TO THE EDITOR
Calcium hydroxylapatite (CaHA) prepackaged injectable filler is often selected for clinical use given its in vivo longevity and biostimulatory effects on neocollagenesis. CaHA-associated adverse events are uncommon but may include nodule formation, infection, product migration, overcorrection, and intravascular occlusion (Alam et al., 2015). Unlike for hyaluronic acid‒based fillers, there is currently no reversal agent for CaHA.
Percutaneous injection of sodium thiosulfate (STS) has been proposed as a potential reversal agent given its utility in other cutaneous calcifying disorders, such as calciphylaxis (Gabel et al., 2021; Strazzula et al., 2013) and calcinosis cutis (Gunasekera et al., 2017; Smith, 2013). However, the mechanism of interaction of STS with CaHA remains unclear. Consequently, we constructed both in vitro and ex vivo models to compare the effect of STS with that of saline on CaHA and to determine to what extent the action of STS, if any, may be attributable to the dissolution of individual CaHA microspheres versus the dispersion of CaHA through a fluid volume effect (this study was reviewed by Northwestern University Institutional Review Board number STU00009443).
In our in vitro experiment, CaHA (Radiesse, Merz North America, Raleigh, NC) microsphere size was assessed after exposure to STS (Sodium Thiosulfate Injection, USP, 12.5 g/50 ml, Hope Pharmaceuticals, Scottsdale, AZ) or saline under room temperature or simulated physiologic (37 °C, 5% carbon dioxide) conditions. Two identical microplates were prepared containing three samples of each CaHA:STS dilution (1:4, 1:2, 1:1, 2:1, 4:1) and one sample of each CaHA:saline dilution (1:4, 1:2, 1:1, 2:1, 4:1). One microplate was incubated at simulated physiologic conditions (37 °C, 5% carbon dioxide) for 24 hours, and the other was stored at 25 °C for 24 hours. Samples were placed on microscope slides. A total of 27 images per STS condition and 3 images per saline condition were captured at ×5 magnification. Microsphere diameter (in μm) was calculated using ImageJ (version 1.53k; National Institutes of Health, Bethesda, MD).
In our ex vivo experiment, a human skin model was used to study CaHA microsphere dispersion after exposure to STS or saline. Four full-thickness tissue samples were collected from eight patients. Three samples were injected with 0.1 ml of CaHA followed by 0.4 ml 25% STS; the remaining sample received 0.1 ml of CaHA followed by 0.4 ml of saline. After incubation in RPMI medium for 24 hours at 37 °C and 5% carbon dioxide, samples were fixed in formalin, embedded en face, sectioned into 4-μm thick sections, and stained with H&E. Microsphere dispersion was assessed by measuring the distance from the central injection point to the furthest radial point at which microspheres were identified using Olympus CellSens Software and a digital micrometer (Olympus, Tokyo, Japan). A maximum dispersion distance was recorded for each of the eight sections per sample. For each sample, an average maximum dispersion distance was calculated. Continuous variables were compared with a Student’s t-test using P < 0.05.
When exposed to STS in both room temperature and simulated physiologic conditions, CaHA microspheres decreased in diameter compared with the saline condition at all STS dilutions, with the exception of the 1:4 dilution in the simulated physiologic condition (28.2 ± 9.3 vs. 27.9 ± 10.2 μm) (Figure 1). CaHA microsphere size was not monotonically associated with increasing or decreasing concentrations of STS or with increasing or decreasing concentrations of saline.
Figure 1.
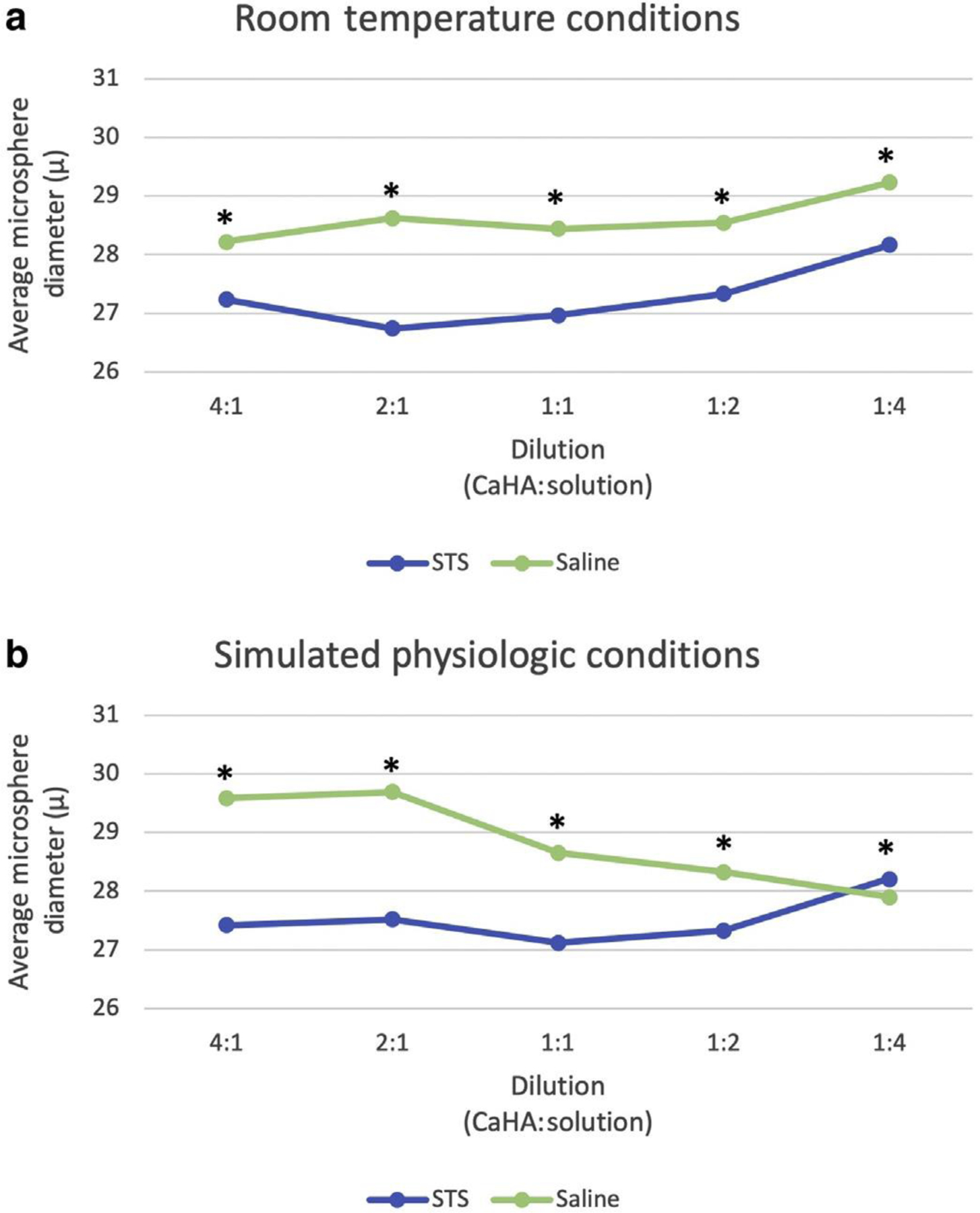
In vitro microsphere diameter after treatment with STS or saline. (a) Average diameters under room temperature conditions; (b) Average diameters under simulated physiologic conditions at 37 °C and 5% carbon dioxide to maintain a pH of 7.4. ∗P < 0.05. STS, sodium thiosulfate.
In our ex vivo human tissue model, the distance traveled (i.e., dispersion) by microspheres from the center of the CaHA bolus after injection of STS was not statistically different from the distance traveled after injection of an equivalent volume of saline. The average maximum distance traveled was 2.87 ± 0.82 mm for STS and 2.88 ± 0.78 mm for saline samples (Table 1).
Table 1.
Microsphere Dispersion Distance in Ex Vivo Human Tissue after 24-Hour Incubation, Tissue Fixation, and Histologic Sectioning
| Solution1 | Average Maximum Dispersion Distance – SD (mm) |
P-Value |
|---|---|---|
| STS (n ¼ 159) | 2865.2 ± 824.0 | 0.8897 |
| Normal saline (n ¼ 51) | 2882.9 ± 780.7 |
Abbreviation: STS, sodium thiosulfate.
Solution: n ¼ number of sections
In summary, our in vitro analysis suggests that STS decreases CaHA microsphere size compared with saline. This effect was notable under both room temperature and simulated physiologic conditions and may be responsible for any apparent effect that occurs in vivo. Our ex vivo analysis showed comparable dispersion of microspheres in samples treated with STS and saline, suggesting that STS clearance may also be dependent on microsphere dispersion due to the volume of solvent. Overall, STS appears to act by reducing the diameter of CaHA microspheres and dispersing the aliquot of CaHA.
Our results add clarity to promising initial reports in which histologic analysis of porcine skin samples injected with CaHA and STS showed an inability to detect CaHA microspheres after 24 hours (Robinson, 2018), with a lack of clarity on whether microspheres had been reduced in size or merely missed owing to tissue processing artifact. Our findings can also be compared with those of an in vitro analysis evaluating CaHA droplet density in cadaveric facial arteries after exposure to STS or saline. Using gray-scale imaging, the authors found increasing disintegration of CaHA with increasing concentrations of STS (Yankova et al., 2021). Similar to Yankova et al. (2021), we found an effect of STS on microsphere size reduction, consistent with a mechanism of disintegration. However, in contrast to the finding of Yankova et al. (2021), we found no linear relationship between STS concentration and CaHA microsphere size reduction. Finally, another in vitro analysis found no CaHA disintegration after exposure to 25% STS (Danysz et al., 2020), but this study investigated only a single concentration of STS and assessed microsphere surface behavior, a less relevant measure than microsphere size and droplet density, which was assessed in this study and that of Yankova et al. (2021), respectively.
Our study limitation was the use of a single concentration of 25% STS in our ex vivo model. Moreover, in living tissue, there may be additional interactions between CaHA and STS over time and with the surrounding biologic environment. Finally, concentrated saline solutions may have led to the aggregation of inorganic salts appearing on image analysis as small microspheres, measurement of which may have led to the apparently reduced average CaHA microsphere size in the saline group of the 1:4 dilution.
In summary, we found that STS reversal of CaHA entails spreading the volume of injected filler and reducing the size of CaHA microspheres. Thus, in addition to its therapeutic potential in the treatment of calciphylaxis and calcinosis cutis, STS may be helpful in reversing injectable CaHA for soft tissue augmentation.
Acknowledgments
The authors would like to acknowledge Shuangni Yang for preparation of histopathology slides. The work of author STE was supported by the Translational and Experimental Skin Testing & Immune Tracing Core of Northwestern University Skin Biology and Diseases Resource-Based Center, grant P30 AR075049. This study was supported by Departmental Research Funds, Department of Dermatology, Northwestern University.
Abbreviations
- CaHA
calcium hydroxylapatite
- STS
sodium thiosulfate
Footnotes
Human studies
The study was approved by the Northwestern University Institutional Review Board, and all participants provided written informed consent.
Conflict of Interest
The authors state no conflict of interest.
Data availability statement
Datasets related to this article can be found at https://doi.org/10.17632/td9kvjvg9g.1, an open-source online data repository hosted at Mendeley Data (Merkel et al., 2022).
References
- Alam M, Kakar R, Nodzenski M, Ibrahim O, Disphanurat W, Bolotin D, et al. Multicenter prospective cohort study of the incidence of adverse events associated with cosmetic dermatologic procedures: lasers, energy devices, and injectable neurotoxins and fillers [published correction appears in JAMA Dermatol 2015;151:236]. JAMA Dermatol 2015;151:271e7. [DOI] [PubMed] [Google Scholar]
- Danysz W, Nowag B, Hengl T, Kreymerman P, Furne C, Madeuf E, et al. Can sodium thiosulfate act as a reversal agent for calcium hydroxylapatite filler? Results of a preclinical study. Clin Cosmet Investig Dermatol 2020;13: 1059e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel CK, Nguyen ED, Dobry AS, Baker O, Garza-Mayers AC, Ko LN, et al. Assessment of outcomes of calciphylaxis lesions treated with intralesional sodium thiosulfate. J Am Acad Dermatol 2021;85:770e3. [DOI] [PubMed] [Google Scholar]
- Gunasekera NS, Maniar LEG, Lezcano C, Laga AC, Merola JF. Intralesional sodium thio- sulfate treatment for calcinosis cutis in the setting of lupus panniculitis. JAMA Dermatol 2017;153:944e5. [DOI] [PubMed] [Google Scholar]
- Robinson DM. In vitro analysis of the degradation of calcium hydroxylapatite dermal filler: a proof-of-concept study. Dermatol Surg 2018;44(Suppl 1):S5e9. [DOI] [PubMed] [Google Scholar]
- Smith GP. Intradermal sodium thiosulfate for exophytic calcinosis cutis of connective tissue disease. J Am Acad Dermatol 2013;69: e146e7. [DOI] [PubMed] [Google Scholar]
- Strazzula L, Nigwekar SU, Steele D, Tsiaras W, Sise M, Bis S, et al. Intralesional sodium thio- sulfate for the treatment of calciphylaxis. JAMA Dermatol 2013;149:946e9. [DOI] [PubMed] [Google Scholar]
- Yankova M, Pavicic T, Frank K, Schenck TL, Beleznay K, Gavril DL, et al. Intraarterial degradation of calcium hydroxylapatite using sodium thiosulfate - an in vitro and cadaveric study. Aesthet Surg J 2021;41:NP226–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets related to this article can be found at https://doi.org/10.17632/td9kvjvg9g.1, an open-source online data repository hosted at Mendeley Data (Merkel et al., 2022).


