]urea breath test have demonstrated an intrafamilial clustering of H. pylori infections in Canada (8), the United States (18), Italy (7, 27), and England (1, 26). It has also been suggested that H. pylori is transmitted from infected parents, especially infected mothers, to children in Germany (4, 28, 29), the United States (10), and Japan (20, 22). H. pylori transmission among siblings has been suggested in Colombia (13) and Japan (22). Previous studies in England using DNA fingerprinting demonstrated a familial infection of a mother, father, and child due to a single H. pylori strain (1).
In this study, we carefully analyzed a case of intrafamilial infection in a Japanese family. We examined 18 to 60 H. pylori isolates in each biopsy specimen. In addition, we used two restriction enzymes (HindIII and HaeIII) in ribotype analysis. HindIII is a six-base cutter and HaeIII is a four-base cutter. Thus, HaeIII will produce many more fragments and ultimately has more power to subtype. For example, when examining the father's isolates, HindIII digestion produced only one ribotype, while HaeIII digestion produced three.
In this family, before treatment the 9-year-old boy (son 1) and the father were infected with the same H. pylori strain and the 7-year-old boy (son 2) and the mother were infected with a different H. pylori strain. The 4-year-old boy (son 3) was not infected with H. pylori.
Son 1, the father, and son 2, who experienced duodenal ulcers, were subjected to simultaneous eradication therapy, resulting in a successful cure 7 weeks after treatment. Thirty-six weeks later, however, son 1 was reinfected with an H. pylori strain with the same ribotyping pattern as one of the mother's isolates. The mother had not been included in the eradication therapy because she displayed a normal gastric mucosa. Since the mother was the only family member who was H. pylori positive during son 1's reinfection, this provides the first direct evidence of mother-to-child transmission within a family. The possibility that other environmental sources are involved in reinfection exists.
Kuipers et al. have shown that genetic drift occurs within H. pylori populations over the course of years of colonization within a single host (17). Indeed, analysis of H. pylori by HaeIII digestion demonstrated variants (subtypes) with genomic mutation. All of the H. pylori subtypes found in son 1's stomach before treatment were also present in the father's stomach, and all of the H. pylori subtypes found in son 1's stomach after reinfection were present in the mother's stomach but not in son 2's. The father, mother, and son 2 had unique H. pylori subtypes, whereas son 1 did not. These findings are consistent with the suggestion that, before treatment son 1 had been infected with the father's H. pylori strain and son 2 had been infected with the mother's H. pylori strain; after eradication therapy, son 1 became infected with the mother's strain. The last mother-to-child transmission must have occurred within a period as short as 29 weeks.
It has been reported that younger children are more susceptible to the acquisition of H. pylori (13). Why only son 1 became reinfected with H. pylori after eradication therapy and son 2 and son 3 did not is not known. However, there is a possibility that in this family, the mother spent much more time with son 1, who was ill, and spent less time with son 2 and son 3, and thus, H. pylori was transmitted from the mother to son 1 (who had more contact with the mother) and not to the other two children. In addition, many older children have their own rooms in Japan, and this may also decrease the possibility of transmission among children in families.
Reinfection by H. pylori in adults as well as children is generally uncommon (3, 12, 31, 37). This report describes only a single family case; however, such mother-to-child transmission after treatment may be significant in Japan.
In this study, we examined biopsy specimens by electron microscopy as well as histologically. This provided the first ultrastructural findings of intrafamilial H. pylori infection. The father, who was the source of the initial H. pylori infection in the boy, had extremely high levels of H. pylori colonization. In the case of another familial infection in which father-to-child transmission occurred, the father again had extremely high levels of H. pylori colonization (unpublished data). In Japan, some fathers may have extremely high levels of H. pylori colonization (probably due to genetic backgrounds or eating habits that may facilitate H. pylori colonization in the stomach by providing cell surface receptors or bacterial growth factors) and may have a high risk of transmitting H. pylori to children within their families.
Thus, factors involved in the parent-to-child transmission of H. pylori in Japan could be the high level of H. pylori colonization in the stomach (e.g., the father) and frequent, close contact with the child (e.g., the mother).
Scanning electron microscopic analysis of the biopsy specimens showed tight H. pylori adherence to the gastric epithelial cell surface. Transmission electron microscopic analysis of the same biopsy specimens, however, showed adherence via filament-like structures in most cases. H. pylori may adhere to the epithelium via a surface layer such as a glycocalix or via pili. At a later stage, H. pylori may adhere intimately to the epithelium (6). Interestingly, in this study, ruffle formation (which resembled that of Shigella) (11) was observed by scanning electron microscopy. Further studies are necessary to gain insight into the precise mechanisms of H. pylori adherence to the human gastric mucosa as well as the circulation of H. pylori within a family.
ACKNOWLEDGMENT
This study was supported in part by a grant from Ohyama Health Foundation Inc.
REFERENCES
- 1.Bamford K B, Bickley J, Collins J S A, Johnston B T, Potts S, Boston V, Owen R J, Sloan J M. Helicobacter pylori: comparison of DNA fingerprints provides evidence for intrafamilial infection. Gut. 1993;34:1348–1350. doi: 10.1136/gut.34.10.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blecker U, McKeithan T W, Hart J, Kirschner B S. Resolution of Helicobacter pylori-associated gastric lymphoproliferative disease in a child. Gastroenterology. 1995;109:973–977. doi: 10.1016/0016-5085(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 3.Borody T, Andrews P, Mancuso N, Jankiewicz E, Brandl S. Helicobacter pylori reinfection 4 years post-eradication. Lancet. 1992;339:1295. doi: 10.1016/0140-6736(92)91622-f. [DOI] [PubMed] [Google Scholar]
- 4.Brenner H, Bode G, Adler G, Rothenbacher D. Does maternal smoking hinder mother-child transmission of Helicobacter pylori infection? Epidemiology. 2000;11:71–75. doi: 10.1097/00001648-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Cave D R. How is Helicobacter pylori transmitted? Gastroenterology. 1997;113(Suppl. 6):S9–S14. doi: 10.1016/s0016-5085(97)80004-2. [DOI] [PubMed] [Google Scholar]
- 6.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 7.Dominici P, Bellentani S, Di Biase A R, Saccoccio G, Le Rose A, Masutti F, Viola L, Balli F, Tiribelli C, Grilli R, Fusillo M, Grossi E. Familial clustering of Helicobacter pylori infection: population based study. BMJ. 1999;319:537–540. doi: 10.1136/bmj.319.7209.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drumm B, Perez-Perez G I, Blaser M J, Sherman P M. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990;322:359–363. doi: 10.1056/NEJM199002083220603. [DOI] [PubMed] [Google Scholar]
- 9.Echeverria P, Seriwatana J, Sethabutr O, Chatkaeomorakot A. Detection of diarrheagenic Escherichia coli using nucleotide probes. In: Macario A J L, de Macario E C, editors. Gene probes for bacteria. San Diego, Calif: Academic Press; 1990. pp. 95–141. [Google Scholar]
- 10.Elitsur Y, Adkins L, Saeed D, Neace C. Helicobacter pylori antibody profile in household members of children with H. pylori infection. J Clin Gastroenterol. 1999;29:178–182. doi: 10.1097/00004836-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 12.Forbes G M, Glaser M E, Cullen D J E, Warren J R, Christiansen K J, Marshall B J, Collins B J. Duodenal ulcer treated with Helicobacter pylori eradication: seven-year follow-up. Lancet. 1994;343:258–260. doi: 10.1016/s0140-6736(94)91111-8. [DOI] [PubMed] [Google Scholar]
- 13.Goodman K J, Correa P. Transmission of Helicobacter pylori among siblings. Lancet. 2000;355:358–362. doi: 10.1016/S0140-6736(99)05273-3. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin C S. Helicobacter pylori gastritis, peptic ulcer, and gastric cancer: clinical and molecular aspects. Clin Infect Dis. 1997;25:1017–1019. doi: 10.1086/516077. [DOI] [PubMed] [Google Scholar]
- 15.Granström M, Tindberg Y, Blennow M. Seroepidemiology of Helicobacter pylori infection in a cohort of children monitored from 6 months to 11 years of age. J Clin Microbiol. 1997;35:468–470. doi: 10.1128/jcm.35.2.468-470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IARC Working Group. IARC monographs on the evaluation of carcinogenic risks to humans. 61. Schistosomes, liver flukes and Helicobacter pylori. Lyon, France: International Agency for Research on Cancer, World Health Organization; 1994. pp. 177–241. [Google Scholar]
- 17.Kuipers E J, Israel D A, Kusters J G, Gerrits M M, Weel J, van der Ende A, van der Hulst R W M, Wirth H P, Höök-Nikanne J, Thompson S A, Blaser M J. Quasispecies development of Helicobacter pylori observed in paired isolates obtained years apart from the same host. J Infect Dis. 2000;181:273–282. doi: 10.1086/315173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malaty H M, Graham D Y, Klein P D, Evans D G, Adam E, Evans D J. Transmission of Helicobacter pylori infection. Studies in families of healthy individuals. Scand J Gastroenterol. 1991;26:927–932. doi: 10.3109/00365529108996244. [DOI] [PubMed] [Google Scholar]
- 19.Malaty H M, Graham D Y, Wattigney W A, Srinivasan S R, Osato M, Berenson G S. Natural history of Helicobacter pylori infection in childhood: 12-year follow-up cohort study in a biracial community. Clin Infect Dis. 1999;28:279–282. doi: 10.1086/515105. [DOI] [PubMed] [Google Scholar]
- 20.Malaty H M, Kumagai T, Tanaka E, Ota H, Kiyosawa K, Graham D Y, Katsuyama T. Evidence from a nine-year birth cohort study in Japan of transmission pathways of Helicobacter pylori infection. J Clin Microbiol. 2000;38:1971–1973. doi: 10.1128/jcm.38.5.1971-1973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendall M A, Goggin P M, Molineaux N, Levy J, Toosy T, Strachan D, Northfield T C. Childhood living conditions and Helicobacter pylori seropositivity in adult life. Lancet. 1992;339:896–897. doi: 10.1016/0140-6736(92)90931-r. [DOI] [PubMed] [Google Scholar]
- 22.Miyaji H, Azuma T, Ito S, Abe Y, Gejyo F, Hashimoto N, Sugimoto H, Suto H, Ito Y, Yamazaki Y, Kohli Y, Kuriyama M. Helicobacter pylori infection occurs via close contact with infected individuals in early childhood. J Gastroenterol Hepatol. 2000;15:257–262. doi: 10.1046/j.1440-1746.2000.02070.x. [DOI] [PubMed] [Google Scholar]
- 23.Murray M G, Thompson W F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nei M, Li W H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–68. [PubMed] [Google Scholar]
- 26.Nwokolo C U, Bickley J, Attard A R, Owen R J, Costas M, Fraser I A. Evidence of clonal variants of Helicobacter pylori in three generations of a duodenal ulcer disease family. Gut. 1992;33:1323–1327. doi: 10.1136/gut.33.10.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oderda G, Vaira D, Holton J, Ainley C, Altare F, Boero M, Smith A, Ansaldi N. Helicobacter pylori in children with peptic ulcer and their families. Dig Dis Sci. 1991;36:572–576. doi: 10.1007/BF01297021. [DOI] [PubMed] [Google Scholar]
- 28.Rothenbacher D, Bode G, Berg G, Knayer U, Gonser T, Adler G, Brenner H. Helicobacter pylori among preschool children and their parents: evidence of parent-child transmission. J Infect Dis. 1999;179:398–402. doi: 10.1086/314595. [DOI] [PubMed] [Google Scholar]
- 29.Rothenbacher D, Bode G, Brenner H. Helicobacter pylori among siblings. Lancet. 2000;355:1998. doi: 10.1016/S0140-6736(05)72939-1. [DOI] [PubMed] [Google Scholar]
- 30.Rowland M. Transmission of Helicobacter pylori: is it all child's play? Lancet. 2000;355:332–333. doi: 10.1016/S0140-6736(99)00427-4. [DOI] [PubMed] [Google Scholar]
- 31.Rowland M, Kumar D, Daly L, O'Connor P, Vaughan D, Drumm B. Low rates of Helicobacter pylori reinfection in children. Gastroenterology. 1999;117:336–341. doi: 10.1053/gast.1999.0029900336. [DOI] [PubMed] [Google Scholar]
- 32.Sarker S A, Mahalanabis D, Hildebrand P, Rahaman M M, Bardhan P K, Fuchs G, Beglinger C, Gyr K. Helicobacter pylori: prevalence, transmission, and serum pepsinogen II concentrations in children of a poor periurban community in Bangladesh. Clin Infect Dis. 1997;25:990–995. doi: 10.1086/516070. [DOI] [PubMed] [Google Scholar]
- 33.Sikorski J, Rossello-Mora R, Lorenz M G. Analysis of genotypic diversity and relationships among Pseudomonas stutzeri strains by PCR-based genomic fingerprinting and multilocus enzyme electrophoresis. Syst Appl Microbiol. 1999;22:393–402. doi: 10.1016/S0723-2020(99)80048-4. [DOI] [PubMed] [Google Scholar]
- 34.Staat M A, Kruszon-Moran D, McQuillan G M, Kaslow R A. A population-based serologic survey of Helicobacter pylori infection in children and adolescents in the United States. J Infect Dis. 1996;174:1120–1123. doi: 10.1093/infdis/174.5.1120. [DOI] [PubMed] [Google Scholar]
- 35.Taylor N S, Fox J G, Akopyants N S, Berg D E, Thompson N, Shames B, Yan L, Fontham E, Janney F, Hunter F M, Correa P. Long-term colonization with single and multiple strains of Helicobacter pylori assessed by DNA fingerprinting. J Clin Microbiol. 1995;33:918–923. doi: 10.1128/jcm.33.4.918-923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tee W. Ribosomal RNA gene restriction pattern analysis (ribotyping) of H. pylori. In: Clayton C L, Mobley H L T, editors. Helicobacter pylori protocols. Totowa, N.J: Humana Press, Inc.; 1997. pp. 89–98. [DOI] [PubMed] [Google Scholar]
- 37.van der Hulst R W M, Rauws E A J, Köycü B, Keller J J, ten Kate F J W, Dankert J, Tytgat G N J, van der Ende A. Helicobacter pylori reinfection is virtually absent after successful eradication. J Infect Dis. 1997;176:196–200. doi: 10.1086/514023. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T, Kaneko M, Changchawalit S, Serichantalergs O, Ijuin S, Echeverria P. Actin accumulation associated with clustered and localized adherence in Escherichia coli isolated from patients with diarrhea. Infect Immun. 1994;62:2917–2929. doi: 10.1128/iai.62.7.2917-2929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto T, Koyama Y, Matsumoto M, Sonoda E, Nakayama S, Uchimura M, Paveenkittiporn W, Tamura K, Yokota T, Echeverria P. Localized, aggregative, and diffuse adherence to HeLa cells, plastic, and human small intestines by Escherichia coli isolated from patients with diarrhea. J Infect Dis. 1992;166:1295–1310. doi: 10.1093/infdis/166.6.1295. [DOI] [PubMed] [Google Scholar]