Abstract
Two largely separate lines of research have documented altered pupillary dynamics in individuals diagnosed with schizophrenia. An older set of studies has demonstrated reductions in the pupillary light reflex (PLR) in individuals with schizophrenia; however, clinical and cognitive correlates of this blunted PLR have been relatively unexplored. More recently, a large body of work has demonstrated reductions in pupillary dilation in response to cognitive demands in individuals with schizophrenia, and the degree of this blunted pupil dilation has been related to more severe cognitive deficits and motivational negative symptoms. These clinically relevant alterations in the cognitive modulation of pupil size have been interpreted as reflecting insufficient information processing resources or inappropriate effort allocation. To begin to bridge these two lines of work, we investigated the PLR in 34 individuals with schizophrenia and 30 healthy controls and related the amplitude of the PLR to motivational negative symptoms and cognitive performance. Consistent with prior work, we found that the PLR was reduced in individuals with schizophrenia, and furthermore, that these measurements were highly reliable across individuals. Blunted constriction was associated with more severe motivational negative symptoms and poorer working memory among individuals with schizophrenia. These observed correlates provide a bridge between older literature documenting an altered PLR and more recent work reporting associations between negative symptoms, cognition, and blunted pupillary dilation in response to cognitive demands in individuals with schizophrenia. We provide possible mechanistic interpretations of our data and consider a parsimonious explanation for reduced cognitive- and light-related modulation of pupil size.
Keywords: Pupillometry, Pupil light reflex, Schizophrenia, Negative symptoms, Working memory
1. Introduction
Investigations into pupillary dynamics have a rich history in schizophrenia research, with documented observations of alterations in the pupil light reflex (PLR) dating back to the early 1900’s (Westphal, as cited in Bach, 1908; Bumke, 1904). As pupil constriction and dilation are under parasympathetic and sympathetic control (Beatty and Lucero-Wagoner, 2000), pupil reactivity has been employed as a valuable, non-invasive window into putative disruptions in the autonomic nervous system and its associated neurotransmitters.
Early clinical observations of altered pupil reactivity in individuals with schizophrenia spectrum disorders were confirmed by quantitative measurements, which revealed reduced constriction in response to light (Bär et al., 2008; Lidsky et al., 1971; Okada et al., 1978; Rubin and Barry, 1976, 1972; Steinhauer et al., 1979). Less consistently reported observations include longer constriction latency (Okada et al., 1978) and velocity (Lidsky et al., 1971), blunted (Rubin and Barry, 1972) and slower (Rubin and Barry, 1976) re-dilation to light offset, and smaller resting pupil diameters (Hakerem et al., 1964; Lidsky et al., 1971; Rubin and Barry, 1976; Steinhauer et al., 1992). Combined, these findings suggest an overall reduction in pupil motility in individuals with schizophrenia and have been framed as reflecting altered autonomic homeostatic balance (Rubin and Barry, 1972).1
A subset of older studies also highlighted marked variability in the PLR among individuals with schizophrenia (Rubin, 1962, 1961, 1960a, 1960b) and suggested that the large within-group variability may render pupil metrics valuable for predicting treatment response. Clinical observations also described marked within-person variability in pupil responsivity (Westphal, 1907), suggesting potential utility as a state maker. However, apart from one study, which found that faster re-dilation following initial constriction predicted relapse (Steinhauer et al., 1992), we are not aware of any studies that have attempted to explain within-group variability in the PLR by measuring relationships between clinical characteristics and either resting pupil diameter or the PLR.
A subsequent line of research investigating cognitive and affective modulation of pupil diameter has revealed relationships with clinical symptoms and cognitive functioning in people with schizophrenia. Cognitive demands and affective contexts modulate pupillary dynamics, and pupil dilation has been linked to, among other things, cognitive load (Einhäuser, 2017). A rich body of work has described reduced cognitive-related pupil dilation in individuals with schizophrenia (Fish and Granholm, 2008; Granholm et al., 2016, 2007, 2000, 1998, 1997; Granholm and Verney, 2004; Karatekin et al., 2010, 2009; Kreis et al., 2020; Minassian et al., 2004; Morris et al., 1997; Thakkar et al., 2018), which has been interpreted as reflecting insufficient information processing resources or inappropriate effort allocation. Consistent with theories that position abnormal allocation of effort at the heart of negative symptoms (Barch et al., 2014; Fervaha et al., 2013; Gold et al., 2013; McCarthy et al., 2016; Treadway et al., 2015), reduced cognitive modulation of pupillary dilation has been related to negative symptoms (Granholm et al., 2007; Minassian et al., 2004; Reddy et al., 2018; Thakkar et al., 2018), defeatist beliefs (Granholm et al., 2016), and cognitive performance (Granholm et al., 2016, 2009; Granholm and Verney, 2004; Minassian et al., 2004), albeit inconsistently (Bartolomeo et al., 2020; Fish and Granholm, 2008; Granholm et al., 2009, 1998; Granholm and Verney, 2004; Kreis et al., 2020; Strauss et al., 2015).
Despite these bodies of work describing an abnormal PLR, on one hand, and abnormal cognitive modulation of pupillary dilation that is related to negative and cognitive symptoms on the other, whether these two sets of findings are rooted in a common mechanism is unknown. A putative common mechanism would provide important insights into the physiological correlates of negative symptoms in schizophrenia. In the current study, we take an initial step toward filling this knowledge gap by measuring the PLR in individuals with schizophrenia and healthy controls and investigating whether PLR dynamics relate to negative symptoms and working memory. We measured working memory for two reasons. First, impairments in working memory are central to schizophrenia. They are among the most robust cognitive deficits (see Lee and Park, 2005 for meta-analysis), present before formal illness onset (reviewed in Fusar-Poli et al., 2012), and predict transition to psychosis in at-risk youth (Eastvold et al., 2007; Pukrop et al., 2007; Seidman et al., 2016). Second, studies investigating cognitive-related dilations in individuals with schizophrenia most commonly operationalize load as working memory load (Fish and Granholm, 2008; Granholm et al., 2016, 2007, 2000, 1997; Karatekin et al., 2009; Kreis et al., 2020; Morris et al., 1997). We focus here on negative symptoms given the aforementioned literature relating the severity of these symptoms to cognitive-related pupil size changes. Therefore, assessing working memory and negative symptoms in this context allows us to forge links between the PLR literature and the literature describing altered cognitive modulation of the pupil in individuals with schizophrenia. We expected to replicate findings of reduced constriction amplitude in response to light. We further hypothesized that if negative symptom severity, particularly motivational symptoms, and working memory deficits are at least partly related to broadly altered pupillary control, then we should observe that the severity of these symptoms is related to a blunted PLR.
2. Methods
2.1. Participants and clinical assessments
34 persons with schizophrenia or schizoaffective disorder (PSZ) and 30 healthy controls (HC) were recruited from outpatient mental health facilities and via community advertisements. Diagnoses were based on results of the NetSCID (Brodey et al., 2016), an electronic version of the Structured Clinical Interview for DSM-5 Axis I disorders, and material collected from medical records and collateral informants, when available. Final diagnosis was reached at a consensus conference with study staff. Exclusion criteria included meeting DSM-5 criteria for moderate or severe substance use disorder within the previous six months, history of neurological disorders, history of head injury with loss of consciousness longer than 1 h, visual acuity that was not normal or corrected-to-normal, or any other reported vision problems. HC were excluded if they had a personal history of any DSM-5 Axis I disorders or a first-degree relative with a schizophrenia spectrum disorder or bipolar disorder.
Clinical symptoms were assessed using the Brief Psychiatric Rating Scale (Overall and Gorham, 1962), Scale for the Assessment of Positive Symptoms (Andreasen, 1984), and Scale for the Assessment of Negative Symptoms (Andreasen, 1983). We were particularly interested in the relationship between pupil dynamics and negative symptoms. To examine this construct in greater detail, three scales were derived based on a factor analytic study of the SANS (Peralta and Cuesta, 1999): Poverty of Affect and Speech, Social Dysfunction, and Inattention, which we used in a prior study relating pupil dynamics to negative symptoms (Thakkar et al., 2018). These first two scales assess expressive and motivational negative symptoms, respectively. Given evidence that anxiety and depression can modulate the pupil response (e.g. Bakes et al., 1990; Laurenzo et al., 2016), we also looked at the sum of items measuring depression and anxiety symptoms on the BPRS. Chlorpromazine (CPZ) equivalent dosages of antipsychotic medication were calculated for each patient (Andreasen et al., 2010). Anticholinergic medications modulate the pupil response; thus anticholinergic activity of medications was calculated with the Anticholinergic Drug Scale (ADS; Carnahan et al., 2006) in order to investigate potentially confounding effects. Premorbid IQ was measured with the Weschler Test for Adult Reading (Wechsler, 2001). Because the digit span task is the most frequently used paradigm in studies investigating cognitive-related pupil dilations in schizophrenia (Fish and Granholm, 2008; Granholm et al., 2016, 1997; Kreis et al., 2020; Morris et al., 1997), working memory was measured using the Digit Span subtest of the Wechsler Adult Intelligence Scale, Fourth Edition (Wechsler, 2008).
One PSZ was excluded due to substantial high frequency noise that was visually observed in the pupil traces. One HC was excluded as a significant outlier (>1.5 times the interquartile range for initial diameter). For the remaining 33 PSZ and 29 HC, demographic characteristics are presented in Table 1, medication details are presented in Supplemental Table 1, and physical conditions are reported in Supplemental Table 2. Groups were matched for age, race, and sex at birth. All subjects gave written informed consent approved by the Michigan State University Institutional Review Board and were compensated for study participation.
Table 1.
Demographic characteristics of the healthy controls (HC) and persons with schizophrenia (PSZ).
| HC (n = 29) Mean (s.d.) | PSZ (n = 33) Mean (s.d.) | Statistic | p | |
|---|---|---|---|---|
|
| ||||
| Age | 35.5 (9.9) | 37.4 (10.4) | t = −0.734 | 0.466 |
| Sex | 18 M/11 F | 19 M/14 F | X2 = −0.130 | 0.719 |
| Gender | 18 M/11 F/0 O | 18 M/14 F/1 O | X2 = −1.107 | 0.575 |
| Race (White/Black/Other) | 19 W/3 B/7 O | 19 W/10 B/4 O | X2 = 4.254 | 0.119 |
| IQ | 109.1 (6.2) | 100.5 (12.3) | t = 3.399 | 0.001 |
| Working memory | 31.2 (5.2) | 26.7 (6.8) | t = 2.773 | 0.008 |
| CPZ equivalent (N = 31) | 348.0 (272.9) | |||
| SAPS | 22.4 (15.9) | |||
| SANS total | 34.3 (21.0) | |||
| SANS poverty of affect and speech | 9.15 (9.2) | |||
| SANS social dysfunction | 11.1 (7.1) | |||
| SANS inattention | 4.0 (3.4) | |||
| BPRS total | 48.0 (13.4) | |||
| BPRS depression/anxiety | 9.8 (5.4) | |||
2.2. Experimental paradigms and procedure
Participants were seated in a dark room (0.2Lux of ambient light). A handheld NeurOptics PLR 3000 pupillometer (30 Hz sampling rate, accuracy±0.03 mm) controlled light presentation and measured pupil diameter. Participants were instructed to keep their eyes open and look straight ahead throughout the 5-second light protocol. Upon the start of the measurement, a 451-lux pulse of light was flashed for 1 s. The light remained off for the remaining 4 s. Duration between measurement periods was not controlled but typically occurred within a few seconds of each other. For most participants, this procedure was repeated twice per eye, resulting in four total measurements. If the pupillometer flagged the data as unusable, the measurement was repeated. In 9 participants (5 PSZ, 4 HC), >4 usable measurements were obtained2.
2.3. Data analysis
Analyses of pupil dynamics were done in Matlab R2018b. Pre-processing is described in Supplemental Methods. Various aspects of the pupil light response were derived given that the parasympathetic and sympathetic systems separately contribute to pupillary control, and their influences might be differentially reflected in different metrics, as detailed in Supplemental Methods (Steinhauer and Hakerem, 1992).
2.3.1. Initial pupil size
Initial pupil diameter was calculated as the mean diameter during the first 0.15 s of the measurement, which is prior to the onset of the pupil response to even an intense light stimulus (Ellis, 1981).
2.3.2. Constriction amplitude
Maximally constricted pupil diameter was identified as the minimum pupil size across the measurement. Constriction amplitude was the absolute value of the difference between maximally constricted pupil diameter and initial diameter.
2.3.3. Constriction latency
The onset of constriction was defined as the first point at which pupil diameter decreased continuously for 0.30 s. Constriction latency was calculated as the time from light onset to constriction onset.
2.3.4. Maximum constriction velocity
Maximum constriction velocity was calculated as the absolute value of the maximum velocity of pupil size change from constriction onset to maximal constriction (larger values indicate faster constriction).
2.3.5. Re-dilation latency
Following the offset of the light, the pupil began to re-dilate. Dilation onset was calculated as the first point following maximal constriction after which pupillary diameter was monotonically increasing for 0.30s. Re-dilation latency was calculated as the time between light offset and dilation onset.
2.3.6. T50
T50 was defined as the latency between the time of maximal constriction to 50 % recovery of the initial diameter.
2.3.7. Statistical analyses
Statistical analyses were conducted using IBM SPSS Statistics. Demographic variables were compared across groups using chi-square test (sex), Fisher’s exact test (race), and independent samples t-test (age, IQ). We examined the reliability of pupillary measurements as follows. For participants with fewer than four viable measurements, missing data were completed using multiple imputation. Data were imputed five times, and Cronbach’s alpha was calculated for each of the imputed databases, then pooled across the five analyses by averaging the output. Then, pupillary measures were averaged across measurements for each participant. Before comparing pupillary measurements across groups, normality of all variables was assessed using a Shapiro-Wilk test. Independent samples t-tests and Mann-Whitney tests were used to compare PSZ and HC on those PLR metrics that were normally and non-normally distributed, respectively. The Bonferroni-Holm procedure (Holm, 1979) was used to correct for multiple group comparisons.
Correlations between pupillary dynamics were evaluated with Spearman’s rho (rs), separately for PSZ and HC. Within PSZ, Spearman’s rho was also computed to evaluate the relationships between clinical symptom scores, working memory scores, and pupillary dynamics. Our a priori hypotheses were that there would be significant correlations between motivational negative symptoms (SANS Social Dysfunction subscale), working memory (WAIS-IV Digit Span Total Score), and those pupil dynamics that significantly differed between HC and PSZ. Alpha-values for all other exploratory correlations were corrected using the Bonferroni-Holm procedure. To explore possible medication confounds, we computed correlations (Spearman’s rho) between CPZ equivalent antipsychotic doses and anticholinergic burden. These medication correlations were not corrected for multiple testing.
3. Results
3.1. Reliability
There was strong internal consistency for initial diameter (α(61) = 0.970), constriction amplitude (α(61) = 0.937), maximum constriction velocity (α(61) = 0.949), and re-dilation latency (α(60) = 0.825), and acceptable consistency for T50 (α(60) = 0.647). Constriction latency had lower internal reliability (α(61) = 0.368).
3.2. Group differences in pupillary dynamics
Fig. 1 depicts average pupil waveforms, and Fig. 2 depicts distributions of pupillary metrics. Shapiro-Wilk tests indicated that constriction latency (W(62) = 0.931, p = 0.002), re-dilation latency (W(61) = 0.901, p < 0.001), and T50 (W(61) = 0.736, p < 0.001) were significantly non-normally distributed, and thus Mann-Whitney tests were used to compare values across groups. The remainder of the measures were compared using independent samples t-tests. Compared to HC, PSZ had significantly smaller constriction amplitude (HC = 2.19 ± 0.22 mm, PSZ = 1.82 ± 0.43 mm, t(48.2) = 4.330, corrected-p < 0.001). Initial pupil diameter (HC = 5.27 ± 0.52 mm, PSZ = 4.97 ± 0.94 mm, t(51.2) = 1.558, uncorrected-p = 0.125), constriction latency (HC = 0.16 ± 0.03 s, PSZ = 0.17 ± 0.03 s, U = 366.500, uncorrected-p = 0.112), maximum constriction velocity (HC = 5.11 ± 1.07 mm/s, PSZ = 5.61 ± 0.84 mm/s, t(60) = − 2.016, uncorrected-p = 0.048), re-dilation latency (HC = 0.25 ± 0.10s, PSZ = 0.17 ± 0.14 s, U = 288.500, uncorrected-p = 0.012), and T50 (HC = 1.06 ± 0.32, PSZ = 0.99 ± 0.24, U = 368.5, uncorrected-p = 0.176) did not differ significantly between groups following correction for multiple comparisons. Within groups, there were significant correlations among our various pupil measures (see Supplemental Tables 3 and 4). While it possible that some of those correlations reflect meaningful physiological associations, others may be trivial (e.g. re-dilation may be slower following a small versus large constriction simply because the pupil starts from a less constricted state).
Fig. 1.

Averaged pupil waveforms relative to initial pupil diameter across time in healthy controls (HC) and individuals with schizophrenia (PSZ), with standard error represented by shaded error bars. Constriction onset time (blue), light offset time (dotted black), and re-dilation latency within groups (orange and grey) are marked by vertical lines. The graphical inset highlights the time period surrounding the dilation onset time.
Fig. 2.

Raincloud plots representing distribution of pupil dynamics in healthy controls (HC) and individuals with schizophrenia (PSZ). Significant group differences are indicated with an asterisk (*corrected-p < 0.05, **corrected-p < 0.01).
3.3. Correlations between pupillary dynamics and clinical measures
Correlations between clinical and cognitive measures and pupillary dynamics are reported in Table 2. We predicted, a priori, that motivational negative symptoms (SANS Social Dysfunction subscale) and working memory (WAIS-IV Digit Span Total Score) would relate to those pupillary dynamics that significantly differed between HC and PSZ (constriction amplitude). Constriction amplitude was significantly negatively correlated with the SANS Social Dysfunction subscale, such that more severe motivational symptoms were associated with smaller constriction amplitude (rs(31) = − 0.362, p = 0.038; Fig. 3A). Supplemental Table 5 reports correlations using more recent conceptualizations of negative symptom structure using SANS items (Strauss et al., 2018). Larger constriction amplitude was also positively correlated with better digit span performance (rs(30) = 0.535, p = 0.002; Fig. 3B). A regression analysis that tested their independent contributions indicated that motivational but not expressive negative symptoms predicted constriction amplitude (motivational: β = − 0.440, p = 0.046; expressive: β = − 0.073, p = 0.731). Working memory and motivational negative symptoms were not significantly correlated with each other (rs(30) = − 0.254, p = 0.161). Finally, we performed exploratory analyses to evaluate correlations between clinical symptoms and those pupil dynamics that did not differ significantly between groups. Following Bonferroni-Holm correction for 34 tests, no relationships remained significant. Medication analyses are reported in Supplemental Results.
Table 2.
Correlations between clinical and cognitive symptoms and PLR metrics within PSZ.
| Initial diameter (N = 33) | Amplitude (N = 33) | Constriction latency (N = 33) | Max constriction velocity (N = 33) | Re-dilation latency (N = 33) | T50 (N = 33) | |
|---|---|---|---|---|---|---|
|
| ||||||
| SAPS total | rs = −0.101 | rs = −0.107 | rs = 0.345 | rs = −0.132 | rs = 0.094 | rs = −0.056 |
| p = 0.575 | p = 0.552 | p = 0.049 | p = 0.464 | p = 0.602 | p = 0.757 | |
| SANS total | rs = −0.032 | rs = −0.296 | rs = 0.452 | rs = −0.399 | rs = −0.005 | rs = 0.087 |
| p = 0.858 | p = 0.095 | p = 0.008 | p = 0.021 | p = 0.976 | p = 0.629 | |
| SANS poverty of affect and speech | rs = −0.008 | rs = −0.207 | rs = 0.432 | rs = −0.341 | rs = 0.084 | rs = 0.039 |
| p = 0.964 | p = 0.248 | p = 0.012 | p = 0.052 | p = 0.644 | p = 0.831 | |
| SANS social dysfunction | rs = −0.140 | rs = −0.362* | rs = 0.414 | rs = −0.366 | rs = −0.143 | rs = −0.041 |
| p = 0.438 | p = 0.038 | p = 0.017 | p = 0.036 | p = 0.426 | p = 0.821 | |
| BPRS depression/anxiety | rs = 0.190 | rs = 0.038 | rs = 0.140 | rs = −0.060 | rs = 0.109 | rs = 0.036 |
| p = 0.289 | p = 0.835 | p = 0.439 | p = 0.742 | p = 0.547 | p = 0.843 | |
| WAIS-IV digit Span (N = 32) | rs = 0.354 | rs = 0.535** | rs = −0.317 | rs = 0.505 | rs = 0.176 | rs = −0.213 |
| p = 0.047 | p = 0.002 | p = 0.077 | p = 0.003 | p = 0.335 | p = 0.242 | |
| CPZ (N = 31) | rs = −0.249 | rs = −0.391* | rs = 0.143 | rs = −0.386 | rs = −0.154 | rs = 0.058 |
| p = 0.177 | p = 0.030 | p = 0.442 | p = 0.032 | p = 0.407 | p = 0.757 | |
| ADS | rs = −0.214 | rs = −0.298 | rs = 0.119 | rs = −0.224 | rs = −0.200 | rs = −0.043 |
| p = 0.232 | p = 0.092 | p = 0.510 | p = 0.210 | p = 0.265 | p = 0.813 | |
Scale for the Assessment of Positive Symptoms (SAPS); Score for the Assessment of Negative Symptoms (SANS); Brief Psychiatric Rating Scale (BPRS); chlorpromazine equivalent dosage (CPZ); Anticholinergic Drug Scale (ADS). Uncorrected-p values are reported for all correlations.
p < 0.05
p < 0.01, bolded values represent those correlations about which we had a priori hypotheses. The remainder are exploratory.
Fig. 3.
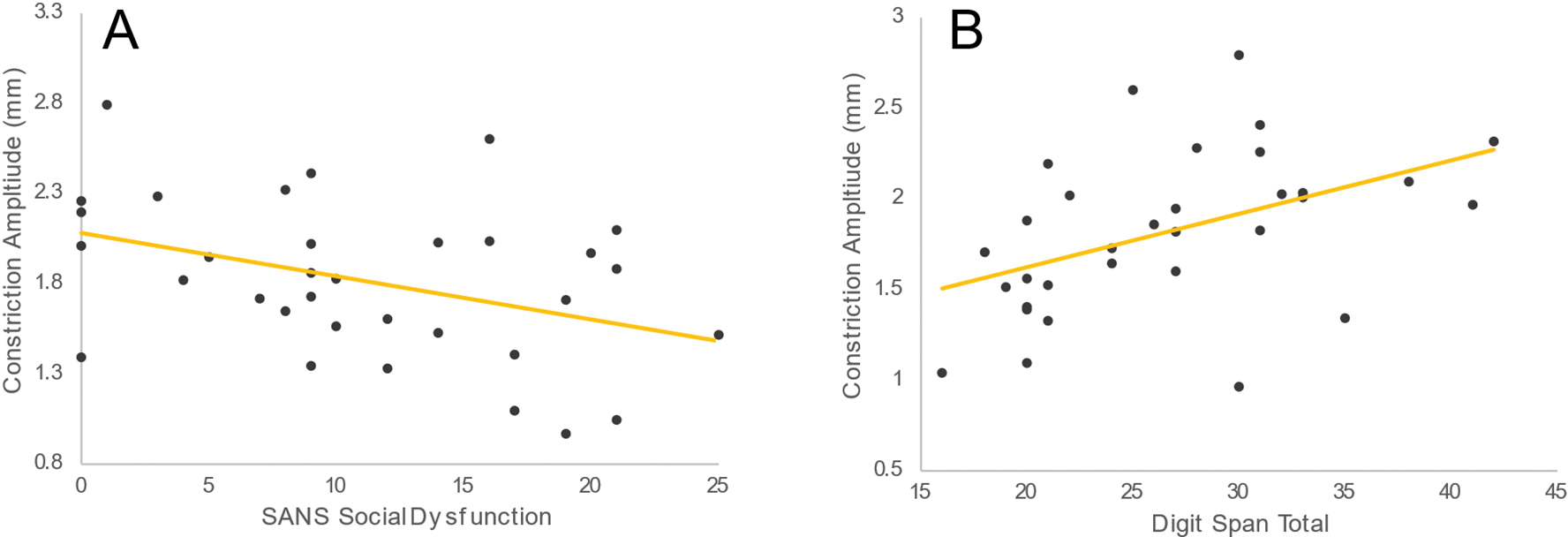
A priori Spearman’s correlations between (A) constriction amplitude and social dysfunction and (B) constriction amplitude and working memory. Constriction amplitude was significantly correlated with the Social Dysfunction subscale, such that more severe social dysfunction was associated with smaller constriction amplitude (rs(31) = − 0.362, p = 0.038). Amplitude was also correlated with working memory, with worse memory being associated with smaller constriction amplitude (rs(30) = 0.535, p = 0.002).
4. Discussion
In the current study, we explored clinical and cognitive correlates of alterations in the pupil light reflex (PLR) in individuals with schizophrenia. First, we observed significant differences in PLR dynamics between groups, such that individuals with schizophrenia had reduced constriction amplitude. These measures were highly reliable within individuals, and results replicate previous findings of PLR abnormalities in persons with schizophrenia (Bär et al., 2008; Lidsky et al., 1971; Okada et al., 1978; Rubin and Barry, 1976, 1972; Steinhauer et al., 1979). Second, we found that blunted constriction was associated with more severe motivational negative symptoms and working memory deficits. These observed motivational and cognitive correlates provide a bridge between an older literature documenting an altered PLR and more recent work reporting associations between negative symptoms, cognition, and blunted pupillary dilation in response to cognitive demands in individuals with schizophrenia. In the following section, we will interpret these findings in the context of the published literature, consider mechanistic explanations for our results, underscore the limitations of this study, and speculate upon the clinical implications of this work and discuss avenues for future studies.
The PLR is mediated by a circuit from the retina to subcortical brain regions to the neurons that innervate the constrictor and dilator muscles of the iris (McDougal and Gamlin, 2008). Retinal ganglion cells receive signals from retinal photoreceptors, and further signals arise directly in intrinsically photoreceptive ganglion cells (ipRGCs). These signals mediate the PLR via the pretectal olivary nucleus, which innervates the parasympathetic Edinger-Westphal nucleus (EW) which, in turn, projects to the sphincter muscle. While this circuit is directly responsible for the PLR, the reflex is also subject to sympathetic modulation. While it is uncertain whether, in humans, sympathetic outflow to the pupil dilator muscles is modulated by light (McDougal and Gamlin, 2008), there is strong evidence that signals from the locus coeruleus (LC), the brain’s main source of noradrenaline and a key node in the arousal and stress response, can directly or indirectly inhibit the EW and thereby diminish the PLR (Bakes et al., 1990; Loewenfield, 1958; Samuels and Szabadi, 2008; Steinhauer et al., 2000; Szabadi, 2012). Response modulation at the level of the EW or the pretectal olivary nucleus may also involve other brain centers aside from the LC, for instance the superior colliculus (Joshi and Gold, 2020; Wang and Munoz, 2015) and the hypothalamus (Barbur, 2004; Wilhelm et al., 2002).
Considering the above, what might explain the blunted PLR in schizophrenia? First, it may reflect retinal abnormalities that reduce the effective luminance of the light stimulus. Indeed, decreasing the luminance of a light pulse reduces amplitude, but not latency, of constriction (Barbur, 2004), consistent with our findings. This notion is further supported by alterations in retinal function in schizophrenia, which has been related to important clinical and cognitive measures (reviewed in Silverstein et al., 2020). An explanation for our PLR findings that centers on retinal dysfunction is further consistent with widespread visual abnormalities in individuals with schizophrenia that have downstream effects on higher-order cognition and functioning (Green et al., 1989; McCleery et al., 2020). While retinal alterations may account for a blunted PLR, however, they cannot explain the blunted cognition-related dilation that has been observed in individuals with schizophrenia.
A second potential explanation of our findings centers on autonomic imbalance. Reduced constriction amplitude could arise from a shift in autonomic balance toward decreased parasympathetic activity and increased sympathetic activity in individuals with schizophrenia. Consistent with such a shift are findings of increased baseline heart rate (Akar et al., 2015; Ardizzi et al., 2016; Bär et al., 2007; Castro et al., 2008; Henry et al., 2010; Kimhy et al., 2017; Mathewson et al., 2012; Quintana et al., 2016) and increased baseline skin conductance (Brekke et al., 1995; Dawson et al., 1994; Green et al., 1989; Maina et al., 1995; Schell et al., 2005) in individuals with schizophrenia. There are roughly two routes through which altered autonomic imbalance might reduce the PLR. First, increased sympathetic outflow to the pupil dilator muscle could counteract pupil constriction in response to light. Second, it could be related to the parasympathetic pathway from EW back to the constrictor muscles. This may be an aspect of disordered parasympathetic function, but it could also result from an increase of inhibition exerted by the sympathetic pathway onto EW. As described earlier, EW receives inhibition from LC that can reduce the amplitude of the PLR (Bakes et al., 1990; Loewenfield, 1958; Samuels and Szabadi, 2008; Szabadi, 2012). LC activity is, itself, augmented by light (Szabadi, 2012). Therefore, blunted PLR in schizophrenia may reflect either increased LC reactivity to light or a broader alteration in LC’s reactivity or inhibition of EW.
A (partial) explanation of the current findings in terms of autonomic function holds the promise of tying together findings of blunted PLR and reduced pupil dilation during cognitive tasks in schizophrenia. Part of this task-related pupil dilation results from increased sympathetic outflow to the pupil dilator muscle, and another part likely reflects an increase of sympathetic inhibition onto the EW (Kardon, 2005; Loewenfield, 1958; Steinhauer and Hakerem, 1992). Despite this appeal, however, it should be noted that no straightforward account in terms of autonomic function seems to explain all available findings. For one, a shift toward increased sympathetic drive would be expected to cause an increase in baseline pupil size (Kardon, 2005; Szabadi, 2012). We did not observe group differences in baseline pupil size, and it has, for the most part, not been reported in previous studies (Hakerem et al., 1964; Okada et al., 1978). In addition, whereas a reduction in PLR suggests a relatively dominant sympathetic system, reduced task-related pupil dilation suggests relative dominance of the parasympathetic system. Thus, while altered autonomic activity could, in principle, result both in reduced constriction to light as well as reduced dilation to cognitive demands, a simple shift in autonomic balance would not have that effect. One possibility here is disordered reactivity of the LC and its inhibitory influence on the EW in individuals with schizophrenia, whereby the LC shows greater reactivity in response to direct external stimuli (e.g. light) and decreased reactivity based on internal goals and challenges (e.g. cognitive demands and action preparation).
Such an explanation would require direct testing, but does relate to work by Nuechterlein, Dawson and colleagues (Dawson et al., 1994; Dawson and Schell, 2002; Nuechterlein and Dawson, 1984), who proposed dysregulated arousal mechanisms in schizophrenia, which interfere with cognitive functioning and manifest in clinical symptoms, whereby chronic hyperarousal may lead to compensatory withdrawal (Dawson et al., 1992; Schell et al., 2005). By extension, the observed correlations between the PLR and motivational and cognitive symptoms may suggest a link between disordered sympathetic action and such symptoms, potentially via its influence on resource/effort allocation (Radulescu et al., 2015). Altered sympathetic regulation in schizophrenia is further supported by findings of reduced heart rate variability (reviewed in Alvares et al., 2016; Montaquila et al., 2015) and skin conductance responsivity (Dawson et al., 1994; Maina et al., 1995; Schell et al., 2005; Tarrier et al., 1979; Williams et al., 2007; Zahn and Pickar, 2005) in individuals with schizophrenia. Interestingly, although baseline differences in heart rate and skin conductance have been observed, compared to variability measures, they have been much less consistently associated with negative symptoms (Boettger et al., 2006; Chang et al., 2011; Chung et al., 2013; Mathewson et al., 2012; Zahn and Pickar, 2005).
Comorbidities and medications may play a confounding role in this study. Antipsychotic (Koller et al., 2020) and anticholinergic (e.g. Bitsios et al., 1999) drugs affect pupil dynamics. Although anticholinergic burden was not significantly related to PLR measures, potentially due to limitations of the measure, we observed a modest relationship between CPZ dose and constriction amplitude. However, motivational negative symptoms and working memory remained significant predictors of constriction amplitude after controlling for CPZ dose. Regardless, it is impossible to rule out medication effects, and future work will be necessary to parse the effect of medication from illness-related effects. Altered PLR may also be due to comorbid mental health conditions, which are prevalent in individuals with schizophrenia (reviewed in Buckley et al., 2009), and which impact retinal functioning (Silverstein et al., 2020) and have been related to blunted PLR (e.g. Bakes et al., 1990).
In conclusion, our findings suggest a relationship between blunted pupillary constriction in response to light and both motivational negative symptoms and working memory impairments. These data attest to the clinical relevance of this simple psychophysiological response. Future studies could test the hypotheses laid out here to examine the mechanisms of blunted constriction to light. For example, dynamics of the PLR may be related to retinal measures in the same participants. In addition, future studies examining the relationship between altered pupillary dynamics to light and to cognitive demands in the same individuals will provide critical data regarding possible shared mechanisms of these two findings. Finally, despite the demonstrated reliability of PLR dynamics, their relationship to important clinical measures, and the ease with which they can be measured, few studies have investigated their clinical utility (Miller et al., 2021). Further research may reveal that the PLR has concurrent and possibly predictive ability to assess the onset and development of both negative symptoms and cognitive deficits. In this way, the PLR could provide a noninvasive and simple tool with practical implications when used in a clinical setting.
Supplementary Material
Acknowledgements
The authors would like to thank Dominic Roberts and Beier Yao for their assistance with clinical interviews, and Dalia Fragoso for her contributions to subject recruitment and data collection. They would also like to thank Ricarda Bothe and Dr. Julia Vogt for their help in translating early documentation of pupillary alterations in individuals with schizophrenia.
Financial support
This work was funded by NIMH R01MH112644 (KT, EA) and R01MH121417 (KT, JWB, EA).
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Jessica Fattal: Conceived and designed the analysis, Collected the data, Performed the analysis, Wrote the paper
Jan W. Brascamp: Conceived and designed the analysis, Other contribution - Edited the paper
Rachael E. Slate: Collected the data, Other contribution - Edited the paper
Matthew Lehet: Conceived and designed the analysis, Other contribution - Edited the paper
Eric D. Achtyes: Conceived and designed the analysis, Other contribution - Edited the paper
Katharine Thakkar: Conceived and designed the analysis, Wrote the paper
But see Loewenfeld and Lowenstein, 1993 for criticism of this proposal.
If a participant had more than four usable values, only the first four were used.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.schres.2022.09.019.
References
- Akar SA, Kara S, Latifoğlu F, Bilgiç V, 2015. Analysis of heart rate variability during auditory stimulation periods in patients with schizophrenia. J. Clin. Monit. Comput. 29, 153–162. 10.1007/s10877-014-9580-8. [DOI] [PubMed] [Google Scholar]
- Alvares GA, Quintana DS, Hickie IB, Guastella AJ, 2016. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J. Psychiatry Neurosci. 41, 89–104. 10.1503/jpn.140217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, 1983. The Scale for the Assessment of Negative Symptoms (SANS). [PubMed]
- Andreasen NC, 1984. The Scale for the assessment of Positive Symptoms (SAPS).
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B-C, 2010. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry 67. 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardizzi M, Ambrosecchia M, Buratta L, Ferri F, Peciccia M, Donnari S, Mazzeschi C, Gallese V, 2016. Interoception and positive symptoms in schizophrenia. Front. Hum. Neurosci. 10, 1–10. 10.3389/fnhum.2016.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach L, 1908. Pupillenlehre: Anatomic, Physiologie und Pathologie. Methodikder Untersuchung. verlag von S. Karger, Berlin. [Google Scholar]
- Bakes A, Bradshaw C, Szabadi E, 1990. Attenuation of the pupillary light reflex in anxious patients. Br. J. Clin. Pharmacol. 30, 377–381. 10.1111/j.1365-2125.1990.tb03787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär KJ, Boettger MK, Koschke M, Schulz S, Chokka P, Yeragani VK, Voss A, 2007. Non-linear complexity measures of heart rate variability in acute schizophrenia. Clin. Neurophysiol. 118, 2009–2015. 10.1016/j.clinph.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Bär KJ, Boettger MK, Schulz S, Harzendorf C, Agelink MW, Yeragani VK, Chokka P, Voss A, 2008. The interaction between pupil function and cardiovascular regulation in patients with acute schizophrenia. Clin. Neurophysiol. 119, 2209–2213. 10.1016/j.clinph.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Barbur JL, 2004. Learning from the pupil - studies of basic mechanisms and clinical application. In: Chalupa LM, Werner JS (Eds.), Visual Neurosciences. MIT Press, Cambridge, MA, pp. 641–656. [Google Scholar]
- Barch DM, Treadway MT, Schoen N, 2014. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. J. Abnorm. Psychol 123 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo LA, Culbreth AJ, Ossenfort KL, Strauss GP, 2020. Neurophysiological evidence for emotion regulation impairment in schizophrenia: the role of visual attention and cognitive effort. J. Abnorm. Psychol 129 10.1037/abn0000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty J, Lucero-Wagoner B, 2000. The pupillary system. In: Cacioppo JT, Tassinary LG, Berntson GG (Eds.), Handbook of Psychophysiology. Cambridge University Press, pp. 142–162. [Google Scholar]
- Bitsios P, Szabadi E, Bradshaw CM, 1999. Comparison of the effects of venlafaxine, paroxetine and desipramine on the pupillary light reflex in man. Psychopharmacology 143, 286–292. 10.1007/s002130050949. [DOI] [PubMed] [Google Scholar]
- Boettger S, Hoyer D, Falkenhahn K, Kaatz M, Yeragani VK, Bär KJ, 2006. Altered diurnal autonomic variation and reduced vagal information flow in acute schizophrenia. Clin. Neurophysiol. 117, 2715–2722. 10.1016/j.clinph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Brekke JS, Raine A, Thomson C, 1995. Cognitive and psychophysiological correlates of positive, negative, and disorganized symptoms in the schizophrenia spectrum. Psychiatry Res. 57, 241–250. 10.1016/0165-1781(95)02668-M. [DOI] [PubMed] [Google Scholar]
- Brodey BB, First M, Linthicum J, Haman K, Sasiela JW, Ayer D, 2016. Validation of the NetSCID: an automated web-based adaptive version of the SCID. Compr. Psychiatry 66, 67–70. 10.1016/j.comppsych.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ, 2009. Psychiatric comorbidities and schizophrenia. Schizophr. Bull. 35, 383–402. 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumke O, 1904. Die Pupillenstörungen Bei Geistes-Und Nervenkrankheiten. Fischer, Germany. [Google Scholar]
- Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR, 2006. The anticholinergic drug scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J. Clin. Pharmacol. 46, 1481–1486. 10.1177/0091270006292126. [DOI] [PubMed] [Google Scholar]
- Castro MN, Vigo DE, Weidema H, Fahrer RD, Chu EM, de Achával D, Nogués M, Leiguarda RC, Cardinali DP, Guinjoan SM, 2008. Heart rate variability response to mental arithmetic stress in patients with schizophrenia. Autonomic response to stress in schizophrenia. Schizophr. Res. 99, 294–303. 10.1016/j.schres.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Chang LR, Lin YH, Kuo TBJ, Chang HCW, Liu CM, Liu CC, Hwu HG, Yang CCH, 2011. Autonomic modulation and health-related quality of life among schizophrenic patients treated with non-intensive case management. PLoS ONE 6. 10.1371/journal.pone.0026378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MS, Yang AC, Lin YC, Lin CN, Chang FR, Shen, Hua S, Ouyang WC, Loh EW, Chiu HJ, 2013. Association of altered cardiac autonomic function with psychopathology and metabolic profiles in schizophrenia. Psychiatry Res. 210, 710–715. 10.1016/j.psychres.2013.07.034. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, 2002. What does electrodermal activity tell us about prognosis in the schizophrenia spectrum? Schizophr. Res. 54 10.1016/S0920-9964(01)00355-3. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Nuechterlein KH, Schell AM, Mintz J, 1992. Concurrent and predictive electrodermal correlates of symptomatology in recent-onset schizophrenic patients. J. Abnorm. Psychol 101 10.1037/0021-843X.101.1.153. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Nuechterlein KH, Schell AM, Gitlin M, Ventura J, 1994. Autonomic abnormalities in schizophrenia. State or trait indicators? Arch. Gen. Psychiatry 51, 813–824. 10.1001/archpsyc.1994.03950100061006. [DOI] [PubMed] [Google Scholar]
- Eastvold AD, Heaton RK, Cadenhead KS, 2007. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophr. Res. 93, 266–277. 10.1016/j.schres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhäuser W, 2017. The pupil as a marker of cognitive processes. In: Zhao Q (Ed.), Computational and Cognitive Neuroscience of Vision. Springer, Singapore, pp. 141–169. 10.1007/978-981-10-0213-7. [DOI] [Google Scholar]
- Ellis CJK, Thomas’s Hospital, 1981. The pupillary light reflex in normal subjects. British Journal of Ophthalmology 65 (11), 754–759. 10.1136/bjo.65.11.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, Remington G, 2013. Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. J. Psychiatr. Res. 47 10.1016/j.jpsychires.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Fish SC, Granholm E, 2008. Easier tasks can have higher processing loads: task difficulty and cognitive resource limitations in schizophrenia. J. Abnorm. Psychol. 117, 355–363. 10.1037/0021-843X.117.2.355. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Deste G, Smieskova R, Barlati S, Yung AR, Howes O, Stieglitz R-D, Vita A, McGuire P, Borgwardt S, 2012. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch. Gen. Psychiatry 69, 562–571. 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ, 2013. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol. Psychiatry 74. 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Verney SP, 2004. Pupillary responses and attentional allocation problems on the backward masking task in schizophrenia. Int. J. Psychophysiol 52 10.1016/j.ijpsycho.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Granholm E, Morris SK, Sarkin AJ, Asarnow RF, Jeste DV, 1997. Pupillary responses index overload of working memory resources in schizophrenia. J. Abnorm. Psychol 106 10.1037//0021-843x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Granholm E, Chock D, Morris S, 1998. Pupillary responses evoked during verbal fluency tasks indicate semantic network dysfunction in schizophrenia. J. Clin. Exp. Neuropsychol 20 10.1076/jcen.20.6.856.1107. [DOI] [PubMed] [Google Scholar]
- Granholm E, Morris S, Asarnow RF, Chock D, Jeste DV, 2000. Accelerated age-related decline in processing resources in schizophrenia: evidence from pupillary responses recorded during the span of apprehension task. J. Int. Neuropsychol. Soc 6 10.1017/s1355617700611049. [DOI] [PubMed] [Google Scholar]
- Granholm E, Verney SP, Perivoliotis D, Miura T, 2007. Effortful cognitive resource allocation and negative symptom severity in chronic schizophrenia. Schizophr. Bull 33 10.1093/schbul/sbl040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Fish SC, Verney SP, 2009. Pupillometric measures of attentional allocation to target and mask processing on the backward masking task in schizophrenia. Psychophysiology 46. 10.1111/j.1469-8986.2009.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Ruiz I, Gallegos-Rodriguez Y, Holden J, Link PC, 2016. Pupillary responses as a biomarker of diminished effort associated with defeatist attitudes and negative symptoms in schizophrenia. Biol. Psychiatry 80. 10.1016/j.biopsych.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Satz P, 1989. The relationship of symptomatology and medication to electrodermal activity in schizophrenia. Psychophysiology 26, 148–157. [DOI] [PubMed] [Google Scholar]
- Hakerem G, Sutton S, Zubin J, 1964. Pupillary reactions to light in schizophrenic patients and normals. Ann. N. Y. Acad. Sci. 105 10.1111/j.1749-6632.1964.tb42965.x. [DOI] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Paulus MP, Geyer MA, Perry W, 2010. Heart rate variability in bipolar mania and schizophrenia. J. Psychiatr. Res. 44, 168–176. 10.1016/j.jpsychires.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S, 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70. [Google Scholar]
- Joshi S, Gold JI, 2020. Pupil size as a window on neural substrates of cognition. Trends Cogn. Sci. 24, 466–480. 10.1016/j.tics.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatekin C, Bingham C, White T, 2009. Regulation of cognitive resources during an n-back task in youth-onset psychosis and attention-deficit/hyperactivity disorder (ADHD). Int. J. Psychophysiol. 73 10.1016/j.ijpsycho.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatekin C, Bingham C, White T, 2010. Oculomotor and pupillometric indices of pro- and antisaccade performance in youth-onset psychosis and attention deficit/hyperactivity disorder. Schizophr. Bull. 36 10.1093/schbul/sbp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon R, 2005. Anatomy and physiology of the autonomic nervous system. In: Miller NR, Newman NJ, Biousse V, Kerrison JB (Eds.), Clinical Neuro-ophthalmology. Lippincott Williams & Wilkins, pp. 649–714. [Google Scholar]
- Kimhy D, Wall MM, Hansen MC, Vakhrusheva J, Choi CJ, Delespaul P, Tarrier N, Sloan RP, Malaspina D, 2017. Autonomic regulation and auditory hallucinations in individuals with schizophrenia: an experience sampling study. Schizophr. Bull. 43, 754–763. 10.1093/schbul/sbw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller D, Saiz-Rodríguez M, Zubiaur P, Ochoa D, Almenara S, Román M, Romero-Palacián D, de Miguel-Cáceres A, Martín S, Navares-Gómez M, Mejía G, Wojnicz A, Abad-Santos F, 2020. The effects of aripiprazole and olanzapine on pupillary light reflex and its relationship with pharmacogenetics in a randomized multiple-dose trial. British J. Clin. Pharmacol. 86 (10), 2051–2062. 10.1111/bcp.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis I, Moritz S, Pfuhl G, 2020. Objective versus subjective effort in schizophrenia. Front. Psychol 11 10.3389/fpsyg.2020.01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenzo SA, Kardon R, Ledolter J, Poolman P, Schumacher AM, Potash JB, Full JM, Rice O, Ketcham A, Starkey C, Fiedorowicz JG, 2016. Pupillary response abnormalities in depressive disorders. Psychiatry Res. 246, 492–499. 10.1016/j.psychres.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Park S, 2005. Working memory impairments in schizophrenia: a meta-analysis. J. Abnorm. Psychol. 114, 599–611. 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lidsky A, Hakerem G, Sutton S, 1971. Pupillary reactions to single light pulses in psychiatric patients and normals. J. Nervous Mental Disord. 153, 286–291. [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE, Lowenstein O, 1993. The Pupil: Anatomy, Physiology, and Clinical Applications. Iowa State University Press, Ames and Wayne State University Press, Detroit. [Google Scholar]
- Loewenfield IE, 1958. Mechanisms of reflex dilatation of the pupil; historical review and experimental analysis. Doc. Ophthalmol. 12, 185–448. 10.1007/BF00913471. [DOI] [PubMed] [Google Scholar]
- Maina G, Barzega G, Bellino S, Bogetto F, Ravizza L, 1995. Type I and type II schizophrenia: relations between tonic electrodermal activity and clinical ratings before and after haloperidol treatment. Psychiatry Res. 57, 49–56. 10.1016/0165-1781(95)02354-Y. [DOI] [PubMed] [Google Scholar]
- Mathewson KJ, Jetha MK, Goldberg JO, Schmidt LA, 2012. Autonomic regulation predicts performance on Wisconsin card sorting test (WCST) in adults with schizophrenia. Biol. Psychol. 91, 389–399. 10.1016/j.biopsycho.2012.09.002. [DOI] [PubMed] [Google Scholar]
- McCarthy JM, Treadway MT, Bennett ME, Blanchard JJ, 2016. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr. Res. 170 10.1016/j.schres.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery A, Wynn JK, Lee J, Reavis EA, Ventura J, Subotnik KL, Green MF, Nuechterlein KH, 2020. Early visual processing is associated with social cognitive performance in recent-onset schizophrenia. Front. Psychiatry 11. 10.3389/fpsyt.2020.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal DH, Gamlin PDR, 2008. Pupillary control pathways. In: Masland RH, Albright TD, Masland RH, Dallos P, Oertel D, Firestein S, Beauchamp GK, Bushnell MC, Basbaum AI, Kaas JH, Gardner EP (Eds.), The Senses: A Comprehensive Reference. Academic Press, Birmingham, AL, pp. 521–536. [Google Scholar]
- Miller BJ, Sareddy S, Rosenquist PB, McCall WV, 2021. Pupillary light reflex markers of suicide risk in a trans-diagnostic sample. Schizophr Res 235. 10.1016/j.schres.2021.06.027. [DOI] [PubMed] [Google Scholar]
- Minassian A, Granholm E, Verney S, Perry W, 2004. Pupillary dilation to simple vs. Complex tasks and its relationship to thought disturbance in schizophrenia patients. Int. J. Psychophysiol. 52. 10.1016/j.ijpsycho.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Montaquila JM, Trachik BJ, Bedwell JS, 2015. Heart rate variability and vagal tone in schizophrenia: a review. J. Psychiatr. Res. 69, 57–66. 10.1016/j.jpsychires.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Morris SK, Granholm E, Sarkin AJ, Jeste D, v., 1997. Effects of schizophrenia and aging on pupillographic measures of working memory. Schizophr. Res. 27 10.1016/S0920-9964(97)00065-0. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, 1984. A heuristic Vulnerability/Stress model of schizophrenic episodes. Schizophr. Bull 10 10.1093/schbul/10.2.300. [DOI] [PubMed] [Google Scholar]
- Okada F, Kase M, Shintomi Y, 1978. Pupillary abnormalities in schizophrenic patients during long-term administration of psychotropic drugs: dissociation between light and near vision reactions. Psychopharmacology 58. 10.1007/BF00427385. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR, 1962. The brief psychiatric rating scale. Psychol. Rep 10 10.2466/pr0.1962.10.3.799. [DOI] [Google Scholar]
- Peralta V, Cuesta MJ, 1999. Dimensional structure of psychotic symptoms: an item-level analysis of SAPS and SANS symptoms in psychotic disorders. Schizophr. Res. 38 10.1016/s0920-9964(99)00003-1. [DOI] [PubMed] [Google Scholar]
- Pukrop R, Ruhrmann S, Schultze-Lutter F, Bechdolf A, Brockhaus-Dumke A, Klosterkötter J, 2007. Neurocognitive indicators for a conversion to psychosis: comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr. Res. 92, 116–125. 10.1016/j.schres.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Quintana DS, Westlye LT, Kaufmann T, Rustan OG, Brandt CL, Haatveit B, Steen NE, Andreassen OA, 2016. Reduced heart rate variability in schizophrenia and bipolar disorder compared to healthy controls. Acta Psychiatr. Scand. 133, 44–52. 10.1111/acps.12498. [DOI] [PubMed] [Google Scholar]
- Radulescu E, Nagai Y, Critchley H, 2015. Mental effort: brain and autonomic correlates in health and disease. In: Handbook of Biobehavioral Approaches to Self-regulation. Springer, New York, New York, NY. 10.1007/978-1-4939-1236-0_16. [DOI] [Google Scholar]
- Reddy LF, Reavis EA, Wynn JK, Green MF, 2018. Pupillary responses to a cognitive effort task in schizophrenia. Schizophr. Res. 199 10.1016/j.schres.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Rubin LS, 1960a. Pupillary reactivity as a measure of adrenergic-cholinergic mechanisms in the study of psychotic behavior. J. Nerv. Ment. Dis 130 10.1097/00005053-196005000-00003. [DOI] [PubMed] [Google Scholar]
- Rubin LS, 1960b. Division of psychology: pupillary reactivity as a measure of autonomic balance in the study of psychotic behavior: a rational approach to chemotherapy. Trans. N. Y. Acad. Sci 22 10.1111/j.2164-0947.1960.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Rubin LS, 1961. Patterns of pupillary dilatation and constriction in psychotic adults and autistic children. J. Nerv. Ment. Dis 133 10.1097/00005053-196108000-00009. [DOI] [PubMed] [Google Scholar]
- Rubin LS, 1962. Patterns of adrenergic-cholinergic imbalance in the functional psychoses. Psychol. Rev 69 10.1037/h0046372. [DOI] [PubMed] [Google Scholar]
- Rubin LS, Barry TJ, 1972. The reactivity of the iris muscles as an index of autonomic dysfunction in schizophrenic remission. J. Nerv. Ment. Dis 155 10.1097/00005053-197210000-00006. [DOI] [PubMed] [Google Scholar]
- Rubin LS, Barry TJ, 1976. Amplitude of pupillary contraction as a function of intensity of illumination in schizophrenia. Biol. Psychiatry 11. [PubMed] [Google Scholar]
- Samuels ER, Szabadi E, 2008. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr. Neuropharmacol 6 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell AM, Dawson ME, Rissling A, Ventura J, Subotnik KL, Gitlin MJ, Nuechterlein KH, 2005. Electrodermal predictors of functional outcome and negative symptoms in schizophrenia. Psychophysiology 42, 483–492. 10.1111/j.1469-8986.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Mathalon DH, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, 2016. Association of Neurocognition with transition to psychosis: baseline functioning in the second phase of the North American prodrome longitudinal study. JAMA Psychiatry 73, 1239–1248. 10.1001/jamapsychiatry.2016.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Fradkin SI, Demmin DL, 2020. Schizophrenia and the retina: towards a 2020 perspective. Schizophr. Res. 219 10.1016/j.schres.2019.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer SR, Hakerem G, 1992. The pupillary response in cognitive psychophysiology and schizophrenia. Ann. N. Y. Acad. Sci. 658 10.1111/j.1749-6632.1992.tb22845.x. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Hakerem G, Spring BJ, 1979. The pupillary response as a potential indicator of vulnerability to schizophrenia. Psychopharmacol. Bull 15. [PubMed] [Google Scholar]
- Steinhauer SR, van Kammen DP, Colbert K, Peters JL, Zubin J, 1992. Pupillary constriction during haloperidol treatment as a predictor of relapse following drug withdrawal in schizophrenic patients. Psychiatry Res 43 10.1016/0165-1781(92)90061-7. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Condray R, Kasparek A, 2000. Cognitive modulation of midbrain function: task-induced reduction of the pupillary light reflex. Int. J. Psychophysiol 39 10.1016/s0167-8760(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Kappenman ES, Culbreth AJ, Catalano LT, Ossenfort KL, Lee BG, Gold JM, 2015. Emotion regulation abnormalities in schizophrenia: directed attention strategies fail to decrease the neurophysiological response to unpleasant stimuli. J. Abnorm. Psychol 124 10.1037/abn0000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Nuñez A, Ahmed AO, Barchard KA, Granholm E, Kirkpatrick B, Gold JM, Allen DN, 2018. The latent structure of negative symptoms in schizophrenia. JAMA Psychiatry 75, 1271–1279. 10.1001/jamapsychiatry.2018.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadi E, 2012. Modulation of physiological reflexes by pain: role of the locus coeruleus. Front. Integr. Neurosci 6 10.3389/fnint.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrier N, Vaughn C, Lader MH, Leff JP, 1979. Bodily reactions to people and events in schizophrenics. Arch. Gen. Psychiatry 36, 311–315. 10.1001/archpsyc.1979.01780030077007. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Brascamp JW, Ghermezi L, Fifer K, Schall JD, Park S, 2018. Reduced pupil dilation during action preparation in schizophrenia. Int. J. Psychophysiol 128 10.1016/j.ijpsycho.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Peterman JS, Zald DH, Park S, 2015. Impaired effort allocation in patients with schizophrenia. Schizophr. Res. 161 10.1016/j.schres.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-A, Munoz DP, 2015. A circuit for pupil orienting responses: implications for cognitive modulation of pupil size. Curr. Opin. Neurobiol. 33, 134–140. 10.1016/j.conb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 2001. Wechsler Test of Adult Reading (WTAR).
- Wechsler D, 2008. Wechsler Adult Intelligence Scale - Fourth Edition. [Google Scholar]
- Westphal A, 1907. Ueber ein katatonischen stupor beobachtetes pupillen-phänomen. Dtsch. Med. Wochenschr. 33, 1080–1084. [Google Scholar]
- Wilhelm BJ, Wilhelm H, Moro S, Barbur JL, 2002. Pupil response components: studies in patients with Parinaud’s syndrome. Brain 125, 2296–2307. 10.1093/brain/awf232. [DOI] [PubMed] [Google Scholar]
- Williams L.(Lea) M., Das P, Liddell BJ, Olivieri G, Peduto AS, David AS, Gordon E, Harris AWF, 2007. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Research - Neuroimaging 155, 29–44. 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Zahn TP, Pickar D, 2005. Autonomic activity in relation to symptom ratings and reaction time in unmedicated patients with schizophrenia. Schizophr. Res. 79, 257–270. 10.1016/j.schres.2005.05.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


