Abstract
Objective:
Black and Hispanic patients have higher rates of chronic limb-threatening ischemia (CLTI) and suffer worse perioperative outcomes after lower extremity bypass compared with White patients. The underlying reasons for these disparities are unclear, and data on 3-year outcomes are limited. Therefore, we examined differences in 3-year outcomes after open infrainguinal bypass for CLTI by race/ethnicity and explored potential factors contributing to these differences.
Methods:
We identified all CLTI patients undergoing primary open infrainguinal bypass in the Vascular Quality Initiative registry from 2003–2017 with linkage to Medicare claims through 2018 for 3-year outcomes. Our primary outcomes were 3-year major amputation, re-intervention, and mortality. We also report 30-day major adverse limb events (MALE) defined as major amputation or re-intervention. We used Kaplan-Meier estimation methods and multivariable Cox regression analyses to evaluate outcomes by race/ethnicity and to identify contributing factors.
Results:
Among 7,108 bypass procedures performed in CLTI patients, 5,599 (79%) were in non-Hispanic White patients, 1,053 (15%) were in Blacks, 48 (1%) were in Asians, and 408 (6%) were in Hispanics. Compared with White patients, Black patients had higher rates of 3-year major amputation (Black: 32% vs White: 19%; hazard ratio (HR):1.9 [95% confidence interval:1.7–2.2]), re-intervention (61% vs 57%; HR:1.2[1.1–1.3]), and 30-day MALE (8.1% vs 4.9%; HR:1.3[1.2–1.4]), but lower mortality (38% vs 42%; HR:0.9[0.8–0.99]). Hispanic patients experienced higher rates of amputation (Hispanic: 27% vs White: 19%; HR:1.6[1.3–2.0]), re-intervention (70% vs 57%; HR:1.4[1.2–1.6]), and MALE (8.7% vs 4.9%; HR:1.5 [1.3–1.7]); however, mortality was similar between the groups (38% vs 42%; HR:0.88[0.76–1.0]). The low number of Asian patients prevented meaningful assessment of amputation (Asian: 20% vs White: 19%; HR:0.93[0.44–2.0]), re-intervention (55% vs 57%; HR:0.79[0.51–1.2]), MALE (8.5% vs 4.9%; HR:0.71[0.46–1.1]), or mortality (36% vs 42%; HR:0.83[0.52–1.3]) in this group. In adjusted analyses, the association of Black race and Hispanic ethnicity with amputation and re-intervention was explained by differences in demographic characteristics (age, sex) and baseline comorbidities (tobacco use, diabetes, renal disease).
Conclusions:
Compared with White patients, Black and Hispanic patients had higher 3-year major amputation and re-intervention rates; however, mortality was lower among Black patients and similar between Hispanic and White patients. Disparities in amputation and re-intervention are partly attributable to demographic characteristics and the higher prevalence of comorbidities in Black and Hispanic patients with CLTI. Future work is necessary to determine if interventions to improve access to care and reduce the burden of comorbidities in these populations confer limb salvage benefits.
Keywords: Race, ethnicity, limb salvage, revascularization, chronic limb threatening ischemia
Introduction:
Chronic limb-threatening ischemia (CLTI) disproportionately affects racial and ethnic minority populations, particularly Black and Hispanic individuals.1,2 The annual incidence of amputation among Black patients with CLTI is 21%, which is nearly double the incidence in White and Hispanic patients.1 Furthermore, Black patients have been shown to have signficantly higher odds of amputation during hospitalization.3
Beyond the physicial, emotional, and socioeconomic toll associated with limb loss, the higher rate of amputation among minority patients is particularly concerning as it has implications for mortality. Studies have shown that CLTI patients who underwent major amputation (i.e., above the ankle) had 3-fold higher odds of in-hospital mortality compared with other CLTI patients,4 and 6-fold higher odds of mortality compared with PAD patients without major amputations.5 Furthermore, longer-term mortality is also affected by amputation. The 1-year mortality rate after CLTI diagnosis was 30% in patients without amputation, and 40% in those who underwent amputation.1
Although many studies have described racial disparities in short-term outcomes for CLTI patients, 3-year mortality is unknown. Futhermore, there are limited data identifying explanatory factors as potential opportunities to reduce disparities. Therefore, the objectives of this study were to examine 3-year amputation, re-intervention, and mortality after infrainguinal bypass for CLTI by race and ethnicity and to determine factors associated with these outcomes.
Methods:
Data source
We performed a retrospective cohort study using the Vascular Implant Surveillance and Interventional Outcomes Network (VISION), which includes data from the Vascular Quality Initiative (VQI), a national clinical registry, with linkage to Medicare claims. The VQI was developed to improve patient care through the collection of clinical data from approximately 700 participating centers with 4,000 physicians in the United States and Canada (www.vqi.org). Patients identified in the VQI registry were linked to the Medicare claims files using a previously described methodology.6 This method combines the advantages of prospectively collected clinical data from the VQI registry with administrative data for 3-year outcomes.
The Beth Israel Deaconess Medical Center Institutional Review Board and VQI Research Advisory Committee approved this study and gave permission to use data without the need for informed consent, given the retrospective and deidentified nature of the data. This study adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) standards for observational studies.7
Patient cohort
We identified all primary infrainguinal bypass procedures performed in patients with CLTI in the VQI from 2003–2017 (n=10,315). Follow up data were available through 2018. We selected this period to ensure that we had at least 1 year of follow-up for each patient. We excluded patients who were not enrolled in Medicare at the time of their index bypass procedure to have complete capture of claims-derived outcomes throughout the study period (n=3,069). Additionally, we excluded patients with unknown or missing race/ethnicity data (n=109) and those of American Indian or Alaskan Native race (n=17), Native Hawaiian or other Pacific Islander race (n=9), and more than 1 race (n=3) as low numbers in these groups precluded meaningful assessment.
In addition, there were 371 (5%) patients who underwent more than 1 index bypass procedure during the study period. To assess if these procedures impacted outcomes, we excluded these patients as a sensitivity analysis.
Variable definitions
Race and ethnicity were defined according to pre-specified variables in the VQI based on definitions from the United States Census Bureau (Supplemental Table I).8 We divided patients into 4 groups according to their race or ethnicity: Non-Hispanic White, Black, Asian, or Hispanic. We calculated BMI using the standard formula of weight(kg)/height(m).2 Underweight and overweight were defined as a BMI <18.5 and BMI ≥30, respectively. Coronary artery disease (CAD) was defined as a history of stable or unstable angina, or myocardial infarction. Congestive heart failure (CHF) was categorized into Class I-IV according to the New York Heart Association Functional Classification.9 Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease (CKD) Epidemiology Collaboration equation without the race correction coefficient. CKD was defined as eGFR <30 mL/min/1.73m2 or requirement for hemodialysis. Preoperative medication use was defined as medications taken within 36 hours of the procedure. Optimal medical therapy was defined as pre-operative aspirin and statin use. Urgent status was assigned if the procedure was required within 72 hours but >12 hours from admission, or emergent if the procedure was required within 12 hours of admission to prevent limb loss. Distal bypass was defined as bypass to an infrapopliteal target.
We calculated physician and center experience by determining the total number of procedures performed within the previous 12 months of the index operation. Volumes were divided into quintiles with the first quintile considered as low volume, quintiles 2–4 considered as medium volume, and quintile 5 considered as high volume.
Outcomes
Our primary outcomes were 3-year major amputation, re-intervention, and mortality. Secondary outcomes were factors associated with these outcomes. We also report 30-day major adverse limb events (MALE) defined as major amputation or re-intervention. Major amputation was defined as amputation at or above the ankle and was determined based on Medicare procedure codes. Re-interventions, also identified using procedure codes, were defined as any relatable open or endovascular revascularization procedure performed after hospital discharge from index bypass procedure (Supplemental Table II). Because procedure codes do not capture laterality, we could not be certain that amputations and re-interventions identified in Medicare were ipsilateral to the index bypass procedure. To understand how this limitation may bias results, we performed a sensitivity analysis to assess how 3-year major amputation and re-intervention changed as we varied the proportion of contralateral events from 0% to 50% across racial/ethnic groups.10 Mortality was determined from the Medicare denominator file.
Statistical analysis
Before performing our primary analyses, we evaluated the age distribution of the VQI-Medicare study cohort. Most patients (79%) were Medicare-eligible based on age ≥65 years, while the remaining patients were <65 years old and had other indications for Medicare eligibility (i.e., permanent dialysis or disability). As in prior work,10 we assessed whether these younger patients may bias our results by testing for an interaction between age<65 years and race/ethnicity with respect to our primary outcomes. We did not find any significant interactions, so we included patients <65 years old in our final study cohort.
We then compared baseline and operative characteristics across racial and ethnic groups using the chi-square test for categorical variables and the Kruskal Wallis test for continuous variables. Categorical variables were presented as percentages and continuous variables were presented as medians with interquartile ranges. We used Kaplan-Meier estimation and unadjusted Cox proportional hazards regression to compare 3-year rates of major amputation, re-intervention, and mortality by race/ethnicity. For non-survival outcomes, patients who died were censored at the date of death, and those who left Medicare fee-for-service were censored on the day of the exit (n=765). We censored patients using this method because we were unable to capture data on amputation or re-interventions after that time point.
We then performed adjusted Cox analyses to identify potential contributory factors to racial/ethnic disparities in major amputation, re-intervention, and mortality. We selected the following covariates for inclusion in the analysis a priori based on previous literature and clinical relevance: demographic factors (sex, age), comorbidities (CAD, tobacco use, diabetes, kidney disease), preoperative optimal medical therapy, center and physician volume, disease severity (presence of tissue loss, urgency status), and operative factors (distal bypass target, conduit type). In the adjusted analyses, we combined all types of vein conduits together (i.e., greater saphenous vein, arm vein, etc.) and all types of prosthetic conduits together (Dacron, polytetrafluoroethylene) to create a binary variable (vein vs prosthetic) rather than having multiple categories to avoid overfitting the model. We used these covariates to create 7 different Cox regression models for 3-year amputation, re-intervention, and mortality. All models included race and ethnicity. To create models 1–6, we incorporated demographic factors, comorbidities, optimal medical therapy, center and physician volume, disease severity factors, and operative factors into the model in a stepwise manner. With the incorporation of each set of covariates, we assessed if there was a change in the association between race/ethnicity and amputation, re-intervention, and mortality. We included all covariates in model 7. In models 4 and 7 for each outcome, which included physician and center volume, we also allowed for clustering by center.
All variables had <5% missing data. All tests were two-sided, and p<0.05 was considered statistically significant. All analyses were performed using Stata version 16 software (StataCorp LP, College Station, Texas, USA).
Results:
Demographic and operative characteristics
Among 7,108 bypasses performed in CLTI patients, 5,599 (79%) were performed in non-Hispanic White patients, 1,053 (15%) in Black patients, 48 (1%) in Asian patients, and 408 (6%) in Hispanic patients (Table I). Compared with White patients, Black and Hispanic patients were younger and more likely to have hypertension, diabetes, and CKD. They were also more likely to undergo infrapopliteal bypass. Hispanic patients were more likely to have tissue loss, prior contralateral lower extremity revascularization, and receive care at high-volume centers. Asian patients were less likely to be current smokers, but more likely to be female and have tissue loss compared with all other groups. Rates of rest pain were similar between White and Black patients, but lower among Hispanics. Black, Hispanic, and Asian patients were more likely than White patients to be on pre-operative antiplatelet and statin, and less likely to be treated by high-volume physicians. Black and Hispanic patients were more likely to have prosthetic grafts used for below the knee reconstructions. Hispanic patients had the highest percentage of venous conduit for bypass (76%) followed by White patients (70%) and Black patients (67%) (Table I).
Table I.
Baseline and operative characteristics for CLTI patients undergoing primary infrainguinal bypass by race/ethnicity.
| N (%) or median (IQR) | Non-Hispanic White (n=5599) | Black (n=1053) | Hispanic (n=408) | Asian (n=48) | P-value |
|---|---|---|---|---|---|
| Age, years | 73 (67,80) | 68 (61,75) | 70 (63,78) | 73 (67,83) | <.001 |
| Female sex | 1998 (36) | 448 (43) | 158 (39) | 26 (54) | <.001 |
| Pre-op ambulatory status | 0.001 | ||||
| Ambulatory | 3441 (62) | 587 (56) | 225 (55) | 27 (56) | |
| BMI | |||||
| Obese (BMI>=30) | 1501 (27) | 313 (30) | 115 (28) | <11 (<23) | 0.07 |
| Underweight (BMI<18.5) | 268 (4.9) | 62 (5.9) | <11 (<2.7) | <11 (<23) | 0.02 |
| Smoker, current Comorbidities | 1662 (30) | 355 (34) | 78 (19) | <11 (<23) | <.001 |
| COPD | 1632 (29) | 216 (21) | 63 (15) | <11 (<23) | <.001 |
| Hypertension | 5077 (91) | 1011 (96) | 377 (92) | >37 (>77) | <.001 |
| Diabetes | 3272 (58) | 711 (68) | 325 (80) | 36 (75) | <.001 |
| Insulin dependent diabetes | 1883 (34) | 470 (45) | 228 (56) | 26 (54) | <.001 |
| CAD | 2822 (50) | 449 (43) | 207 (51) | 23 (48) | <.001 |
| CHF | 1391 (25) | 283 (27) | 91 (22) | 12 (25) | 0.3 |
| Prior cardiac intervention | 2205 (39) | 344 (33) | 171 (42) | 20 (43) | <.001 |
| CKD (eGFR<30) | 848 (15) | 378 (36) | 124 (31) | 17 (35) | <.001 |
| Dialysis dependent | 528 (9.4) | 314 (30) | 105 (26) | 16 (33) | <.001 |
| Prior contralateral lower extremity revascularization | 2500 (45) | 511 (49) | 217 (53) | 25 (52) | <.001 |
| Prior major amputation | 337 (6.0) | 101 (9.6) | 36 (8.8) | <11 (<23) | <.001 |
| Preoperative medication | |||||
| Statin | 3815 (68) | 749 (71) | 275 (67) | >37 (>77) | 0.03 |
| Any antiplatelet therapy | 4325 (77) | 846 (80) | 332 (72) | >37 (>77) | 0.05 |
| Aspirin | 4010 (72) | 749 (71) | 295 (72) | 33 (69) | 0.9 |
| P2Y12 antagonist | 1369 (25) | 339 (32) | 157 (39) | 16 (33) | <.001 |
| Any antiplatelet and statin | 3187 (57) | 647 (61) | 241 (59) | 32 (67) | 0.03 |
| Annual surgeon volume | <.001 | ||||
| Low (quintile 1) <=4 | 1741 (31) | 348 (33) | 155 (38) | 23 (48) | |
| Medium (quintile 2–4) 4–15 | 2818 (50) | 591 (56) | 201 (49) | 23 (48) | |
| High (quintile 5) >15 | 1040 (19) | 114 (11) | 52 (13) | <11 (<23) | |
| Annual center volume | <.001 | ||||
| Low (quintile 1) <= 19 | 1476 (26) | 305 (29) | 105 (26) | 24 (50) | |
| Medium (quintile 2–4) 19–62 | 3018 (54) | 662 (63) | 161 (40) | 23 (48) | |
| High (quintile 5) >62 | 1105 (20) | 86 (8.2) | 142 (35) | <11 (<23) | |
| Disease Severity | <.001 | ||||
| Rest pain | 1614 (29) | 309 (29) | 87 (21) | <11 (<23) | |
| Tissue loss | 3985 (71) | 744 (71) | 321 (79) | >37 (>77) | |
| Urgency | <.001 | ||||
| Elective | 4336 (78) | 839 (80) | 298 (76) | 35 (73) | |
| Urgent | 1204 (22) | 191 (18) | 102 (25) | 11 (23) | |
| Emergent | 55 (1.0) | 22 (2.1) | <11 (<2.7) | <11 (<23) | |
| Conduit | <.001 | ||||
| Dacron/PTFE | 1593 (29) | 327 (31) | 86 (21) | 15 (32) | |
| Below knee | 1032 (65) | 229 (70) | 68 (79) | < 11 (<23) | |
| Any vein* | 3661 (70) | 648 (67) | 271 (76) | 26 (63) | |
| Reversed GSV | 1480 (27) | 361 (35) | 160 (39) | 15 (32) | |
| In situ GSV | 1105 (20) | 124 (12) | 48 (12) | <11 (<23) | |
| Non-reversed transposed GSV | 802 (14) | 138 (13) | 50 (12) | <11 (<23) | |
| Other | 587 (11) | 93 (8.8) | 63 (15) | <11 (<23) | |
| Infrapopliteal bypass | 1999 (35) | 461 (44) | 208 (51) | 20 (42) | <.001 |
Abbreviations: IQR = interquartile range; COPD = chronic obstructive pulmonary disease; BMI = body mass index; CAD = coronary artery disease; CKD = chronic kidney disease; GSV = great saphenous vein; PTFE = Polytetrafluoroethylene;
*includes GSV, small saphenous vein, arm vein, or composite vein
Unadjusted 3-year outcomes
Amputation.
At 3 years, rates of major amputation were highest in Black patients at 32% followed by 27% in Hispanics, 20% in Asians, and 19% in Whites. Compared with White patients, Black patients had a higher hazard of amputation at 3 years (hazard ratio [HR]:1.9[95% confidence interval:1.7–2.2]), as did Hispanic patients (HR:1.6[1.3–2.0]). There was no significant difference between White and Asian patients in the hazard of 3-year amputation (HR:0.93[0.44–2.0]); however, the low number of Asian patients prevented meaningful assessment of amputation in this group (Table II; Figure IA).
Table II.
Unadjusted Cox regression for 3-year outcomes after open infrainguinal bypass by race/ethnicity with non-Hispanic White race as the reference group.
| Outcome | 3-year event rates (%) | uHR (95% CI) | P-value |
|---|---|---|---|
|
Amputation Non-Hispanic White Black Hispanic Asian |
19 32 27 20 |
Ref 1.9 (1.7–2.2) 1.6 (1.3–2.0) 0.93 (0.4–2.0) |
Ref <.001 <.001 .849 |
|
Re-intervention Non-Hispanic White Black Hispanic Asian |
57 61 70 55 |
Ref 1.2 (1.1–1.3) 1.4 (1.2–1.6) 0.79 (0.51–1.2) |
Ref .001 <.001 .298 |
|
Mortality Non-Hispanic White Black Hispanic Asian |
42 38 38 36 |
Ref 0.90 (0.81–0.99) 0.88 (0.76–1.0) 0.83 (0.52–1.3) |
Ref .026 .095 .423 |
|
MALE* Non-Hispanic White Black Hispanic Asian |
4.9 8.1 8.7 8.5 |
Ref 1.3 (1.2–1.4) 1.5 (1.3–1.7) 0.71 (0.46–1.1) |
<.001 <.001 0.12 |
*Data for MALE represent 30-day event rates and hazard ratios.
Abbreviations: uHR = unadjusted hazard ratio; CI = confidence interval; MALE = major adverse limb events
Figure I.
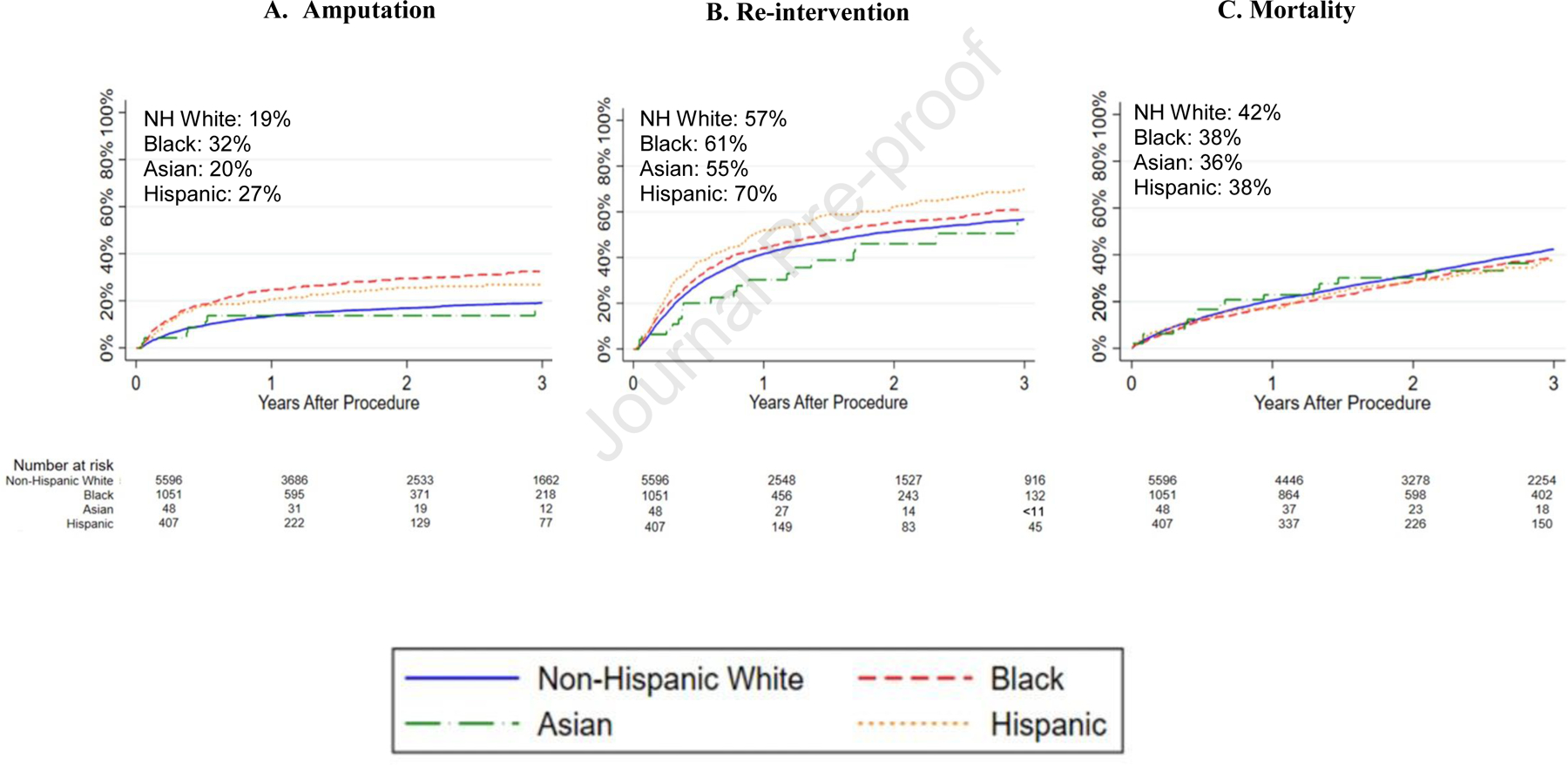
Kaplan-Meier estimates for 3-year amputation, re-intervention, and mortality after open infrainguinal bypass by race/ethnicity. All standard errors are <10%.
Re-intervention.
The re-intervention rates at 3 years were highest in Hispanic patients at 70% followed by 61% in Blacks, 57% in Whites, and 55% in Asians. Compared with White patients, both Black (HR:1.2[1.1–1.3]) and Hispanic patients (HR:1.4[1.2–1.6]) had a higher hazard of 3-year re-intervention. The low number of Asian patients prevented meaningful assessment of re-intervention rates in this group (HR:0.79[0.51–1.2]) (Table II; Figure IB).
Mortality.
At 3 years, the mortality rate was highest in White patients at 42% followed by Black and Hispanics at 38%, and Asians at 36%. Compared with White patients, Black patients had a lower hazard of mortality (38% vs 42%; HR:0.90[0.81–0.99]). However, the hazard of mortality was similar between Hispanic and White patients (HR:0.88[0.76–1.0]). The low number of Asian patients prevented meaningful assessment of mortality in this group (HR:0.83[0.52–1.3]) (Table II; Figure IC).
MALE.
At 30 days, MALE was highest among Hispanics (8.7%) followed by Asians (8.5%), Blacks (8.1%) and Whites (4.9%). The hazard of MALE was higher for Blacks (HR:1.3[1.2–1.4]) and Hispanics (HR:1.5[1.3–1.7]). The low number of Asian patients prevented meaningful assessment of MALE in this group (HR:0.71[0.46–1.1]) (Table II).
Adjusted analyses
Amputation.
In adjusted analyses, the associations between Black race and Hispanic ethnicity and amputation were attenuated after adjustment for demographic characteristics such as female sex and age, baseline comorbidities including insulin-dependent diabetes and CKD, and operative characteristics such as infrapopliteal bypass and prosthetic conduit. For Hispanic patients in particular, the disparity in 3-year amputation rates was partly explained by differences in disease severity including tissue loss and urgency status. After including all covariates in model 7, the association between Black race and amputation was attenuated, and the association between Hispanic ethnicity and amputation was no longer significant (Table III).
Table III.
Adjusted Cox regression models for 3-year major amputation after infrainguinal bypass. Race/ethnicity was included in all models, with non-Hispanic White as the reference group. For prosthetic conduit, vein conduit was used as the reference group. The second column shows the unadjusted hazard ratio (uHR) for each variable with respect to amputation. Subsequent columns present the adjusted HRs (aHR) for covariates included in each model. 95% confidence intervals are reported after HRs.
| Covariates | uHR | Model 1 aHR | Model 2 aHR | Model 3 aHR | Model 4 aHR | Model 5 aHR | Model 6 aHR | Model 7 aHR |
|---|---|---|---|---|---|---|---|---|
|
Race/ethnicity Black Hispanic Asian |
1.9 (1.7–2.2) 1.6 (1.3–2.0) 0.93 (0.44–2.0) |
1.7 (1.5–2.0) 1.5 (1.2–1.9) 0.96 (0.5–2.0) |
1.5 (1.3–1.7) 1.2 (1.0–1.5) 0.70 (0.33–1.5) |
1.9 (1.7–2.2) 1.6 (1.3–2.0) 0.93 (0.45–2.0) |
1.9 (1.7–2.2) 1.6 (1.3–1.9) 0.96 (0.46–2.0) |
1.9 (1.7–2.2) 1.5 (1.2–1.9) 0.83 (0.40–1.8) |
1.8 (1.6–2.1) 1.6 (1.3–2.0) 0.91 (0.41–2.0) |
1.4 (1.2–1.6) 1.2 (0.96–1.5) 0.69 (0.33–1.5) |
|
Demographic factors Female sex Age (years) |
0.91 (0.81–1.0) 0.98 (0.97–0.98) |
0.91 (0.8–1.0) 0.98 (0.98–0.99) |
– |
– |
– |
– |
– |
0.95 (0.83–1.1) 0.98 (0.98–0.99) |
|
Comorbidities Current smoker Diabetes Diabetes, insulin dependent CKD Preoperative dialysis |
0.96 (0.86–1.1) 1.7 (1.5–1.9) 1.8 (1.6–2.0) 2.3 (2.1–2.6) 2.7 (2.4–3.1) |
– |
1.1 (0.97–1.2) 1.3 (1.1–1.5) 1.4 (1.2–1.6) 1.3 (1.04–1.6) 1.7 (1.3–2.2) |
– | – | – | – |
1.0 (0.87–1.2) 1.2 (1.04–1.5) 1.2 (1.02–1.4) 1.3 (0.95–1.8) 1.4 (1.0–2.1) |
|
Optimal medical therapy Aspirin and statin |
0.92 (0.83–1.0) |
– |
– |
0.90 (0.81–1.01) |
– |
– |
– |
0.87 (0.78–0.98) |
|
Environmental factorsa Center volume Low center volume Medium center volume High center volume Surgeon volume Low surgeon volume Medium surgeon volume High surgeon volume |
Ref 1.1 (0.90–1.3) 1.1 (0.86–1.4) Ref 0.98 (0.86–1.1) 0.99 (0.86–1.1) |
– | – | – |
Ref 1.1 (0.92–1.3) 1.2 (0.97–1.4) Ref 0.95 (0.83–1.1) 0.97 (0.81–1.2) |
– | – |
Ref 1.0 (0.85–1.2) 1.0 (0.82–1.3) Ref 0.98 (0.85–1.2) 0.99 (0.82–1.3) |
|
Disease severity Rest pain Tissue loss Urgency status Elective Urgent Emergent |
Ref 1.7 (1.5–2.0) Ref 1.4 (1.3–1.6) 2.1 (1.4–3.1) |
– | – | – | – |
Ref 1.7 (1.5–1.9) Ref 1.4 (1.2–1.5) 2.0 (1.4–3.0) |
– |
Ref 1.4 (1.2–1.6) Ref 1.3 (1.1–1.5) 2.2 (1.6–3.2) |
|
Operative factors Infrapopliteal bypass Prosthetic conduit |
1.6 (1.4–1.7) 1.0 (0.92–1.2) |
– | – | – | – | – |
1.6 (1.4–1.8) 1.2 (1.04–1.4) |
1.4 (1.3–1.6) 1.3 (1.1–1.5) |
Re-intervention.
The higher rates of re-intervention for Black and Hispanic patients were also partly explained by demographic characteristics and comorbidities noted above. Additionally, for Hispanic patients, differences in center and surgeon volume, and operative factors partly explained the disparity in re-intervention rates. After including all covariates in model 7, Black race was no longer significantly associated with re-intervention. For Hispanic patients, this association was attenuated (Table IV).
Table IV.
Adjusted Cox regression models for 3-year re-intervention after infrainguinal bypass. Race/ethnicity was included in all models, with non-Hispanic White as the reference group. For prosthetic conduit, vein conduit was used as the reference group. The second column shows the unadjusted hazard ratio (uHR) for each variable with respect to re-intervention. Subsequent columns present the adjusted HRs (aHR) for covariates included in each model. 95% confidence intervals are reported after HRs.
| Covariates | uHR | Model 1 aHR | Model 2 aHR | Model 3 aHR | Model 4 aHR | Model 5 aHR | Model 6 aHR | Model 7 aHR |
|---|---|---|---|---|---|---|---|---|
|
Race/ethnicity Black Hispanic Asian |
1.2 (1.1–1.3) 1.4 (1.2–1.6) 0.79 (0.51–1.2) |
1.1 (1.02–1.2) 1.3 (1.2–1.5) 0.80 (0.51–1.2) |
1.1 (0.97–1.2) 1.3 (1.1–1.5) 0.73 (0.47–1.1) |
1.2 (1.1–1.3) 1.4 (1.2–1.6) 0.79 (0.51–1.2) |
1.2 (1.1–1.3) 1.3 (1.1–1.6) 0.78 (0.5–1.2) |
1.2 (1.1–1.3) 1.4 (1.1–1.6) 0.78 (0.51–1.2) |
1.2 (1.05–1.3) 1.3 (1.1–1.6) 0.80 (0.50–1.3) |
1.0 (0.94–1.2) 1.2 (1.01–1.4) 0.73 (0.47–1.1) |
|
Demographic factors Female sex Age (years) |
1.0 (0.93–1.1) 0.99 (0.99–0.99) |
1.01 (0.94–1.1) 0.99 (0.99–1.0) |
– |
– |
– |
– |
– |
1.03 (0.95–1.1) 0.99 (0.99–1.0) |
|
Comorbidities Current smoker Diabetes Diabetes, insulin dependent CKD Preoperative dialysis |
0.96 (0.90–1.0) 1.1 (1.1–1.2) 1.2 (1.1–1.3) 1.3 (1.2–1.5) 1.5 (1.3–1.6) |
– |
1.0 (0.93–1.1) 1.0 (0.94–1.1) 1.1 (1.0–1.2) 1.1 (0.91–1.2) 1.3 (1.1–1.6) |
– | – | – | – |
0.95 (0.88–1.03) 0.99 (0.89–1.1) 1.1 (0.96–1.2) 1.0 (0.91–1.2) 1.3 (1.1–1.5) |
|
Optimal medical therapy Aspirin and statin |
1.1 (1.02–1.2) |
– |
– |
1.1 (1.02–1.2) |
– |
– |
– |
1.1 (1.03–1.2) |
|
Environmental factorsa Center volume Low center volume Medium center volume High center volume Surgeon volume Low surgeon volume Medium surgeon volume High surgeon volume |
Ref 0.96 (0.88–1.1) 1.0 (0.80–1.2) Ref 0.93 (0.86–1.0) 0.82 (0.72–0.94) |
– | – | – |
Ref 1.0 (0.93–1.1) 1.1 (0.97–1.3) Ref 0.92 (0.83–1.01) 0.78 (0.67–0.92) |
– | – |
Ref 1.0 (0.91–1.1) 1.1 (0.97–1.3) Ref 0.92 (0.84–1.0) 0.78 (0.67–0.92) |
|
Disease severity Rest pain Tissue loss Urgency status Elective Urgent Emergent |
Ref 1.0 (0.97–1.1) Ref 1.1 (0.97–1.1) 1.4 (1.01–1.8) |
– | – | – | – |
Ref 1.0 (0.96–1.1) Ref 1.0 (0.97–1.1) 1.3 (0.99–1.8) |
– |
Ref 0.98 (0.91–1.1) Ref 1.0 (0.96–1.1) 1.4 (1.03–1.9) |
|
Operative factors Infrapopliteal bypass Prosthetic conduit |
1.1 (1.1–1.2) 0.86 (0.80–0.93) |
– | – | – | – | – |
1.1 (1.02–1.2) 0.89 (0.82–0.96) |
1.2 (1.00–1.2) 0.88 (0.79–0.98) |
Mortality.
The lower mortality in Black patients was partly explained by female sex and age. The association was further attenuated after adjusting for comorbidities. After including all covariates in model 7, the association between Black race and mortality remained significantly lower. Although there was no association between Hispanic ethnicity and mortality, after adjusting for comorbidities and disease severity, Hispanic ethnicity was associated with lower mortality. After including all covariates in model 7, Hispanic ethnicity remained associated with lower mortality (Table V).
Table V.
Adjusted Cox regression models for 3-year mortality. Race/ethnicity was included in all models, with non-Hispanic White as the reference group. For prosthetic conduit, vein conduit was used as the reference group. The second column shows the unadjusted hazard ratio (uHR) for each variable with respect to mortality. Subsequent columns present the adjusted HRs (aHR) for covariates included in each model. 95% confidence intervals are reported after HRs.
| Covariates | uHR | Model 1 aHR | Model 2 aHR | Model 3 aHR | Model 4 aHR | Model 5 aHR | Model 6 aHR | Model 7 aHR |
|---|---|---|---|---|---|---|---|---|
|
Race/ethnicity Black Hispanic Asian |
0.90 (0.81–0.99) 0.88 (0.76–1.0) 0.83 (0.52–1.3) |
1.0 (0.92–1.1) 0.96 (0.82–1.1) 0.80 (0.50–1.3) |
0.72 (0.65–0.80) 0.72 (0.62–0.84) 0.63 (0.40–1.0) |
0.90 (0.82–0.99) 0.88 (0.75–1.0) 0.83 (0.48–1.4) |
0.90 (0.82–0.99) 0.88 (0.75–1.03) 0.84 (0.49–1.4) |
0.90 (0.81–0.99) 0.83 (0.72–0.97) 0.73 (0.46–1.2) |
0.88 (0.80–0.98) 0.88 (0.75–1.03) 0.80 (0.48–1.3) |
0.77 (0.68–0.87) 0.77 (0.66–0.88) 0.46 (0.25–0.87) |
|
Demographic factors Female sex Age (years) |
0.92 (0.86–0.98) 1.03 (1.02–1.03) |
0.87 (0.81–0.93) 1.03 (1.02–1.03) |
– |
– |
– |
– |
– |
0.93 (0.86–01.01) 1.04 (1.03–1.04) |
|
Comorbidities CAD Current smoker Diabetes Diabetes, insulin dependent CKD Preoperative dialysis |
1.5 (1.4–1.6) 0.75 (0.70–0.80) 1.2 (1.2–1.3) 1.2 (1.1–1.3) 2.5 (2.3–2.7) 2.6 (2.4–2.9) |
– |
1.3 (1.3–1.4) 0.85 (0.79–0.91) 1.1 (0.97–1.1) 0.95 (0.88–1.0) 1.9 (1.7–2.1) 1.5 (1.3–1.8) |
– | – | – | – |
1.3 (1.2–1.4) 1.2 (1.1–1.3) 1.1 (0.98–1.2) 1.1 (0.96–1.1) 1.7 (1.5–1.9) 2.1 (1.8–2.4) |
|
Optimal medical therapy Aspirin and statin |
0.91 (0.86–0.97) |
– |
– |
0.92 (0.86–0.98) |
– |
– |
– |
0.91 (0.84–0.98) |
|
Environmental factorsa Center volume Low center volume Medium center volume High center volume Surgeon volume Low surgeon volume Medium surgeon volume High surgeon volume |
Ref 1.0 (0.95–1.1) 1.1 (0.98–1.2) Ref 1.0 (0.94–1.1) 1.0 (0.95–1.1) |
– | – | – |
Ref 1.0 (0.95–1.1) 1.1 (0.95–1.2) Ref 1.0 (0.93–1.1) 1.0 (0.90–1.1) |
- | – |
Ref 1.0 (0.95–1.1) 1.0 (0.90–1.1) Ref 1.0 (0.93–1.1) 0.98 (0.87–1.1) |
|
Disease severity Rest pain Tissue loss Urgency status Elective Urgent Emergent |
Ref 1.8 (1.7–1.9) Ref 1.2 (1.1–1.3) 1.6 (1.2–2.1) |
– | – | – | – |
Ref 1.8 (1.7–1.9) Ref 1.2 (1.1–1.3) 1.6 (1.2–2.2) |
– |
1.5 (1.4–1.7) Ref 1.2 (1.1–1.3) 2.2 (1.5–3.0) |
|
Operative factors Infrapopliteal bypass Prosthetic conduit |
1.1 (0.99–1.1) 1.2 (1.2–1.3) |
– | – | – | – | – |
1.1 (1.03–1.2) 1.3 (1.2–1.4) |
0.99 (0.92–1.1) 1.3 (1.2–1.4) |
Sensitivity analyses
We performed 3 sensitivity analyses to assess the robustness of our findings. First, when we excluded patients who underwent more than 1 index bypass procedure during the study period, the associations between race/ethnicity and each primary outcome were unchanged (Supplemental Table III). Second, when we varied the proportion of contralateral amputation or re-intervention from 0% to 50% equally across racial/ethnic groups, the associations between these events and Black race or Hispanic ethnicity were unchanged. Third, when we varied the proportion of contralateral amputation from 0% to 50% differentially across racial/ethnic groups, the association between amputation and Black race or Hispanic ethnicity remained significant until there was at least a 30% difference in the proportion of contralateral amputation between groups. When we varied the proportion of contralateral re-intervention from 0% to 50% differentially across racial/ethnic groups, the association between re-intervention and Black race was no longer significant after at least a 10% difference in the proportion of contralateral re-intervention. The association between re-intervention and Hispanic ethnicity was no longer significant after at least a 20% difference in the proportion of contralateral re-intervention (Supplemental Table IV).
Discussion:
In this observational study of VQI-Medicare patients who underwent infrainguinal bypass for CLTI, we identified disparities in 3-year outcomes by race and ethnicity. Compared with White patients, Black and Hispanic patients experienced higher rates of major amputation and re-intervention at 3 years. However, 3-year mortality was lower in Black patients compared with White patients, and there was no significant difference in mortality between White and Hispanic patients. Racial and ethnic disparities in amputation and re-intervention rates were partly attributable to differences in demographic characteristics and the higher prevalence of comorbidities in Black and Hispanic patients. Overall, our findings suggest that the higher burden of comorbidities in Black and Hispanic patients with CLTI was associated with higher 3-year amputation and re-intervention after infrainguinal bypass.
Our data are consistent with previous studies documenting disparities in amputation rates among CLTI patients. In a study using 2011–2015 Medicare data, Black patients with an initial primary CLTI diagnosis more frequently underwent primary major amputation compared with Whites (10% vs 4%; p<0.001), which was partly explained by a higher prevalence of gangrene in Black patients (36% vs 22%; p<0.001).2 In our study, we observed higher rates of major amputation in Black patients compared with White patients even among CLTI patients who underwent infrainguinal bypass. Our adjusted analyses demonstrated that tissue loss partly explained the higher rate of 3-year amputation in Hispanic patients, but not in Black patients. Among Black CLTI patients, the disparity in amputation rates was partly explained by demographic characteristics and comorbidities. Disparities remained after adjustment for all covariates, suggesting that there are other factors contributing to these disparities that we could not assess in this study.11 Further research is needed to determine the relative contribution of various factors to racial/ethnic disparities in amputation rates.
We also observed that Black and Hispanic patients had higher rates of re-intervention compared with White patients. In a study assessing outcomes after limb salvage procedures among 16,249 CLTI patients, Black patients were found to have higher 2-year re-intervention rates compared with White patients (34% vs 32%; p=0.05), but there was no difference between Hispanic and White patients (32% vs 30%; p=0.3).12 This study included CLTI patients who underwent open and endovascular revascularizations which may explain the differences from our current findings. It remains unclear if the higher rates of re-intervention in these populations represent higher rates of graft failure, or progressive disease above or below the graft. However, the finding that Black and Hispanic patients had higher rates of 3-year major amputation despite having higher rates of re-intervention represents an important disparity. Further research is needed to assess if differences in intervention strategy or graft surveillance across groups contribute to disparities in rates of re-intervention among CLTI patients.
Importantly, we found that Black patients had lower 3-year mortality after revascularization for CLTI compared with White patients. After adjusting for female sex and age, the lower hazard of 3-year mortality in Black patients was no longer significant. In contrast, after adjusting for comorbidities, the hazard of mortality for Black patients was even lower. These data suggest that Black CLTI patients may have lower mortality because they are more likely to be female and diagnosed with CLTI at younger ages. The finding that mortality is lower in Black patients despite higher amputation is paradoxical. Several prior studies have demonstrated that Black patients have lower mortality despite also having higher rates of amputation after lower extremity revascularization.13–15 This difference was attributed to Black patients being younger and more likely to be female. It was also attributed to differences in the distribution of comorbidities, as Black patients were more likely to have diabetes, but less likely to have vascular disease in other territories.13,14 Our study confirms these findings and demonstrates that this paradox continues to exist 3 years after infrainguinal bypass.
Previous work has demonstrated that access to preventative care and multidisciplinary care including podiatry and vascular surgery may reduce amputation rates.16–18 Furthermore, the concept of evidence-based revascularization (EBR) has been developed as part of the Global Vascular Guidelines to improve the quality of vascular care and reduce disparities in treatment and outcomes. To standardize the approach for caring for CLTI patients, EBR consists of a structured management framework based on patient risk, limb severity, and anatomic patterns of disease.19 An evidence-based approach is fundamental to reducing outcome disparities for CLTI patients.
Black and Hispanic patients had higher rates of comorbidities despite being more likely to be on antiplatelet and statin therapy. Although we do not have information on how well comorbidities are managed, previous work has highlighted the undertreatment of comorbidities such as hypertension and diabetes in racial/ethnic minority patients.20–22 The higher rates of comorbidities could reflect inadequate medication dosing or underutilization of prescribed medications; however, we were unable to assess these factors in the current database. Given that the disparities in limb outcomes observed in our study were partly explained by the higher rate of comorbidities among Black and Hispanic patients, interventions to strengthen the medical management of these conditions may improve outcomes.
Our study was subject to certain limitations. First, the VQI is a quality improvement registry with voluntary physician and center participation and may not be nationally representative.23 This selection bias may lead to underrepresentation of certain racial/ethnic groups and/or underestimation of disparities in outcomes compared with a more general database. Second, these data may not be generalizable to young patients, as Medicare only includes patients over age 65, and select patients under age 65. Third, due to the low number of Asian patients, we were unable to meaningfully assess outcomes in this population, and these data should be updated as more patients become available. Fourth, we were unable to assess the degree to which comorbidities were adequately managed (e.g., HbA1c), or the severity of tissue loss as these data were not available. We were also unable to determine laterality of amputations and re-interventions as these data are not available. Our sensitivity analyses showed that the findings with respect to amputation were robust despite this limitation. For re-intervention, the effect measures were smaller. Therefore, differences in contralateral events may impact these results. Of note, 5% of patients underwent multiple index bypass procedures, which could indicate more aggressive disease. Because we could not determine the chronology of the procedures, we excluded these patients as a sensitivity analysis, and our outcomes were unchanged. Lastly, although we adjusted for relevant covariates that may impact outcomes, we were unable account for individual surgeon decision making or important social determinants of health such as socioeconomic status, geographical location, education level, or food insecurity.24,25
Conclusions:
In conclusion, compared with White patients, Black and Hispanic patients had higher 3-year amputation and re-intervention rates after infrainguinal bypass for CLTI in the VQI-Medicare linked population. However, mortality was lower among Black patients and similar between Hispanic and White patients. Disparities in amputation and re-intervention rates were partly attributable to demographic characteristics and the higher prevalence of comorbidities in Black and Hispanic patients with CLTI. Future work is necessary to determine if interventions to improve access to care and reduce the burden of comorbidities in these populations confer limb salvage benefits.
Supplementary Material
Supplemental Table I. Vascular Quality Initiative (VQI) variable definitions
Supplemental Table II. Current procedural terminology (CPT) codes for re-intervention
Supplemental Table III. Unadjusted Cox regression for long-term outcomes after open infrainguinal bypass by race/ethnicity with non-Hispanic White race as the reference group. Patients who underwent more than 1 index bypass procedure during the study period were excluded.
Supplemental Table IV. Unadjusted Cox regression for 3-year amputation and re-intervention with the rate of contralateral major adverse limb events ranging from 0–50%, with Non-Hispanic White race as reference group. uHR = adjusted hazard ratio; CI = confidence interval.
ARTICLE HIGHLIGHTS.
Type of Research:
Retrospective cohort study of prospectively collected data from the Vascular Quality Initiative registry with Medicare-linkage for 3-year outcomes
Key Findings:
From 2003–2017, 7,108 bypass procedures were performed in CLTI patients (79% White, 15% Black, 6% Hispanic, 1% Asian). Compared with White patients, Black and Hispanic patients had higher 3-year major amputation and re-intervention rates; however, mortality was lower among Black patients and similar between Hispanic and White patients.
Take home Message:
Disparities in major amputation and re-intervention rates after infrainguinal bypass are partly attributable to patients’ age and sex, and the higher prevalence of comorbidities in Black and Hispanic patients with CLTI.
Table of Contents Summary:
Compared with White patients, Black and Hispanic patients had higher 3-year major amputation and re-intervention after infrainguinal bypass for CLTI. Mortality was lower among Blacks and similar between Hispanics and Whites. Future work is necessary to determine if reducing the burden of comorbidities in these populations confer limb salvage benefits.
ACKNOWLEDGEMENTS
Data for this project was generated by the Vascular Implant Surveillance and Interventional Outcomes Network (VISION) (http://mdepinet.org/vision-crn/), a partnership between the Vascular Quality Initiative and the Medical Device Epidemiology Network (MDEpiNet), a Food and Drug Administration supported initiative aimed at developing distributed research networks. VISION links VQI data to Medicare claims to enhance long-term follow-up data in the VQI. Use of the VQI-Medicare linked data is governed by CMS Data Use Agreement 52144 and the Society for Vascular Surgery Patient Safety Organization.
FUNDING INFORMATION:
CM is supported by grant number F32HS027285 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. PP and SW are supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant 5T32HL007734. The study was partially supported by The Assistant Secretary for Planning and Evaluation (ASPE) and Patient-Centered Outcomes Research Trust Fund (PCORTF) of the U.S. Department of Health and Human Services under Interagency Agreement #750119PE060048, through the U.S. Food and Drug Administration (FDA) Grant (#U01FD006936). The funder had no influence on design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
PRESENTATION INFORMATION:
This study was accepted for a podium presentation at the 2022 Vascular Annual Meeting, Boston, MA, June 15–18, 2022.
CONFLICTS OF INTEREST:
None reported
References
- 1.Baser O, Verpillat P, Gabriel S, Wang L. Prevalence, incidence, and outcomes of critical limb ischemia in the US Medicare population. Vasc Dis Manag 2013;10(2):E26–36. [Google Scholar]
- 2.Mustapha JA, Katzen BT, Neville RF, Lookstein RA, Zeller T, Miller LE, et al. Determinants of Long-Term Outcomes and Costs in the Management of Critical Limb Ischemia: A Population-Based Cohort Study. J Am Heart Assoc 2018;7(16):e009724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. J Vasc Surg 2011. Feb;53(2):330–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal S, Pitcavage JM, Sud K, Thakkar B. Burden of Readmissions Among Patients With Critical Limb Ischemia. J Am Coll Cardiol 2017. Apr 18;69(15):1897–908. [DOI] [PubMed] [Google Scholar]
- 5.Malyar NM, Freisinger E, Meyborg M, Lüders F, Fürstenberg T, Kröger K, et al. Low Rates of Revascularization and High In-Hospital Mortality in Patients With Ischemic Lower Limb Amputation: Morbidity and Mortality of Ischemic Amputation. Angiology 2016;67(9):860–9. [DOI] [PubMed] [Google Scholar]
- 6.Mao J, Moore KO, Columbo JA, Mehta KS, Goodney PP, Sedrakyan A. Validation of an indirect linkage algorithm to combine registry data with Medicare claims. J Vasc Surg 2022. Feb 15. [DOI] [PMC free article] [PubMed]
- 7.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014. Dec;12(12):1495–9. [DOI] [PubMed] [Google Scholar]
- 8.de Guerre LEVM, Rice J, Cheng J, Li C, Dansey KD, Marcaccio C, et al. Racial Differences in Isolated Aortic, Concomitant Aortoiliac, and Isolated Iliac Aneurysms: This is a Retrospective Observational Study. Ann Surg 2020. Dec 29. [DOI] [PubMed]
- 9.Criteria Committee NYHAI. Diseases of the Heart and Blood Vessels. Nomenclature and Criteria for diagnosis 6th ed. Boston; 1964. 114 p. [Google Scholar]
- 10.Marcaccio CL, Patel PB, Wang S, Rastogi V, Moreira CC, Siracuse JJ, et al. Effect of postoperative antithrombotic therapy on lower extremity outcomes after infrapopliteal bypass for chronic limb-threatening ischemia. J Vasc Surg 2022. Jan 21. [DOI] [PubMed]
- 11.Flanagin A, Frey T, Christiansen SL, AMA Manual of Style Committee. Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA 2021;326(7):621–7. [DOI] [PubMed] [Google Scholar]
- 12.Mathlouthi A, Zarrintan S, Khan M-A, Malas M, Barleben A. Contemporary Outcomes of Limb-Salvage Procedures Using Vascular Quality Initiative-Medicare Linked Data: Racial and Ethnic Disparities Persist. J Vasc Surg 2022. Feb 8. [DOI] [PubMed]
- 13.O’Donnell TFX, Powell C, Deery SE, Darling JD, Hughes K, Giles KA, et al. Regional variation in racial disparities among patients with peripheral artery disease. J Vasc Surg 2018;68(2):519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brothers TE, Zhang J, Mauldin PD, Tonnessen BH, Robison JG, Vallabhaneni R, et al. Better survival for African and Hispanic/Latino Americans after infrainguinal revascularization in the Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg 2017. Apr;65(4):1062–73. [DOI] [PubMed] [Google Scholar]
- 15.Brothers TE, Robison JG, Sutherland SE, Elliott BM. Racial differences in operation for peripheral vascular disease: results of a population-based study. Cardiovasc Surg 1997. Feb;5(1):26–31. [DOI] [PubMed] [Google Scholar]
- 16.Larsson J, Apelqvist J, Agardh CD, Stenström A. Decreasing incidence of major amputation in diabetic patients: a consequence of a multidisciplinary foot care team approach? Diabet Med 1995. Sep;12(9):770–6. [DOI] [PubMed] [Google Scholar]
- 17.Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and Risk of Amputation in Patients With Diabetes Mellitus and Peripheral Artery Disease. Arterioscler Thromb Vasc Biol 2020;40(8):1808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pohlman FW, Ford CB, Weissler EH, Smerek MM, Hardy NC, Narcisse DI, et al. Impact of risk factor control on peripheral artery disease outcomes and health disparities. Vasc Med 2022. Apr 7;1358863X221084360. [DOI] [PMC free article] [PubMed]
- 19.Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg 2019. Jun;69(6S):3S–125S.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon SSS, Carroll MD, Fryar CD. Hypertension Prevalence and Control Among Adults: United States, 2011–2014. NCHS Data Brief 2015. Nov;(220):1–8. [PubMed] [Google Scholar]
- 21.Smalls BL, Ritchwood TD, Bishu KG, Egede LE. Racial/Ethnic Differences in Glycemic Control in Older Adults with Type 2 Diabetes: United States 2003–2014. Int J Environ Res Public Health 2020;17(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soden PA, Zettervall SL, Deery SE, Hughes K, Stoner MC, Goodney PP, et al. Black patients present with more severe vascular disease and a greater burden of risk factors than white patients at time of major vascular intervention. J Vasc Surg 2018;67(2):549–556.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dansey KD, de Guerre LEVM, Swerdlow NJ, Li C, Lu J, Patel PB, et al. A comparison of administrative data and quality improvement registries for abdominal aortic aneurysm repair. J Vasc Surg 2021;73(3):874–88. [DOI] [PubMed] [Google Scholar]
- 24.Cerdeña JP, Plaisime MV, Tsai J. From race-based to race-conscious medicine: how anti-racist uprisings call us to act. Lancet 2020;396(10257):1125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Social Determinants of Health. Healthy People 2020 Updated 06/02/2022. Available from: https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table I. Vascular Quality Initiative (VQI) variable definitions
Supplemental Table II. Current procedural terminology (CPT) codes for re-intervention
Supplemental Table III. Unadjusted Cox regression for long-term outcomes after open infrainguinal bypass by race/ethnicity with non-Hispanic White race as the reference group. Patients who underwent more than 1 index bypass procedure during the study period were excluded.
Supplemental Table IV. Unadjusted Cox regression for 3-year amputation and re-intervention with the rate of contralateral major adverse limb events ranging from 0–50%, with Non-Hispanic White race as reference group. uHR = adjusted hazard ratio; CI = confidence interval.


