Abstract
Achieving safe, independent, and efficient walking is a top priority for stroke survivors to enable quality of life and future health.
This narrative review explores the state of the science in walking recovery after stroke and potential for development.
The importance of targeting walking capacity and performance is explored in relation to individual stroke survivor gait recovery, applying a common language, measurement, classification, prediction, current and future intervention development, and healthcare delivery. Findings are summarised in a model of current and future stroke walking recovery research and a mission statement is set for researchers and clinicians to drive the field forward to improve the lives of stroke survivors and their carers.
Graphical Abstract
The importance of gait recovery
Walking problems are common after stroke experienced by approximately 80% of stroke survivors. (1) Achieving safe, independent, effective, and efficient walking are top priorities for stroke survivors to enable quality of life and future health. (2) Approximately one quarter of stroke survivors, however, do not achieve independent walking by three months post-stroke. (3) For those who do achieve independent walking, persistent gait abnormalities such as reduced gait speed and endurance and early discharge from rehabilitation before potential is met result in many stroke survivors not achieving community ambulation status. (4, 5)
Stroke survivors cite walking independence, speed, endurance, and quality are all important factors in walking recovery and as such should be considered in research and practice to ensure person-centred care. (2) Gait speed and endurance are important outcomes for stroke survivors (2) and are commonly measured in stroke research. Reduced gait speed is a predictor of all-cause mortality and is associated with lower survival rate, increased risk of falls and reduced quality of life. (6) Reduced endurance is also associated with poor health outcomes, (7) and increasing endurance can enable participation in the community following stroke. (8)
But measures of gait speed and endurance only capture walking capacity after stroke which does not always translate into performance or ‘real-world walking’. Stroke survivors report the importance of real-world walking in creating freedom from dependence which can lead to significant psychological and health benefits. Whilst gait speed and endurance are important measures of walking capacity after stroke, it is imperative that we also capture walking performance to enable insight into gait recovery in real-world settings to guide research and practice.
In this review we will comprehensively explore the state of the science and practice in walking recovery after stroke considering both walking capacity and performance. We will firstly define a common language for walking recovery, then translate this language into measurement, classification, and prediction. The concepts of walking capacity and performance will be explored in current guidelines, future interventions, and current healthcare models. The review will conclude by summarising current knowledge in a model and provide a mission statement for future research and practice to improve the lives of stroke survivors and their carers.
Defining walking recovery
To explore walking recovery after stroke and drive research and practice forward in this field it is essential that a common language is employed. This language is already defined by the World Health Organisation’s International Classification of Functioning, Disability and Health (ICF). The language of the ICF should be placed at the centre of walking recovery research as it encompasses aspects of gait recovery voiced by stroke survivors as important.
The ICF defines walking as “Moving along a surface on foot, step by step, so that one foot is always on the ground, such as when strolling, sauntering, walking forwards, backwards, or sideways” (9) and is classified under Activities and Participation within the ICF model. We argue it is essential to also consider two important ICF qualifiers; capacity and performance as both are important components of walking recovery. (10) Capacity is the ability to execute a task or action, at a given moment and is typically assessed in a structured, clinical or research laboratory environment. Performance describes what a person does in his/her current environment, including the societal context, and thus performance can be considered the real-life behaviour. Thus, adequate measurement tools, prediction models, and treatment guidelines should be considered by clinicians in relation to both walking capacity and performance.
The ICF allows the systematic consideration of the multiple factors that may influence an individual’s walking recovery after stroke to allow individualised care plans. This includes their health condition (e.g., stroke type, location, and size) and related sensorimotor, visual, balance, pain, mood and cognitive impairments.
The ICF also encompasses personal and environmental factors which are critical to consider during gait recovery. Personal factors such multimorbidity, pre stroke walking status, age, motivation, mood, and support systems impact upon walking recovery. (11) As can environmental factors which are often not considered in intervention development or rehabilitation, but impact upon walking performance. These environmental factors include weather, different terrains, carrying loads, negotiating roads and traffic, responding to attentional demands and postural transitions to enable turning, opening doors, etc. (12) Only more recently have the impact and interventions targeting these contextual factors begun to be explored.
Recovery after stroke is a non-linear process with most recovery observed in the first weeks to months with possible stagnation and even deterioration in the chronic stage and at five years after stroke. (13, 14) The walking domain increasingly benefits from understanding subgroups of patients. Indeed, recent work evaluating functional mobility indicated different recovery patterns for mild and moderately affected patients; mild patients showed a relative mobility improvement in the first year, moderately affected patients demonstrated most recovery in the first three months and then declined significantly at one year. (15) Other factors that are associated with reduced walking recovery such as age, diabetes, type, and location of stroke also need to be considered. (3)
Measuring walking recovery
Stroke survivors report independence, speed, endurance and quality are all important aspects of gait recovery. (2) Gait recovery measurement tools should therefore be available to accurately capture these four domains from both a capacity and a performance perspective. To date recommended tools for measuring walking capacity in terms of dependence, speed and endurance exist, (16-18) but measures of quality are less well defined and there is no consensus for performance measures.
The Functional Ambulation Category is commonly used as a capacity measure of walking independence, (17) whereas the mobility items of a self-reported questionnaire such as the Stroke Impact Scale (19) could be used to measure performance of independent walking. For the approximately three quarters of people who get back onto their feet following stroke, measures such as speed, endurance and quality then become more important. The 10 Metre Walk Test (10MWT) (16, 18), the 6 Minute Walk Test (6MWT) (16) and Functional Gait Assessment (16) are recommended capacity-based measures of speed, endurance and postural stability during walking tasks. Novel applications like iWalkAssess (http://www.iwalkassess.com) aid clinical interpretation of these measures by allowing comparison with normative and community ambulation values. Measures of performance or “real world walking”, however, are as, or arguably more important, than capacity-based measures as they translate into what an individual does in their current environment.
A common example of a walking performance measure is the number of steps taken per day measured by a wearable device such as an activity monitor or pedometer. The Step Activity Monitor is the most accurate for measuring steps per day after stroke, however this is a research grade monitor with costs prohibiting use in clinical practice. (20) Limitations to pedometers and wrist/upper limb and waist worn activity monitors for measuring steps/day in the stroke population have been noted at all stages post-stroke (20) and greater accuracy is evident with monitors worn on the lower limb. (21) This can be achieved clinically with relatively inexpensive activity monitors. However, accuracy of these monitors has not been established in the real world where stepping is not consistent in terms of cadence, step length, and direction. Further limitations to steps/day as a measure of performance are that it only considers the frequency domain of walking and does not consider intensity (e.g., sedentary time and light, moderate or vigorous walking) and duration (e.g., time spent walking, length of short and long bouts of walking) domains or the context of the walking activity which could add meaningful information and enable targeted intervention for optimal health benefits.
Alongside independence, speed, and endurance, stroke survivors also cite walking quality as important. Traditionally walking quality has been measured in gait laboratories using ‘gold standard’ camera-based systems with a force plate that can measure temporal-spatial and force aspects of gait. (22) More recently low-cost systems incorporating accelerometers, (22) and advanced signal processing techniques have been shown to be valid and reliable in capturing some of these measures in the home and community.
Although a stroke survivor may have the physical capacity to walk, it does not necessarily translate to increased walking performance. Many studies have demonstrated that even relatively mild stroke survivors do not meet daily step recommendations. (23) This alludes to factors broader than physical capacity alone being important for walking performance – for example self-efficacy, motivation, and an accessible environment. Currently subjective walking performance is measured via self-report questionnaires. There are sub-sections of global questionnaires on physical activity or overall health that relate to walking, (20) and preliminary evidence that Walk-12 may be a suitable generic self-report measure of walking performance in neurological conditions. (24) At present, however there is no consensus on use of self-report questionnaires for walking recovery after stroke.
Rather than relying on subjective report, which is subject to recall bias, a ‘real world measure’ of walking endurance via a 6MWT may help to capture how personal and environmental factors impact upon performance. Alternatively, combining objective measurement with subjective report may help to understand why capacity often does not relate to performance and how to target this. Ecological momentary assessment (EMA), where real time self-report data is used to provide information on the persons behaviour, cognitions, affect, context and other experiences, (25) could be combined with other technologies including accelerometers and GPS (26, 27) to provide a more holistic measure of walking performance.
At present although we have recommended measures of capacity, they are often not implemented in clinical practice, (28) and we do not have the tools to comprehensively measure performance in research and practice, limiting standardization of delivery. Currently, no one performance tool can quantify both quantity (e.g., frequency, duration and intensity of walking) and quality of walking and contextualise this information and this should be the focus of future research.
Alongside enhancing and standardising measurement of walking performance, the field would benefit from development of data collection systems to improve understanding. A biobank of data could drive analysis of large datasets and stratification into subgroups for the purposes of prediction and treatment which may spearhead meaningful breakthroughs in stroke rehabilitation interventions. This has been explored in other conditions (29) and learning from this research could be applied to stroke.
Classifying and predicting walking recovery
Because the recovery of walking ability is a primary goal for and a major component of rehabilitation for people with stroke (2, 30) it is important to be able to classify different levels of walking ability and to predict return of walking function. The ability to predict and classify walking ability assists researchers and clinicians with setting goals, interpreting change, selecting interventions, monitoring progress, and revising the plan of care. It is important to distinguish between predicting future walking ability and classifying walking ability. Although many studies indicate that their findings predict walking ability, these are actually cross-sectional studies that predict what factors categorize people with stroke into different functional walking classifications. (30, 31) Findings from these studies are useful to understand what factors may play a role in walking and have been used as primary end points for rehabilitation trials targeting walking. (32) True predictive studies predict walking ability at some future date based on factors gathered earlier after stroke. (33)
Expert opinion (34) and self-report (30) have been widely used to develop categories of walking in order to classify people with stroke as home or community ambulators. Recently the amount of real-world walking performance has been used to classify people with stroke as home or community ambulators. Fulk and colleagues (31) used steps/day, measured with an activity monitor, as a way to classify walking ability where 100-2499 steps/day was classified as home ambulator, 2,500-7,499 steps/day was classified as limited community ambulator, >=7,500 steps/day was classified as unlimited community ambulator. They found that the distance walked during a 6MWT was able to accurately distinguish between the different categories of walking function (home ambulator: 6MWT distance <=205 meters, limited community ambulator: 206-287 meters, and unlimited community ambulator: >=288 meters). Gait speed could also distinguish between these categories (<=0.49 m/s, 0.50-0.93 m/s, >=0.94 m/s) but it was not as accurate as the 6MWT. Recent studies have highlighted the impact of personal (self-efficacy (11, 35)) and environmental factors (social and physical environments (11)) on walking performance in the real world.
Predicting who and when an individual post-stroke will be able to walk independently without physical assistance is important. This is an outcome that is important to people with stroke (2) and this knowledge assists clinicians with discharge planning and goal setting. Several measures of physical capacity early after stroke have been identified as predictors of independent walking ability. A number of studies have demonstrated that trunk control/balance and strength/motor control measured less than 1-month post-stroke are able to accurately predict independent walking ability at 2-6 months post stroke. (36) Other factors such as younger age, (36) less severe stroke, (3, 37, 38) continence, (36) stroke location and lesion size, (3, 36, 39) and hemianopsia (39) have also been found to be significant predictors of independent walking.
Other studies have identified factors that predict walking capacity measured by gait speed (34) and self-reported walking ability. (30) Balance (40, 41) and initial gait speed (40, 42) early after stroke (<1 month) are valid predictors of walking capacity at six months after stroke. Gait speed, (43, 44) lower extremity strength, (43) and self-efficacy (44) at 1-3 months post stroke are predictive of self-reported walking performance at >6 months post stroke. Only one study, to the best of our knowledge, has identified predictors of real-world walking performance (steps/day). Handlery and colleagues (45) found that balance at 2 months post-stroke predicted steps/day at 12 months after stroke. A limitation of this study was that it was a secondary analysis of data from an intervention study.
Although there is strong evidence that good trunk control/balance and other factors listed above early after stroke are significant predictors of independent ambulation at ~6 months after stroke a major limitation in these findings is that none of the predictive models have been externally validated. Additionally, the initial measurement and end point of many of these studies are different making comparisons difficult.
As demonstrated, we already have numerous capacity-based prediction measures, but where the field is especially lacking is identification of factors early after stroke that predict performance of walking in the real world and further research should be focused here. Expanding understanding beyond clinical outcomes and surrogate markers using biomarkers that measure the underlying molecular / cellular processes such as structural and functional imaging could aid prediction of walking recovery and treatment response. Consensus-based recommendations for the use of certain motor biomarkers for clinical trials already exist (e.g., corticospinal tract indexed by diffusion tensor imaging or by lesion overlap), (46) but further developments to understand how factors such as lesion type, location and volume influence walking recovery are recommended.
Walking recovery interventions
The importance of measuring both capacity and performance to monitor progress and classify and predict walking recovery outcome is clear, but how best do we target these important measures? To date the efficacy of walking recovery interventions has predominantly been measured in terms of capacity not performance. This is demonstrated in a summary of best practice clinical guidelines for walking recovery after stroke from four different areas across the world (Table S1) where guidelines are predominantly based upon measurement of capacity. (47-50) Primary research applied within these guidelines has also often focused on the chronic stages of stroke, therefore we have less understanding of the acute and sub-acute phases when motor recovery is more likely to take place. (13) It also should be noted that the summarised guidelines all come from higher income countries, however, most guideline-based interventions could also be delivered in low and middle-income countries.
The methodology for the Australian and New Zealand, Canadian, and UK guidelines presented in Table S1 differs and the guidelines were developed at different time points. But despite these differences, commonalities exist across the guideline recommendations. All the guidelines strongly recommend tailored repetitive practice of walking and a variety of forms of delivering this practice including, treadmill training with / without body weight support, circuit class training and cardiorespiratory fitness training to improve capacity measures. Tailored repetitive task practice is supported by motor learning literature which emphasises the importance of specificity of practice to enhance neuroplasticity and motor learning and tailoring of the walking intervention to individual goals. (51) The recommendation also encompasses the principle of dose via the repetitive nature of the practice. Neuroplastic changes have been observed with repetition of skilled movement versus unskilled movement in animal studies (52) and clinical studies support repetitive training to improve gait recovery. (53)
Intensity is another important component of dose measured via power output, rate of work / workload or heart rate response. Intensity is emerging as an important factor in walking recovery after stroke evidenced by the inclusion of intensity within The American Academy of Neurologic Physical Therapy Clinical Practice Guidelines. (48) The guidelines strongly recommend moderate to high intensity walking training for chronic stroke survivors (> six months) based upon the preponderance of findings and quality of single randomized controlled trials with a primary or secondary goal to improve walking speed and timed distance.
Recent studies have provided further evidence for the influence of repetition and intensity on walking recovery after stroke. For example, a recent phase 2 multi-center trial in inpatient stroke rehabilitation (N=75, 6-sites) found that increasing the amount of walking practice and aerobic intensity (from low to moderate/vigorous) during one-hour physical therapy sessions resulted in better walking and quality of life outcomes than usual care. (54) Meanwhile, doubling the duration of the higher intensity physical therapy sessions appeared to have minimal effect on outcomes, (54) suggesting that intensity is a key dosing parameter.
Treadmill training is strongly recommended as a mode of delivering repetitive task practice in the guidelines. Treadmill training allows for the systematic progression of speed, individuals can potentially work at higher aerobic intensities and the use of a body weight support system can reduce the need for manual support from the therapist for patients who require assistance for ambulation. However, the guidelines recommend treadmill training should only be an adjunct to overground walking or used when overground walking is not available as speed gains on the treadmill do not always translate to the overground environment. Recent preliminary findings support combining both treadmill and overground training to improve walking recovery, (55) but these findings require further exploration and translation to practice would be reliant on access to a treadmill.
When individuals cannot walk independently, or would not otherwise practice walking, electromechanically assisted gait training is weakly recommended. But if an individual can walk without physical assistance motor learning literature supports independent practice as physical guidance can potentially impede learning. (51) Other weakly recommended walking recovery interventions based on the synthesis of the guidelines can be viewed in Table S1.
Importantly, walking recovery clinical guidelines focus upon interventions targeting walking capacity, measured often by speed and endurance (e.g., 10MWT, 6MWT) rather than walking performance. A systematic review and metanalysis on interventions to improve real world walking (measured via self-report, pedometer or step watch activity monitor) demonstrated a small effect size and sustained benefit. (56) A subgroup analysis highlighted interventions that included a behaviour change technique were more likely to be effective. Goal setting, barrier identification and self-monitoring were the techniques with the largest effect sizes. It is imperative that further research is conducted in this field to inform recommendations on targeting walking performance, alongside walking capacity.
Promising research directions targeting walking capacity
Although the predominance of evidence to date in gait recovery has focused upon improving capacity, one could argue we still do not know how best to optimise capacity. Targeting capacity is important as a certain level of capacity is required for performance in the home and community. Optimizing intensity, neuromodulation, targeting biomechanical impairments, enhancing motor learning, and predicting responsiveness are all interventions that show promise in improving walking capacity after stroke and will be discussed below.
While the evidence reviewed in the U.S. guidelines supports task-specific locomotor training, (48) it includes a very wide intensity range, and there is limited knowledge to help clinicians decide between moderate and vigorous intensity. Preliminary studies suggest that vigorous intensity (e.g., above the threshold for blood lactate accumulation ~60% heart rate reserve) could yield substantially better walking outcomes than moderate intensity (40-60% heart rate reserve) with a comparable safety profile. (57, 58) However, further research is needed to fully evaluate the risk/benefit ratio. In addition, therapists have reported that vigorous intensity is difficult to achieve among stroke survivors using traditional methods (59) and some studies have had difficulty sustaining vigorous intensity exercise among persons with motor impairment. (60) Locomotor high-intensity interval training (HIIT) is one intervention currently under investigation to address these challenges. (61) This strategy uses bursts of fast walking alternated with recovery periods with the goal of sustaining higher training intensities for longer durations.
Transcranial magnetic stimulation, direct current stimulation & deep brain stimulation all have the potential to modulate locomotor training responsiveness. (62, 63) However, the optimal target region(s) for stimulation remain unclear due to limited knowledge about the neural underpinnings of locomotor recovery. Current conceptual models in post-stroke motor rehabilitation largely focus on the ipsilesional corticospinal tract, (64, 65) but evidence suggests that other pathways (e.g. bilateral cortico-reticulospinal) may be at least as important for gait function and recovery. (66, 67) A better understanding of the neural mechanisms of walking recovery would allow more physiologically informed stimulation-targeting and a more systematic progression of research in this promising area.
Additional supplemental treatments using specific exercises or neuromuscular electrical stimulation to enhance the effects of task-specific locomotor training are also under investigation. These interventions target specific biomechanical impairments that limit walking capacity for many stroke survivors, like impaired paretic propulsion force. (68) Examples of these promising interventions include high-velocity strength training of propulsion-generating muscles, (69, 70) and fast walking with concurrent functional electrical stimulation of the plantar flexors and dorsiflexors timed to the gait cycle. (71)
Another promising class of interventions being tested to improve post-stroke walking capacity are those that aim to enhance motor learning during locomotor training. For example, virtual/augmented reality systems may enhance motivation and/or feedback delivery during training, (48) and novel portable rhythmic auditory stimulation systems could augment gait training and facilitate increased volumes of walking practice outside of clinical settings for appropriate patients. (72)
While several studies have attempted to predict overall recovery of walking capacity post-stroke (i.e., combined effects of spontaneous recovery and variable rehabilitation intervention/dosing), few have aimed to predict responsiveness to specific interventions. Further, such analyses have generally been done on an exploratory post-hoc basis (high risk of false positive and false negative findings) (73) using data from a single group. Without control group data, it is impossible to distinguish general prognostic factors (e.g. predictors of spontaneous recovery or repeated testing effects) from predictors of response to a specific intervention. (73) To enable personalized evidence-based rehabilitation, we need to identify patient characteristics that predict differential response to a specific treatment versus control or one treatment strategy versus another (i.e., treatment by characteristic interactions). To date, the only reproducible differential response predictor identified has been baseline self-selected gait speed, where ≥0.4 m/s appears to predict better walking capacity improvement after locomotor training. (74)
Promising research directions targeting walking performance
As noted above, there is evidence to support task-specific interventions that focus on walking at a high enough dosage result in improvements in walking capacity (i.e., gait speed and endurance). However, improvements in walking capacity alone do not always transfer to more walking performance in the real world. (31) Walking capacity seems to account for less than 50% of the variance in daily stepping activity. (35) Complementary interventions that address other factors that are associated with walking activity in the real world may be necessary to support people with stroke to translate their improved capacity to walking in the real world.
Early studies that incorporate behavior change techniques including goal setting, barrier identification, social support and self-monitoring, show promise in targeting walking performance (56, 75) Using mobile technologies to self-monitor and provide feedback on physical activity in neurologically intact individuals can positively influence physical activity levels. (76) Preliminary findings indicate that this strategy may support increased walking activity in people with stroke. (77, 78) There is a large randomized controlled trial utilizing this type of intervention that is currently near completion. (79) Similarly, Ecological Momentary Assessment combined with Ecological Momentary Intervention may provide contextually relevant interventions delivered at the point of performing the walking activity in the real world to support persons with stroke to better translate walking capacity to walking performance. (25)
With the issues related to access to physical therapy services raised by the COVID-19 pandemic and continued improvements in technologies there has been an increase in studies examining the use of digital health measures to improve stroke outcomes. (80) Although preliminary studies indicate that various types of telerehabilitation interventions may support improved outcomes after stroke, these studies are mainly feasibility studies with a high potential for bias with small sample sizes. (81-83) Further research in this area is needed to understand the specific impact of digital health interventions on walking performance after stroke.
Future research directions
We would argue that although we have some understanding of how to measure and target walking capacity after stroke, the field is still very much in its infancy, and this is even more apparent for walking performance. It is also important to remember that what we think we know about how to maximize walking recovery after stroke is based upon interventions developed by researchers, tested on relatively small single-site samples (N<100), with variable methodological rigor, done in research laboratories with selected patient samples.
To address these issues future research should be driven and guided in partnership with stroke survivors and their caregivers’ preferences, with this approach showing promise in other areas of stroke rehabilitation. (84, 85) Intervention design needs to include and report on intervention components using standardized frameworks such as the TIDieR. (86) This will enable the development of targeted interventions that: encompass the best available evidence (why?); are personalized (who?); are delivered at the right time (when?); address environmental factors (where?); are at the appropriate dosage (how much?); use appropriate forms of delivery (how?); allow for tailoring (tailoring?); and can be delivered as intended (how well?). Standardised capacity and performance outcome assessment and more robust systems for data pooling are required to inform clinical practice. International collaboration in the field of stroke gait recovery is growing (87) but should continue to advance to allow larger, more generalizable studies and aid consensus-based recommendations.
Implementation of current best evidence in gait recovery after stroke
The preceding sections have reviewed current research in stroke gait recovery. But are our current healthcare services facilitating the delivery of this evidence and if not, how can this be enabled to allow stroke survivors the best potential for walking recovery?
Our strongest evidence to date supports the use of tailored repetitive practice of walking, or the components of walking. But although this evidence exists, there are many barriers to consistent implementation of these clinical guidelines into practice. Current recommendations for therapy delivered to stroke survivors are frequently not met, (88) with low doses of balance and walking practice observed within these sessions (89, 90) and minimal walking practice outside of therapy. (91) The healthcare systems and models of delivery of care present significant barriers to implementation.
Non-modifiable patient characteristics and staffing impact upon therapy delivery, (92) but interestingly have not been found to be the main determinant of frequency and intensity of inpatient therapy. (93) Time spent on information exchange appears more important with units structured so timetables enable increased practice appearing the most successful in terms of dose. Supporting staff knowledge of the underpinning evidence behind amount and frequency of therapy could also impact upon walking recovery therapy provision. (93)
Another area where there is varied implementation of evidence-based practice is in walking recovery measurement. This is vital to classify and predict activity, measure progress and outcome, and increase patient engagement, however, clinicians often rely on experience to guide decision-making. As previously discussed, there is a need for further research and standardisation of walking performance measures to aid implementation. In contrast we have recommended walking capacity measures, but these are still not implemented. Knowledge translation interventions which target barrier identification, knowledge and skills training, social influences and environmental issues have shown increased adherence to stroke walking recovery capacity measurement. (28) These strategies could be applied to increase implementation and systems developed to capture this rich data-source to inform practice.
Changing healthcare professional behaviour is complex and indeed a whole field of science has been built around implementation. Within the stroke recovery community, we need to employ and investigate techniques to enable implementation of walking recovery research to determine which are the most successful. (94) Using consensus-based behaviour change and implementation taxonomies will allow structured understanding in this field. (95, 96) Investing in people could aid the implementation of research via: adequate staffing; research-informed teaching at undergraduate level; (97) and the development of adequately funded stroke recovery clinical academic career pathways to aid translation of research to practice. (98)
We also need to invest in environments and service delivery pathways that promote walking recovery. Research is emerging to support the importance of environmental enrichment to enable stroke recovery. (99) Unfortunately however, many stroke units have been structurally altered at the expense of rehabilitation facilities and potential for walking practice due to many factors. These factors include: the ever-increasing bed shortage in hospitals; (100) a focus on medicalisation of stroke units in response to the rapid development of thrombolysis and thrombectomy procedures; enhanced infection control procedures particularly since the pandemic; (101) and falls risks procedures creating risk adverse staff cultures. (102) We need to fight for stroke unit environments that enable walking practice and rehabilitation. This means having the floorspace to practice walking, deliver circuit classes and undertake recommended measurement protocols, (e.g., 6MWT) and having equipment to enable general (e.g., treadmills) and targeted walking practice (e.g., leg sled). It also means the creation of environments that replicate real world walking (e.g., different terrain, slopes, doors, steps) and areas to undertake independent practice and encourage habitual activity e.g., activity and dining rooms.
But one might argue the best environment to target walking recovery is in the home and nearby community where practice is more meaningful. Early discharge from hospital could also support other important neuroprotective factors such as sleep (103) and nutrition which can be negatively impacted in hospital settings. (104) Appropriately resourced early supported discharge (ESD) has been found to reduce length of stay and disability (105) and walking activity has been shown to be higher in ESD versus inpatient settings. (106) ESD services however vary considerably and are not available in some areas.
There will always be a limit to the amount of face-to-face therapy, so it is essential that stroke survivors are supported to self-manage walking practice, creating long-term positive walking behaviours, and potentially increasing self-efficacy. Often, however, stroke survivors are discharged from therapy services before potential has been reached (5) and without strategies to enable continuation or maintenance of walking. Promising long-term behaviour change strategies have been identified for enabling long-term physical activity (75) and interventions developed, (107) but these interventions do not specifically target walking. There may be a need for more therapist support to observe and clinically reason around specific impairments that may be influencing walking activity to tailor exercise plans, rather than simply adopting a basic ‘move more, sit less’ strategy.
Although there are multiple promising applications of technology within stroke walking research, often even simple technology such as a stopwatch and counter are not used in practice. (108) The COVID 19 pandemic, however, led the necessity to use technologies such as telehealth when face-to-face rehabilitation was prohibited. Lessons learned during this period now need to be applied and further explored to determine if this approach is safe during activities requiring high levels of balance such as walking. (80)
Conclusion
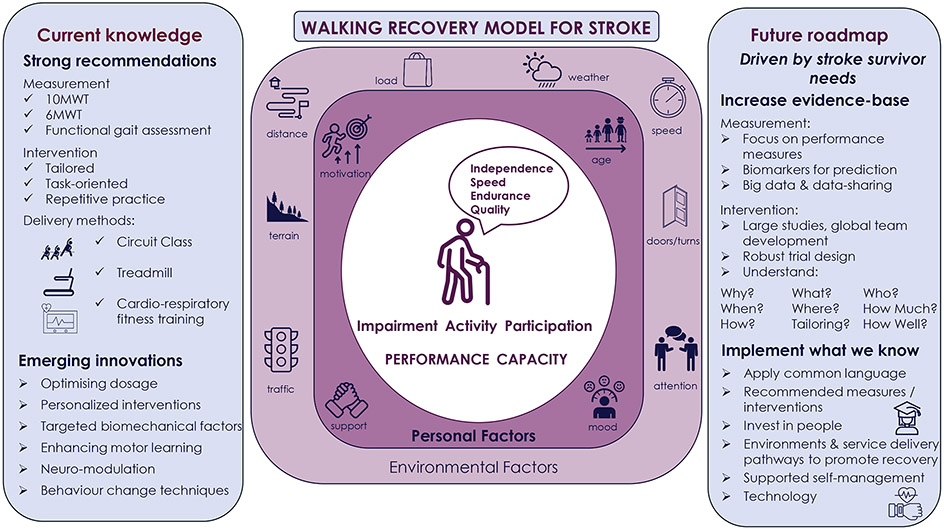
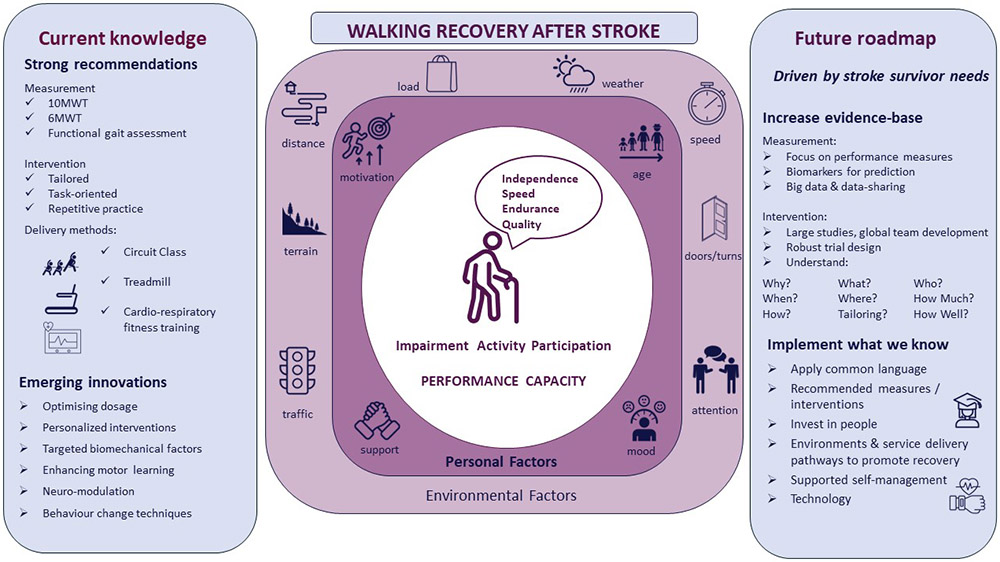
In this review we present current evidence, promising interventions, future research and implementation of research to practice in the field of walking recovery after stroke. Key messages from the review focus on measuring and targeting both walking capacity and performance after stroke, increasing the evidence base and implementing what we know to practice. The themes of the review are summarised in our model of walking recovery (Figure 1). The model places stroke survivor needs centrally and emphasizes the use of the ICF to consider the multiple factors that influence individual stroke survivor walking recovery to guide research and clinical care. In Figure 2 we present a mission statement to enhance walking recovery after stroke. Researchers and clinicians need to collectively work to achieve this mission and in doing so improve the lives of stroke survivors and their carers.
Figure 1.
Walking recovery model for stroke
Figure 2.
Mission statement to enhance walking recovery after stroke
Supplementary Material
Acknowledgments:
We would like to thank the following for their contribution: Professor Julie Bernhardt and Professor Pam Duncan for inviting the review and reviewing the paper as members of the editorial board and Professor Lynn Rochester and Dr Sue Lord for support developing the concept for the paper.
Non-standard abbreviations and acronyms
- 10MWT
10 Metre Walk Test
- 6MWT
6 Minute Walk Test
- EMA
Ecological momentary assessment
- ESD
Early Supported Discharge
- FAC
Functional Ambulation Category
- FES
Functional Electrical Stimulation
Footnotes
Disclosures: None.
Contributor Information
Sarah A Moore, Northumbria University and Newcastle University, UK.
Pierce Boyne, University of Cincinnati, USA.
George Fulk, Emory University, USA.
Geert Verheyden, KU Leuven – University of Leuven, Belgium.
Natalie A. Fini, The University of Melbourne, Australia..
References
- 1.Algurén B, Lundgren-Nilsson Å, Sunnerhagen KS. Functioning of stroke survivors–a validation of the ICF core set for stroke in Sweden. Disability and rehabilitation. 2010;32:551–9. [DOI] [PubMed] [Google Scholar]
- 2.Bohannon RW, Morton MG, Wikholm JB. Importance of four variables of walking to patients with stroke. International journal of rehabilitation research. 1991;14:246–50. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy C, Bernhardt J, Churilov L, Collier JM, Ellery F, Rethnam V, Carvalho LB, Donnan GA, Hayward KS. Factors associated with time to independent walking recovery post-stroke. Journal of neurology, neurosurgery, and psychiatry. 2021;92:702–8. [DOI] [PubMed] [Google Scholar]
- 4.Beyaert C, Vasa R, Frykberg GE. Gait post-stroke: Pathophysiology and rehabilitation strategies. Neurophysiologie clinique = Clinical neurophysiology. 2015;45:335–55. [DOI] [PubMed] [Google Scholar]
- 5.Blennerhassett JM, Levy CE, Mackintosh A, Yong A, McGinley JL. One-quarter of people leave inpatient stroke rehabilitation with physical capacity for community ambulation. Journal of Stroke and Cerebrovascular Diseases. 2018;27:3404–10. [DOI] [PubMed] [Google Scholar]
- 6.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility—giving mobility clinical visibility: a Mobility Working Group recommendation. Jama. 2014;311:2061–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivey FM, Macko R, Ryan A, Hafer-Macko C. Cardiovascular health and fitness after stroke. Topics in stroke rehabilitation. 2005;12:1–16. [DOI] [PubMed] [Google Scholar]
- 8.Pang MY, Eng JJ, Miller WC. Determinants of satisfaction with community reintegration in older adults with chronic stroke: role of balance self-efficacy. Physical therapy. 2007;87:282–91. [DOI] [PubMed] [Google Scholar]
- 9.International Classification of Functioning, Disability and Health (ICF) 2022. [Available from: https://icd.who.int/dev11/l-icf/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1550548595]
- 10.World Health Organization. Towards a common language for functioning, disability and health. Geneva: World Health Organization; 2002. [Google Scholar]
- 11.Miller A, Pohlig RT, Wright T, Kim HE, Reisman DS. Beyond Physical Capacity: Factors Associated With Real-world Walking Activity After Stroke. Archives of physical medicine and rehabilitation. 2021;102:1880–7. [DOI] [PubMed] [Google Scholar]
- 12.Shumway-Cook A, Patla AE, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental demands associated with community mobility in older adults with and without mobility disabilities. Physical therapy. 2002;82:670–81. [PubMed] [Google Scholar]
- 13.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–702. [DOI] [PubMed] [Google Scholar]
- 14.Meyer S, Verheyden G, Brinkmann N, Dejaeger E, De Weerdt W, Feys H, Gantenbein AR, Jenni W, Laenen A, Lincoln N, et al. Functional and motor outcome 5 years after stroke is equivalent to outcome at 2 months: follow-up of the collaborative evaluation of rehabilitation in stroke across Europe. Stroke. 2015;46:1613–9. [DOI] [PubMed] [Google Scholar]
- 15.Buvarp D, Rafsten L, Sunnerhagen KS. Predicting longitudinal progression in functional mobility after stroke: a prospective cohort study. Stroke. 2020;51:2179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore JL, Potter K, Blankshain K, Kaplan SL, O'Dwyer LC, Sullivan JE. A core set of outcome measures for adults with neurologic conditions undergoing rehabilitation: a clinical practice guideline. Journal of Neurologic Physical Therapy. 2018;42:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehrholz J, Wagner K, Rutte K, Meissner D, Pohl M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Archives of physical medicine and rehabilitation. 2007;88:1314–9. [DOI] [PubMed] [Google Scholar]
- 18.Kwakkel G, Lannin NA, Borschmann K, English C, Ali M, Churilov L, Saposnik G, Winstein C, van Wegen EE, Wolf SL. et al. Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. International journal of stroke : official journal of the International Stroke Society. 2017;12:451–61. [DOI] [PubMed] [Google Scholar]
- 19.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–40. [DOI] [PubMed] [Google Scholar]
- 20.Fini NA, Holland AE, Keating J, Simek J, Bernhardt J. How is physical activity monitored in people following stroke? Disability and rehabilitation. 2015;37:1717–31. [DOI] [PubMed] [Google Scholar]
- 21.Klassen TD, Semrau JA, Dukelow SP, Bayley MT, Hill MD, Eng JJ. Consumer-Based Physical Activity Monitor as a Practical Way to Measure Walking Intensity During Inpatient Stroke Rehabilitation. Stroke. 2017;48:2614–7. [DOI] [PubMed] [Google Scholar]
- 22.Moore SA, Hickey A, Lord S, Del Din S, Godfrey A, Rochester L. Comprehensive measurement of stroke gait characteristics with a single accelerometer in the laboratory and community: a feasibility, validity and reliability study. Journal of NeuroEngineering and Rehabilitation. 2017;14:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fini NA, Holland AE, Keating J, Simek J, Bernhardt J. How Physically Active Are People Following Stroke? Systematic Review and Quantitative Synthesis. Physical therapy. 2017;97:707–17. [DOI] [PubMed] [Google Scholar]
- 24.Holland A, O'Connor RJ, Thompson AJ, Playford ED, Hobart JC. Talking the talk on walking the walk: a 12-item generic walking scale suitable for neurological conditions? Journal of neurology. 2006;253:1594–602. [DOI] [PubMed] [Google Scholar]
- 25.Demers M, Winstein CJ. A perspective on the use of ecological momentary assessment and intervention to promote stroke recovery and rehabilitation. Topics in Stroke Rehabilitation. 2021;28:594–605. [DOI] [PubMed] [Google Scholar]
- 26.Lonini L, Shawen N, Hoppe-Ludwig S, Deems-Dluhy S, Mummidisetty CK, Eisenberg Y, Jayaraman A. Combining Accelerometer and GPS Features to Evaluate Community Mobility in Knee Ankle Foot Orthoses (KAFO) Users. IEEE Trans Neural Syst Rehabil Eng. 2021;29:1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanke TA, Hwang S, Keller S, Zielke D, Hailey T, Nathaniel K, Evans CC. Measuring Community Mobility in Survivors of Stroke Using Global Positioning System Technology: A Prospective Observational Study. J Neurol Phys Ther. 2019;43:175–85. [DOI] [PubMed] [Google Scholar]
- 28.Moore JL, Virva R, Henderson C, Lenca L, Butzer JF, Lovell L, Roth E, Graham ID, Hornby TG. Applying the Knowledge-to-Action Framework to Implement Gait and Balance Assessments in Inpatient Stroke Rehabilitation. Archives of physical medicine and rehabilitation. 2022:103:S230–S245. [DOI] [PubMed] [Google Scholar]
- 29.Mazzà C, Alcock L, Aminian K, Becker C, Bertuletti S, Bonci T, Brown P. Brozgol M. Buckley E. Carsin E. et al. Technical validation of real-world monitoring of gait: a multicentric observational study. BMJ Open. 2021;11:e050785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Archives of physical medicine and rehabilitation. 2004;85:234–9. [DOI] [PubMed] [Google Scholar]
- 31.Fulk GD, Reynolds C, Mondal S, Deutsch JE. Predicting home and community walking activity in people with stroke. Archives of physical medicine and rehabilitation. 2010;91:1582–6. [DOI] [PubMed] [Google Scholar]
- 32.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, Dobkin BH, Rose DK, Tilson JK, Cen S, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364:2026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stinear CM, Smith M-C, Byblow WD. Prediction Tools for Stroke Rehabilitation. Stroke. 2019;50:3314–22. [DOI] [PubMed] [Google Scholar]
- 34.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–9. [DOI] [PubMed] [Google Scholar]
- 35.Danks KA, Pohlig RT, Roos M, Wright TR, Reisman DS. The relationship between walking capacity, biopsychosocial factors, self-efficacy and walking activity in individuals post stroke. Journal of neurologic physical therapy: JNPT. 2016;40:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preston E, Ada L, Stanton R, Mahendran N, Dean CM. Prediction of independent walking in people who are nonambulatory early after stroke: a systematic review. Stroke. 2021;52:3217–24. [DOI] [PubMed] [Google Scholar]
- 37.Langerak AJ, McCambridge AB, Stubbs PW, Fabricius J, Rogers K, de Oliveira CQ, Nielson JF. Verhagen AP. Externally validated model predicting gait independence after stroke showed fair performance and improved after updating. Journal of clinical epidemiology. 2021;137:73–82. [DOI] [PubMed] [Google Scholar]
- 38.Kwah LK, Harvey LA, Diong J, Herbert RD. Models containing age and NIHSS predict recovery of ambulation and upper limb function six months after stroke: an observational study. J Physiother. 2013;59:189–97. [DOI] [PubMed] [Google Scholar]
- 39.Craig LE, Wu O, Bernhardt J, Langhorne P. Predictors of poststroke mobility: systematic review. International journal of stroke. 2011;6:321–7. [DOI] [PubMed] [Google Scholar]
- 40.Louie DR, Eng JJ. Berg Balance Scale score at admission can predict walking suitable for community ambulation at discharge from inpatient stroke rehabilitation. Journal of Rehabilitation Medicine. 2018;50:37–44. [DOI] [PubMed] [Google Scholar]
- 41.Bland MD, Sturmoski A, Whitson M, Connor LT, Fucetola R, Huskey T, Corbetta M, Lang CE. Prediction of discharge walking ability from initial assessment in a stroke inpatient rehabilitation facility population. Archives of physical medicine and rehabilitation. 2012;93:1441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harari Y, O’Brien MK, Lieber RL, Jayaraman A. Inpatient stroke rehabilitation: prediction of clinical outcomes using a machine-learning approach. Journal of neuroengineering and rehabilitation. 2020;17:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulder M, Nijland RH, van de Port IG, van Wegen EE, Kwakkel G. Prospectively classifying community walkers after stroke: who are they? Archives of physical medicine and rehabilitation. 2019;100:2113–8. [DOI] [PubMed] [Google Scholar]
- 44.Rosa MC, Marques A, Demain S, Metcalf CD. Fast gait speed and self-perceived balance as valid predictors and discriminators of independent community walking at 6 months post-stroke–a preliminary study. Disability and rehabilitation. 2015;37:129–34. [DOI] [PubMed] [Google Scholar]
- 45.Handlery R, Regan EW, Stewart JC, Pellegrini C, Monroe C, Hainline G, Handlery K, Fritz SL. Predictors of Daily Steps at 1-Year Poststroke: A Secondary Analysis of a Randomized Controlled Trial. Stroke. 2021;52:1768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, Carter AR, Leff AP, Copland DA, Carey LM et al. Biomarkers of Stroke Recovery: Consensus-Based Core Recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabilitation and neural repair. 2017;31:864–76. [DOI] [PubMed] [Google Scholar]
- 47.Stroke Foundation. Clinical Guidelines for Stroke Management Melbourne Australia: Stroke Foundation and Inform Me; 2021. [Available from: https://informme.org.au/guidelines/clinical-guidelines-for-stroke-management]. [Google Scholar]
- 48.Hornby TG, Reisman DS, Ward IG, Scheets PL, Miller A, Haddad D, Fox EJ, Fritz NE, Hawkins K, Henderson CE et al. Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury. Journal of neurologic physical therapy : JNPT. 2020;44:49–100. [DOI] [PubMed] [Google Scholar]
- 49.Royal College Of Physicians Intercollegiate Stroke Working Party. National clinical guideline for stroke; 5th Edition 2016. [Available from: https://www.strokeaudit.org/SupportFiles/Documents/Guidelines/2016-National-Clinical-Guideline-for-Stroke-5t-(1).aspx]. [Google Scholar]
- 50.Teasell R, Salbach NM, Foley N, Mountain A. Cameron JI. De Jong A, Acerra NE, Bastasi D, Carter SL,.et al. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke; 6th Edition Update 2019. International journal of stroke. 2020;15:763–788 [DOI] [PubMed] [Google Scholar]
- 51.Maier M, Ballester BR, Verschure PFMJ. Principles of Neurorehabilitation After Stroke Based on Motor Learning and Brain Plasticity Mechanisms. Frontiers in Systems Neuroscience. 2019;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiology of learning and memory. 2000;74:27–55. [DOI] [PubMed] [Google Scholar]
- 53.French B, Thomas LH, Coupe J, McMahon NE, Connell L, Harrison J, Sutton CJ, Tishkovskaya S, Watkins CL. Repetitive task training for improving functional ability after stroke. The Cochrane database of systematic reviews. 2016;11:Cd006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klassen TD, Dukelow SP, Bayley MT, Benavente O, Hill MD, Krassioukov A, Liu-Ambrose T, Pooyania S, Poulin MJ, Schneeberg A et al. Higher Doses Improve Walking Recovery During Stroke Inpatient Rehabilitation. Stroke. 2020;51(9):2639–48. [DOI] [PubMed] [Google Scholar]
- 55.Boyne P, Doren S, Scholl V, Staggs E, Whitesel D, Carl D, Shatz R, Sawyer R, Awosika OO, Reisman DS, et al. Preliminary Outcomes of Combined Treadmill and Overground High-Intensity Interval Training in Ambulatory Chronic Stroke. Frontiers in Neurology. 2022;13:812875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stretton CM, Mudge S, Kayes NM, McPherson KM. Interventions to improve real-world walking after stroke: a systematic review and meta-analysis. Clinical rehabilitation. 2017;31(3):310–8. [DOI] [PubMed] [Google Scholar]
- 57.Boyne P, Dunning K, Carl D, Gerson M, Khoury J, Rockwell B, Keeton G, Westover J, Williams A, McCarthy M et al. High-intensity interval training and moderate-intensity continuous training in ambulatory chronic stroke: feasibility study. Physical therapy. 2016;96:1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivey FM, Stookey AD, Hafer-Macko CE, Ryan AS, Macko RF. Higher Treadmill Training Intensity to Address Functional Aerobic Impairment after Stroke. J Stroke Cerebrovasc Dis. 2015;24:2539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boyne P, Billinger S, MacKay-Lyons M, Barney B, Khoury J, Dunning K. Aerobic exercise prescription in stroke rehabilitation: a web-based survey of United States physical therapists. Journal of neurologic physical therapy: JNPT. 2017;41:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Billinger SA, Boyne P, Coughenour E, Dunning K, Mattlage A. Does aerobic exercise and the FITT principle fit into stroke recovery? Current neurology and neuroscience reports. 2015;15(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crozier J, Roig M, Eng JJ, MacKay-Lyons M, Fung J, Ploughman M, Bailey DM, Sweet SN, Giacomantonio N, Thiel A, et al. High-intensity interval training after stroke: an opportunity to promote functional recovery, cardiovascular health, and neuroplasticity. Neurorehabilitation and neural repair. 2018;32:543–56. [DOI] [PubMed] [Google Scholar]
- 62.Tien H-H, Liu W-Y, Chen Y-L, Wu Y-C, Lien H-Y. Transcranial direct current stimulation for improving ambulation after stroke: a systematic review and meta-analysis. International journal of rehabilitation research. 2020;43:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Fan J, Yang J, He C, Li S. Effects of repetitive transcranial magnetic stimulation on walking and balance function after stroke: a systematic review and meta-analysis. American journal of physical medicine & rehabilitation. 2018;97:773–81. [DOI] [PubMed] [Google Scholar]
- 64.de Paz RH, Serrano-Muñoz D, Pérez-Nombela S, Bravo-Esteban E, Avendaño-Coy J, Gómez-Soriano J. Combining transcranial direct-current stimulation with gait training in patients with neurological disorders: a systematic review. Journal of NeuroEngineering and Rehabilitation. 2019;16:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim B-R, Moon W-J, Kim H, Jung E, Lee J. Transcranial magnetic stimulation and diffusion tensor tractography for evaluating ambulation after stroke. Journal of stroke. 2016;18(2):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cleland BT, Madhavan S. Ipsilateral motor pathways to the lower limb after stroke: Insights and opportunities. Journal of neuroscience research. 2021;99:1565–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jang SH, Chang CH, Lee J, Kim CS, Seo JP, Yeo SS. Functional role of the corticoreticular pathway in chronic stroke patients. Stroke. 2013;44:1099–104. [DOI] [PubMed] [Google Scholar]
- 68.Awad LN, Binder-Macleod SA, Pohlig RT, Reisman DS. Paretic propulsion and trailing limb angle are key determinants of long-distance walking function after stroke. Neurorehabilitation and neural repair. 2015;29:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams G, Hassett L, Clark R, Bryant A, Olver J, Morris ME, Ada L. Improving walking ability in people with neurologic conditions: a theoretical framework for biomechanics-driven exercise prescription. Archives of physical medicine and rehabilitation. 2019;100:1184–90. [DOI] [PubMed] [Google Scholar]
- 70.Hendrey G, Clark RA, Holland AE, Mentiplay BF, Davis C, Windfeld-Lund C, Raymond MJ, Williams G. Feasibility of ballistic strength training in subacute stroke: a randomized, controlled, assessor-blinded pilot study. Archives of physical medicine and rehabilitation. 2018;99:2430–46. [DOI] [PubMed] [Google Scholar]
- 71.Awad LN, Reisman DS, Pohlig RT, Binder-Macleod SA. Reducing the cost of transport and increasing walking distance after stroke: a randomized controlled trial on fast locomotor training combined with functional electrical stimulation. Neurorehabilitation and neural repair. 2016;30:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spencer J, Wolf SL, Kesar TM. Biofeedback for Post-stroke Gait Retraining: A Review of Current Evidence and Future Research Directions in the Context of Emerging Technologies. Front Neurol. 2021;12:637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hingorani AD, van der Windt DA, Riley RD, Abrams K, Moons KG, Steyerberg EW, Schroter S, Sauerbrei W, Altman DG, Hemingway H. Prognosis research strategy (PROGRESS) 4: stratified medicine research. Bmj. 2013;346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dean CM, Ada L, Lindley RI. Treadmill training provides greater benefit to the subgroup of community-dwelling people after stroke who walk faster than 0.4 m/s: a randomised trial. Journal of physiotherapy. 2014;60:97–101. [DOI] [PubMed] [Google Scholar]
- 75.Moore SA, Hrisos N, Flynn D, Errington L, Price C, Avery L. How should long-term free-living physical activity be targeted after stroke? A systematic review and narrative synthesis. International Journal of Behavioral Nutrition and Physical Activity. 2018;15:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fanning J, Mullen SP, McAuley E. Increasing physical activity with mobile devices: a meta-analysis. Journal of medical Internet research. 2012;14:e2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Danks KA, Pohlig R, Reisman DS. Combining fast-walking training and a step activity monitoring program to improve daily walking activity after stroke: a preliminary study. Archives of physical medicine and rehabilitation. 2016;97:S185–S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waddell KJ, Patel MS, Clark K, Harrington TO, Greysen SR. Effect of Gamification With Social Incentives on Daily Steps After Stroke: A Randomized Clinical Trial. JAMA Neurology. 2022;79:528–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wright H, Wright T, Pohlig RT, Kasner SE, Raser-Schramm J, Reisman D. Protocol for promoting recovery optimization of walking activity in stroke (PROWALKS): a randomized controlled trial. BMC neurology. 2018;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramage ER, Fini N, Lynch EA, Marsden DL, Patterson AJ, Said CM, English C. Look Before You Leap: Interventions Supervised via Telehealth Involving Activities in Weight-Bearing or Standing Positions for People After Stroke—A Scoping Review. Physical therapy. 2021;101(6):pzab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Podury A, Raefsky SM, Dodakian L, McCafferty L, Le V, McKenzie A, See J, Zhou RJ, Nguyen T, Vanderschelden B et al. Social Network Structure Is Related to Functional Improvement From Home-Based Telerehabilitation After Stroke. Front Neurol. 2021;12:603767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Odetunde MO, Binuyo OT, Maruf FA, Ayenowowon SO, Okonji AM, Odetunde NA, Mbada CE. Development and Feasibility Testing of Video Home Based Telerehabilitation for Stroke Survivors in Resource Limited Settings. Int J Telerehabil. 2020;12:125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jonsdottir J, Baglio F, Gindri P, Isernia S, Castiglioni C, Gramigna C, Palumbo G, Pagliari C, Di Tella S, Perini G et al. Virtual Reality for Motor and Cognitive Rehabilitation From Clinic to Home: A Pilot Feasibility and Efficacy Study for Persons With Chronic Stroke. Front Neurol. 2021;12:601131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramage ER, Burke M, Galloway M, Graham ID, Janssen H, Marsden DL, Patterson AJ, Pollack M, Said CM, Lynch EA et al. Fit for purpose. Co-production of complex behavioural interventions. A practical guide and exemplar of co-producing a telehealth-delivered exercise intervention for people with stroke. Health research policy and systems. 2022;20:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hall J, Morton S, Hall J, Clarke DJ, Fitzsimons CF, English C, Forster A, Mead GE, Lawton R. A co-production approach guided by the behaviour change wheel to develop an intervention for reducing sedentary behaviour after stroke. Pilot and feasibility studies. 2020;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ : British Medical Journal. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 87.Bernhardt J, Borschmann KN, Kwakkel G, Burridge JH, Eng JJ, Walker MF, Bird ML, Cramer SC, Hayward KS, O'Sullivan MJ et al. Setting the scene for the Second Stroke Recovery and Rehabilitation Roundtable. International journal of stroke 2019;14:450–6. [DOI] [PubMed] [Google Scholar]
- 88.Intercollegiate Stroke working Party. Sentinel Stroke National Audit Programme (SSNAP). London: Royal College of Physicians. 2015. [Google Scholar]
- 89.Lang CE, MacDonald JR, Reisman DS, Boyd L, Jacobson Kimberley T, Schindler-Ivens SM, Hornby TG, Ross SA, Scheets PL. Observation of Amounts of Movement Practice Provided During Stroke Rehabilitation. Archives of physical medicine and rehabilitation. 2009;90:1692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tyson SF, Woodward-Nutt K, Plant S. How are balance and mobility problems after stroke treated in England? An observational study of the content, dose and context of physiotherapy. Clinical rehabilitation. 2018;32:1145–52. [DOI] [PubMed] [Google Scholar]
- 91.West T, Bernhardt J. Physical activity in hospitalised stroke patients. Stroke Res Treat. 2012;2012:813765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gittins M, Vail A, Bowen A, Lugo-Palacios D, Paley L, Bray B, Gannon B, Tyson S. Factors influencing the amount of therapy received during inpatient stroke care: an analysis of data from the UK Sentinel Stroke National Audit Programme. Clinical rehabilitation. 2020;34:981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clarke DJ, Burton L-J, Tyson SF, Rodgers H, Drummond A, Palmer R, Hoffman A, Prescott M, Tyrrell P, Brkic L. et al. Why do stroke survivors not receive recommended amounts of active therapy? Findings from the ReAcT study, a mixed-methods case-study evaluation in eight stroke units. Clinical rehabilitation. 2018;32:1119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morris JH, Bernhardsson S, Bird M-L, Connell L, Lynch E, Jarvis K, Kayes NM, Miller K, Mudge S, Fisher R. Implementation in rehabilitation: a roadmap for practitioners and researchers. Disability and rehabilitation. 2020;42:3265–74. [DOI] [PubMed] [Google Scholar]
- 95.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, Eccles MP, Cane J, Wood CE. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of behavioral medicine. 2013;46:81–95. [DOI] [PubMed] [Google Scholar]
- 96.Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, Proctor EK, Kirchner JE. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implementation Science. 2015;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Higher Education Academy, University Alliance. What does research-informed teaching look like? York: University Alliance and Higher Education Academy; 2017. [Google Scholar]
- 98.Baltruks D, Callaghan P. Nursing, midwifery and allied health clinical academic research careers in the UK. London: Council of Deans of Health; 2018. [Google Scholar]
- 99.McDonald MW, Hayward KS, Rosbergen I, Jeffers MS, Corbett D. Is environmental enrichment ready for clinical application in human post-stroke rehabilitation? Frontiers in behavioral neuroscience. 2018;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ewbank L, Thompson J, McKenna H, Anandaciva S, Ward D. NHS hospital bed numbers: past, present, future. London: The KingsFund; 2021. [Google Scholar]
- 101.Anåker A, von Koch L, Heylighen A, Elf M. “It’s Lonely”: Patients’ Experiences of the Physical Environment at a Newly Built Stroke Unit. HERD. 2018;12:141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luker J, Lynch E, Bernhardsson S, Bennett L, Bernhardt J. Stroke survivors' experiences of physical rehabilitation: a systematic review of qualitative studies. Archives of physical medicine and rehabilitation. 2015;96:1698–708. e10. [DOI] [PubMed] [Google Scholar]
- 103.Eugene AR, Masiak J. The neuroprotective aspects of sleep. MEDtube science. 2015;3(1):35. [PMC free article] [PubMed] [Google Scholar]
- 104.Wesselius HM, Van Den Ende ES, Alsma J, Ter Maaten JC, Schuit SC, Stassen PM, et al. Quality and quantity of sleep and factors associated with sleep disturbance in hospitalized patients. JAMA internal medicine. 2018;178:1201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Langhorne P, Baylan S, Trialists ESD. Early supported discharge services for people with acute stroke. Cochrane Database of Systematic Reviews. 2017(7):CD000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kerr A, Rowe P, Esson D, Barber M. Changes in the physical activity of acute stroke survivors between inpatient and community living with early supported discharge: an observational cohort study. Physiotherapy. 2016;102:327–31. [DOI] [PubMed] [Google Scholar]
- 107.Moore SA, Avery L, Price CI, Flynn D. A feasibility, acceptability and fidelity study of a multifaceted behaviour change intervention targeting free-living physical activity and sedentary behaviour in community dwelling adult stroke survivors. Pilot and Feasibility Studies. 2020;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Langan J, Subryan H, Nwogu I, Cavuoto L. Reported use of technology in stroke rehabilitation by physical and occupational therapists. Disability and rehabilitation Assistive technology. 2018;13:641–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.