Abstract
Background:
Tuberculosis (TB) preventive therapy (TPT) is recommended for people with HIV infection, including during pregnancy. The impact of TPT exposure at conception and during pregnancy is poorly documented.
Methods:
We report pregnancy outcomes among South African women with HIV enrolled in a randomized trial of 4 TPT regimens (two 3-month rifamycin-isoniazid regimens, isoniazid for 6 months, or isoniazid continuously). Descriptive statistics and risk ratios were assessed to examine relationships between study regimens and outcomes.
Results:
216/896 women (24%) conceived during the study. Women who conceived were younger (27.9 vs 31.3 years) and had higher CD4 counts (589.1 vs 536.7). The odds of pregnancy were higher in women in the rifamycin-isoniazid arms than the isoniazid arms (3HP: 1.73, p = 0.001; 3HR: 1.55, p = 0.017) despite increased contraceptive use compared to the standard 6H therapy. 34 women became pregnant while taking preventive treatment (8 rifamycin, 26 isoniazid monotherapy). Pregnancy outcomes in these women were: 17 (50%) mother/baby healthy, 3 (9%) spontaneous abortions, 6 (18%) elective abortions, 1 (3%) premature delivery, 2 (6%) neonatal deaths [1 rifamycin-isoniazid and 1 isoniazid], and 5 (15%) unknown.
Conclusions:
Pregnancy was common in women who had received TPT and more frequent in women who had received rifamycin-isoniazid-based regimens.
Keywords: HIV, tuberculosis, pregnancy, preventive therapy, rifapentine, rifampin, isoniazid
Introduction
Tuberculosis (TB) preventive therapy (TPT) is recommended for people living with HIV infection (PLHIV) 1. Short-course rifamycin-based regimens are recommended as an alternative to longer courses of isoniazid to enhance adherence. Although the World Health Organization (WHO) recommends TPT for pregnant women in high TB burden countries, most studies of TPT regimens in PLHIV have included women of reproductive age but have excluded pregnant women and recommended contraception to avoid conception during treatment. Thus, the frequency of incident pregnancies and pregnancy outcomes of women exposed to TPT regimens early during their pregnancy (i.e. periconception and 1st trimester) are not well described.
A recent large trial of isoniazid preventive therapy (IPT) in pregnant PLHIV found increased adverse outcomes in women who initiated IPT during the second trimester or third trimester compared to those who deferred IPT initiation until the postpartum period 2. Another recent study analyzing first trimester exposure to IPT observed a higher incidence of spontaneous abortions/pregnancy loss than those without IPT exposure 3. Other studies of pregnancy outcomes in women receiving TPT have not documented increased risk of adverse pregnancy outcomes 4–7. We assessed the frequency of incident pregnancies and pregnancy outcomes among women enrolled in a TPT trial in PLHIV in South Africa 8.
Methods
Study Population
The study population consisted of South African adults with HIV infection who were tuberculin skin test (TST)-positive and not receiving antiretroviral therapy (ART) at entry 8. Enrollment began in September 2002 and ended in June 2005 and follow up ended in November 2008. Treatment for HIV infection was not widely available in South Africa during the trial, but in 2004 ART became available to PLHIV whose CD4 was ≤ 200 cells/mm3.
The trial randomized 1148 adults to four treatment arms: rifapentine (900 mg) and isoniazid (900 mg) weekly for 12 weeks, directly observed (3HP), rifampin (600 mg) and isoniazid (900 mg) twice weekly for 12 weeks, directly observed (3HR), isoniazid (300 mg) daily throughout follow up, self-administered (H-Cont), and isoniazid (300 mg) daily self-administered for 6 months (6H), the control regimen. All patients also received pyridoxine (Vitamin B6) 25 mg with each isoniazid dose. Pregnancy was an exclusion criterion, and all women underwent a pregnancy test at enrollment and were encouraged to use a barrier form of contraception instead of or in addition to hormonal contraceptives. Women who conceived while receiving TPT, including both isoniazid and rifamycins, were offered continuation of isoniazid treatment to complete six months of TPT. Participants were followed through the end of the study to assess the primary outcome of TB free survival.
Pregnancy was considered a serious adverse event according to the NIAID/DAIDS adverse experience reporting system. We restricted this analysis to women younger than 50 years of age at entry since that is the approximate age of menopause and thus natural infertility9. Reports of missed menstruation prompted urine pregnancy tests. The date of the adverse event (pregnancy) report was the initial diagnosis of pregnancy, and the date of delivery was also recorded. Pregnancy outcomes were obtained by patient interview and review of medical records.
Definitions
Adverse pregnancy outcomes were defined as: spontaneous abortion (spontaneous termination of pregnancy < 24 weeks estimated gestational age (EGA)), elective abortion (chosen termination of pregnancy < 24 EGA), premature birth (EGA ≤ 37 weeks at birth), and early infant death (mortality within 28 days of delivery). Maternal death or serious morbidity related to pregnancy would have been included as adverse pregnancy outcomes. However, we had no instances of these during the trial.
Statistical Analyses
Continuous variables were analyzed using t-tests with Welch’s correction, not assuming equal SDs between the comparison groups. Bivariate relative risk ratios were calculated for variables of a priori interest, with p-values calculated using 2-sided Fisher’s exact test. Comparison groups included those who became pregnant throughout the trial vs those who did not and those who became pregnant between the 4 treatment arms, using 6H as the control. Covariate analysis on the frequency of pregnancy events between treatment arms was adjusted for age at enrollment, baseline CD4, and form of contraceptive method. Kaplan Meier curves were generated to analyze the timing of pregnancy events from study onset. Adverse outcomes were only collected for women who conceived while on study drug or within 2 weeks of finishing treatment. Analysis of pregnancy outcomes during the post-treatment phase was not performed due to incomplete pregnancy outcome data. Because of the small number of adverse outcomes, multivariable logistic regression models were not performed. Analyses were conducted using GraphPad Prism version 8.4.3 (GraphPad Software, San Diego, California USA).
Ethical considerations
The protocol was approved by the institutional review boards of Johns Hopkins Medicine and the University of the Witwatersrand. All participants provided written informed consent in English or their native language, based on their preference. The trial is registered at https://clinicaltrials.gov/ct2/show/NCT00057122.
Results
Of 1148 PLHIV, TST-positive participants enrolled, 896 (78.0%) were women <50 years of age without tubal ligation or hysterectomy. Most women were unmarried, Black Africans; most were unemployed and lived in a house or a shack (Table 1). Fewer than one-quarter of women reported consuming alcohol on a regular basis. None were on ART at baseline, and the mean CD4 count of women was 549.4 cell/mm3. In all of treatment arms, less than one-quarter of women started ART during the trial. Approximately 70% of women in each treatment arm reported contraceptive use throughout the trial. The leading form of contraceptive was barrier methods, followed by an injectable contraceptive.
Table 1.
Baseline Characteristics by Treatment Arm
| Participant Characteristics | 3HP N = 256 (%) | 3HR N = 252 (%) | 6H N = 260 (%) | H-Cont N = 128 (%) |
|---|---|---|---|---|
| Age, years, Mean (SD) | 29.9 (6.1) | 30.0 (5.5) | 30.3 (6.2) | 29.5 (5.6) |
| Baseline CD4, cell/mm3, Mean (SD) | 546 (262) | 569 (260) | 540 (237) | 537 (269) |
| Weight, kg, Mean (SD) | 67.3 (14.6) | 67.7 (13.9) | 67.5 (14.4) | 69.0 (15.0) |
| Height, cm, Mean (SD) | 158.9 (5.9) | 158.5 (5.5) | 159.3 (6.3) | 159.6 (6.8) |
| BMI, Mean (SD) | 26.5 (5.5) | 26.8 (5.2) | 26.8 (5.4) | 27.0 (5.6) |
| Race | ||||
| African/Black | 256 (100.0) | 251 (99.6) | 259 (99.6) | 128 (100.0) |
| Colored | 0 (0.0) | 1 (0.4) | 1 (0.4) | 0 (0.0) |
| Education | ||||
| None or Primary | 13 (5.1) | 12 (4.8) | 7 (2.7) | 11 (8.6) |
| Secondary and above | 162 (63.3) | 164 (65.1) | 159 (61.2) | 73 (57.0) |
| Grade 12/ Degree/ Diploma | 81 (31.6) | 76 (30.2) | 94 (36.1) | 44 (34.4) |
| Marital status | ||||
| Married/Living Together | 50 (19.6) | 54 (21.4) | 41 (15.8) | 22 (17.1) |
| Divorced/Separated/Widowed | 8 (3.1) | 11 (4.3) | 9 (3.5) | 4 (3.1) |
| Single | 198 (77.3) | 187 (74.2) | 210 (80.8) | 102 (79.7) |
| Separated | 2 (0.8) | 3 (1.2) | 2 (0.8) | 0 (0.0) |
| Employment Status | ||||
| Employed | 28 (10.9) | 28 (11.1) | 26 (10.0) | 12 (9.4) |
| Unemployed/Other | 228 (89.1) | 224 (88.9) | 234 (90.0) | 116 (90.6) |
| Smoking | 43 (16.8) | 35 (13.9) | 49 (18.8) | 20 (15.6) |
| Alcohol Consumption | 45 (17.6) | 45 (17.9) | 66 (25.4) | 29 (22.7) |
| Contraceptive Use | ||||
| Overall | 173 (67.6) | 169 (67.1) | 190 (73.1) | 89 (69.5) |
| Injectable | 104 (40.6) | 92 (36.5) | 108 (41.5) | 57 (44.5) |
| Oral contraceptive | 11 (4.3) | 12 (4.8) | 12 (4.6) | 3 (2.3) |
| Loop/IUCD | 1 (0.4) | 1 (0.4) | 2 (0.8) | 1 (0.8) |
| Barrier (condom, cervical cap, diaphragm) | 136 (53.1) | 132 (52.4) | 151 (58.1) | 74 (57.8) |
| ART | ||||
| Overall | 45 (17.6) | 46 (18.3) | 46 (17.7) | 26 (20.3) |
| ART during pregnancy | 4 (1.6) | 9 (3.6) | 6 (2.3) | 2 (1.6) |
| ART at contraception | 2 (0.8) | 6 (2.4) | 4 (1.5) | 2 (1.6) |
During follow up, there were 235 pregnancies among 213 women (22.3%); 22 women conceived twice. Few (1.6%) women were on ART at conception and 2.3% of women who became pregnant during the trial received ART during their pregnancy (Table 1). Women who became pregnant were younger (27.9 vs 31.3 years, p < 0.001) and had higher mean baseline CD4 counts (589 vs 537, p = 0.011) than women who did not (Table 2). Women in the 3HP and 3HR arms were more likely to become pregnant than those in the 6H or H-Cont arms, using 6H as the reference category (3HP RR = 1.73, p = 0.001; 3HR: RR = 1.55, p = 0.003; Table 3). After adjusting for age at enrollment, baseline CD4, and type of contraceptive method, the increased risk of pregnancy in the rifamycin-isoniazid arms remained statistically significant, though most pregnancies occurred after study medication had been stopped.
Table 2.
Characteristics of Pregnancy vs No Pregnancy Events
| Pregnancy Events Mean (N) | No Pregnancy Events Mean (N) | 95% CI (no event – event) | P Value | |
|---|---|---|---|---|
| Age (years) | 27.87 (212) | 30.64 (684) | 2.02, 3.52 | <0.001 |
| Baseline CD4 (cell/mm3) | 588.8 (212) | 537.1 (682) | −92.48, −10.96 | 0.013 |
| Weight (kilos) | 67.37 (212) | 67.78 (684) | −1.86, 2.68 | 0.723 |
| Height (cm) | 159.0 (183) | 159.0 (581) | −0.95, 1.07 | 0.906 |
| BMI | 26.71 (183) | 26.76 (581) | −0.91, 0.99 | 0.931 |
Table 3.
Relative Risk of Pregnancy Events among Treatment Arms
| Treatment Arm | Pregnancy Events n/N (%) | RR (95% CI) | P value | Adjusted** RR (95% CI) | P value |
|---|---|---|---|---|---|
| All Participants | 212/896 (23.6%) | -- | -- | -- | -- |
|
| |||||
| 6H* | 44/260 (16.9%) | -- | -- | -- | -- |
| 3HP | 75 /256 (29.3%) | 1.73 (1.25, 2.41) | 0.001 | 1.69 (1.23, 2.34) | 0.001 |
| 3HR | 66/252 (26.2%) | 1.55 (1.10, 2.18) | 0.013 | 1.51 (1.08, 2.10) | 0.017 |
| H-Cont | 27/128 (21.1%) | 1.25 (0.81, 1.90) | 0.331 | 1.21 (0.79, 1.85) | 0.375 |
Reference Category
Adjusted for age at enrollment; baseline CD4; injectable, oral, barrier contraceptive methods. Based on 894 women; two were missing baseline CD4.
Supplemental Table 1 depicts the number of women in each treatment arm who became pregnant while using some form of contraception. There were no appreciable differences in pregnancy events between the treatments arm when further stratified by contraceptive type.
To calculate the relative risk of pregnancy events, the proportion of pregnancy events in each treatment arm was compared to the 6H arm, the standard therapy, via Fisher’s exact test. Stratified relative risk ratios for each arm were also generated for pregnancy events that occurred for women who used contraceptives, and then by contraceptive subtype via the same comparison to the 6H standard therapy (Figure 1). Pregnancy events in both rifamycin-isoniazid combination arms were associated with increased overall contraceptive use (3HP vs 6H: RR = 1.76 (95% CI 1.21-2.59), p = 0.004; 3HR: RR = 1.53 (95% CI 1.03-2.28), p = 0.04). When stratified by contraceptive method, only barrier contraceptive use in the 3HP arm was associated with an increased risk of pregnancy (RR = 1.75 (95% CI 1.14-2.70), p = 0.012). However, few women reported using intrauterine devices or oral contraceptives.
Figure 1.

Forest plot of relative risk of pregnancy by treatment arm and type of contraception compared to the control arm, 6H.
Analysis of women who became pregnant while taking TPT
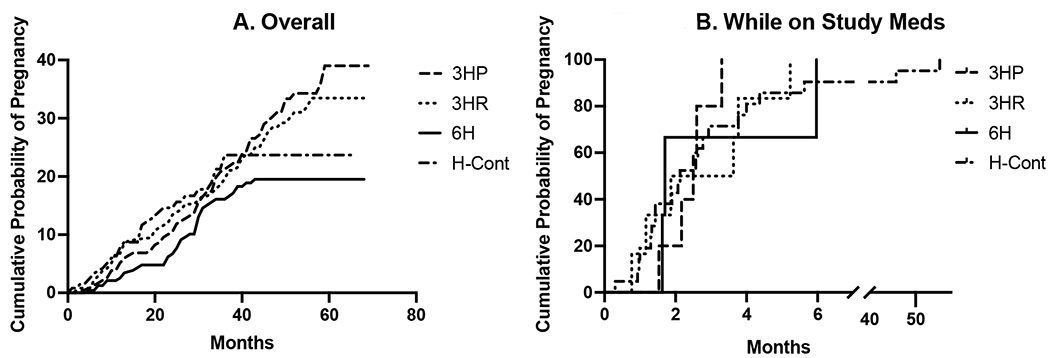
The median time to pregnancy from randomization was 25 months (IQR 24.0 months) across all treatment arms (Supplemental Table 2). Although the trial excluded pregnant women, 9 women appeared to have conceived before the date of enrollment (4 in 3HP; 3 in 3HR, 1 in 6H, 1 in H-cont). A total of 34 women were taking TPT while pregnant, including those who conceived before enrollment. The time from study onset to pregnancy events is depicted in Supplemental Figure 1A, for all women and Supplemental Figure 1B for women who were receiving TPT at the time of conception. For the women receiving TPT at the time of conception, the median time on study medication was 2.1 months.
Of the 34 (15.7%) pregnancies where conception likely occurred while women were receiving a TPT regimen, pregnancy outcomes are known for 29. Women with unknown pregnancy outcomes were similar to women with known outcomes in terms of age, baseline CD4, weight, height, BMI and months of isoniazid after conception. Seventeen (50%) women had healthy deliveries, 3 (8.8%) women had spontaneous abortions, 6 (17.6%) had elective abortions, 1 infant (2.9%) was premature, and 2 (5.9%) infants died – one each in the 3HR arm and H-Cont arm. Six women had adverse pregnancy outcomes by the above definition. When stratified by treatment arm, no adverse pregnancy outcomes occurred in the 3HP arm, 2 in the 3HR arm, 1 in the 6H arm, and 3 in the H-Cont arm (Table 4). No congenital anomalies were reported. None of the pregnant women had prior TB nor subsequently developed TB.
Table 4.
Delivery Outcomes for Pregnancies Occurring During Study Treatment by Arm
| Count of Delivery | Delivery Results | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Arm | Healthy | Spontaneous Abortion | Elective Abortion | Premature birth | Infant Death | Unknown | Grand Total |
| 3HP | 1 (2.9%) | 2 (5.9%) | 1 (2.9%) | 4 (11.8%) | |||
| 3HR | 2 (5.9%) | 1 (2.9%) | 1 (2.9%) | 4 (11.8%) | |||
| 6H | 1 (2.9%) | 1 (2.9%) | 1 (2.9%) | 3 (8.8%) | |||
| H-Cont | 13 (38.2%) | 2 (5.9%) | 3 (8.8%) | 1 (2.9%) | 4 (11.8%) | 23 (67.6%) | |
|
| |||||||
| Grand Total | 17 (50.0%) | 3 (8.8%) | 6 (17.6%) | 1 (2.9%) | 2 (5.9%) | 5 (14.7%) | 34 (100%) |
The average duration of exposure to isoniazid after conception was three months. There was no statistically significant difference between post-conception INH exposure by arm. There was also no difference between women with known and unknown pregnancy outcomes in term of age, baseline CD4, weight, height, BMI, and months of isoniazid after conception. Treatment arm was not associated with knowing pregnancy outcomes.
Discussion
This secondary analysis of the pregnancy in PLHIV enrolled in a randomized trial of TPT found that women receiving rifapentine- or rifampin- containing regimens had higher rates of subsequent pregnancy than women receiving isoniazid alone, though few pregnancies occurred while on study treatment. This could be caused by an increase in fertility associated with rifamycin-isoniazid regimens or a decrease in fertility associated with isoniazid only regimens. If women attempted conception once treatment was completed, the shorter administration period of rifamycin-isoniazid regimens compared to isoniazid alone would have increased the time in which they could have gotten pregnant during the trial. However, the higher rate of pregnancy persisted beyond the six months of treatment with the control isoniazid regimen, as well. Also, drug-drug interactions between rifamycins and hormonal contraceptives could have resulted in lower hormone concentrations which may have affected fertility, though this effect reverses within several weeks of stopping rifamycins.10
Alternatively, this could be driven by a decrease in fertility associated with more prolonged exposure to isoniazid. Some human and animal studies have reported observed fetal demise in association with earlier gestational exposure to IPT, which may have played a role in the observed differences in fertility rates, especially for women in the H-Cont arm 2,3,11. Furthermore, some pregnancies may have been missed altogether due to early trimester loses, especially if the pregnancy and loss occurred between follow up appointments. However, the literature is controversial and further large-scale analyses on the safety of IPT during the different trimesters of pregnancy are needed.
The proportion of fetal loss of 8.8%, as defined by spontaneous abortion, was lower than the 13-14% reported by Moro and colleagues for women enrolled in two other 3HP trials 5, and less than that reported for pregnancies among women with HIV in the USA 12. Although findings from the Tshepiso Cohort, an observational study of TB and HIV co-infection in pregnant women from Soweto 4, did not find that IPT exposure during pregnancy was associated with adverse pregnancy outcomes, other studies have. For example, women with HIV infection taking isoniazid at the time of conception in the BRIEF-TB study had a doubling of the risk of spontaneous abortion compared to women who became pregnant after isoniazid was completed 3. The APPRISE study, which enrolled women in their second trimester and beyond, also reported an increased risk of adverse pregnancy outcomes largely due to fetal demise, when isoniazid-based TPT was given during pregnancy as opposed to postpartum 2. Others found that neither long term isoniazid TPT nor ART exposure during pregnancy were significantly associated with adverse pregnancy outcomes, but these were non-randomized studies 13. Given the challenges with ascertaining early pregnancy loss in general and the small number of reported pregnancies that occurred on TPT in our study, it is difficult to confirm the reason(s) for observed differences in incidence of pregnancies between the rifamycin-isoniazid arms and the isoniazid alone arms.
The safety and efficacy of rifapentine and rifampin prophylaxis in pregnant women has not been as thoroughly studied as isoniazid prophylaxis. Several safety analyses studies of pregnant women revealed that 3HP was not associated with an increased risk of adverse pregnancy outcomes 5,14,15. In terms of interactions with hormonal contraceptives, rifamycins were associated with increased frequency of ovulation and reduced estrogen/progestin exposure, although none of the studies directly measured pregnancy risk 16 17. Rifamycin upregulation of cytochrome p450 enzymes could be driving this effect 6. Future exploration would be to study fecundity associated with different TB treatment drugs.
Although most clinical trials exclude women who are pregnant or planning pregnancy and require female participants to be on barrier forms of birth control, women do conceive during clinical trials 18. Our trial saw a particularly high rate of pregnancies, 22%. Other studies of TPT have seen rates of pregnancies of 10% 19 - 19% 13. Given this, it is no longer appropriate to actively exclude or prohibit pregnant women from being evaluated during these trials. Instead, it is time to recognize that pregnancy will occur and take measures to directly study how TPT directly affects these women and their infants.
This study gives unique insight on the effect of four different TPT arms during pregnancy. The most prominent limitation was the low sample size. The TPT regimens in this trial differed by duration of preventive treatment, with some women receiving up to four years of isoniazid and others only 3 months. This naturally increased the likelihood of women becoming pregnant while on study medication in the isoniazid continuous arm, contributing to the different proportions seen in each treatment arm. Most women used barrier or injectable contraceptives, limiting the conclusions on the impact of OCPs or IUD contraceptives. In addition, pregnancy outcomes were not able to be collected from all women who conceived; therefore, treatment vs post-treatment pregnancy analyses were not performed. The women with unknown outcomes were proportionally spread out amongst the three treatment arms. Contraception data was also gathered via verbal reports and specific information regarding the forms of contraception was limited. Another limitation was that the study data was collected several years ago, early in the ART era. Since then, there may have been changes to the South African health care system that may impact birth outcomes. ART therapy was not treated as a potential confounder due to limited sample size.
Conclusions
Our study of women living with HIV, many of whom were not receiving ART, revealed that women who received rifamycin-isoniazid based TB preventive treatment regimens are associated with increased risk of pregnancy events compared to isoniazid regimens. Given the high rate of pregnancy seen in several TPT trials, the exclusion of pregnant women and/or prohibition of pregnancy from trials may not be effective, but instead, be directly studied in this population. To prevent TB disease, safer shorter preventive treatments such as the 1- to 3- month rifamycin-based regimens should be given to women living with HIV, including those who are pregnant.
Supplementary Material
Figure 2.
Timing of Pregnancy Events
Acknowledgements
We would like to thank the participants in the trial for their contribution to research that transformed global clinical practice. We thank the staff of the Perinatal HIV Research Unit for their efforts on this study; Barbara Laughon, PhD, National Institute of Allergy and Infectious Diseases, for her support. This study was supported by NIH grants AI048526, AI01637, P30AI094189, D43 TW000010-21S1, and 5U2RTW007370, and the U.S. Agency for International Development grant GHS-A-00-03-00019-00.
Footnotes
Competing Interests
There were no competing interests. Rifapentine was donated by Sanofi.
References
- 1.Information NC for B, Pike USNL of M 8600 R, MD B, Usa 20894. Executive Summary. World Health Organization; 2020. Accessed September 23, 2021. https://www.ncbi.nlm.nih.gov/books/NBK554959/ [Google Scholar]
- 2.Gupta A. Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. NEJM. Published online 2019:1333–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Hughes M, Leon-Cruz J. ADVERSE PREGNANCY OUTCOMES AMONG HIV-INFECTED WOMEN EXPOSED TO ISONIAZID IN BRIEF-TB. CROI Conference. Accessed September 23, 2021. https://www.croiconference.org/abstract/adverse-pregnancy-outcomes-among-hiv-infected-women-exposed-to-isoniazid-in-brief-tb/ [Google Scholar]
- 4.Salazar-Austin N, Cohn S, Lala S, et al. Isoniazid Preventive Therapy and Pregnancy Outcomes in Women Living With Human Immunodeficiency Virus in the Tshepiso Cohort. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;71(6):1419–1426. doi: 10.1093/cid/ciz1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moro RN, Scott NA, Vernon A, et al. Exposure to Latent Tuberculosis Treatment during Pregnancy. The PREVENT TB and the iAdhere Trials. Ann Am Thorac Soc. 2018;15(5):570–580. doi: 10.1513/AnnalsATS.201704-326OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skolnick JL, Stoler BS, Katz DB, Anderson WH. Rifampin, Oral Contraceptives, and Pregnancy. JAMA. 1976;236(12):1382–1382. doi: 10.1001/jama.1976.03270130044027 [DOI] [PubMed] [Google Scholar]
- 7.LaCourse SM, Wagner AD, Cranmer LM, et al. High programmatic isoniazid preventive therapy (IPT) use in pregnancy among HIV-infected women. J Acquir Immune Defic Syndr 1999. 2019;82(1):41–45. doi: 10.1097/QAI.0000000000002086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinson N. New regimens to prevent tuberculosis in adults with HIV infection. NEJM. Published online 2011. doi: 10.1056/NEJMoa1005136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKinlay S, Jefferys M, Thompson B. An investigation of the age at menopause. J Biosoc Sci. 1972;4(2):161–173. doi: 10.1017/S0021932000008464 [DOI] [PubMed] [Google Scholar]
- 10.Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404. doi: 10.1016/j.contraception.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bharathi KN, Natesh TS, Ashwitha Reddy A. Prenatal exposure to anti-tubercular drugs and postnatal effect on growth, development and cognitive ability in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):203–209. doi: 10.1016/j.pnpbp.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 12.Cates J, Westreich D, Edmonds A, et al. The Effects of Viral Load Burden on Pregnancy Loss among HIV-Infected Women in the United States. Infect Dis Obstet Gynecol. 2015;2015:1–9. doi: 10.1155/2015/362357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor AW, Mosimaneotsile B, Mathebula U, et al. Pregnancy Outcomes in HIV-Infected Women Receiving Long-Term Isoniazid Prophylaxis for Tuberculosis and Antiretroviral Therapy. Infect Dis Obstet Gynecol. 2013;2013:e195637. doi: 10.1155/2013/195637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snider DE, Layde PM, Johnson MW, Lyle MA. Treatment of tuberculosis during pregnancy. Am Rev Respir Dis. 1980;122(1):65–79. doi: 10.1164/arrd.1980.122.1.65 [DOI] [PubMed] [Google Scholar]
- 15.Mathad JS, Savic R, Britto P, et al. Pharmacokinetics and Safety of 3 Months of Weekly Rifapentine and Isoniazid for Tuberculosis Prevention in Pregnant Women. Clin Infect Dis Off Publ Infect Dis Soc Am. 2022;74(9):1604–1613. doi: 10.1093/cid/ciab665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons KB, Haddad LB, Nanda K, Curtis KM. Drug interactions between rifamycin antibiotics and hormonal contraception: a systematic review. BJOG Int J Obstet Gynaecol. 2018;125(7):804–811. doi: 10.1111/1471-0528.15027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickinson BD, Altman RD, Nielsen NH, Sterling ML, Council on Scientific Affairs, American Medical Association. Drug interactions between oral contraceptives and antibiotics. Obstet Gynecol. 2001;98(5 Pt 1):853–860. doi: 10.1016/s0029-7844(01)01532-0 [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Hughes MD, Garcia-Prats AJ, McIntire K, Hesseling AC. Inclusion of key populations in clinical trials of new antituberculosis treatments: Current barriers and recommendations for pregnant and lactating women, children, and HIV-infected persons. PLOS Med. 2019;16(8):e1002882. doi: 10.1371/journal.pmed.1002882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swindells S, Ramchandani R, Gupta A, et al. One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis. N Engl J Med. 2019;380(11):1001–1011. doi: 10.1056/NEJMoa1806808 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


