Abstract
An increasing share of urinary tract infections (UTIs) are caused by extraintestinal pathogenic Escherichia coli (ExPEC) lineages that have also been identified in poultry and hogs with high genetic similarity to human clinical isolates. We investigated industrial food animal production as a source of uropathogen transmission by examining relationships of hog and poultry density with emergency department (ED) visits for UTIs in North Carolina (NC). ED visits for UTI in 2016-2019 were identified by ICD-10 code from NC’s ZIP code-level syndromic surveillance system and livestock counts were obtained from permit data and aerial imagery. We calculated separate hog and poultry spatial densities (animals/km2) by Census block with a 5km buffer on the block perimeter and weighted by block population to estimate mean ZIP code densities. Associations between livestock density and UTI incidence were estimated using a reparameterized Besag-York-Mollié (BYM2) model with ZIP code population offsets to account for spatial autocorrelation. We excluded metropolitan and offshore ZIP codes and assessed effect measure modification by calendar year, ZIP code rurality, and patient sex, age, race/ethnicity, and health insurance status. In single-animal models, hog exposure was associated with increased UTI incidence (rate ratio [RR]: 1.21, 95% CI: 1.07-1.37 in the highest hog-density tertile), but poultry exposure was associated with reduced UTI rates (RR: 0.86, 95% CI: 0.81-0.91). However, the reference group for single-animal poultry models included ZIP codes with only hogs, which had some of the highest UTI rates; when compared with ZIP codes without any hogs or poultry, there was no association between poultry exposure and UTI incidence. Hog exposure was associated with increased UTI incidence in areas that also had medium to high poultry density, but not in areas with low poultry density, suggesting that intense hog production may contribute to increased UTI incidence in neighboring communities.
Graphical Abstract
1. Introduction
Urinary tract infections (UTIs)—bacterial infections of the urethra, bladder, ureters, or kidneys—are a substantial burden on human health and health care systems (Medina and Castillo-Pino, 2019). Over 400 million people worldwide are estimated to have had UTIs in 2019, resulting in nearly a quarter of a million deaths, and global age-standardized UTI incidence rates have notably increased since 1990 (Zeng et al., 2022; Zhu et al., 2021). In the United States, 3.6 million outpatient office visits and 2.9 million emergency department visits were due to UTIs in 2018, representing 0.4% of all office and 2.2% of ED visits, respectively, with estimated societal costs, including health care and lost work time, of $3.5 billion annually (Cairns et al., 2021; Flores-Mireles et al., 2015; Santo and Okeyode, 2021). UTIs occurring in otherwise healthy individuals are considered clinically uncomplicated and are generally community-acquired, while complicated UTIs arise due to defects in the urinary tract or host defense and are one of the most common hospital-acquired infections, most often due to catheterization (Flores-Mireles et al., 2015; Tandogdu and Wagenlehner, 2016). Community-acquired UTI risk increases with age and predominantly affects women, over half of whom will experience at least one UTI during adulthood, with up to half of UTIs recurring within a year (Foxman, 2014; Medina and Castillo-Pino, 2019; Tandogdu and Wagenlehner, 2016).
By far the most common infectious agents, particularly in uncomplicated UTIs, are extraintestinal pathogenic Escherichia coli (ExPEC) strains (Flores-Mireles et al., 2015; Foxman, 2010; Medina and Castillo-Pino, 2019). E. coli is typically a commensal gut bacterium in humans and other warm-blooded animals, though a number of serotypes are known to cause enteric disease (Holcomb and Stewart, 2020; Jesser and Levy, 2020; Tenaillon et al., 2010). Unlike the commensal and enteropathogenic E. coli strains that reside in the gastrointestinal tract, ExPEC strains exhibit a heterogenous collection of adaptations that aid colonization of the urinary tract or bloodstream (Flores-Mireles et al., 2015; Foxman, 2010; Riley, 2020). In recent decades, E. coli clonal lineage ST131 has become established as the predominate ExPEC associated with community-acquired UTIs globally (Manges et al., 2019; Riley, 2014). ST131 is notable for a robust virulence profile and strong inherent anti-microbial resistance (AMR), including extended-spectrum β-lactamase (ESBL) production, which challenge effective treatment and increase morbidity and mortality associated with extraintestinal infections (Butcher et al., 2019; Nicolas-Chanoine et al., 2014; Shaik et al., 2017).
The existence of pandemic uropathogen strains raises important questions about the mechanisms and risk factors of ExPEC transmission in community-acquired UTIs (Butcher et al., 2019; Foxman, 2014; Riley, 2014). Environmental transmission of intestinal E. coli through the fecal-oral route has long been recognized, but foodborne exposures have increasingly been implicated in ExPEC transmission as well (Holcomb et al., 2021; Hossain et al., 2021; Manges, 2016; Montealegre et al., 2020; Nordstrom et al., 2013). Consumption of meat, particularly poultry, has been associated with higher UTI risk (Fibke et al., 2019; Manges, 2016). ExPEC are abundant in the feces of UTI patients (Owrangi et al., 2018), suggesting that ingestion of contaminated meat may lead to transient gut colonization by ExPEC followed by opportunistic invasion of the urinary tract, though the temporal dynamics of this proposed mechanism remain poorly characterized (Manges, 2016; Sarowska et al., 2019). High genetic similarity has been observed between ExPEC strains, particularly ST131, and bacteria isolated from companion animals, livestock, and retail meat (Platell et al., 2011; Reid et al., 2019; Riley, 2020; Roer et al., 2019; van Hoek et al., 2016). Two population-based studies found that clinical ExPEC isolates exhibited substantial genetic similarity with bacterial isolates from poultry and pork meat sampled contemporaneously from retailers in the same communities, including shared avian-associated plasmids in both poultry meat and human clinical isolates (Liu et al., 2018; Yamaji et al., 2018). The detection of avian-associated ExPEC lineages in urban wastewater further suggests at least transient colonization of human populations by microbes of animal origin (Hayashi et al., 2019).
Putative ExPEC have also been reported in surface waters globally, although a recent systematic review did not identify any studies that have assessed the UTI risk associated with waterborne ExPEC exposure (Graham et al., 2021). However, improved drinking water supply reduced UTI incidence in Galapagos, Ecuador, suggesting that uropathogens could be transmitted indirectly through other environmentally mediated pathways in addition to direct foodborne exposures (Houck et al., 2019). Colonization of farm workers by livestock-associated bacteria has been widely documented, including by ESBL-producing E. coli that may include ExPEC strains (Dahms et al., 2015; Dohmen et al., 2015; Founou et al., 2019; Nadimpalli et al., 2014; Rinsky et al., 2013; van Hoek et al., 2016). Airborne exposures have been proposed as a possible transmission route from livestock to workers (Dohmen et al., 2017), and livestock-associated microbes and allergens have been detected in residential air near animal feeding operations as well as in surrounding surface waters and other environmental compartments (Bai et al., 2022; Dame-Korevaar et al., 2019; de Rooij et al., 2019; George et al., 2020; Hatcher et al., 2016; Wiesner-Friedman et al., 2022; Williams et al., 2011). Dissemination of microbes off-farm may help explain observed associations between residential proximity to animal production facilities and plausibly livestock-associated bacterial infections among individuals without known animal contacts (Anker et al., 2018; Beresin et al., 2017; Carrel et al., 2014; Casey et al., 2014). Numerous other adverse health outcomes have been associated with proximity to industrial animal agriculture, including other foodborne bacterial infections, influenza, respiratory and gastrointestinal illnesses, hypertension, pediatric cancers, and mortality (Anderson et al., 2017; Booth et al., 2017; Casey et al., 2015; Hooiveld et al., 2016; Kravchenko et al., 2018; Quist et al., 2022b; Shaw et al., 2016; Son et al., 2021a). To our knowledge, however, no previously published research has investigated relationships between proximity to livestock production and community-acquired UTIs. We investigated industrial food animal production as a potential source of community uropathogen transmission by examining relationships of hog and poultry density with rates of ED visits for UTIs in North Carolina (NC) over a four-year period from 2016-2019.
2. Methods
2.1. Outcome
We used statewide ED surveillance data to identify UTI episodes in North Carolina by 5-digit ZIP code, the finest geographic resolution available for these data. ED visits with ICD-10 diagnosis codes N10 (acute pyelonephritis), N30.0x (acute cystitis), or N39.0 (urinary tract infection, site not specified) were extracted from the North Carolina Disease Event Tracking and Epidemiologic Collection Tool (NC DETECT) (Bruxvoort et al., 2019; Waller et al., 2011). We considered all ED visits for the same individual within 30 days of the initial visit to be a single UTI episode (Bruxvoort et al., 2019). We calculated the annual UTI incidence in 2016 – 2019 by ZIP code, stratified by patient sex, age group, race, Hispanic ethnicity, and health insurance type, as reported in NC DETECT. This study was exempted from institutional review board review by the University of North Carolina Office of Human Research Ethics (Study #: 19-1860).
2.2. Exposure
Hog and poultry concentrated animal feeding operation (CAFO) data were provided by the Environmental Working Group (EWG, ewg.org) and Waterkeeper Alliance (waterkeeper.org). Hog CAFO locations and head counts were derived from NC Department of Environmental Quality (DEQ) 2019 permit data (https://deq.nc.gov/cafo-map) and manually corrected by EWG to match barn locations determined using satellite imagery (Graddy et al., 2020). Because poultry CAFO permit data are not publicly available in NC, EWG visually identified poultry CAFO locations using North Carolina Orthophotography, RapidEye 5m, and 2019 Planet Scope 3m satellite imagery (Graddy et al., 2020). Locations were confirmed using 3-meter resolution Planet Scope imagery and imagery from the National Agriculture Imagery Program (NAIP), both from 2019. The date of poultry operation construction was approximated by cross checking against 2016, 2018, and 2019 NAIP vintages. EWG digitized imagery of 104 barns from the 25 most poultry-intense NC counties to obtain a mean barn area of ~2270 m2; the mean area was assumed for all NC poultry barns and industry standards for recommended area per broiler, layer, or turkey were applied to approximate the poultry count in each barn, as described by Graddy et al. (2020). Poultry counts at each location were estimated from the number of barns and the county-level distribution of poultry types in the USDA 2017 Census of Agriculture.
Because the outcome data were available only by ZIP code, we estimated human population-weighted ZIP code spatial densities (count/km2) of hogs and poultry to represent livestock exposures. Census block hog and poultry densities were estimated as the sum of head counts for all operations within a 5 km buffer of the 2010 block boundary (the most recent vintage to the study period), divided by the combined area of the block and surrounding buffer. Census blocks were associated with the 2017 ZIP code polygon (ESRI, Redlands, CA, USA; 2017 selected as the study midpoint to which the polygons for other years could be uniquely associated) containing the 2010 block centroid. We summed the 2010 block populations by ZIP code to estimate 2010 ZIP code populations and used the fraction of the ZIP code population residing in each block to calculate population-weighted mean hog and poultry densities for each ZIP code. As a sensitivity analysis, we also considered buffers of 0km (no buffer), 1km, 3km, 10km, and 15km around Census blocks, as well as calculating unweighted ZIP code spatial densities using only operations within the ZIP code polygons (see Supplementary Text S4).
Poultry densities were calculated separately for 2016, 2018, and 2019, as different numbers of operations were identified in NAIP imagery for each of these years (Graddy et al., 2020). Poultry density was constant from 2016 to 2018 in the majority of ZIP codes (393/620, 63%); for ZIP codes where poultry density changed, we assumed half the change occurred in 2017 to estimate 2017 poultry densities, as NAIP imagery was unavailable for that year. Comparable annual hog numbers were unavailable, but the total state hog inventory remained similar across the study period at ~9 million head and ~19 million hogs processed each year (NASS, 2020). Since 2007, NC has instituted a permanent moratorium on new hog operations that use traditional waste management systems, such that nearly all operations in the 2019 permit data likely existed prior to 2016, when our study period began (N.C. GS., 2014). We therefore assumed constant hog density in each ZIP code across the study period while allowing ZIP code population size and other covariates to vary by year.
2.3. Covariates
We obtained American Community Survey (ACS) 5-year estimates of Census block group population counts by sex, age, race, Hispanic ethnicity, health insurance type, and household income for end years 2016 – 2019 (Manson et al., 2021; Walker and Herman, 2020). Although we conducted this analysis at the ZIP code level, we did not access Census data for ZIP Code Tabulation Areas (ZCTA) due to known spatiotemporal misalignment with ZIP codes (Grubesic and Matisziw, 2006; Krieger et al., 2002). Rather, the block group population in each category was apportioned among constituent Census blocks according to the 2010 population distribution and aggregated to the 2017 ESRI ZIP code polygon containing the block centroid (Quist et al., 2022b). We estimated ZIP code median household income using the R package binsmooth to fit mean-constrained cumulative distribution functions (CDFs) to household income categories, where mean household income was obtained by dividing aggregate ZIP code income by the number of households (von Hippel et al., 2017). The degree of ZIP code rurality was represented by a continuous isolation scale, which indicates access to the resources necessary for human thriving (such as food, healthcare, and economic opportunities afforded by internet access) that receive disproportionate capital investment in more urban settings in the United States (Doogan et al., 2018). We obtained isolation distance scores by Census tract (http://doogan.us/isolation/GeoIso.csv), assigned the tract score to the constituent Census blocks, and calculated 2010 population-weighted mean isolation scores for the overlying ZIP codes.
2.4. Statistical analysis
Associations between livestock density and UTI incidence were estimated using a Besag-York-Mollié (BYM) model to account for spatial dependence in the outcome among neighboring ZIP codes (Besag et al., 1991). The BYM model includes both spatial and unstructured random effects for each areal unit to account for overdispersion under the log-Poisson likelihood, with the spatial component structured as an intrinsic conditional autoregressive (ICAR) prior distribution to pool information across neighboring areas (Morris et al., 2019). We used the Riebler et al. (2016) reparameterization (BYM2), which estimates a single standard deviation parameter σ for the combined spatial and unstructured random effects and also includes a mixing parameter ρ corresponding to the spatial fraction of the overall variance (Morris et al., 2019; Riebler et al., 2016). The variances of the spatial and unstructured random effect components must both ≈1 for σ to be the valid standard deviation of the combined effects, which can be accomplished by computing a generalized variance from the adjacency matrix for a given dataset to use as a scaling factor on the spatial variance proportion ρ (Riebler et al., 2016).
We characterized associations between ZIP code-level density of hogs or poultry and UTI incidence using the rate ratio (RR) as the measure of effect, summarized as the mean and central 95% probability interval (95% CI) of the posterior samples. ZIP code ACS population for each year was used as an offset. Density was expressed both continuously, using a log10(density + 1) transformation to account for zero-density ZIP codes, and categorically using tertiles of non-zero density and a fourth category for unexposed (zero-density) observations. We restricted the analysis to contiguous ZIP codes on the NC mainland, excluding 16 ZIP codes off the coast where CAFOs are impractical. We also excluded metropolitan ZIP codes, which typically have limited CAFO exposure and may have different ED utilization practices than less dense areas (Greenwood-Ericksen and Kocher, 2019). We applied a previously developed, North Carolina-specific threshold of < 5.6 to the Doogan et al. (2018) isolation score to identify metropolitan ZIP codes that predominantly lack hog CAFOs (Quist et al., 2022b). Models were adjusted for ZIP code-level median household income, continuous isolation distance score, and percent of the non-institutionalized civilian population lacking health insurance, the minimally sufficient adjustment set identified from a directed acyclic graph (DAG, Supplementary Figure S1) using the R package daggity (Textor et al., 2017). In addition to single animal models, we also fit mutually adjusted models that included separate terms for both hog and poultry exposure. We used a reduced model featuring only the combined random effect and an intercept term to examine overdispersion and the spatial dependency of UTI rates in the study area.
We assessed effect measure modification (EMM) by calendar year, patient sex, age, race/ethnicity, and ZIP code rurality. Age was represented categorically as under 5, 5-17, 18-34, 35-64, and over 65 years of age (Bruxvoort et al., 2019; Zhu et al., 2021). We collapsed race and ethnicity into a combined race/ethnicity variable for which patients with recorded Hispanic ethnicity were assigned to the Hispanic race/ethnicity group and all other patients (including those missing ethnicity information) were assigned to the race/ethnicity groups corresponding to their recorded race. The Pacific Islander and Other Race groups were combined due to low case counts, resulting in 6 race/ethnicity groups: non-Hispanic American Indian, Asian, Black, White, and Other Race, and Hispanic of any race. Approximate quartiles of isolation distance scores for all 730 mainland NC ZIP code polygons with covariate data were used to define four rurality categories from least isolated to most isolated: metropolitan, suburban, small town, and rural (Doogan et al., 2018). We adjusted the metropolitan (least isolated) category to include only ZIP codes with isolation distance score < 5.6, where we had previously observed a natural break in hog CAFO exposures in NC, and considered all ZIP codes with isolation distances ≥ 5.6 but less than the median (7.6) to be suburban (Quist et al., 2022b). EMM was assessed with product-term interactions between the exposure and modifier variables using group-specific ZIP code population counts for each year as an offset. UTI episodes missing data for specific patient attributes were excluded from analyses of EMM by that particular characteristic only, as group-specific population offsets could not be constructed from census data for missing groups. We also assessed the effects of joint hog and poultry exposure using product-term interactions for both as continuous, log10-transformed density or the four-group categorical density separately for each animal.
Adapting the approach of Morris et al. (2019) and the BYM2 implementation in the R package brms (Bürkner, 2017), we coded our model in the probabilistic programming language Stan (see Supplemental Material for Stan code) and fit models using the default No U-Turn Sampler (NUTS) through the rstan package (Stan Development Team, 2020). ZIP code neighbors were determined by queen contiguity using the spdep package (Bivand and Wong, 2018) and were further processed using functions provided by Morris et al. (2019) (https://github.com/stan-dev/example-models/blob/master/knitr/car-iar-poisson/nb_data_funs.R) and the INLA package (Bakka et al., 2018) to assess disconnected sub-graphs and calculate the geometric mean of the neighborhood graph marginal variances, which served as the scaling factor for the spatial variance proportion ρ (Riebler et al., 2016). All continuous predictors were standardized (mean-centered, standard deviation-scaled) to facilitate selection of prior distributions. We assigned regularizing normal(0, 2.5) and normal(0, 1) priors to the intercept and fixed effects, respectively. We used half-normal(0, 1) priors for the standard deviation of the combined random effects, σ, and the standard deviation of the non-spatial random effect, θ. To aid convergence, we placed a standard normal prior on logit(ρ), the log-odds of the spatial proportion parameter. All analyses were conducted in R version 4.0.3 (R Core Team, 2020).
3. Results
3.1. ZIP code characteristics
Of the 750 populated ZIP code polygons in 2019, 734 were contiguously located on the NC mainland. Four ZIP codes lacked median income data due to insufficient household counts and were excluded. Of the remaining 730 ZIP codes, 110 were considered metropolitan (isolation distance < 5.6) and were also excluded, for a total of 620 contiguous, non-metropolitan ZIP codes with complete covariate information included in the main analysis. The estimated total 2019 population of the included areas was 6,596,383.
Hog density ranged from 0 – 1099 hogs/km2, with half of ZIP codes (n=324) unexposed to hogs (Table 1). Population age and sex distributions were similar across hog density exposure categories. However, Black individuals were over-represented in medium hog density (4 - 36 hogs/km2) and high hog density (36 – 1099 hogs/km2) ZIP codes relative to both unexposed ZIP codes and the statewide population. In contrast, non-Hispanic Whites disproportionately resided in unexposed ZIP codes. Health insurance coverage displayed a similar pattern, with increasing shares of publicly insured and uninsured populations and decreasing private insurance coverage with increasing hog density. Household incomes were similar between areas without hogs and with low hog exposure (<4 hogs/km2), but the median income was about $6000 lower on average in medium hog density ZIP codes and $10,000 lower in areas of high hog exposure.
Table 1.
Characteristics of hog and poultry density exposure categories in 2019, excluding metropolitan and offshore ZIP codes. Low, medium, and high exposure categories correspond to tertiles of non-zero density values.
| Hog exposure category | |||||
|---|---|---|---|---|---|
| Unexposed | Low | Medium | High | All | |
| Hog densitya (count/km2), range | 0 | >0 - 4 | >4 - 36 | >36 - 1099 | 0 - 1099 |
| Poultry densitya (count/km2), median (IQR) | 54 (13668) | 5028 (98599) | 15894 (232876) | 23740 (527045) | 3280 (79762) |
| Total populationb, N | 3557461 | 1363184 | 922266 | 753472 | 6596383 |
| Sex, N (%) | |||||
| Female | 1814769 (51) | 701520 (51) | 471227 (51) | 379193 (50) | 3366709 (51) |
| Male | 1742708 (49) | 661668 (49) | 451043 (49) | 374281 (50) | 3229700 (49) |
| Age (years), N (%) | |||||
| < 5 | 193849 (5) | 79591 (6) | 59004 (6) | 46637 (6) | 379081 (6) |
| 5-17 | 587126 (17) | 228395 (17) | 161275 (17) | 127389 (17) | 1104185 (17) |
| 18-34 | 695769 (20) | 274306 (20) | 197651 (21) | 168461 (22) | 1336187 (20) |
| 35-64 | 1434316 (40) | 535662 (39) | 360514 (39) | 284425 (38) | 2614917 (40) |
| 65+ | 646408 (18) | 245226 (18) | 143825 (16) | 126568 (17) | 1162027 (18) |
| Combined race/ethnicityc, N (%) | |||||
| American Indian | 29155 (1) | 18287 (1) | 24785 (3) | 28267 (4) | 100494 (2) |
| Asian | 60670 (2) | 17765 (1) | 7498 (1) | 5185 (1) | 91118 (1) |
| Black | 401753 (11) | 274689 (20) | 275521 (30) | 200948 (27) | 1152911 (17) |
| White | 2717300 (76) | 902123 (66) | 503311 (55) | 413861 (55) | 4536595 (69) |
| Hispanic | 274755 (8) | 116954 (9) | 87815 (10) | 85528 (11) | 565052 (9) |
| Other race | 73839 (2) | 33372 (2) | 23354 (3) | 19699 (3) | 150264 (2) |
| Health insurance status, N (%) | |||||
| Private | 2363057 (68) | 877667 (66) | 556492 (62) | 412367 (57) | 4209583 (65) |
| Public | 770287 (22) | 318584 (24) | 239283 (27) | 215511 (30) | 1543665 (24) |
| Uninsured | 362639 (10) | 139751 (10) | 105859 (12) | 95240 (13) | 703489 (11) |
| Median incomeb (x1000 USD), median (IQR) | 51 (17) | 51 (16) | 45 (14) | 41 (12) | 48 (15) |
| ZIP codesd,e, N (%) | 324 (52) | 99 (16) | 99 (16) | 98 (16) | 620 (100) |
| Suburban | 157 (48) | 46 (46) | 32 (32) | 20 (20) | 255 (41) |
| Small town | 82 (25) | 32 (32) | 32 (32) | 36 (37) | 182 (29) |
| Rural | 85 (26) | 21 (21) | 35 (35) | 42 (43) | 183 (30) |
| Total ED visitsf, N (rate per 1000) | 3308856 (465) | 1447224 (528) | 1151499 (610) | 902923 (622) | 6810502 (516) |
| UTI episodesg | 120196 (17) | 53435 (20) | 45746 (24) | 37067 (26) | 256444 (19) |
| UTI patientsh | 110202 (15) | 48524 (18) | 41319 (22) | 33267 (23) | 233312 (18) |
| Poultry exposure category | |||||
| Unexposed | Low | Medium | High | All | |
| Poultry densitya (count/km2), range | 0 | >0 - 887 | >887 - 4833 | >4833 - 39499 | 0 - 39499 |
| Hog densitya (count/km2), median (IQR) | 0 (0) | 0 (9) | 2 (39) | 24 (221) | 0 (20) |
| Total populationb, N | 1340879 | 2153133 | 1968293 | 1134078 | 6596383 |
| Sex, N (%) | |||||
| Female | 683544 (51) | 1110417 (52) | 998963 (51) | 573785 (51) | 3366709 (51) |
| Male | 657350 (49) | 1042718 (48) | 969332 (49) | 560300 (49) | 3229700 (49) |
| Age (years), N (%) | |||||
| < 5 | 71233 (5) | 126279 (6) | 116337 (6) | 65232 (6) | 379081 (6) |
| 5-17 | 198285 (15) | 373771 (17) | 338988 (17) | 193141 (17) | 1104185 (17) |
| 18-34 | 268945 (20) | 428251 (20) | 403311 (20) | 235680 (21) | 1336187 (20) |
| 35-64 | 523229 (39) | 862889 (40) | 783149 (40) | 445650 (39) | 2614917 (40) |
| 65+ | 279205 (21) | 361945 (17) | 326508 (17) | 194369 (17) | 1162027 (18) |
| Combined race/ethnicityc, N (%) | |||||
| American Indian | 14257 (1) | 12160 (1) | 27902 (1) | 46175 (4) | 100494 (2) |
| Asian | 19717 (1) | 28371 (1) | 33552 (2) | 9478 (1) | 91118 (1) |
| Black | 130279 (10) | 463564 (22) | 362025 (18) | 197043 (17) | 1152911 (17) |
| White | 1055637 (79) | 1418534 (66) | 1332653 (68) | 729771 (64) | 4536595 (69) |
| Hispanic | 92683 (7) | 178702 (8) | 165083 (8) | 128584 (11) | 565052 (9) |
| Other race | 28320 (2) | 51812 (2) | 47096 (2) | 23036 (2) | 150264 (2) |
| Health insurance status, N (%) | |||||
| Private | 897317 (69) | 1409457 (67) | 1236110 (64) | 666699 (60) | 4209583 (65) |
| Public | 270430 (21) | 495723 (23) | 474487 (25) | 303025 (27) | 1543665 (24) |
| Uninsured | 136791 (10) | 214189 (10) | 209501 (11) | 143008 (13) | 703489 (11) |
| Median incomeb (x1000 USD), median (IQR) | 52 (16) | 49 (19) | 47 (15) | 45 (12) | 48 (15) |
| ZIP codesd,e, N (%) | 183 | 142 | 145 | 150 | 620 |
| Suburban | 73 (40%) | 80 (56%) | 77 (53%) | 25 (17%) | 255 (41) |
| Small town | 45 (25%) | 25 (18%) | 35 (24%) | 77 (51%) | 182 (29) |
| Rural | 65 (36%) | 37 (26%) | 33 (23%) | 48 (32%) | 183 (30) |
| Total ED visitsf, N (rate per 1000) | 1123818 (419) | 2184162 (507) | 2171455 (556) | 1331067 (580) | 6810502 (516) |
| UTI episodesg | 40560 (15) | 83389 (19) | 80706 (21) | 51789 (23) | 256444 (19) |
| UTI patientsh | 37294 (14) | 75621 (18) | 73644 (19) | 46753 (20) | 233312 (18) |
Hog and poultry densities derived from 2019 location and count data provided by Environmental Working Group and Water Keeper Alliance
Population and income data derived from 2019 5-year American Community Survey Block Group estimates
Individuals of any race reporting Hispanic ethnicity were included in a combined Hispanic race/ethnicity group; all other race/ethnicity groups contained only individuals of non-Hispanic ethnicity, with the Other race group comprising individuals of two or more races and Pacific Islanders
Contiguous North Carolina mainland ZIP codes identified from 2017 ESRI ZIP code polygons
ZIP code rurality categories derived from Doogan et al. (2018) isolation distance scores
Emergency department visits derived from 2019 NCDETECT data
All ED visits with a UTI diagnosis code for the same individual within 30 days of the first UTI diagnosis considered a single UTI episode
Any individual with one or more UTI episodes in 2019 considered a single UTI patient
Poultry were absent from 183 (30%) of ZIP codes and ranged in density from 0 – 39,499 birds/km2 (Table 1). Demographic patterns similar to those described above held across poultry exposure categories, although ZIP codes unexposed to poultry had slightly higher proportions of older and White residents and the demographic differences between low, medium, and high poultry exposure ZIP codes were somewhat attenuated relative to the corresponding hog exposure categories. The highest hog exposures were most common in rural ZIP codes, while high poultry exposures occurred most frequently in small towns; while rural ZIP codes also frequently had high poultry exposure, they more commonly were unexposed to poultry.
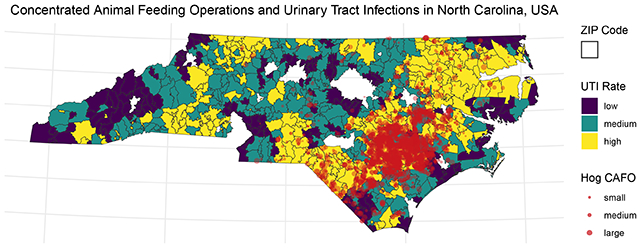
After excluding metropolitan areas, a plurality of ZIP codes (n=273, 44%) were exposed to both hogs and poultry, concentrated in the eastern half of the state (Figure 1). A quarter of ZIP codes, located primarily in the mountain west and coastal east of the state, were unexposed to either hogs or poultry (n=153) and another quarter were exposed only to poultry (n=158), largely in NC’s central and western areas. Least common was exposure to hogs alone, observed in only 36 ZIP codes (6%) that primarily border the concentrated area of joint exposure to the east.
Figure 1.
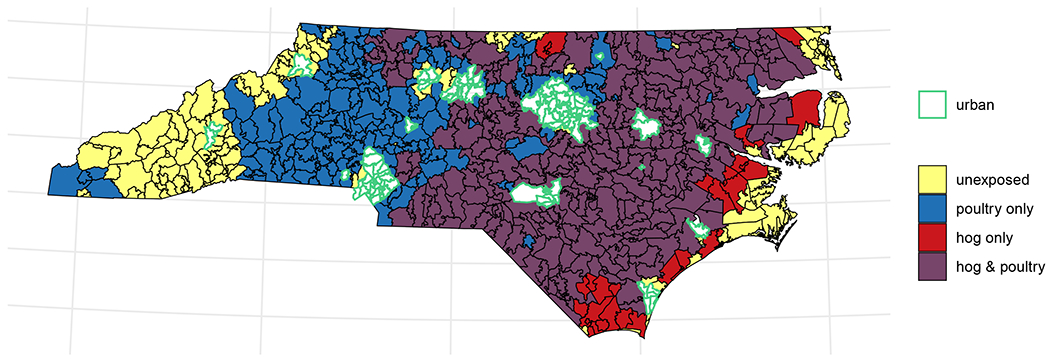
Map of joint binary hog and poultry exposure in North Carolina ZIP codes in 2019. Unexposed ZIP codes (yellow) had zero density of both hogs and poultry. Blue areas contained poultry (in any amount) but no hogs; red areas, hogs but no poultry. Purple areas had non-zero densities of both hogs and poultry. Metropolitan ZIP codes (defined as isolation score < 5.6), outlined in green, were largely unexposed and were excluded from all analyses.
3.2. UTI episodes
Excluding metropolitan and offshore areas, the remaining 620 ZIP codes recorded 13,545,812 ED visits in 2016-2019, of which 557,382 received UTI diagnosis codes for a total of 528,067 UTI episodes among 425,281 unique patients. Patient sex and age were available from nearly all ED records, the distributions of which among UTI episodes notably differed from among all ED visits and from the general population (Table 2). Female patients accounted for a higher proportion of ED visits (57%) and a substantially larger share of UTI episodes (82%) than of the general population (51%). Patients with ED visits and UTI episodes both skewed older than the general population, although 18–34-year-olds also comprised a slightly larger proportion of both UTI episodes and ED visit patients. Individuals 65 and older accounted for a much larger share of UTI episodes (37%) than both ED visits (21%) and the population overall (15%). More than 4% of patients were missing information on race/ethnicity among both UTI episodes and ED visits overall. Non-Hispanic American Indian, Black, and White patients experienced a slightly higher proportion of UTI episodes relative to the general population, while Asian and Hispanic patients experienced a smaller share of UTI episodes. Proportions of UTI episodes and ED visits by race/ethnicity were largely similar, although non-Hispanic Black patients represented a slightly smaller share of UTI episodes than ED visits overall and non-Hispanic Whites a somewhat larger fraction of UTIs than ED visits. A far larger share of ED visits (55%) and especially UTI episodes (63%) were covered by public health insurance than the proportion of the civilian, non-institutionalized population with public coverage (22%). Private health insurance was correspondingly much more common among the wider population (67%) than covered ED visits (25%) or UTIs (20%). UTI episodes and ED visits were also more common among individuals without health insurance than the proportion of the population lacking health insurance. Other payment methods, such as workers’ compensation, were reported in 2% of UTI episodes and 3% of ED visits; these methods were treated as missing and excluded from analyses of EMM by health insurance, as they have no corollary in the ACS data and group-specific population offsets could not be computed. An additional 6% of UTI episodes lacked payment information entirely and were similarly dropped from health insurance EMM analyses.
Table 2.
Demographic composition of UTI episodes, all ED visits, and the general population in 2016-2019, excluding metropolitan and offshore ZIP codes. Percentages were calculated excluding missing observations for specific variables except when calculating percent missing, for which all observations were included in the denominator.
| Characteristic | UTI episodes | Total ED visits | Population |
|---|---|---|---|
| Sex | |||
| Female | 81.7% | 56.7% | 51.3% |
| Male | 18.3% | 43.3% | 48.7% |
| Missing | 0.2% | 0.2% | |
| Age (years) | |||
| 0 - 4 | 2.1% | 6.6% | 6% |
| 5 - 17 | 5.7% | 10% | 16.7% |
| 18 - 34 | 25.6% | 25.3% | 22.8% |
| 35 - 64 | 29.9% | 37.3% | 39.2% |
| 65+ | 36.6% | 20.8% | 15.3% |
| Missing | 0% | 0% | |
| Race/ethnicity | |||
| American Indian | 2.1% | 1.8% | 1.1% |
| Asian | 0.4% | 0.4% | 2.7% |
| Black | 24.4% | 26.5% | 21.1% |
| White | 66.6% | 63.6% | 63.5% |
| Hispanic | 4.6% | 5.4% | 9.2% |
| Other race | 1.9% | 2.4% | 2.4% |
| Missing | 4.3% | 4.5% | |
| Health insurance | |||
| Private | 20.3% | 25% | 66.6% |
| Public | 63.2% | 54.5% | 21.6% |
| Uninsured | 16.5% | 20.5% | 11.8% |
| Other paymenta | 2.2% | 3.4% | |
| Missing | 6.3% | 6.9% |
ED visits with no charge or payment through workers compensation or other insurance; these categories have no corollary in the ACS population estimates and were, therefore, excluded from health insurance EMM analyses.
3.3. Spatial structure of UTI incidence rates
The ZIP code neighborhood graph had a generalized variance of 0.761, calculated as the geometric mean of the unitless adjacency matrix marginal variances (Riebler et al., 2016). We used this generalized neighborhood graph variance as the scaling factor for the spatial variance proportion ρ in all models. The mean ZIP code ED UTI incidence rate was 0.0189 episodes/person/year (95% CI: 0.0187, 0.0191) but the overall variance of the combined random effect was 1.37 (95% CI: 1.31, 1.43), indicating substantial overdispersion of UTI rates between ZIP codes (Morris et al., 2019). UTI rates exhibited strong spatial correlation, with the spatially structured component accounting for 96% (95% CI: 92%, 99%) of the overall variance between ZIP code observations. The residual variance decreased slightly when conditioned on hog or poultry density, other covariates, and effect measure modifiers, but the spatially structured proportion remained comparably high under all model specifications (Table S1). This manifests as greater similarity in rates among neighboring ZIP codes, as can be observed on maps of both the empirical and model-estimated average annual UTI rates (Figure 2). Modeled rates were estimated using an intercept-only BYM2 model that incorporated the combined spatial and unstructured random effects for each ZIP code. By excluding all animal exposure variables and other predictors, the reduced model illustrates the degree to which the BYM2 structural components captured spatial dependencies in the data and induced spatial smoothing in the resulting estimates. The observed annual rates (simple averages across the four-year study period) were quite similar to the modeled rates, with nearly identical values for the mean (observed: 21.1, estimated: 20.9), median (19.7, 19.7), 10th percentile (10.5, 10.8), and 90th percentile (32.7, 32.4) rates per 1000 population. Model-induced smoothing was observed at the extremes of the rate distribution, notably in reducing the maximum observed rate of 144 UTI episodes per 1000 to a modeled rate of 75 (and the maximum modeled rate reduced from the 120 UTI episodes per 1000 observed in that ZIP code to 105), along with less substantial increases in the minimum modeled rate (0.72) from the minimum observed rate of 0.39 UTI episodes per 1000. Spatial smoothing can be observed in Figure 2 at certain small-area ZIP codes, particularly in the west of the state, with observed rates much higher than the surrounding ZIP codes, for which the estimated rates have been somewhat attenuated to better align with their neighbors. The accurate reproduction of spatial patterns of incidence and the concurrent taming of extreme rates (which can reflect poor measurement precision in small populations) suggests the BYM2 model structure successfully accounted for spatial dependencies in the outcome (Hampton et al., 2011).
Figure 2.
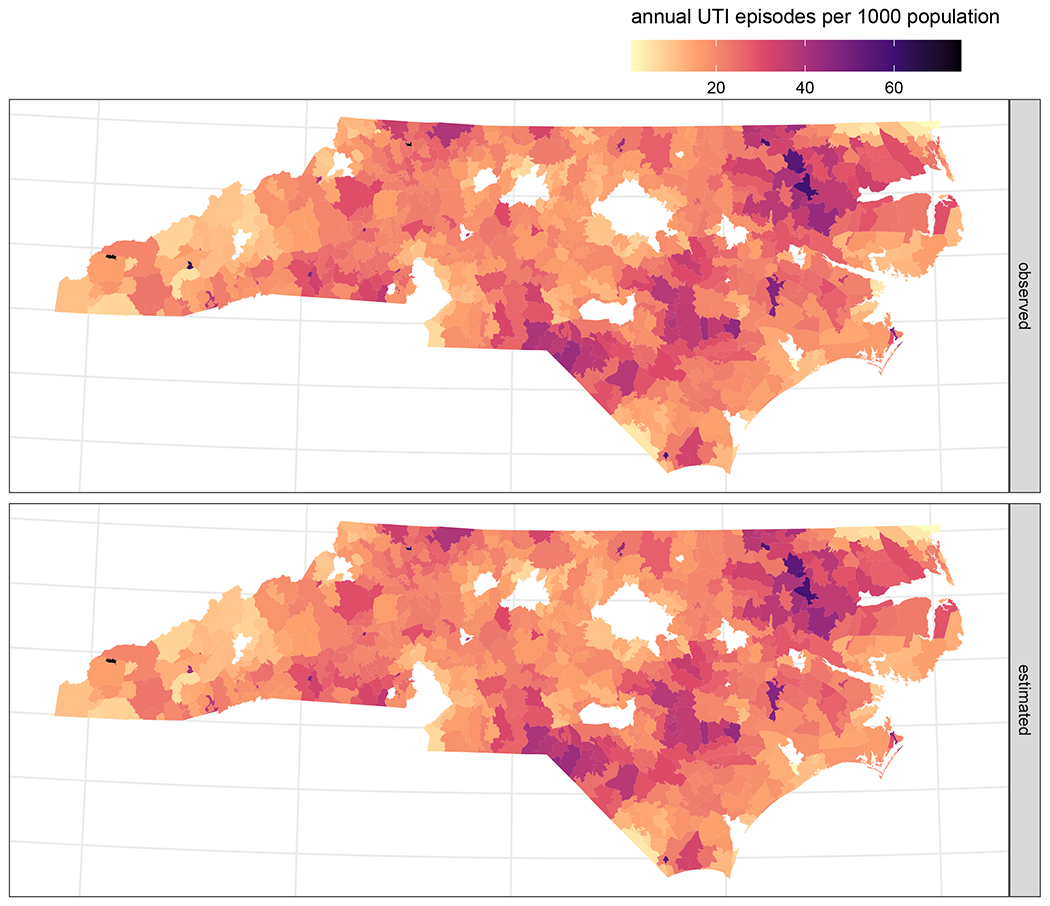
Mean annual observed (top panel) and estimated (bottom panel) UTI incidence rates per 1000 residents by ZIP code in 2016 – 2019. Rates were estimated using a reduced BYM2 model that included only the combined random effect (with both spatial and unstructured components) for each ZIP code and an overall intercept. To aid visualization, high rates have been truncated at 75 for display, affecting observed rates in three ZIP codes (original values: 144, 120, and 111 UTI episodes per 1000 population) and the estimated rate in one ZIP code (originally 105 UTI episodes per 1000).
3.4. UTI associations with animal density
All categories of hog exposure were associated with elevated UTI incidence compared to hog-free areas, including a 21% increase in UTI rate for the highest hog density tertile (RR: 1.21, 95% CI: 1.06, 1.37; Table 3). The rate ratios increased somewhat in magnitude with increasing categorical hog density, in keeping with an estimated 8% increase in UTI rate (RR: 1.08, 95% CI: 1.01, 1.16) for each 1-log10 increase in hog density. However, poultry exposure was associated with reduced incidence of UTIs, particularly at low poultry density (RR: 0.86, 95% CI: 0.81, 0.91). The apparent inverse association was somewhat attenuated at medium and high poultry density, while a 1-log10 increase in poultry density was associated with a 4% reduction in UTIs, but did not exclude the null. Mutually adjusting for both hogs and poultry minimally impacted the rate ratio estimates.
Table 3.
UTI rate ratio estimates for exposure to hogs and poultry from single-animal and mutually adjusted dual-animal models. Low, medium, and high exposure categories correspond to tertiles of non-zero density values, with zero-density ZIP codes serving as the reference group. Models were adjusted for ZIP code isolation score, median household income, and percent lacking health insurance.
| Exposure | Density (count/km2) | ZIP codesa (observations) | UTI episodes (outcome) | Person-years (offset) | Single-animal RR (95% CI) | Mutually adjusted dual-animal RR (95% CI) |
|---|---|---|---|---|---|---|
| Hog | ||||||
| Unexposed | 0 | 324 | 253202 | 14016758 | (ref) | (ref) |
| Low | >0 - 4 | 99 | 109814 | 5375323 | 1.09 (0.99, 1.19) | 1.09 (1.00, 1.19) |
| Medium | >4 - 36 | 99 | 89350 | 3648783 | 1.14 (1.02, 1.27) | 1.16 (1.04, 1.29) |
| High | >36 - 1099 | 98 | 75701 | 3015979 | 1.21 (1.06, 1.37) | 1.23 (1.08, 1.40) |
| Continuous | log10 increase | 620 | 528067 | 26056843 | 1.08 (1.01, 1.16) | 1.11 (1.03, 1.19) |
| Poultry | ||||||
| Unexposed | 0 | 184 | 85070 | 5375900 | (ref) | (ref) |
| Low | >0 - 887 | 145 | 172628 | 8531930 | 0.86 (0.81, 0.91) | 0.85 (0.81, 0.90) |
| Medium | >887 - 4833 | 146 | 169125 | 7716740 | 0.92 (0.86, 0.98) | 0.91 (0.85, 0.97) |
| High | >4833 - 39499 | 145 | 101244 | 4432273 | 0.91 (0.84, 0.98) | 0.89 (0.81, 0.96) |
| Continuous | log10 increase | 620 | 528067 | 26056843 | 0.96 (0.91, 1.00) | 0.94 (0.89, 0.99) |
mean number of ZIP codes in each exposure category, 2016 – 2019
3.5. Effect measure modification
Associations with UTI rates increased somewhat over time for hog exposures (Figure 3a), but calendar year was not a meaningful modifier of the effects of poultry exposure (Figure 3b). Conversely, we did not observe EMM by sex for hog exposure, while the inverse association for poultry exposure was more pronounced for males but not significant for females other than in the lowest category of poultry exposure. Associations with hog exposure were most strongly elevated among the age 35-64 group, while UTI rates associated with poultry exposure were most reduced among both children (age 5-17 years) and older adults (age 65 and above). UTI rates among the privately insured were 39% higher (RR: 1.39, 95% CI: 1.22, 1.58) in the highest hog density category and 27% higher (RR: 1.27, 95% CI: 1.17, 1.38) in the highest poultry density areas. UTI rate ratios were lower among those with public insurance, with no association with hog exposure and inverse associations with poultry exposure (highest poultry density RR: 0.69, 95% CI: 0.63, 0.75). While the magnitude of associations was generally strongest in suburban areas and weakest in rural areas, EMM by rurality was relatively limited compared with other modifiers assessed, particularly at higher-density exposures.
Figure 3.
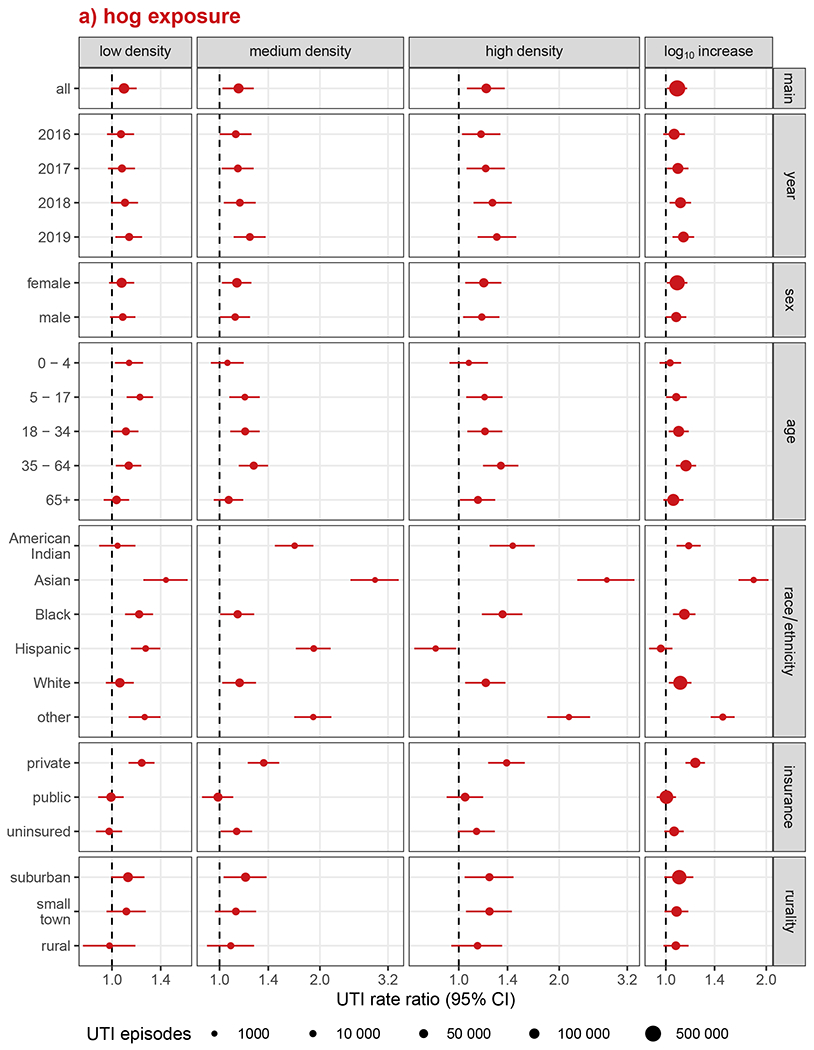
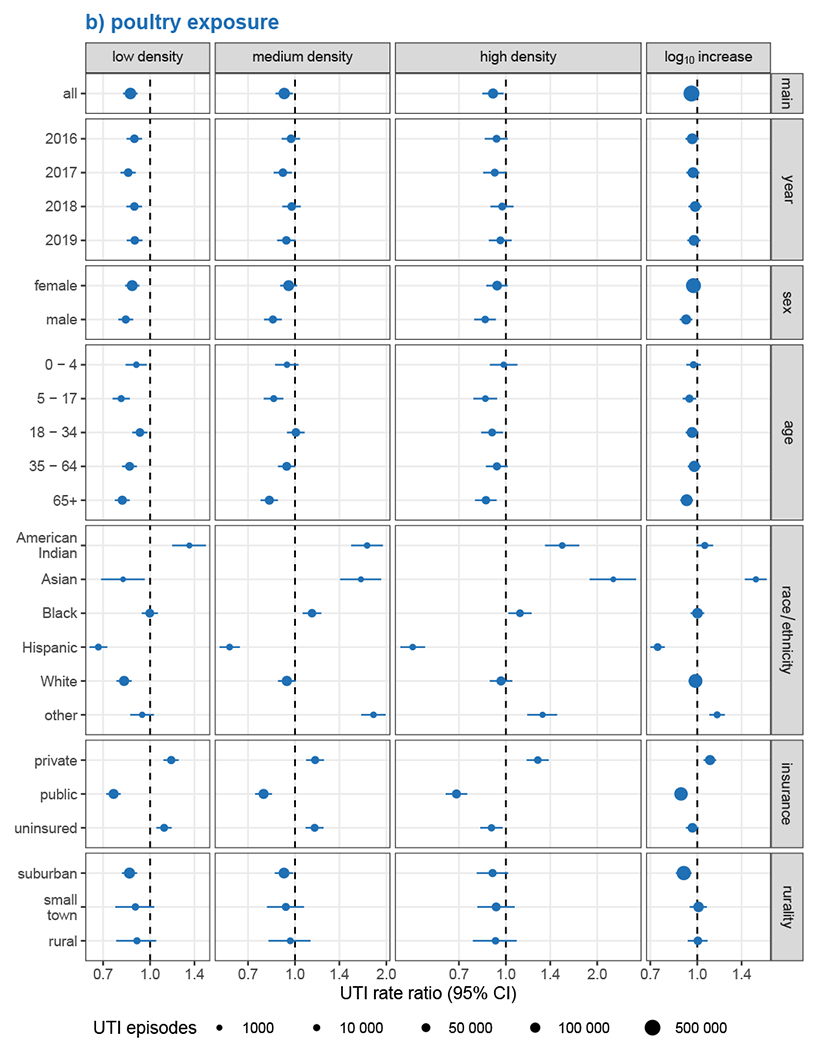
Analysis of effect measure modification of UTI incidence associations with a) hog exposure and b) poultry exposure, by calendar year, sex, age, race/ethnicity, health insurance, and rurality. Modification was assessed on the multiplicative scale in single-animal models using product-term interactions between exposure group and the effect measure modifier and rate ratios as the measure of effect. Low, medium, and high exposure categories correspond to tertiles of non-zero density values, with zero-density ZIP codes serving as the reference group. Race and ethnicity were collapsed into a single variable with Hispanic individuals of any race forming one group and non-Hispanic individuals forming groups according to their recorded race. Models were adjusted for ZIP code isolation score, median household income, and percent lacking health insurance, except for models of EMM by rurality, which were not adjusted for isolation score, and EMM by health insurance, which were not adjusted for uninsured population.
Strong EMM by race/ethnicity was observed for both animal exposures. Associations were notably elevated among Asian populations exposed to hogs, with nearly three-times the UTI rate among Asian residents of medium (RR: 2.93, 95% CI: 2.47, 3.45) and high (RR: 2.78, 95% CI: 2.27, 3.36) hog-density ZIP codes. Despite inverse associations with UTI rates for the general population overall, exposure to medium- and high-density poultry was also associated with a marked increase in UTI rates among Asians, though with an inverse-association for low-density poultry. The Other race/ethnicity group exhibited similar, though generally somewhat attenuated, patterns of association as Asians across animals and exposure levels. Poultry exposures were also consistently associated with increased UTI rates among American Indian residents, even at the low densities that were inversely associated with UTIs among Asian and Other race/ethnicity groups. Among American Indian residents, medium- and high-density, but not low-density, hog exposures were associated with elevated UTI rates. Poultry exposure had the strongest inverse associations with UTI rates among Hispanic populations, with UTI rates reduced by nearly one-third in low-density ZIP codes (RR: 0.68, 95% CI: 0.63, 0.72) and more than halved in the highest-density ZIP codes (RR: 0.49, 95% CI: 0.45, 0.54). Paradoxically, among Hispanic residents, UTI rates were substantially elevated in both low- and medium-density hog areas, but were reduced in high-density hog areas. UTI rate ratios among Black and White residents were largely similar to the unstratified estimates across animals and exposure levels, though they tended to be slightly higher among Black populations.
3.6. Joint exposures
We observed the highest UTI rates in ZIP codes that contained hogs but no poultry, which accounted for only 5% (n=30) of all ZIP codes and 21066 total UTI episodes, with a 51% increase (RR: 1.51, 95% CI: 1.19, 1.89; 1245 total UTIs) in the 3 ZIP codes with the highest hog density and zero poultry density, relative to the 154 ZIP codes that were unexposed to both hogs and poultry (Figure 4). Similarly elevated UTI rates were seen in low- and medium-density hog areas with zero poultry density. Conversely, we found no evidence in these analyses that poultry exposure at any density was associated with UTI rates, in contrast to the apparently reduced UTI rates associated with all poultry levels in the single exposure models. In low and medium hog density areas, increasing poultry density largely attenuated the relationship between hog exposure and UTI rates. The presence of poultry in the highest hog density areas also reduced the magnitude of the association but UTI rates remained elevated, particularly in the 31 high hog, medium poultry density ZIP codes, where UTIs rates were 28% higher (RR: 1.28, 95% CI: 1.10, 1.48; 30048 total UTIs) than in areas exposed to neither.
Figure 4.
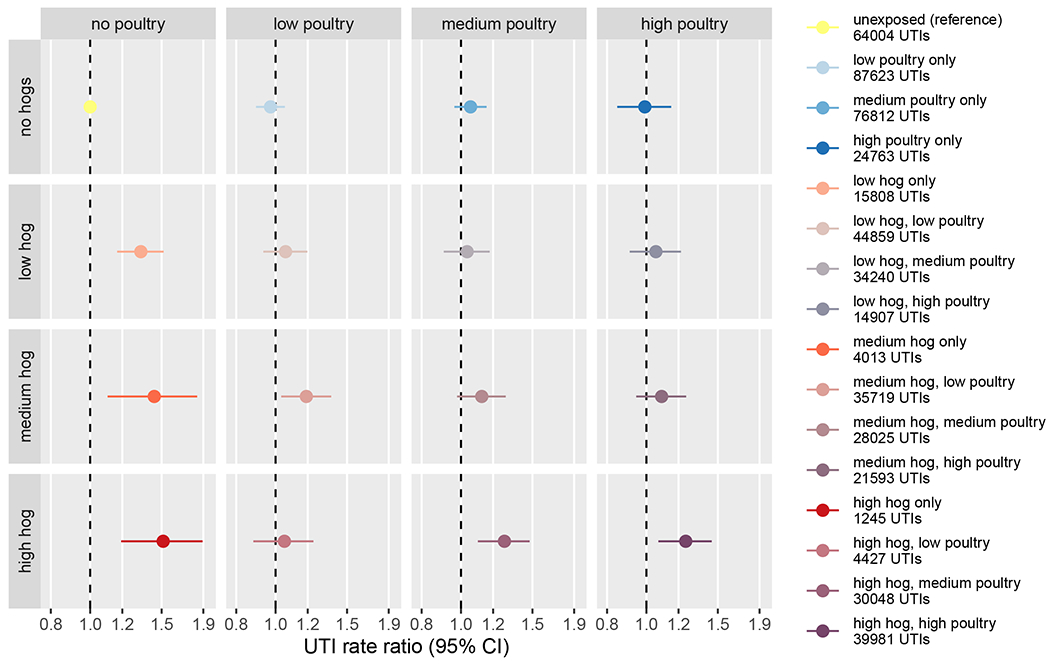
UTI rate ratio estimates for joint categorical exposure to hogs and poultry relative to areas with no exposure to either animal. Low, medium, and high exposure categories correspond to tertiles of non-zero density values. Models were adjusted for ZIP code isolation score, median household income, and percent lacking health insurance.
4. Discussion
We observed elevated rates of ED visits for UTIs in ZIP codes exposed to industrial hog production, with the largest relative increase in UTI rates among ZIP codes with high hog density (>36 hogs/km2). However, hog exposure associations with UTI rates were strongly modified by the presence and intensity of concurrent poultry exposure. Notably, poultry was observed to reduce UTI rates relative to poultry-free ZIP codes, particularly at low poultry densities (<877 birds/km2), but exhibited no relationship with UTI rates when compared only to ZIP codes free of both hogs and poultry. The relationship between low-density hog exposure and increased UTI rates was largely attenuated by any amount of poultry co-exposure, but high-density hog exposure remained associated with elevated UTI rates at both medium- and high-density poultry co-exposure; only at low-density poultry co-exposure were high hog densities not associated with increased UTIs. The spatial distributions of these two exposures in North Carolina differ considerably: hog production is concentrated in the eastern portion of the state, while poultry production is common throughout, including across the central and western areas of the state that largely lack hog operations. However, the eastern hog producing regions are also home to intense poultry production, with very few ZIP codes exposed to hogs alone. Besides metropolitan areas, only the mountains in the far west and coastal areas in the far east of the state are largely free from either animal, likely reflecting topography or other geographic characteristics not conducive to industrial livestock production. Some of the highest UTI rates were observed in areas exposed to hogs alone, while the much more common poultry-only ZIP codes exhibited a broader range of UTI rates that were more representative of the state overall. The apparent reduction in UTI rates in poultry-exposed areas compared with poultry-free areas was likely, in part, an artifact of including high UTI-incidence hog-only ZIP codes in the comparison group, as poultry exposure did not demonstrate any clear relationship with UTI rates when the reference set was limited to areas free of both hogs and poultry. While the strongest positive associations with UTI rates were observed for ZIP codes with hogs but no poultry, such areas were uncommon and home to a small fraction (under 4%) of the population at risk in our analyses. Unknown features of these locations that contributed to the unusual siting of hog operations but not poultry may have also influenced UTI rates upwards, potentially resulting in residual confounding of the hog-UTI relationship that could not be accounted for in this analysis. Nevertheless, co-exposure to higher densities of both hogs and poultry—a more typical scenario affecting over 10% of the study population—was associated with an approximate 25% increase in UTI rates. The consistent increase in UTI rates in high animal density ZIP codes suggests an overall relationship between intense livestock production and UTI incidence.
The relative effect of hog exposure on UTI rates was substantially modified by race/ethnicity, which serves as a proxy for historically rooted economic, behavioral, and environmental processes that shape social inequalities in the present-day context of the United States (Gravlee, 2009; VanderWeele and Robinson, 2014). We examined EMM by race/ethnicity to assess racial disparities in the relationship between hog exposure and UTI rates; however, differences in the prevalence and intensity of exposure or incidence of the outcome by race/ethnicity may contribute to disparity regardless of the particular association of the exposure and outcome within each group (Ward et al., 2019). As the two largest race/ethnicity groups by population as well as number of ED visits and UTI episodes, non-Hispanic Black and non-Hispanic White residents had UTI rate ratio estimates that were broadly similar to the unstratified estimates. The proportion of UTI episodes among both groups was about 3 percentage points higher than their respective shares of the general population (21% Black, 64% White). The highest magnitude associations were observed among non-Hispanic Asian residents, who comprised 2.7% of the study population but only 0.4% of ED visits and UTI episodes during the study period, a more than six-fold underrepresentation in the ED visits relative to population share. The proportion of residents who were Asian decreased with increasing hog density, with the large majority of Asians residing in hog-free ZIP codes (Quist et al., 2022b). The relatively low ED utilization and small hog-exposed population suggests that ED visits for UTI by comparably few Asian residents from high-exposure areas may have been sufficient to produce the elevated UTI rates observed. In contrast, non-Hispanic American Indians accounted for twice the share of UTI episodes (2.1%) than the proportion of the study population that was American Indian, the majority of whom resided in medium and high hog-exposed ZIP codes. UTI rates remained elevated among American Indian residents of hog-exposed areas but to a lesser extent than among Asian residents, potentially due to larger exposed populations and increased ED utilization. Although Hispanic residents also comprised a slightly larger share of medium (10%) and high (11%) hog-density ZIP code populations than of low- and no-exposure ZIP codes (8-9%), the majority of Hispanics lived in unexposed and low exposure ZIP codes and were underrepresented by half, relative to total population share, in ED visits and UTI episodes. For all other groups and the population overall, the lowest ED rates for any cause were observed in unexposed ZIP codes and the highest ED rates in high hog-exposure areas (Table S6). While the lowest ED rates among Hispanic residents were also seen in unexposed areas (290 per 1000 Hispanic residents in 2019, substantially lower than the 465 ED visits per 1000 total population), ED rates in high hog density areas were similarly low (298 per 1000) with correspondingly low UTI episode rates (8 per 1000). Reduced ED utilization by Hispanic populations, relative to non-Hispanic Whites, has been well documented and associated with lower acculturation and fear of discovery of undocumented status (Allen and Cummings, 2016; Maldonado et al., 2013; Quist et al., 2022a). The severely reduced ED utilization by Hispanic residents of high hog-density areas, likely due to characteristics of these areas that could not be directly accounted for in this analysis, may have been largely responsible for the apparent inverse association between high hog exposure and UTI rates among Hispanic patients.
Although bacteria with high genetic similarity to pandemic ExPEC lineages have been isolated from both swine and poultry sources, the existing literature largely focuses on the role of poultry meat in foodborne UTI transmission (Manges, 2016). However, previous research has primarily considered proximal exposures such as meat consumption and animal contact in occupational settings, while we investigated residential proximity to animal production as a proxy for a range of potential exposure pathways from CAFOs to neighboring communities. One possible community exposure pathway is through manure, which is produced in large quantities at CAFOs and must be disposed of regularly (Casey et al., 2015). Poultry manure is primarily handled as dry litter, which can be piled onsite and transported relatively economically by road for disposal or other use (Kelleher et al., 2002). In contrast, hogs produce large volumes of liquid waste that is typically stored in open lagoons onsite and disposed of by spraying on nearby fields (Christenson and Serre, 2017). Community exposure to hog manure may be particularly exacerbated by the increasingly frequent flooding from extreme weather events in the eastern part of the state where hog CAFOs are disproportionately located. At least two major storms, Hurricane Matthew in 2016 and Hurricane Florence in 2018, led to widespread flooding and breaching of hog fecal waste lagoons during our four-year study period (Quist et al., 2022a). Such flooding events in eastern NC have been associated with both hog-associated fecal bacteria in surface waters and increased incidence of gastrointestinal illness (Harris et al., 2021; Quist et al., 2022a). Future work should investigate whether UTI incidence is likewise elevated following extreme weather events, particularly in regions of intense food animal production. Extreme weather is not required to mobilize livestock-associated microbes off-farm, however, as more typical precipitation events have also been associated with both increased surface water contamination and higher rates of gastrointestinal illness in high hog-density areas (Christenson et al., 2022; Heaney et al., 2015; Quist et al., 2022b).
We characterized spatial density of industrial hog and poultry production at fine spatial resolution using overlapping 5 km buffers around Census blocks to estimate population-weighted mean ZIP code exposures (Quist et al., 2022b; Son et al., 2021b). Averaging exposures at the ZIP code-level was necessitated by the spatial resolution of the ED outcome data. Our approach balanced considerations of population representativeness, by weighting exposures to population at a finer spatial scale, and generalizability through the use of a directly interpretable metric—spatial density as animals per km2—as a proxy for the intensity of food animal production. The resulting metrics represent average exposure across an arbitrary area and should not be interpreted as the exposure experienced by individuals within the ZIP code, which have the potential to vary greatly (Briant et al., 2010; Wakefield, 2007). If the reasons for within-ZIP code variation in animal density exposures are different than the causes of within-ZIP code variation in UTIs (and specifically those presenting at EDs), the resulting effect estimates for the aggregate exposure on the aggregate outcome can differ greatly from the corresponding estimates for individual exposures and outcomes, known as ecological bias (Gelman et al., 2001). We accounted for spatial dependencies in the residuals of the aggregate exposure-outcome model, which may help mitigate ecological bias to some extent, but in practice within-area variability in exposure and (potentially unmeasured) confounders present a greater threat to inference about individual risk from aggregate data that can be fully addressed only by incorporating individual-level data (Wakefield, 2003). Accordingly, our results demonstrated that ZIP codes with higher spatial hog density had elevated UTI rates, but not necessarily that increasing hog density in proximity to an individual raised their UTI risk.
The use of ED surveillance allowed us to ascertain UTI incidence with high geographic coverage throughout the state over a four-year period. While most UTIs do not result in ED visits and therefore would not be captured by this surveillance system, UTI is nevertheless one of the most common diagnoses at EDs (Bruxvoort et al., 2019; Foxman, 2010). We extracted over half a million ED diagnoses for UTI in non-metropolitan, mainland NC in 2016-2019, nearly 4% of the 13.6 million ED visits recorded in total. We were limited to all-cause UTI as the outcome because the vast majority of records lacked information on infectious agent or antimicrobial resistance profiles. This is a broad outcome that encompasses a range of potential causes besides food animal-related exposures, but studies consistently find that about three-quarters of UTIs are attributable to E. coli and increasingly dominated by a small number of globally pandemic clonal lineages with putative animal origins, so it is likely that a substantial proportion of UTIs recorded at NC EDs were related to such uropathogens (Manges et al., 2019; Riley, 2014; Tandogdu and Wagenlehner, 2016). Because extensive AMR is a notable feature of pandemic ExPEC lineages, access to comprehensive urine culture data may provide valuable insight into the role of food animal production and other environmental exposure routes on increasing UTI morbidity for future research efforts (Bruxvoort et al., 2019; Graham et al., 2021; Zeng et al., 2022).
Supplementary Material
5. Acknowledgments
This study was supported by the National Institute of Environmental Health Sciences T32 Training Grant (T32ES007018). The North Carolina Disease Event Tracking and Epidemiologic Collection Tool (NC DETECT) is an advanced, statewide public health surveillance system. NC DETECT is supported by the North Carolina Division of Public Health through a federal Public Health Emergency Preparedness Grant and is managed through a collaboration between NC DPH and the University of North Carolina at Chapel Hill Department of Emergency Medicine’s Carolina Center for Health Informatics. The findings and conclusions in this publication are those of the authors and do not necessarily represent the views of the North Carolina Department of Health and Human Services, Division of Public Health.
Footnotes
Declaration of Competing Financial Interests
The authors declare they have no actual or potential competing financial interests.
6 References
- Allen L, Cummings J, 2016. Emergency Department Use Among Hispanic Adults: The Role of Acculturation. Med. Care 54, 449–456. 10.1097/MLR.0000000000000511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ, Rojas LF, Watson S, Knelson LP, Pruitt S, Lewis SS, Moehring RW, Sickbert Bennett EE, Weber DJ, Chen LF, Sexton DJ, the CDC Prevention Epicenters Program, 2017. Identification of novel risk factors for community-acquired Clostridium difficile infection using spatial statistics and geographic information system analyses. PLOS ONE 12, e0176285. 10.1371/journal.pone.0176285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JCH, Koch A, Ethelberg S, Mølbak K, Larsen J, Jepsen MR, 2018. Distance to pig farms as risk factor for community-onset livestock-associated MRSA CC398 infection in persons without known contact to pig farms-A nationwide study. Zoonoses Public Health 65, 352–360. 10.1111/zph.12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, He L-Y, Wu D-L, Gao F-Z, Zhang M, Zou H-Y, Yao M-S, Ying G-G, 2022. Spread of airborne antibiotic resistance from animal farms to the environment: Dispersal pattern and exposure risk. Environ. Int 158, 106927. 10.1016/j.envint.2021.106927 [DOI] [PubMed] [Google Scholar]
- Bakka H, Rue H, Fuglstad G-A, Riebler A, Bolin D, Krainski E, Simpson D, Lindgren F, 2018. Spatial modelling with R-INLA: A review. ArXiv180206350 Stat. URL http://arxiv.org/abs/1802.06350 (accessed 4.23.21).
- Beresin GA, Wright JM, Rice GE, Jagai JS, 2017. Swine exposure and methicillin-resistant Staphylococcus aureus infection among hospitalized patients with skin and soft tissue infections in Illinois: A ZIP code-level analysis. Environ. Res 159, 46–60. 10.1016/j.envres.2017.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besag J, York J, Mollié A, 1991. Bayesian image restoration, with two applications in spatial statistics. Ann. Inst. Stat. Math 43, 1–20. 10.1007/bf00116466 [DOI] [Google Scholar]
- Bivand RS, Wong DWS, 2018. Comparing implementations of global and local indicators of spatial association. TEST 27, 716–748. 10.1007/s11749-018-0599-x [DOI] [Google Scholar]
- Booth BJ, Jones RR, Turyk ME, Freels S, Patel DM, Stayner LT, Ward MH, 2017. Livestock and poultry density and childhood cancer incidence in nine states in the USA. Environ. Res 159, 444–451. 10.1016/j.envres.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briant A, Combes P-P, Lafourcade M, 2010. Dots to boxes: Do the size and shape of spatial units jeopardize economic geography estimations? J. Urban Econ 67, 287–302. 10.1016/j.jue.2009.09.014 [DOI] [Google Scholar]
- Bruxvoort KJ, Bider-Canfield Z, Casey JA, Qian L, Pressman A, Liang AS, Robinson S, Jacobsen SJ, Tartof SY, 2019. Outpatient Urinary Tract Infections in an Era of Virtual Healthcare: Trends From 2008 to 2017. Clin. Infect. Dis ciz764. 10.1093/cid/ciz764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkner P-C, 2017. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw 80. 10.18637/jss.v080.i01 [DOI] [Google Scholar]
- Butcher CR, Rubin J, Mussio K, Riley LW, 2019. Risk Factors Associated with Community-Acquired Urinary Tract Infections Caused by Extended-Spectrum β-Lactamase-Producing Escherichia coli: a Systematic Review. Curr. Epidemiol. Rep 6, 300–309. 10.1007/s40471-019-00206-4 [DOI] [Google Scholar]
- Cairns C, Kang K, Santo L, 2021. National Hospital Ambulatory Medical Care Survey: 2018 Emergency Department Summary Tables. National Center for Health Statistics. URL https://www.cdc.gov/nchs/data/nhamcs/web_tables/2018-ed-web-tables-508.pdf [Google Scholar]
- Carrel M, Schweizer ML, Sarrazin MV, Smith TC, Perencevich EN, 2014. Residential Proximity to Large Numbers of Swine in Feeding Operations Is Associated with Increased Risk of Methicillin-Resistant Staphylococcus aureus Colonization at Time of Hospital Admission in Rural Iowa Veterans. Infect. Control Hosp. Epidemiol 35, 190–192. 10.1086/674860 [DOI] [PubMed] [Google Scholar]
- Casey JA, Kim BF, Larsen J, Price LB, Nachman KE, 2015. Industrial Food Animal Production and Community Health. Curr. Environ. Health Rep 2, 259–271. 10.1007/s40572-015-0061-0 [DOI] [PubMed] [Google Scholar]
- Casey JA, Shopsin B, Cosgrove SE, Nachman KE, Curriero FC, Rose HR, Schwartz BS, 2014. High-Density Livestock Production and Molecularly Characterized MRSA Infections in Pennsylvania. Environ. Health Perspect 122, 464–470. 10.1289/ehp.1307370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson E, Wickersham L, Jacob M, Stewart J, 2022. A watershed study assessing effects of commercial hog operations on microbial water quality in North Carolina, USA. Sci. Total Environ 838, 156085. 10.1016/j.scitotenv.2022.156085 [DOI] [PubMed] [Google Scholar]
- Christenson EC, Serre ML, 2017. Integrating remote sensing with nutrient management plans to calculate nitrogen parameters for swine CAFOs at the sprayfield and sub-watershed scales. Sci. Total Environ 580, 865–872. 10.1016/j.scitotenv.2016.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahms C, Hubner NO, Kossow A, Mellmann A, Dittmann K, Kramer A, 2015. Occurrence of ESBL-producing Escherichia coli in livestock and farm workers in Mecklenburg-Western Pomerania, Germany. PLoS ONE 10, 1–13. 10.1371/journal.pone.0143326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame-Korevaar A, Fischer EAJ, van der Goot J, Stegeman A, Mevius D, 2019. Transmission routes of ESBL/pAmpC producing bacteria in the broiler production pyramid, a literature review. Prev. Vet. Med 162, 136–150. 10.1016/j.prevetmed.2018.12.002 [DOI] [PubMed] [Google Scholar]
- de Rooij MMT, Hoek G, Schmitt H, Janse I, Swart A, Maassen CBM, Schalk M, Heederik DJJ, Wouters IM, 2019. Insights into Livestock-Related Microbial Concentrations in Air at Residential Level in a Livestock Dense Area. Environ. Sci. Technol 53, 7746–7758. 10.1021/acs.est.8b07029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen W, Bonten MJM, Bos MEH, van Marm S, Scharringa J, Wagenaar JA, Heederik DJJ, 2015. Carriage of extended-spectrum β-lactamases in pig farmers is associated with occurrence in pigs. Clin. Microbiol. Infect 21, 917–923. 10.1016/j.cmi.2015.05.032 [DOI] [PubMed] [Google Scholar]
- Dohmen W, Schmitt H, Bonten M, Heederik D, 2017. Air exposure as a possible route for ESBL in pig farmers. Environ. Res 155, 359–364. 10.1016/j.envres.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Doogan NJ, Roberts ME, Wewers ME, Tanenbaum ER, Mumford EA, Stillman FA, 2018. Validation of a new continuous geographic isolation scale: A tool for rural health disparities research. Soc. Sci. Med 215, 123–132. 10.1016/j.socscimed.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibke CD, Croxen MA, Geum HM, Glass M, Wong E, Avery BP, Daignault D, Mulvey MR, Reid-Smith RJ, Parmley EJ, Portt A, Boerlin P, Manges AR, 2019. Genomic Epidemiology of Major Extraintestinal Pathogenic Escherichia coli Lineages Causing Urinary Tract Infections in Young Women Across Canada. Open Forum Infect. Dis 6, ofz431. 10.1093/ofid/ofz431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ, 2015. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol 13, 269–284. 10.1038/nrmicro3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Founou LL, Founou RC, Ntshobeni N, Govinden U, Bester LA, Chenia HY, Djoko CF, Essack SY, 2019. Emergence and spread of extended spectrum β-lactamase producing enterobacteriaceae (ESBL-PE) in pigs and exposed workers: A multicentre comparative study between Cameroon and South Africa. Pathogens 8. 10.3390/pathogens8010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman B, 2014. Urinary Tract Infection Syndromes. Infect. Dis. Clin. North Am 28, 1–13. 10.1016/j.idc.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Foxman B, 2010. The epidemiology of urinary tract infection. Nat. Rev. Urol 7, 653–660. 10.1038/nrurol.2010.190 [DOI] [PubMed] [Google Scholar]
- Gelman A, Park DK, Ansolabehere S, Price PN, Minnite LC, 2001. Models, assumptions and model checking in ecological regressions. J. R. Stat. Soc. Ser. A Stat. Soc 164, 101–118. 10.1111/1467-985X.00190 [DOI] [Google Scholar]
- George AN, Stewart JR, Evans JC, Gibson JM, 2020. Risk of Antibiotic-Resistant Staphylococcus aureus Dispersion from Hog Farms: A Critical Review. Risk Anal. risa 13495. 10.1111/risa.13495 [DOI] [PubMed] [Google Scholar]
- Graddy S, Simon E, Rundquist S, 2020. UPDATE: Exposing Fields of Filth: Factory Farms Disproportionately Threaten Black, Latino and Native American North Carolinians. The Environmental Working Group and Waterkeeper Alliance, Washington, D.C. URL https://www.ewg.org/interactive-maps/2020-fields-of-filth/#methodology (accessed 9.1.22). [Google Scholar]
- Graham JP, Amato HK, Mendizabal-Cabrera R, Alvarez D, Ramay BM, 2021. Waterborne Urinary Tract Infections: Have We Overlooked an Important Source of Exposure? Am. J. Trop. Med. Hyg 105, 12–17. 10.4269/ajtmh.20-1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravlee CC, 2009. How race becomes biology: Embodiment of social inequality. Am. J. Phys. Anthropol 139, 47–57. 10.1002/ajpa.20983 [DOI] [PubMed] [Google Scholar]
- Greenwood-Ericksen MB, Kocher K, 2019. Trends in Emergency Department Use by Rural and Urban Populations in the United States. JAMA Netw. Open 2, e191919–e191919. 10.1001/jamanetworkopen.2019.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubesic TH, Matisziw TC, 2006. On the use of ZIP codes and ZIP code tabulation areas (ZCTAs) for the spatial analysis of epidemiological data. Int. J. Health Geogr 5, 58. 10.1186/1476-072X-5-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton KH, Serre ML, Gesink DC, Pilcher CD, Miller WC, 2011. Adjusting for sampling variability in sparse data: geostatistical approaches to disease mapping. Int. J. Health Geogr 10, 54. 10.1186/1476-072X-10-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AR, Fidan EN, Nelson NG, Emanuel RE, Jass T, Kathariou S, Niedermeyer J, Sharara M, de los Reyes FL, Riveros-Iregui DA, Stewart JR, 2021. Microbial Contamination in Environmental Waters of Rural and Agriculturally-Dominated Landscapes Following Hurricane Florence. ACS EST Water. 10.1021/acsestwater.1c00103 [DOI] [Google Scholar]
- Hatcher SM, Myers KW, Heaney CD, Larsen J, Hall D, Miller MB, Stewart JR, 2016. Occurrence of methicillin-resistant Staphylococcus aureus in surface waters near industrial hog operation spray fields. Sci. Total Environ 565, 1028–1036. 10.1016/j.scitotenv.2016.05.083 [DOI] [PubMed] [Google Scholar]
- Hayashi W, Tanaka H, Taniguchi Y, Iimura M, Soga E, Kubo R, Matsuo N, Kawamura K, Arakawa Y, Nagano Y, Nagano N, 2019. Acquisition of mcr-1 and Cocarriage of Virulence Genes in Avian Pathogenic Escherichia coli Isolates from Municipal Wastewater Influents in Japan. Appl. Environ. Microbiol 85, 1–11. 10.1128/AEM.01661-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney CD, Myers K, Wing S, Hall D, Baron D, Stewart JR, 2015. Source tracking swine fecal waste in surface water proximal to swine concentrated animal feeding operations. Sci. Total Environ 511, 676–683. 10.1016/j.scitotenv.2014.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb DA, Knee J, Capone D, Sumner T, Adriano Z, Nalá R, Cumming O, Brown J, Stewart JR, 2021. Impacts of an Urban Sanitation Intervention on Fecal Indicators and the Prevalence of Human Fecal Contamination in Mozambique. Environ. Sci. Technol 55, 11667–11679. 10.1021/acs.est.1c01538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb DA, Stewart JR, 2020. Microbial Indicators of Fecal Pollution: Recent Progress and Challenges in Assessing Water Quality. Curr. Environ. Health Rep 7, 311–324. 10.1007/s40572-020-00278-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooiveld M, Smit LAM, van der Sman-de Beer F, Wouters IM, van Dijk CE, Spreeuwenberg P, Heederik DJJ, Yzermans CJ, 2016. Doctor-diagnosed health problems in a region with a high density of concentrated animal feeding operations: a cross-sectional study. Environ. Health 15, 24. 10.1186/s12940-016-0123-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain ZZ, Sultana R, Begum A, Jensen PKM, 2021. Investigation of the Domestic Reservoirs of Diarrheagenic Escherichia coli in Diarrhea Case Households of Urban Bangladesh. Curr. Microbiol 10.1007/s00284-021-02506-9 [DOI] [PubMed] [Google Scholar]
- Houck KM, Terán E, Ochoa J, Zapata GN, Gomez AM, Parra R, Dvorquez D, Stewart JR, Bentley ME, Thompson AL, 2019. Drinking water improvements and rates of urinary and gastrointestinal infections in Galápagos, Ecuador: Assessing household and community factors. Am. J. Hum. Biol 1–12. 10.1002/ajhb.23358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesser KJ, Levy K, 2020. Updates on defining and detecting diarrheagenic Escherichia coli pathotypes. Curr. Opin. Infect. Dis 33. 10.1097/qco.0000000000000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher BP, Leahy JJ, Henihan AM, O’Dwyer TF, Sutton D, Leahy MJ, 2002. Advances in poultry litter disposal technology – a review. Bioresour. Technol 83, 27–36. 10.1016/S0960-8524(01)00133-X [DOI] [PubMed] [Google Scholar]
- Kravchenko J, Rhew SH, Akushevich I, Agarwal P, Lyerly HK, 2018. Mortality and Health Outcomes in North Carolina Communities Located in Close Proximity to Hog Concentrated Animal Feeding Operations. N. C. Med. J 79, 278–288. 10.18043/ncm.79.5.278 [DOI] [PubMed] [Google Scholar]
- Krieger N, Waterman P, Chen JT, Soobader M-J, Subramanian SV, Carson R, 2002. Zip Code Caveat: Bias Due to Spatiotemporal Mismatches Between Zip Codes and US Census–Defined Geographic Areas—The Public Health Disparities Geocoding Project. Am. J. Public Health 92, 1100–1102. 10.2105/AJPH.92.7.1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CM, Stegger M, Aziz M, Johnson TJ, Waits K, Nordstrom L, Gauld L, Weaver B, Rolland D, Statham S, Horwinski J, Sariya S, Davis GS, Sokurenko E, Keim P, Johnson JR, Price LB, 2018. Escherichia coli ST131-H22 as a Foodborne Uropathogen. mBio 9, 1–11. 10.1128/mbio.00470-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado CZ, Rodriguez RM, Torres JR, Flores YS, Lovato LM, 2013. Fear of Discovery Among Latino Immigrants Presenting to the Emergency Department. Acad. Emerg. Med 20, 155–161. 10.1111/acem.12079 [DOI] [PubMed] [Google Scholar]
- Manges AR, 2016. Escherichia coli and urinary tract infections: the role of poultry-meat. Clin. Microbiol. Infect 22, 122–129. 10.1016/j.cmi.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Manges AR, Geum HM, Guo A, Edens TJ, Fibke CD, Pitout JDD, 2019. Global Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. Clin. Microbiol. Rev 32, e00135–18. 10.1128/CMR.00135-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson S, Schroeder J, Riper DV, Kugler T, Ruggles S, 2021. IPUMS National Historical Geographic Information System: Version 16.0 [dataset]. URL 10.18128/D050.V16.0 [DOI] [Google Scholar]
- Medina M, Castillo-Pino E, 2019. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol 11, 175628721983217. 10.1177/1756287219832172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montealegre MC, Talavera Rodríguez A, Roy S, Hossain MI, Islam MA, Lanza VF, Julian TR, 2020. High Genomic Diversity and Heterogenous Origins of Pathogenic and Antibiotic-Resistant Escherichia coli in Household Settings Represent a Challenge to Reducing Transmission in Low-Income Settings. mSphere 5, 1–17. 10.1128/msphere.00704-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Wheeler-Martin K, Simpson D, Mooney SJ, Gelman A, DiMaggio C, 2019. Bayesian hierarchical spatial models: Implementing the Besag York Mollié model in stan. Spat. Spatio-Temporal Epidemiol 31, 100301. 10.1016/j.sste.2019.100301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadimpalli M, Rinsky J, Wing S, 2014. Persistence of livestock-associated antibiotic-resistant Staphylococcus aureus among industrial hog operation workers in North Carolina over 14 days. Occup. … 1–10. 10.1136/oemed-2014-102095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASS, 2020. North Carolina Agricultural Statistics (No. 220). USDA National Agricultural Statistics Service and North Carolina Department of Agriculture & Consumer Services. URL https://www.nass.usda.gov/Statistics_by_State/North_Carolina/Publications/Annual_Statistical_Bulletin/AgStat/NCAgStatBook.pdf [Google Scholar]
- N.C. GS., 2014. § 143–215.10I. Performance standards for animal waste management systems that serve swine farms; lagoon and sprayfield systems prohibited. URL https://www.ncleg.net/EnactedLegislation/Statutes/pdf/BySection/Chapter_143/GS_143-215.10I.pdf
- Nicolas-Chanoine M-H, Bertrand X, Madec J-Y, 2014. Escherichia coli ST131, an Intriguing Clonal Group. Clin. Microbiol. Rev 27, 543–574. 10.1128/CMR.00125-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom L, Liu CM, Price LB, 2013. Foodborne urinary tract infections: a new paradigm for antimicrobial-resistant foodborne illness. Front. Microbiol 4, 1–6. 10.3389/fmicb.2013.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owrangi B, Masters N, Kuballa A, O’Dea C, Vollmerhausen TL, Katouli M, 2018. Invasion and translocation of uropathogenic Escherichia coli isolated from urosepsis and patients with community-acquired urinary tract infection. Eur. J. Clin. Microbiol. Infect. Dis 37, 833–839. 10.1007/s10096-017-3176-4 [DOI] [PubMed] [Google Scholar]
- Platell JL, Johnson JR, Cobbold RN, Trott DJ, 2011. Multidrug-resistant extraintestinal pathogenic Escherichia coli of sequence type ST131 in animals and foods. Vet. Microbiol 153, 99–108. 10.1016/j.vetmic.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Quist AJL, Fliss MD, Wade TJ, Delamater PL, Richardson DB, Engel LS, 2022a. Hurricane flooding and acute gastrointestinal illness in North Carolina. Sci. Total Environ 809, 151108. 10.1016/j.scitotenv.2021.151108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist AJL, Holcomb DA, Fliss MD, Delamater PL, Richardson DB, Engel LS, 2022b. Exposure to industrial hog operations and gastrointestinal illness in North Carolina, USA. Sci. Total Environ 830, 154823. 10.1016/j.scitotenv.2022.154823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ [Google Scholar]
- Reid CJ, McKinnon J, Djordjevic SP, 2019. Clonal ST131-H22 Escherichia coli strains from a healthy pig and a human urinary tract infection carry highly similar resistance and virulence plasmids. Microb. Genomics 5. 10.1099/mgen.0.000295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebler A, Sørbye SH, Simpson D, Rue H, 2016. An intuitive Bayesian spatial model for disease mapping that accounts for scaling. Stat. Methods Med. Res 25, 1145–1165. 10.1177/0962280216660421 [DOI] [PubMed] [Google Scholar]
- Riley LW, 2020. Extraintestinal Foodborne Pathogens. Annu. Rev. Food Sci. Technol 11, annurev-food-032519-051618. 10.1146/annurev-food-032519-051618 [DOI] [PubMed] [Google Scholar]
- Riley LW, 2014. Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin. Microbiol. Infect 20, 380–390. 10.1111/1469-0691.12646 [DOI] [PubMed] [Google Scholar]
- Rinsky JL, Nadimpalli M, Wing S, Hall D, Baron D, Price LB, Larsen J, Stegger M, Stewart J, Heaney CD, 2013. Livestock-Associated Methicillin and Multidrug Resistant Staphylococcus aureus Is Present among Industrial, Not Antibiotic-Free Livestock Operation Workers in North Carolina. PLoS ONE 8, e67641. 10.1371/journal.pone.0067641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roer L, Overballe-Petersen S, Hansen F, Johannesen TB, Stegger M, Bortolaia V, Leekitcharoenphon P, Korsgaard HB, Seyfarth AM, Mossong J, Wattiau P, Boland C, Hansen DS, Hasman H, Hammerum AM, Hendriksen RS, 2019. ST131 fimH22 Escherichia coli isolate with a blaCMY-2/IncI1/ST12 plasmid obtained from a patient with bloodstream infection: Highly similar to E. coli isolates of broiler origin. J. Antimicrob. Chemother 74, 557–560. 10.1093/jac/dky484 [DOI] [PubMed] [Google Scholar]
- Santo L, Okeyode T, 2021. National Ambulatory Medical Care Survey: 2018 National Summary Tables. National Center for Health Statistics. URL https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2018-namcs-web-tables-508.pdf [Google Scholar]
- Sarowska J, Futoma-Koloch B, Jama-Kmiecik A, Frej-Madrzak M, Ksiazczyk M, Bugla-Ploskonska G, Choroszy-Krol I, 2019. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathog. 11, 10. 10.1186/s13099-019-0290-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaik S, Ranjan A, Tiwari SK, Hussain A, Nandanwar N, Kumar N, Jadhav S, Semmler T, Baddam R, Islam MA, Alam M, Wieler LH, Watanabe H, Ahmed N, 2017. Comparative Genomic Analysis of Globally Dominant ST131 Clone with Other Epidemiologically Successful Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. mBio 8, 1–15. 10.1128/mBio.01596-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KS, Cruz-Cano R, Jiang C, Malayil L, Blythe D, Ryan P, Sapkota AR, 2016. Presence of animal feeding operations and community socioeconomic factors impact salmonellosis incidence rates: An ecological analysis using data from the Foodborne Diseases Active Surveillance Network (FoodNet), 2004-2010. Environ. Res 150, 166–172. 10.1016/j.envres.2016.05.049 [DOI] [PubMed] [Google Scholar]
- Son J-Y, Miranda ML, Bell ML, 2021a. Exposure to concentrated animal feeding operations (CAFOs) and risk of mortality in North Carolina, USA. Sci. Total Environ 799, 149407. 10.1016/j.scitotenv.2021.149407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J-Y, Muenich RL, Schaffer-Smith D, Miranda ML, Bell ML, 2021b. Distribution of environmental justice metrics for exposure to CAFOs in North Carolina, USA. Environ. Res 195, 110862. 10.1016/j.envres.2021.110862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan Development Team, 2020. RStan: the R interface to Stan. URL http://mc-stan.org/
- Tandogdu Z, Wagenlehner FME, 2016. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis 29, 73–79. 10.1097/QCO.0000000000000228 [DOI] [PubMed] [Google Scholar]
- Tenaillon O, Skurnik D, Picard B, Denamur E, 2010. The population genetics of commensal Escherichia coli. Nat. Rev. Microbiol 8, 207–217. 10.1038/nrmicro2298 [DOI] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH, 2017. Robust causal inference using directed acyclic graphs: the R package ‘dagitty.’ Int. J. Epidemiol dyw341. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- van Hoek AHAM, Stalenhoef JE, van Duijkeren E, Franz E, 2016. Comparative virulotyping of extended-spectrum cephalosporin-resistant E. coli isolated from broilers, humans on broiler farms and in the general population and UTI patients. Vet. Microbiol 194, 55–61. 10.1016/j.vetmic.2016.04.008 [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ, Robinson WR, 2014. On the Causal Interpretation of Race in Regressions Adjusting for Confounding and Mediating Variables. Epidemiology 25, 473–484. 10.1097/EDE.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel P, Hunter D, Drown M, 2017. Better Estimates from Binned Income Data: Interpolated CDFs and Mean-Matching. Sociol. Sci 4, 641–655. 10.15195/v4.a26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield J, 2007. Disease mapping and spatial regression with count data. Biostatistics 8, 158–183. 10.1093/biostatistics/kxl008 [DOI] [PubMed] [Google Scholar]
- Wakefield J, 2003. Sensitivity Analyses for Ecological Regression. Biometrics 59, 9–17. 10.1111/1541-0420.00002 [DOI] [PubMed] [Google Scholar]
- Walker K, Herman M, 2020. tidycensus: Load US Census Boundary and Attribute Data as “tidyverse” and “sf”-Ready Data Frames. URL https://CRAN.R-project.org/package=tidycensus [Google Scholar]
- Waller AE, Scholer M, Ising AI, Travers DA, 2011. Using Emergency Department Data For Biosurveillance: The North Carolina Experience, in: Castillo-Chavez C, Chen H, Lober WB, Thurmond M, Zeng D (Eds.), Infectious Disease Informatics and Biosurveillance: Research, Systems and Case Studies. Springer US, Boston, MA, pp. 45–66. 10.1007/978-1-4419-6892-0_3 [DOI] [Google Scholar]
- Ward JB, Gartner DR, Keyes KM, Fliss MD, McClure ES, Robinson WR, 2019. How do we assess a racial disparity in health? Distribution, interaction, and interpretation in epidemiological studies. Ann. Epidemiol 29, 1–7. 10.1016/j.annepidem.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner-Friedman C, Beattie RE, Stewart JR, Hristova KR, Serre ML, 2022. Characterizing Differences in Sources of and Contributions to Fecal Contamination of Sediment and Surface Water with the Microbial FIT Framework. Environ. Sci. Technol 10.1021/acs.est.2c00224 [DOI] [PubMed] [Google Scholar]
- Williams DL, Breysse PN, McCormack MC, Diette GB, McKenzie S, Geyh AS, 2011. Airborne cow allergen, ammonia and particulate matter at homes vary with distance to industrial scale dairy operations: an exposure assessment. Environ. Health 10, 72. 10.1186/1476-069X-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji R, Friedman CR, Rubin J, Suh J, Thys E, McDermott P, Hung-Fan M, Riley LW, 2018. A Population-Based Surveillance Study of Shared Genotypes of Escherichia coli Isolates from Retail Meat and Suspected Cases of Urinary Tract Infections. mSphere 3, 1–12. 10.1128/mSphere.00179-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Zhan J, Zhang K, Chen H, Cheng S, 2022. Global, regional, and national burden of urinary tract infections from 1990 to 2019: an analysis of the global burden of disease study 2019. World J. Urol 40, 755–763. 10.1007/s00345-021-03913-0 [DOI] [PubMed] [Google Scholar]
- Zhu C, Wang D-Q, Zi H, Huang Q, Gu J-M, Li L-Y, Guo X-P, Li F, Fang C, Li X-D, Zeng X-T, 2021. Epidemiological trends of urinary tract infections, urolithiasis and benign prostatic hyperplasia in 203 countries and territories from 1990 to 2019. Mil. Med. Res 8, 64. 10.1186/s40779-021-00359-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


