Abstract
There has been significant progress in the study of extracellular vesicles (EVs) since the 2017 American Gastroenterological Association-sponsored Freston conference Extracellular Vesicles: Biology, Translation and Clinical Application in GI Disorders. The burgeoning interest in this field stems from the increasing recognition that EVs represent an understudied form of cell-to-cell communication and contain cargo replete with biomarkers and therapeutic targets. This short review will highlight recent advances in the field with an emphasis on colorectal cancer (CRC). Following a short introduction to secreted particles, we will describe how our lab became interested in EVs, which led to refined methods of isolation and identification of two secreted nanoparticles. We will then summarize the cargo found in small (s)EVs released from CRC cells and other cells in the tumor microenvironment (TME), as well as those found in the circulation of CRC patients. Finally, we will consider the continuing challenges and future opportunities in this rapidly evolving field.
Keywords: Extracellular Vesicles, Exosomes, Colorectal Cancer, Tumor Microenvironment, Cancer Biomarkers, Neutrophils, Cancer-Associated Fibroblasts, Macrophages, Microbiome
EVs as a class of secreted particles
EVs are lipid bilayer-enclosed vesicles derived from the cell membrane either through direct budding or through cell surface fusion of an endosomally-derived multivesicular body (MVB)1. Figure 1 depicts several specialized classes of EVs, as well as other categories of secreted mediators like lipoproteins and two recently described nanoparticles, exomeres and supermeres2–4. While many of these particles have been studied for decades, exomeres and supermeres are newly discovered amembranous nanoparticles of unknown biogenesis that are smaller than extracellular vesicles with distinct cargo and functional properties3–4. There are many different ways in which EVs are formed: apoptotic bodies bleb from the plasma membrane during cell death processes, microvesicles bud outward from the plasma membrane, and exosomes arise from inward invagination and pinching off within a subclass of late endosomes to form multiple vesicles within an MVB2, 5. In the process of this inward budding, the topology of transmembrane proteins in these intraluminal vesicles is reoriented with the ectodomain facing outwards6. MVBs can fuse with the plasma membrane rather than lysosomes, releasing their cargo in a signaling-competent manner with the extracellular domain facing the extracellular environment and cytoplasmic components enclosed within the exosome7. Classical exosomes have a distinct set of commonly associated proteins reflecting their endosomal-linked biogenesis, including tetraspanins such as CD63 and CD81, endosomal sorting complexes required for transport (ESCRT)-related proteins like TSG101, and scaffold proteins like syntenin-1 that are less typical of other types of EVs or nanoparticles1, 4, 8–10. It is important to note that exosomes represent only a subset of EVs. Given the heterogeneity of EVs, the International Society of Extracellular Vesicles currently recommends the terms small (s) and large (l)EVs, which have a diameter of < 200 nm and > 200 nm, respectively1. For this review, we will focus on sEVs and have chosen to use the term exosomes for sEVs that contain tetraspanins and/or other well-accepted exosomal markers. In general, EVs, regardless of their cargo or biogenesis, are released from cells and can interact locally with other cells and travel to distant sites to impart cellular changes.
Figure 1. Schematic of secreted particles with typical size ranges.
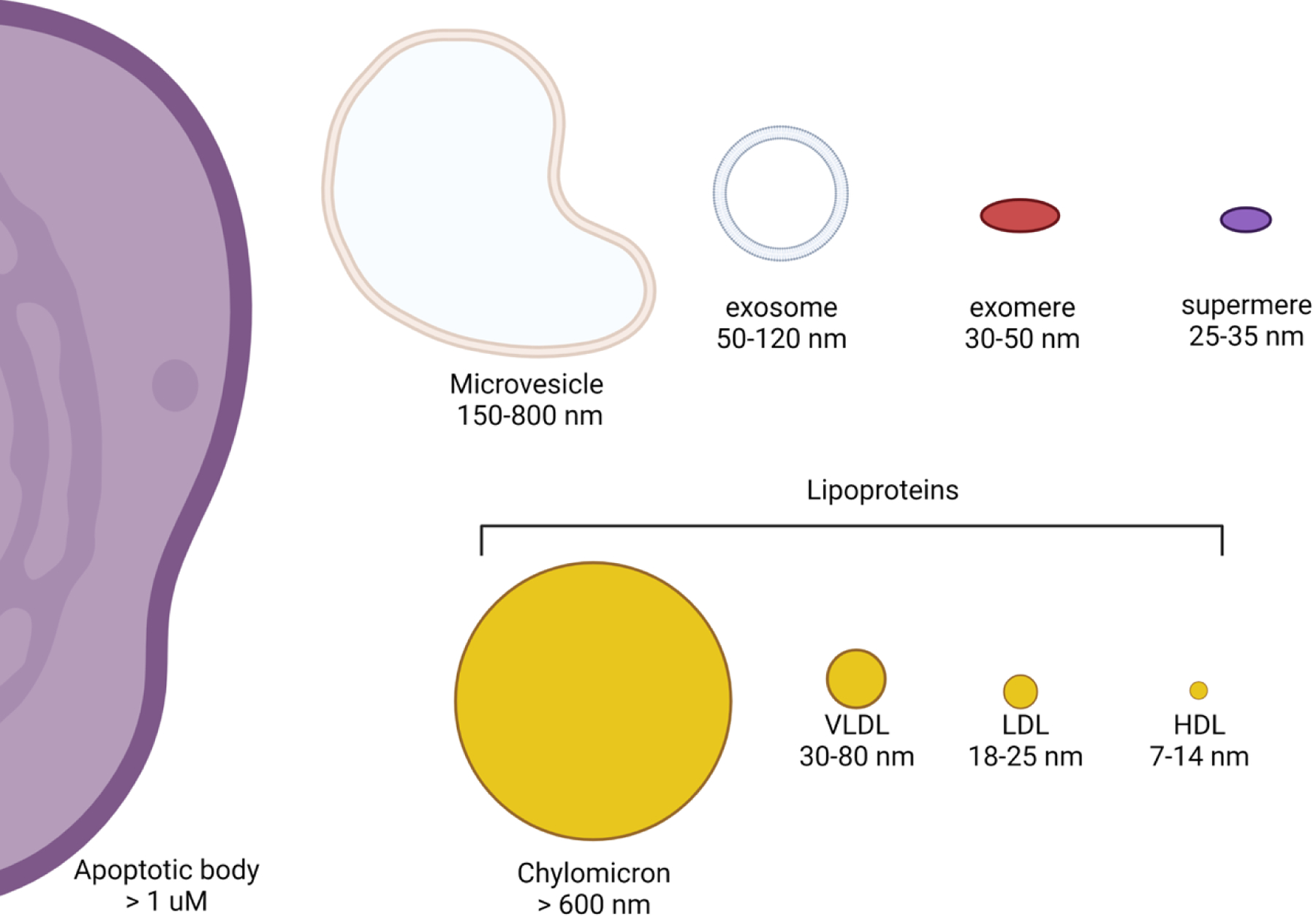
Extracellular vesicles have been classified by their origin and size into apoptotic bodies, microvesicles, and exosomes. Additional secreted particles include lipoproteins and two recently described amembranous nanoparticles, exomeres and supermeres.
Our entrée into the EV field and refinement of isolation methods
Our initial interest in EVs arose from our long-standing studies of EGFR ligand trafficking in polarized epithelial cells11–16. We discovered that these type I transmembrane ligands are not only trafficked to the plasma membrane but are also packaged in exosomes, building on previous work that identified EGFR as an EV cargo17. Amphiregulin (AREG), one of the seven mammalian EGFR ligands, shows a propensity for being endocytosed and packaged in intraluminal vesicles within MVBs with its ectodomain facing outwards. Signaling competent, AREG-containing vesicles are released during MVB-plasma membrane fusion. We found that AREG-containing exosomes were much more potent in enhancing invasiveness of recipient breast cancer cells than equivalent amounts of recombinant AREG18. The effects of these exosomes were mediated, at least in part, by binding to EGFR, leading us to introduce exosomal targeted receptor activation (ExTRAcrine) as a new mode of EGFR ligand signaling.
After this initial foray into the EV field, a major focus became the impact of mutant KRAS on the sEV cargo produced by CRC cells. In a series of reports, we systematically examined the sEV protein and RNA content that was differentially produced by KRASG13D DLD-1 cells and their isogenic derivatives engineered to express only a single wild-type KRAS allele (DKs-8) or a single mutant KRAS allele (DKO-1)19–23. In contrast to mutant KRAS cells, DKs-8 cells no longer exhibit a transformed phenotype. We were able to detect mutant KRAS in sEVs isolated from DLD-1 and DKO-1 cells and confirmed that transfer of mutant KRAS via sEVs could lead to transformation of DKs-8 and rat intestinal epithelial cells19.
We subsequently reported that the glucose transporter GLUT-1 (SLC2A1) was enriched in sEVs isolated from DKO-1 and DLD-1 cells, but not DKs-8 cells, and was functional in these mutant KRAS-derived sEVs as they were able to take up 18F-fluorodeoxyglucose (FDG)20. Addition of these sEVs to wild-type KRAS cells led to increased aerobic glycolysis and growth in the recipient cells. More recent reports highlight exosomal circular RNAs, which will be elaborated on as potential biomarkers in this review, as mediating enhanced glycolysis in CRC24, 25. Our results identified a cell-nonautonomous effect of mutant KRAS, suggesting that mutant KRAS-derived sEVs may “seed the soil” by altering the composition and metabolis state of cells within the tumor microenvironment. This work builds upon the pioneering contributions of Richard Simpson in the proteomic analysis of CRC EVs26. Another important contribution by Dr. Simpson and his colleague Suresh Mathivanan was the creation of early searchable EV databases, EVpedia and ExoCarta, as well as Vesiclepedia, which is a web-based repository of proteins, RNA, lipids and metabolites identified in EVs that acts as a critical resource for the EV community28–30.
As the work matured, we began to develop more refined methods of EV isolation. A wide range of isolation options exist, including ultracentrifugation (UC) with or without a density gradient, size-exclusion chromatography (SEC), immunoaffinity capture methods, microfluidic isolation, high-performance liquid chromatography (HPLC), and polymer precipitation31–33. In 2019, our lab described rigorous methods for exosome isolation from non-vesicular material by generating a high-speed ultracentrifugation exosomal pellet (EV-P), loading it at the bottom of an optimized iodixanol gradient, performing another high-speed ultracentrifugation step, and collecting individual fractions for large scale proteomic and RNA profiling8. This work complemented and extended proteomic studies performed by Clotilde Théry’s group10. Our ongoing refinement of methods of isolation and parsing of particles led to our characterization of exomeres and supermeres3, 4. David Lyden’s group was the first to identify exomeres using a costly, low-yield asymmetric flow field fractionation (AF4) method; however, we found exomeres could be isolated by simply performing high-speed ultercentrifugation of the supernatant from the EV-P34. We recently identified yet another amembranous nanoparticle, the supermere, by performing a higher speed ultracentifugation of the supernatant from the exomere pellet4. We conducted a comprehensive protein and RNA profiling of supermeres, exomeres and sEVs from a CRC cell line, DiFi, along with correlating these findings to other cancer cell lines and human plasma4. While the field is consistently challenged by the heterogeneity intrinsic to EVs and other particles, we have made important strides towards characterization of these secreted mediators in CRC that will be useful for further functional studies as well as biomarker discovery.
EVs as a source of CRC biomarkers
With advances in isolation and characterization, a shift in thinking has occurred from DNA being in exosomes to its presence on the outside of EVs or in non-vesicular fractions8. We do not detect DNA within exosomes. Rather we have found that amphisomes, representing a fusion of autophagasomes and MVBs, are a source of extracellular dsDNA8. Major sources of circulating DNA biomarkers are thought to be dying cancer cells releasing naked DNA or circulating tumor cells themselves. However, a recent report found mutant KRAS and BRAF DNA on the outside of exosomes isolated from the plasma of CRC patients whose tumors harbor these mutations35. The amount of either wild-type or mutant KRAS associated with exosomes was reported to be a prognostic marker for CRC36.
KRAS protein also can affect micro (mi)RNA sorting into exosomes with miR-100 being preferentially packaged and released in exosomes from mutant KRAS CRC cells 21. Other reported miRNA biomarkers span from miR-17–5p and 125b-5p being associated with liver metastasis to a combination of let7b-3p and miR-145–3p predicting early-stage CRC37, 38. A recent report used a bioinformatics approach of TCGA data to determine a CRC-specific, exosomal miRNA-mRNA network, and found that five miRNA hubs, encompassing miRs 141, 126, 139, 29c, and 423, were predictive for CRC39. Along with miRNAs, circular RNAs (circRNAs) are attracting the attention of the EV research community. CircRNAs are single-stranded RNAs that form a closed loop and have gene regulatory roles. In CRC in particular, ciRs-7 has been shown to be a biomarker and therapeutic target, acting as a sponge for a tumor-suppressing miRNAs, leading to EGFR activation and association with poor patient survival40. Circ-PNN, circ_0005963, and circ_0000338 have been shown to be EV-related circRNA biomarkers with the first being isolated from plasma exosomes and the latter two having a role in chemoresistance24, 41, 42. Important considerations for RNAs as functional biomarkers include how many molecules are present in an EV, what quantity is needed to be functionally active, are the necessary RNA-binding proteins present, and how can isolation and quantification methods be improved to reduce the time, cost, and processing for clinical translation.
In addition to nucleic acids serving as EV biomarkers in CRC, a number of groups have examined the utility of EV-associated proteins for this purpose. Hakho Lee and co-workers have developed a technique that uses an integrated magneto-electrochemical device to capture and analyze EVs from 20 microliters of plasma within an hour43. When this approach was applied to a bank of CRC plasma samples, it revealed that multi-marker combinations of EGFR, EpCAM, GPA33 and CD24 led to a 98% accuracy in diagnosing CRC43. In our own studies, we have identified DPEP1, a dipeptidase normally expressed in the kidney, as a potential CRC biomarker44. It is the most abundant protein in sEVs from a CRC line, DiFi, and is highly enriched in a subset of exosomes sorted on the basis of CD81 and EGFR positivity by fluorescence-activated vesicle sorting (FAVS)4. In addition to EGFR, this subset also contained EpCAM and GPA33, as well as CEACAM5 and CD73, highlighting an overlap with Hakho Lee’s group that fosters confidence that these proteins may be relevant CRC biomarkers4. Using a clinically well-annotated tissue microarray of over 150 CRC samples, we found that over 70% of CRCs express DPEP1 and that diffuse staining portended a worse overall and progression-free survival4. By examining DPEP1/CEACAM5 double-positive vesicles in plasma samples from three CRC patients and three healthy individuals, we have preliminary evidence that EV-bound DPEP1 could be a clinically relevant biomarker4. Figure 2 is a cartoon of a CRC exosome with classical tetraspainins and recently reported potential protein and RNA CRC biomarkers and Table 1 annotates their reported functions45. One striking feature is that a number of these proteins are GPI-linked, which make them attractive biomarkers based on their efficient sorting into exosomes, cell surface localization, and overexpression in CRC46. Isolating EVs from a biofluid is advantageous to simply profiling the overall patient sample since proteins such as albumin or other contaminants are so abundant that they can frustrate one’s ability to identify clinically relevant biomarkers. EVs also have other markers that can help determine the cell type of origin and can increase overall stability of the biomarker. Comparing clinical samples to validated reference materials and an adherence to accepted purification protocols will be necessary for clinical translation of basic research findings. Overall, profiling EVs is a tractable method for minimally invasive liquid biopsies, and further parsing of EV populations offers promise as a strategy to identify potential diagnostic, prognostic and predictive biomarkers.
Figure 2. Representative exosome with classical tetraspanins and cargo identified as potential CRC biomarkers from the recent literature.
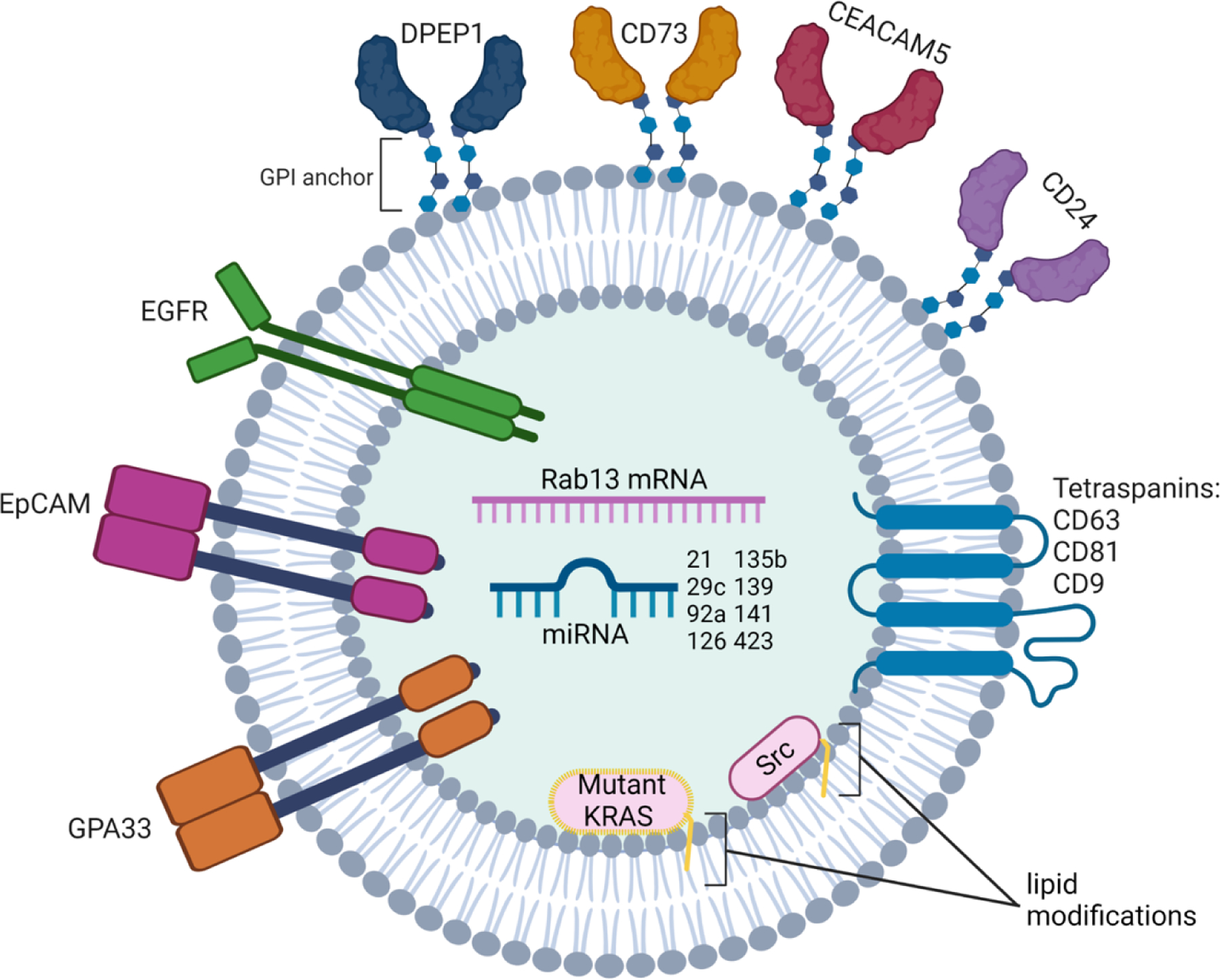
Tetraspanins listed are in order of their specificity to classical exosomes13. Lipid modifications depicted can be farnesylation and geranylgeranylation for KRAS and myristoylation and palmitoylation for Src.
Table 1.
Colorectal Biomarkers Found in EVs and Their Disease Relevance
| Biomarker category | Biomarker | Source | EV Isolation Method | Function in CRC* | References |
|---|---|---|---|---|---|
| Protein | GPA33 | DiFi cells, human CRC plasma | Ultracentrifugation and FAVS | barrier function, biomarker | 4,50 |
| EpCAM | DiFi cells, human CRC plasma | Ultracentrifugation and FAVS | proliferation, migration, EMT, cancer stemness | 4,51 | |
| EGFR | DiFi cells, human CRC plasma | Ultracentrifugation and FAVS | proliferation, invasion, metastasis | 4, 52 | |
| DPEP1 | DiFi cells, human CRC plasma | Ultracentrifugation and FAVS | proliferation, metastasis | 4, 53, 54 | |
| CD73 | DiFi cells | Ultracentrifugation and FAVS | immunosuppression, metastasis | 4, 55 | |
| CEACAM5 | DiFi cells, human CRC plasma | Ultracentrifugation and FAVS | blocks differentiation, inhibits apoptosis | 4, 56 | |
| CD24 | human CRC plasma | Ultracentrifugation and HiMEX | proliferation, invasion | 43, 57 | |
| Mutant KRAS | DKO-1 cells, DLD-1 cells | Ultracentrifugation | proliferation, cetuximab resistance | 19, 20, 58 | |
| Src | HT29 cells, HCT116 cells | Ultracentrifugation | proliferation, tumor formation | 59 | |
| miRNA | miR-21 | LS174, human CRC plasma | Ultracentrifugation | proliferation, invasion, 5-FU resistance | 38, 60 |
| miR-29c | human CRC plasma | Ultracentrifugation | inhibits EMT, migration, invasion | 39, 61 | |
| miR-92a | human CRC plasma | Ultracentrifugation | proliferation, invasion migration | 39, 62 | |
| miR-126 | human CRC plasma | Ultracentrifugation | inhibits proliferation, invasion, and migration | 39, 63 | |
| miR-135b | human CRC plasma | Ultracentrifugation and exosome precipitation | proliferation, inhibits apoptosis, chemoresistance | 64, 65 | |
| miR-139 | human CRC plasma | Ultracentrifugation | inhibits EMT, enhances drug sensitivity, inhibits proliferation and invasion | 38, 66 | |
| miR-141 | human CRC plasma | Ultracentrifugation | reduces migration and metastasis | 39, 67 | |
| miR-423 | human CRC plasma | Ultracentifugation | cell growth | 39, 68 | |
| mRNA | Rab13 | DKO-1 cells | Density-gradient ultracentrifugation | regulates sEV secretion | 22 |
| circRNA | circ-PNN | human CRC plasma | Exoquick exosome precipitation | no reported function | 41 |
| circ_0000338 | SW480 cells, HCT116 cells, human CRC plasma | Ultracentrifugation | 5-FU resistance | 42 | |
| DNA | mutant KRAS (outside surface) | human CRC plasma | Ultracentrifugation and exosome precipitation | production of mutant KRAS protein | 35 |
| mutant BRAF (outside surface) | human CRC plasma | Ultracentrifugation and exosome precipitation | production of mutant BRAF protein | 35 |
Functions in CRC are based on cellular function and not consequences of EV transfer
Cancer-promoting properties of CRC EVs
EVs are not only an indicator of the state of a cell, but also functional entities that can impart cellular changes on recipient cells. Cargo transfer, cell-EV surface interactions to activate signaling cascades, and EV-mediated decoy mechanisms are all processes that can promote cancer. CRC cells can release EVs into the tumor microenvironment to modulate themselves or other stromal components for cancer promotion and progression47. Typically, cancer cells secrete more EVs than normal cells, and in CRC a variety of factors contribute to altered secretion patterns48. Activated Wnt signaling through loss-of-function mutations in APC, as well as collagen deposition, increases CRC sEV release49. RAB27B, a regulator of the late endocytic pathway, has been implicated as a downstream target of Wnt signaling that promotes EV secretion as well as SNAP23, a component of the SNARE complex69, 70. Wnt signaling not only stimulates EV release but also can be activated by transferred cargo. Mutant β-catenin was transferred by CRC EVs to recipient cells, leading to nuclear localization of β-catenin and activated Wnt signaling27. This transfer led to increased tumor burden, highlighting potential resistance mechanisms and sEV-mediated tumor heterogeneity27.
Another important signaling hub in CRC is initiated by EGFR activation71. Regulators of EGFR ligand sorting into EVs may be clinically relevant as this could activate EGFR signaling throughout a tumor. A recent study discovered that loss of tetraspanin 6 (Tspan6) increased packaging of TGF-α, an EGFR ligand, into exosomes, resulting in increased EGFR signaling72. The mechanism involves syntenin-1, a common exosome marker, linking Tspan6 to TGF-α72. High levels of Tspan6 in CRC patients led to a better response to cetuximab, an EGFR neutralizing monoclonal antibody, and portended a better prognosis for mutant KRAS patients72. If confirmed, these findings have important treatment implications since individuals with mutant KRAS CRC are excluded from receiving anti-EGFR monoclonal antibodies.
Yet another characteristic of most CRCs is an immunosuppressive immune environment. Roughly 15% of CRC patients have a deficient mismatch repair system and exhibit microsatellite instability (MSI), resulting in an influx of immune cells that recognize neoantigens, although the majority of CRCs are microsatellite stable (MSS) with an overall immunosuppressive phenotype73. EVs, particularly exosomes, are critical contributors to these TME differences as serum exosomal miR-146a levels are correlated with decreased CD8+ T-cell numbers and increased neutrophil counts69. CD8+ T-cell exclusion is associated with a worse prognosis, and neutrophil infiltration is emerging as a potential marker of CRC progression74. A handful of studies have equated neutrophil infiltration, and in some cases subsequent lymphocyte exclusion, with the onset or progression of CRC 75, 76. In terms of mechanism, neutrophils can secrete MMP9, which, in turn, activates TGF-β to suppress T-cell proliferation77. While the connection between neutrophils and tumor progression is beginning to take shape, much less is known about how neutrophil EVs contribute. One model involves exosomes from CRC stem cells migrating to the bone marrow where RNA transfer led to prolonged neutrophil survival and conversion to a pro-tumorigenic phenotype78. Yet another study discovered that exosomes from mutant KRAS CRC cells promote IL-8 and Neutrophil Extracellular Trap (NET) production in neutrophils that subsequently increases CRC proliferation, migration, and invasion79.
CRC EVs not only modulate neutrophil behavior, but also fibroblast and macrophage function. CRC sEVs have been shown to educate fibroblasts towards a cancer-associated fibroblast (CAF) phenotype through enhanced Rho-Fak signaling80. Another group reported that CRC EVs transferred integrin beta-like 1(ITGBL1) to liver and lung fibroblasts, leading to their activation and NFκB-mediated release of pro-inflammatory cytokines for metastatic niche formation81. These investigators detected ITGBL1 in the EV-P so further work is needed to confirm that ITGBL1 is in EVs rather than non-vesicular material; however, they did confirm the previous observation by David Lyden’s group that plasma CRC EVs enriched for ITGαv/ITGβ5 and ITGα6/ITGβ4 or ITGα6/ITGβ1 correlated with liver and lung metastasis, respectively82. CRC EVs can also induce pro-inflammatory cytokine production and PD-L1 induction in macrophages83. In summation, CRC EVs can travel throughout circulation to interact with a variety of cell types to promote tumor progression.
Stromal cell-derived EVs promotion of CRC
Along with cancer cells releasing EVs for tumor progression, cells within the TME can also release EVs. In this review, we have chosen to focus on well-studied components that contribute to CRC such as CAFs and macrophages, as well as emerging players such as EVs derived from neutrophils and bacteria. Although CAFs are a heterogenous population of cells with no one distinct marker, a number of studies have established their ability to promote CRC84. This also applies to CAF-derived exosomes as one study attributes metastatic capacity, EMT, and oxaliplatin and 5-fluoroacil resistance to a single exosomal miRNA, miR-92a-3p85. Evidence for sEVs producing these cancer-promoting changes is supported by studies showing that sEVs derived from CAFs with lncRNA H19 enhance CRC stemness and chemoresistance, and CAF sEVs containing circEIF3K and miR-224–5p increase invasion and proliferation of CRC cells86–88. AREG, which we have found in CRC exosomes, is present in CAF sEVs and can increase CRC proliferation, giving an alternative source of an EGFR ligand from a stromal component18, 89.
Another cell type known to have roles in CRC is the macrophage90. Recent studies regarding how macrophage sEVs impact CRC characteristics are focused on miRNA cargo and the M2 pro-tumorigenic subset91, 92. miR-21–5p and miR-155–5p from M2 macrophage-derived exosomes regulate the levels of BRG1, a chromatin remodeling component, to increase metastatic capacity of recipient CRC cells, suggesting that stromal-derived exosomes influence CRC cell plasticity91. Another miRNA, miR-183–5p, was reported to be upregulated and released in exosomes from M2 macrophages92. This miRNA was reported to increase migration, invasion, colony formation, and reduce apoptosis in vitro as well as increase tumor volume and number of lung metastasis in vivo92.
A recent study reported that yet another stromal component, activated neutrophils, release exosomes enriched in neutrophil elastase (NE), a serine protease with broad substrate specificity93. Exosomal NE was resistant to inhibition and could degrade extracellular matrix in the lung, triggering emphysema93. It is intriguing to consider how activated neutrophils might act in a similar way in CRC to degrade matrix, leading to tumor cell invasion and metastasis. Another study described a feedforward loop whereby mRNA for the transcription factor salmonella pathogenicity island 1 (SPI1/PU.1) is released by neutrophil sEVs and taken up by CRC cells where it works in concert with SPI1-related protein (SPIB) to increase expression of hexose kinase 2 (HK2) and phosphoglycerate kinase 1 (PGK1)94. This results in aerobic glycolysis with increased lactate production by CRC cells that drives neutrophils to become tumor-promoting94.
Bacteria such as E. coli, Fusobacterium nucleatum, and Bacteroides fragilis have been associated with CRC incidence, presumed to be due to inflammation and oxidative stress95. Mechanisms underlying bacterial tumor promotion are an active area of investigation and might include a role for bacterial EVs. EVs released by gut bacteria differ in composition and diversity between CRC and normal patients, as well as in early and late-stage disease with EVs from Firmicutes and Proteobacteria phyla being significantly changed96, 97. Not only does CRC impact the microbiome, but bacterial vesicles can impact CRC progression. Fusobacterium nucleatum releases outer membrane vesicles that stimulate proinflammatory cytokine production in colonic epithelial cells, which could contribute to a pro-tumorigenic microenvironment98. Bacterial EVs have been reported since the 1960s, but true characterization and functional studies have lagged due to difficulties in purification and a lack in consensus for common identifying markers99, 100. Expanding the study of EVs to include the tumor microenvironment and microbiome will enhance our understanding of how EVs promote CRC.
Challenges and Future Opportunities
The EV field continues to be confronted by issues of isolation, characterization, and nomenclature. One outstanding issue within the field is the concept of physiological relevant concentrations. While there is no consensus about reporting absolute particle numbers or protein amount when adding EVs to cells, a larger issue looms. Hundreds of micrograms of EV protein are often used to observe effects in vivo, raising the concern that non-phyiological amounts of material are being introduced101, 102. Studies are needed to determine the local concentrations of EVs released from cells and the concentrations that exist in circulation to better inform both in vitro and in vivo experiments. A variety of approaches are being taken to address these issues. From bioluminescence resonance energy transfer (BRET) imaging to pH-sensitive reporters coupled with dynamic correlative light-electron microscopy and TIRF to defining optical sigantures of EVs using label-free methods, technological innovations are being combined to address concentration concerns. It is anticipated that progress will be made as the field continues to grapple with the definition of a physiologically-relevant amount of EVs103–105.
Use of the EV pellet, which contains non-vesicular material, has been used to identify a variety of extracellular biomarkers in cancer, but this broad stroke approach obscures relevant biomarkers that are seen only in a subpopulation of EVs or nanoparticles106. By only analyzing top proteomic hits from a crude starting material, rare proteins that are enriched in specific subclasses of extracellular particles may be lost in the milieu. We have demonstrated by FAVS that parsing of sEVs into further subsets is of value as it not only informs the cell biology of cargo that are sorted together but also allows for identification of new biomarkers previously unrecognized as important for the initiation and progression of CRC4, 107. These biomarkers though require rigorous validation for clinical translation. Our present labor-intensive and time-consuming methods do not lend themselves to easy scaling. A biomarker confirmation process specific to EVs should include verifying the biomarker in the tumor in comparison to normal samples and other inflammatory non-neoplastic states, as well as its presence in circulating EVs, and its reduction in plasma levels post-resection or after an effective therapeutic intervention108. This validation process also hints at the clinical application of biomarkers for assessing CRC disease management, where a blood draw followed by isolation of the biomarker-containing EV population could be used to track the course of disease or response to therapy. Strategies that reduce the amount of starting material needed or specifically enrich for a certain biomarker may lead to improved disease monitoring.
Further technological developments and refinements, as demonstrated by the isolation of exomeres first by AF4, but eventually by our group by a simple sequential centrifugation spin of the supernatant from an EV-P, will lead to parsing of the heterogeneity present in EV and nanoparticle classes for accurate subclass categorization3, 34. Another improvement is the use of microfluidics devices that require as little as 20 microliters of plasma sample in order to quantify miRNAs in sEVs109. Since many commercial flow cytometers are not designed to detect sEVs and related nanoparticles, the focus of round 2 of the Extracellular RNA Communication Consortium (ERCC), of which we are a part, is to design EV-centric devices, such as a flow cytometer designed for the purpose of analyzing and sorting EVs. Our own studies have shown that further refinements often lead to reassessing carrier contents as we have recently shown that supermeres, and not exomeres, as originally reported by Lyden and our group, are a more abundant source of metabolic cargo and glycolytic enzymes3, 4, 34. Furthermore, at least in some contexts, superemeres contain a higher percentage of secreted RNAs including miRNAs than do EVs or exomeres, highlighting the possibility that effects ascribed to secreted RNAs will be greater when measuring their function within supermeres4. As this field evolves and matures, further refinements in nomenclature and parsing of complex EV fractions will lead to a clearer analysis when attributing function to a particular EV class.
Acknowledgements
We apologize in advance for work we have not cited in this brief review. We thank Jeffrey L Franklin for helpful discussions. Figures were created with Biorender.com. SEG is supported by the National Science Foundation Graduate Research Fellowship Program 1937963 and National Cancer Institute Training Grant CA 009582. RJC is supported by NCI R35 CA197570, UH3 CA241685, P01 CA229123 and P50 CA236733. We acknowledge the generous support of the Nicholas Tierney Memorial GI Cancer Fund.
References
- 1.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zijlstra A, Di Vizio D. Size matters in nanoscale communication. Nature Cell Biology 2018;20:228–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Q, Higginbotham JN, Jeppesen DK, et al. Transfer of Functional Cargo in Exomeres. Cell Rep 2019;27:940–954 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, Jeppesen DK, Higginbotham JN, et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nature Cell Biology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caruso S, Poon IKH. Apoptotic Cell-Derived Extracellular Vesicles: More Than Just Debris. Frontiers in Immunology 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couch Y, Buzas EI, Vizio DD, et al. A brief history of nearly EV-erything - The rise and rise of extracellular vesicles. J Extracell Vesicles 2021;10:e12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurung S, Perocheau D, Touramanidou L, et al. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal 2021;19:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of Exosome Composition. Cell 2019;177:428–445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathieu M, Nevo N, Jouve M, et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun 2021;12:4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 2016;113:E968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damstrup L, Kuwada SK, Dempsey PJ, et al. Amphiregulin acts as an autocrine growth factor in two human polarizing colon cancer lines that exhibit domain selective EGF receptor mitogenesis. British Journal of Cancer 1999;80:1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempsey PJ, Meise KS, Coffey RJ. Basolateral sorting of transforming growth factor-α precursor in polarized epithelial cells: characterization of cytoplasmic domain determinants. Experimental Cell Research 2003;285:159–174. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Franklin JL, Graves-Deal R, et al. Myristoylated Naked2 escorts transforming growth factor to the basolateral plasma membrane of polarized epithelial cells. Proceedings of the National Academy of Sciences 2004;101:5571–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C, Hao M, Cao Z, et al. Naked2 Acts as a Cargo Recognition and Targeting Protein to Ensure Proper Delivery and Fusion of TGF-α–containing Exocytic Vesicles at the Lower Lateral Membrane of Polarized MDCK Cells. Molecular Biology of the Cell 2007;18:3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gephart JD, Singh B, Higginbotham JN, et al. Identification of a novel mono-leucine basolateral sorting motif within the cytoplasmic domain of amphiregulin. Traffic 2011;12:1793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh B, Coffey RJ. Trafficking of Epidermal Growth Factor Receptor Ligands in Polarized Epithelial Cells. Annual Review of Physiology 2014;76:275–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanderson MP, Keller S, Alonso A, et al. Generation of novel, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via metalloprotease-dependent ectodomain shedding and exosome secretion. Journal of cellular biochemistry 2008;103:1783–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higginbotham JN, Demory Beckler M, Gephart JD, et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol 2011;21:779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demory Beckler M, Higginbotham JN, Franklin JL, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics 2013;12:343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Jeppesen DK, Higginbotham JN, et al. Mutant KRAS Exosomes Alter the Metabolic State of Recipient Colonic Epithelial Cells. Cellular and Molecular Gastroenterology and Hepatology 2018;5:627–629.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha DJ, Franklin JL, Dou Y, et al. KRAS-dependent sorting of miRNA to exosomes. Elife 2015;4:e07197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinger SA, Abner JJ, Franklin JL, et al. Rab13 regulates sEV secretion in mutant KRAS colorectal cancer cells. Scientific Reports 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abner JJ, Franklin JL, Clement MA, et al. Depletion of METTL3 alters cellular and extracellular levels of miRNAs containing m6A consensus sequences. Heliyon 2021;7:e08519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Zhang H, Yang H, et al. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Molecular Oncology 2020;14:539–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi B, Dai K, Yan Z, et al. Circular RNA PLCE1 promotes epithelial mesenchymal transformation, glycolysis in colorectal cancer and M2 polarization of tumor-associated macrophages. Bioengineered 2022;13:6243–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji H, Greening DW, Kapp EA, et al. Secretome-based proteomics reveals sulindac-modulated proteins released from colon cancer cells. Proteomics Clin Appl 2009;3:433–51. [DOI] [PubMed] [Google Scholar]
- 27.Kalra H, Gangoda L, Fonseka P, et al. Extracellular vesicles containing oncogenic mutant beta-catenin activate Wnt signalling pathway in the recipient cells. J Extracell Vesicles 2019;8:1690217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pathan M, Fonseka P, Chitti SV, et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res 2019;47:D516–D519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DK, Lee J, Kim SR, et al. EVpedia: a community web portal for extracellular vesicles research. Bioinformatics 2015;31:933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol 2016;428:688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Witwer KW, Buzas EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gardiner C, Vizio DD, Sahoo S, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. Journal of Extracellular Vesicles 2016;5:32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan K, Martin K, FitzGerald SP, et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep 2020;10:1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Freitas D, Kim HS, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 2018;20:332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galbiati S, Damin F, Brambilla D, et al. Small EVs-Associated DNA as Complementary Biomarker to Circulating Tumor DNA in Plasma of Metastatic Colorectal Cancer Patients. Pharmaceuticals (Basel) 2021;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucchetti D, Zurlo IV, Colella F, et al. Mutational status of plasma exosomal KRAS predicts outcome in patients with metastatic colorectal cancer. Sci Rep 2021;11:22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sur D, Balacescu L, Cainap SS, et al. Predictive Efficacy of MiR-125b-5p, MiR-17–5p, and MiR-185–5p in Liver Metastasis and Chemotherapy Response Among Advanced Stage Colorectal Cancer Patients. Front Oncol 2021;11:651380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Min L, Zhu S, Chen L, et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: a comparison with plasma total miRNAs. J Extracell Vesicles 2019;8:1643670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma J, Wang P, Huang L, et al. Bioinformatic analysis reveals an exosomal miRNA-mRNA network in colorectal cancer. BMC Med Genomics 2021;14:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weng W, Wei Q, Toden S, et al. Circular RNA ciRS-7—A Promising Prognostic Biomarker and a Potential Therapeutic Target in Colorectal Cancer. Clinical Cancer Research 2017;23:3918–3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie Y, Li J, Li P, et al. RNA-Seq Profiling of Serum Exosomal Circular RNAs Reveals Circ-PNN as a Potential Biomarker for Human Colorectal Cancer. Frontiers in Oncology 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hon KW, Ab-Mutalib NS, Abdullah NMA, et al. Extracellular Vesicle-derived circular RNAs confers chemoresistance in Colorectal cancer. Scientific Reports 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J, Park JS, Huang CH, et al. An integrated magneto-electrochemical device for the rapid profiling of tumour extracellular vesicles from blood plasma. Nat Biomed Eng 2021;5:678–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau A, Chung H, Komada T, et al. Renal immune surveillance and dipeptidase-1 contribute to contrast-induced acute kidney injury. J Clin Invest 2018;128:2894–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hikita T, Kuwahara A, Watanabe R, et al. Src in endosomal membranes promotes exosome secretion and tumor progression. Sci Rep 2019;9:3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Exosomes Vidal M. and GPI-anchored proteins: Judicious pairs for investigating biomarkers from body fluids. Advanced Drug Delivery Reviews 2020;161–162:110–123. [DOI] [PubMed] [Google Scholar]
- 47.Sheehan C, D’Souza-Schorey C. Tumor-derived extracellular vesicles: molecular parcels that enable regulation of the immune response in cancer. J Cell Sci 2019;132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bebelman MP, Janssen E, Pegtel DM, et al. The forces driving cancer extracellular vesicle secretion. Neoplasia 2021;23:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szvicsek Z, Oszvald A, Szabo L, et al. Extracellular vesicle release from intestinal organoids is modulated by Apc mutation and other colorectal cancer progression factors. Cell Mol Life Sci 2019;76:2463–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams BB, Tebbutt NC, Buchert M, et al. Glycoprotein A33 deficiency: a new model of impaired intestinal epithelial barrier function and inflammatory disease. Disease Models & Mechanisms 2015;8:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boesch M, Spizzo G, Seeber A. Concise Review: Aggressive Colorectal Cancer: Role of Epithelial Cell Adhesion Molecule in Cancer Stem Cells and Epithelial-to-Mesenchymal Transition. STEM CELLS Translational Medicine 2018;7:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koveitypour Z, Panahi F, Vakilian M, et al. Signaling pathways involved in colorectal cancer progression. Cell & Bioscience 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park SY, Lee SJ, Cho HJ, et al. Dehydropeptidase 1 promotes metastasis through regulation of E-cadherin expression in colon cancer. Oncotarget 2016;7:9501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Q, Deng J, Yang C, et al. DPEP1 promotes the proliferation of colon cancer cells via the DPEP1/MYC feedback loop regulation. Biochemical and Biophysical Research Communications 2020;532:520–527. [DOI] [PubMed] [Google Scholar]
- 55.Harvey JB, Phan LH, Villarreal OE, et al. CD73’s Potential as an Immunotherapy Target in Gastrointestinal Cancers. Frontiers in Immunology 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan CHF, Stanners CP. Recent Advances in the Tumour Biology of the GPI-Anchored Carcinoembryonic Antigen Family Members CEACAM5 and CEACAM6. Current Oncology 2007;14:70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su N, Peng L, Xia B, et al. Lyn is involved in CD24-induced ERK1/2 activation in colorectal cancer. Molecular Cancer 2012;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng M, Zhong K, Jiang T, et al. The current understanding on the impact of KRAS on colorectal cancer. Biomedicine & Pharmacotherapy 2021;140:111717. [DOI] [PubMed] [Google Scholar]
- 59.Hikita T, Kuwahara A, Watanabe R, et al. Src in endosomal membranes promotes exosome secretion and tumor progression. Scientific Reports 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun L-H, Tian D, Yang Z-C, et al. Exosomal miR-21 promotes proliferation, invasion and therapy resistance of colon adenocarcinoma cells through its target PDCD4. Scientific Reports 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang JX, Mai SJ, Huang XX, et al. MiR-29c mediates epithelial-to-mesenchymal transition in human colorectal carcinoma metastasis via PTP4A and GNA13 regulation of β-catenin signaling. Annals of Oncology 2014;25:2196–2204. [DOI] [PubMed] [Google Scholar]
- 62.Chen E, Li Q, Wang H, et al. MiR-92a promotes tumorigenesis of colorectal cancer, a transcriptomic and functional based study. Biomedicine & Pharmacotherapy 2018;106:1370–1377. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Zhou Y, Feng X, et al. MicroRNA-126 functions as a tumor suppressor in colorectal cancer cells by targeting CXCR4 via the AKT and ERK1/2 signaling pathways. International Journal of Oncology 2014;44:203–210. [DOI] [PubMed] [Google Scholar]
- 64.Jin G, Liu Y, Zhang J, et al. A panel of serum exosomal microRNAs as predictive markers for chemoresistance in advanced colorectal cancer. Cancer Chemotherapy and Pharmacology 2019;84:315–325. [DOI] [PubMed] [Google Scholar]
- 65.Li J, Liang H, Bai M, et al. miR-135b Promotes Cancer Progression by Targeting Transforming Growth Factor Beta Receptor II (TGFBR2) in Colorectal Cancer. PLOS ONE 2015;10:e0130194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Q, Liang X, Wang Y, et al. miR-139–5p Inhibits the Epithelial-Mesenchymal Transition and Enhances the Chemotherapeutic Sensitivity of Colorectal Cancer Cells by Downregulating BCL2. Scientific Reports 2016;6:27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Long ZH, Bai ZG, Song JN, et al. miR-141 Inhibits Proliferation and Migration of Colorectal Cancer SW480 Cells. Anticancer Res 2017;37:4345–4352. [DOI] [PubMed] [Google Scholar]
- 68.Li H-T, Zhang H, Chen Y, et al. MiR-423–3p Enhances Cell Growth Through Inhibition of p21Cip1/Waf1 in Colorectal Cancer. Cellular Physiology and Biochemistry 2015;37:1044–1054. [DOI] [PubMed] [Google Scholar]
- 69.Cheng WC, Liao TT, Lin CC, et al. RAB27B-activated secretion of stem-like tumor exosomes delivers the biomarker microRNA-146a-5p, which promotes tumorigenesis and associates with an immunosuppressive tumor microenvironment in colorectal cancer. Int J Cancer 2019;145:2209–2224. [DOI] [PubMed] [Google Scholar]
- 70.Liu YD, Zhuang XP, Cai DL, et al. Let-7a regulates EV secretion and mitochondrial oxidative phosphorylation by targeting SNAP23 in colorectal cancer. J Exp Clin Cancer Res 2021;40:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther 2020;5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andrijes R, Hejmadi RK, Pugh M, et al. Tetraspanin 6 is a regulator of carcinogenesis in colorectal cancer. Proc Natl Acad Sci U S A 2021;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138:2073–2087 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Idos GE, Kwok J, Bonthala N, et al. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci Rep 2020;10:3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turhan VB, Unsal A, Gok HF, et al. Predictive Value of Preoperative Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratio in Determining the Stage of Colon Tumors. Cureus 2021;13:e18381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karaman H, Karaman A, Erden A, et al. Relationship between colonic polyp type and the neutrophil/ lymphocyte ratio as a biomarker. Asian Pac J Cancer Prev 2013;14:3159–61. [DOI] [PubMed] [Google Scholar]
- 77.Germann M, Zangger N, Sauvain MO, et al. Neutrophils suppress tumor-infiltrating T cells in colon cancer via matrix metalloproteinase-mediated activation of TGFbeta. EMBO Mol Med 2020;12:e10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hwang WL, Lan HY, Cheng WC, et al. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J Hematol Oncol 2019;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shang A, Gu C, Zhou C, et al. Exosomal KRAS mutation promotes the formation of tumor-associated neutrophil extracellular traps and causes deterioration of colorectal cancer by inducing IL-8 expression. Cell Commun Signal 2020;18:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clerici SP, Peppelenbosch M, Fuhler G, et al. Colorectal Cancer Cell-Derived Small Extracellular Vesicles Educate Human Fibroblasts to Stimulate Migratory Capacity. Front Cell Dev Biol 2021;9:696373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ji Q, Zhou L, Sui H, et al. Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nat Commun 2020;11:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoshino A, Costa-Silva B, Shen T-L, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pucci M, Raimondo S, Urzi O, et al. Tumor-Derived Small Extracellular Vesicles Induce Pro-Inflammatory Cytokine Expression and PD-L1 Regulation in M0 Macrophages via IL-6/STAT3 and TLR4 Signaling Pathways. Int J Mol Sci 2021;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gieniec KA, Butler LM, Worthley DL, et al. Cancer-associated fibroblasts-heroes or villains? Br J Cancer 2019;121:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu JL, Wang W, Lan XL, et al. CAFs secreted exosomes promote metastasis and chemotherapy resistance by enhancing cell stemness and epithelial-mesenchymal transition in colorectal cancer. Molecular Cancer 2019;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ren J, Ding L, Zhang D, et al. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics 2018;8:3932–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang K, Zhang J, Bao C. Exosomal circEIF3K from cancer-associated fibroblast promotes colorectal cancer (CRC) progression via miR-214/PD-L1 axis. BMC Cancer 2021;21:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng Y, Zeng J, Lin D, et al. Extracellular vesicles derived from cancer-associated fibroblast carries miR-224–5p targeting SLC4A4 to promote the proliferation, invasion and migration of colorectal cancer cells. Carcinogenesis 2021;42:1143–1153. [DOI] [PubMed] [Google Scholar]
- 89.Oszvald A, Szvicsek Z, Papai M, et al. Fibroblast-Derived Extracellular Vesicles Induce Colorectal Cancer Progression by Transmitting Amphiregulin. Front Cell Dev Biol 2020;8:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang H, Tian T, Zhang J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int J Mol Sci 2021;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lan J, Sun L, Xu F, et al. M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Research 2019;79:146–158. [DOI] [PubMed] [Google Scholar]
- 92.Zhang S, Li D, Zhao M, et al. Exosomal miR-183–5p Shuttled by M2 Polarized Tumor-Associated Macrophage Promotes the Development of Colon Cancer via Targeting THEM4 Mediated PI3K/AKT and NF-kappaB Pathways. Front Oncol 2021;11:672684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Genschmer KR, Russell DW, Lal C, et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019;176:113–126 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Wang X, Guo Y, et al. Therapeutic targeting of SPIB/SPI1-facilitated interplay of cancer cells and neutrophils inhibits aerobic glycolysis and cancer progression. Clin Transl Med 2021;11:e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng Y, Ling Z, Li L. The Intestinal Microbiota and Colorectal Cancer. Front Immunol 2020;11:615056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park J, Kim NE, Yoon H, et al. Fecal Microbiota and Gut Microbe-Derived Extracellular Vesicles in Colorectal Cancer. Front Oncol 2021;11:650026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim DJ, Yang J, Seo H, et al. Colorectal cancer diagnostic model utilizing metagenomic and metabolomic data of stool microbial extracellular vesicles. Sci Rep 2020;10:2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Engevik MA, Danhof HA, Ruan W, et al. Fusobacterium nucleatum Secretes Outer Membrane Vesicles and Promotes Intestinal Inflammation. mBio 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nahui Palomino RA, Vanpouille C, Costantini PE, et al. Microbiota-host communications: Bacterial extracellular vesicles as a common language. PLoS Pathog 2021;17:e1009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amatya SB, Salmi S, Kainulainen V, et al. Bacterial Extracellular Vesicles in Gastrointestinal Tract Cancer: An Unexplored Territory. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pinilla-Macua I, Grassart A, Duvvuri U, et al. EGF receptor signaling, phosphorylation, ubiquitylation and endocytosis in tumors in vivo. eLife 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiley HS. How low can you go? eLife 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hikita T, Miyata M, Watanabe R, et al. In vivo imaging of long-term accumulation of cancer-derived exosomes using a BRET-based reporter. Scientific Reports 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Verweij FJ, Bebelman MP, Jimenez CR, et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. Journal of Cell Biology 2018;217:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.You S, Barkalifa R, Chaney EJ, et al. Label-free visualization and characterization of extracellular vesicles in breast cancer. Proceedings of the National Academy of Sciences 2019;116:24012–24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoshino A, Kim HS, Bojmar L, et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020;182:1044–1061.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Higginbotham JN, Zhang Q, Jeppesen DK, et al. Identification and characterization of EGF receptor in individual exosomes by fluorescence-activated vesicle sorting. J Extracell Vesicles 2016;5:29254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Y, Wu T, Zhu Z, et al. An integrated workflow for biomarker development using microRNAs in extracellular vesicles for cancer precision medicine. Semin Cancer Biol 2021;74:134–155. [DOI] [PubMed] [Google Scholar]
- 109.Ramshani Z, Zhang C, Richards K, et al. Extracellular vesicle microRNA quantification from plasma using an integrated microfluidic device. Communications Biology 2019;2. [DOI] [PMC free article] [PubMed] [Google Scholar]


