Abstract
Background and Purpose:
Microglia are important brain immune cells. However, it is difficult to differentiate microglia from monocyte-derived macrophages. To visualize microglia changes following intracerebral hemorrhage (ICH), we utilized a genetic knock-in mouse line, Tmem119-enhanced green fluorescent protein (EGFP), which expresses EGFP specifically in microglia.
Methods:
There were two parts in this study. First, autologous blood was injected into the right basal ganglia to model ICH in Tmem119-EGFP mice. Mice were euthanized at 4 hours, days 1, 3, and 7 after ICH. Sham animals were used as controls. Second, Tmem119-EGFP mice were injected with iron or thrombin, factors involved in ICH-induced injury, and were euthanized at 4 hours. Naïve mice were controls. Brains were harvested for histology.
Results:
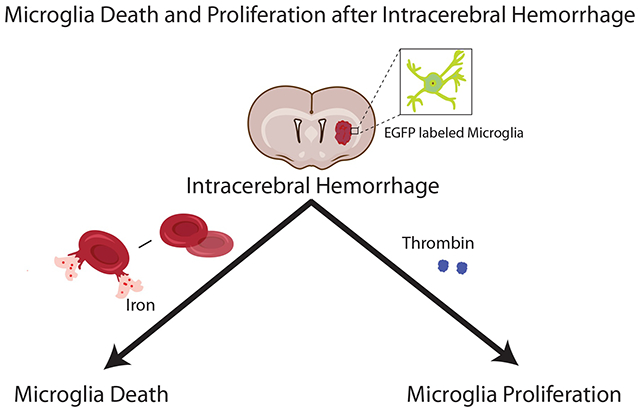
The number of perihematomal microglia significantly decreased 1 day after ICH, but markedly increased by days 3 and 7. Microglia death was also induced by intracerebral iron injection while microglia proliferation was found with intracerebral thrombin injection.
Conclusions:
Perihematomal microglia death and proliferation after ICH are visualized in vivo with a Tmem119-EGFP transgenic mouse line. Iron and thrombin may contribute to ICH-induced microglia death and proliferation, respectively.
Keywords: intracerebral hemorrhage, iron, microglia, mouse, thrombin
Graphical Abstract
Introduction
Microglia are prominent immune cells in the central nervous system, accounting for ~10% of all brain cells. Microglia originate from primitive macrophages and have auto-proliferate ability without being replaced by circulating monocytes.1 Besides microglia, there are other central nervous system phagocytes, such as leptomeningeal macrophages, perivascular macrophages, and epiplexus cells.2 Over the past decade, microglia/monocyte-derived macrophages have been reported to play roles in both brain injury and brain recovery after intracerebral hemorrhage (ICH).3 However, the role of microglia specifically in ICH-induced brain is not well delineated.
Microglia, non-microglia brain resident macrophages and monocyte-derived infiltrating macrophages are believed to participate in phagocytosis and hematoma clearance after ICH.4 It is common to refer to the macrophage/microglia response, and differences between macrophages and microglia are rarely discussed. One reason is the lack of ideal microglia-specific markers. Tmem119 is a transmembrane protein explicitly expressed in parenchymal microglia but not in circulating monocytes.5 Tmem119 is a transmembrane protein with a relatively diffuse localization pattern in cell body and processes, and together with the microglia spider-like morphology, this makes immunostaining and interpretation challenging.
This study utilized the Tmem119-EGFP mouse line with an enhanced green fluorescent protein(EGFP) gene inserted into the stop codon of the Tmem119 locus using CRISPR/case9 endonuclease-mediated genome editing6. This is designed to have resident microglia fluorescent labeled without interfering with their function. These mice were used to visualize microglia death and proliferation after experimental ICH.
Material and Methods
The data supporting this study’s findings are available from the corresponding author, G.X., upon reasonable request.
Animals and ICH Model
Animal protocols were approved by the University of Michigan Committee on the Use and Care of Animals. The study followed ARRIVE guidelines for reporting in vivo animal experiments. Forty-nine male Tmem119-EGFP (C57BL/6-Tmem119em2Gfng/J) mice (The Jackson Laboratory, strain #:031823), aged 2–4 months, were used. The ICH model was established using autologous arterial blood injection into the basal ganglia as previously described7. At 4 hours, 1, 3, and 7 days after ICH, mice were euthanized, and harvested brains fixed in 4% paraformaldehyde. Sham surgeries omitted the injection and mice were euthanized at day 1. Ferrous chloride (0.2mM, 15μl, Sigma, 372870) in saline(n=6) or rat thrombin in saline (0.5U, 10μl, Sigma, T5772, n=6) was also injected into the basal ganglia and mice were euthanized at 4 hours. Naïve animals were used as controls(n=6). Cryosections(18μm) were observed with or without immunostaining for caspase-3, receptor-interacting protein kinase 1(RIPK1) and heme oxygenase-1(HO-1), and Ki67 as described previously.8 Additional experimental details are provided in the Supplemental Material.
Data and Statistical Analysis
Cell counting was conducted on coronal brain sections by a blinded researcher. All data are expressed as means±standard deviation(SD) and analyzed by Student’s t test or one-way ANOVA and a Tukey post hoc test. Significant levels were set at p<0.05. Statistics were done using Prism.
Results
EGFP fluorescence expressed in microglia in different brain regions
In naïve EGFP mice, EGPF positive cells were detected throughout the cortex, corpus callosum, and basal ganglia but not in choroid plexus (Supplemental Figure1A–B). In striatum, most of the EGFP-positive cells were also IBA1 positive (Supplemental Figure1C). The location of EGFP positive cells relative to neurons, white matter tracts, and blood vessels in the striatum are shown in Supplemental Figure1D.
Microglia death and proliferation after experimental ICH
Four hours after ICH, there was a non-significant 30% decrease in EGFP positive cell number in the immediate peri-hematoma area (162±15 vs. 241±18 cells/mm2 in the sham group, n=6, p=0.225) (Figure1A–B). However, more microglia loss was found on day 1 (130±21 cells/mm2, p<0.05 vs. the sham group, n=6, Figure1A–B, Supplemental Figure2A–B). The number of IBA1 positive cells followed a similar trend of changes in TMEM119-GFP mice 4 hours and 1 day after ICH, but the changes versus sham group were not significant (Supplemental Figure3A–B).
Figure 1:
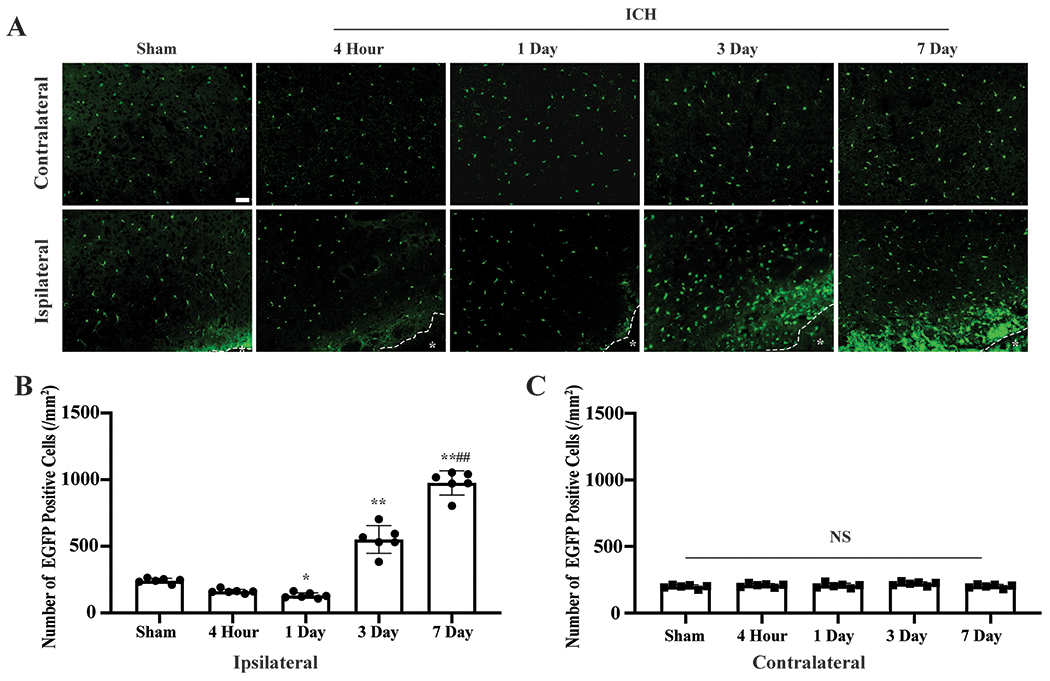
ICH induced microglia death and proliferation. (A) Representative EGFP (green) fluorescence images of Tmem119-EGFP after ICH. *indicates hematoma or needle track. (B) Quantification data on perihematomal (ipsilateral) microglia, and (C) contralateral. Sham mice received a needle insertion. *P<0.05, **P<0.001 vs. the sham group, ##P<0.001 vs. 3 days. Values are means±SD; n=6 per group. Scale bar is 50μm.
Three days after ICH, peri-hematoma microglia significantly increased (551±104 cells/mm2, n=6, p<0.001 vs. Sham group), and microglia proliferation was even more profound by day 7 (976±91cells/mm2, p<0.001 vs. sham group, p<0.001 vs. ICH day 3, n=6, Figure1A–B, Supplemental Figure2C–D). Microglia death and proliferation were not observed in the contralateral hemisphere (p>0.05, Figure1A,C).
Microglia colocalized with cell death/stress markers
To explore possible mechanisms underlying microglia death, immunostaining with cell death markers was examined. At 1 day after ICH, some Tmem19-EGFP microglia expressed cleaved caspase 3, an apoptosis marker, and RIPK1, a protein playing a role in apoptosis and necroptosis. HO-1, a protein involved in oxidative stress response and heme metabolism also colocalized to microglia (Figure2A, Supplemental Figure4A–D). At day 3 after ICH, there was colocalization EGFP with a cell proliferation marker, Ki67, in the peri-hematoma region (Figure2B, Supplemental Figure4E–F).
Figure 2:
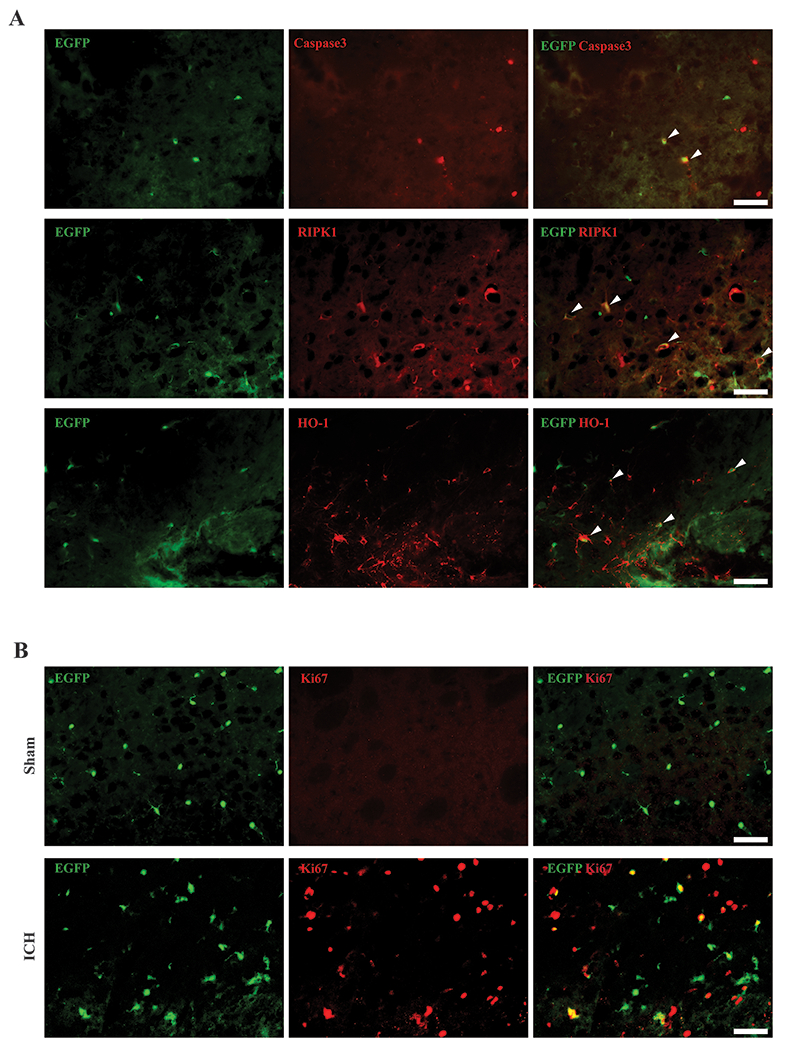
Microglia colocalized with cell death/stress markers and Ki67. (A) Representative cleaved caspase 3 (red), RIPK1 (red), and HO-1 (red) immunoreactivity colocalized with Tmem119-EGFP (green) 1 day after ICH. Arrowheads indicate double positive cells. (B) Representative fluorescence images of Ki67 immunoreactivity colocalization with Tmem119-EGFP in sham and 3 days after ICH. Scale bars are 50μm.
Microglia after thrombin or iron intracerebral injection
In proof-of-concept studies, two blood components, thrombin and iron, were injected into the basal ganglia. At 4 hours post-injection, pronounced microglia death was found around the iron injection site compared to naïve Tmen119-EGFP mice (88±29 vs. 219±5 cells/mm2, p<0.001, Figure 3A–B) and RIPK1 was colocalized to microglia (Supplemental Figure5A). In contrast, microglial proliferated 1.5-fold 4 hours after thrombin injection compared to naïve mice (341±23 cells/mm2, p<0.001 vs. naïve mice, Figure3A–B) and Ki67 was colocalized to microglia (Supplemental Figure5B).
Figure 3:
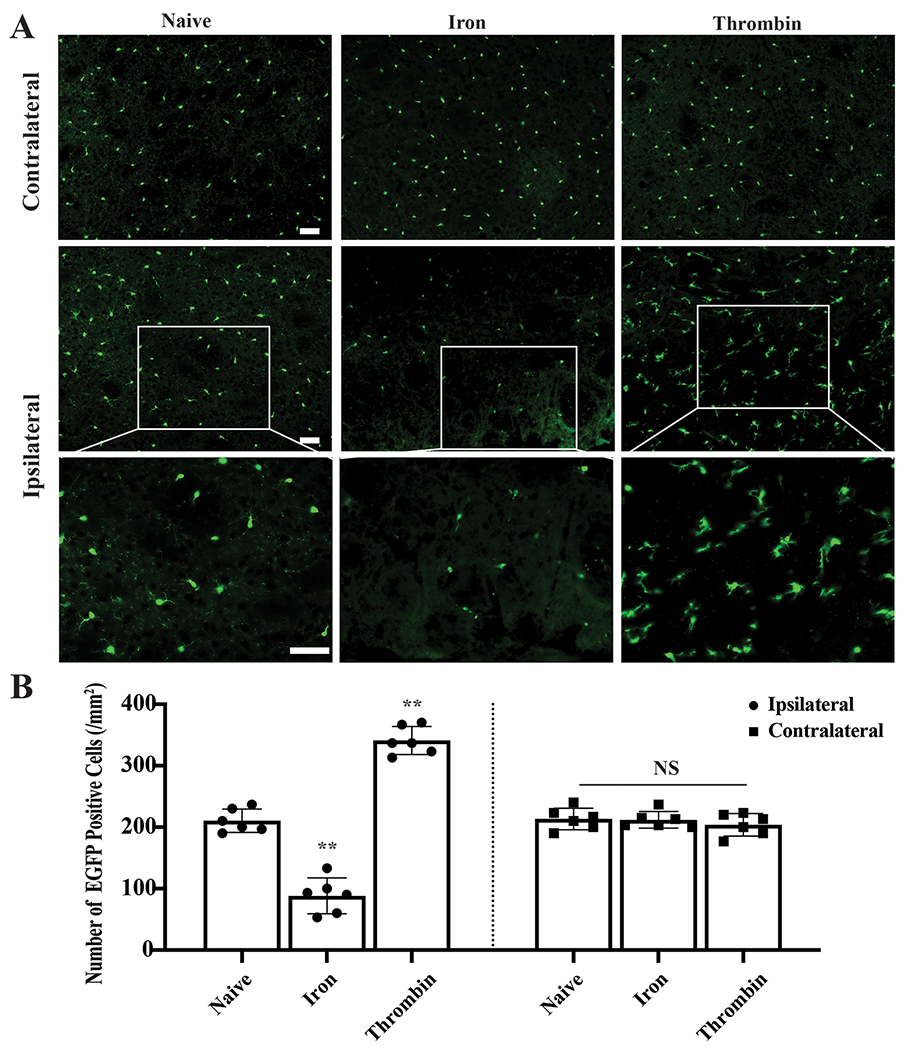
Induction of microglia death and proliferation by blood components. (A) Representative fluorescence images of Tmem119-EGFP (green) in naïve mice and 4 hours after iron and thrombin injection. (B) Quantification data of EGFP positive cells. **P<0.001 vs. naïve mice. Values are means±SD; n=6 per group. Scale bars are 50μm.
Discussion
The major findings in this study are: (1) Perihematomal microglia death happens hours after ICH followed by significant microglia proliferation, which persists for days. (2) Microglia death after ICH may be due to iron release from the hematoma and may involve necroptosis and apoptosis. (3) Microglia proliferation may be induced by thrombin formation after ICH.
Microglia have become a centerpiece of central nervous system disorder research. However, the lack of methods that efficiently discriminate microglia from brain resident non-microglia macrophages and blood infiltrating monocytes make it difficult to investigate these cells’ individual properties. Here, we present data on a new tool that allowed us to study microglial behavior after ICH in vivo. We were able to detect perihematomal microglia death and microglia proliferation. Initially the former predominates, with a loss of microglia, whereas by day 3 and 7 after ICH, microglial numbers rebound above normal. Microglial activation also occurs as early as one hour after ICH and persists for up to 4 weeks9. Overall microglial function will depend on a balance between activation and cell death/proliferation which may vary with distance from the hematoma and time. This study only focused on the immediate perihematomal zone.
The underlying mechanisms of microglia death warrant further investigation but we showed colocalization of cleaved caspase 3 with EGFP fluorescence 1 day post hemorrhage, suggesting some apoptosis. In addition, a key mediator in necroptosis, RIPK110, also colocalized with EGFP suggesting this pathway is also involved. There was also colocalization with HO-1, a protein involved in oxidative stress and heme metabolism, suggesting microglia were under stress 1 day after ICH. Intracerebral iron injection also caused microglia death within 4 hours, suggesting iron release from red blood cells may contribute to microglia death after ICH. How blood components cause microglia death remains obscure, our findings provide a novel perspective and evidence of how brain hemorrhage causes brain injury.
In normal adult mouse brain, microglia are long-lived with a low self-renewal rate.11 In contrast, ICH induced marked proliferation at day 3 and 7, effects mimicked by thrombin. Whether thrombin is the major inducer of ICH-induced proliferation needs further study.
Limitations of this study include only using adult male mice, and only four time points were tested. However, the study does show that the recently generated genetic engineered Tmem119-EGFP mouse provides researchers with a novel tool for studying the microglia response to ICH.
Supplementary Material
Sources of Funding
YH, RFK and GX were supported by grants NS-096917, NS-106746, NS-112394 and NS-116786 from the National Institutes of Health.
Disclosures
Drs. Hua, Keep and Xi were supported by grants from the National Institutes of Health. Other authors declare no conflicts of interest.
Non-standard Abbreviations and Acronyms
- ICH
intracerebral hemorrhage
- EGFP
enhanced green fluorescent protein
- RIPK1
receptor-interacting protein kinase 1
- HO-1
heme oxygenase-1
References
- 1.Augusto-Oliveira M, Arrifano GP, Lopes-Araujo A, Santos-Sacramento L, Takeda PY, Anthony DC, et al. What do microglia really do in healthy adult brain? Cells. 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prinz M, Masuda T, Wheeler MA, Quintana FJ. Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu Rev Immunol. 2021;39:251–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkinson DA, Keep RF, Hua Y, Xi G. Hematoma clearance as a therapeutic target in intracerebral hemorrhage: From macro to micro. J Cereb Blood Flow Metab. 2018;38:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang CF, Goods BA, Askenase MH, Beatty HE, Osherov A, DeLong JH, et al. Divergent functions of tissue-resident and blood-derived macrophages in the hemorrhagic brain. Stroke. 2021;52:1798–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, et al. New tools for studying microglia in the mouse and human cns. Proc Natl Acad Sci U S A. 2016;113:E1738–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaiser T, Feng G. Tmem119-egfp and tmem119-creert2 transgenic mice for labeling and manipulating microglia. eNeuro. 2019;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jing C, Bian L, Wang M, Keep RF, Xi G, Hua Y. Enhancement of hematoma clearance with cd47 blocking antibody in experimental intracerebral hemorrhage. Stroke. 2019;50:1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye F, Hua Y, Keep RF, Xi G, Garton HJL. Cd47 blocking antibody accelerates hematoma clearance and alleviates hydrocephalus after experimental intraventricular hemorrhage. Neurobiol Dis. 2021;155:105384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren H, Han R, Chen X, Liu X, Wan J, Wang L, et al. Potential therapeutic targets for intracerebral hemorrhage-associated inflammation: An update. J Cereb Blood Flow Metab. 2020;40:1752–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan J, Amin P, Ofengeim D. Necroptosis and ripk1-mediated neuroinflammation in cns diseases. Nat Rev Neurosci. 2019;20:19–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain cns microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


