Abstract
Background
Resection of colorectal liver metastasis (CLM) is beneficial when feasible. However, the benefit of second hepatectomy for hepatic recurrence in CLM remains unclear.
Methods
The Colorectal Liver Operative Metastasis International Collaborative retrospectively examined 1004 CLM cases from 2000 to 2018 from a total of 953 patients. Hepatic recurrence after initial hepatectomy was identified in 218 patients. Kaplan–Meier analysis was performed for overall survival (OS) and recurrence‐free survival (RFS). Propensity score matching (PSM) was performed to offset selection bias. Cox proportional‐hazards regression was performed to identify risk factors associated with OS.
Results
A total of 51 patients underwent second hepatectomy. Unadjusted median OS was 60.1 months in repeat‐hepatectomy versus 38.3 months in the single‐hepatectomy group (p = 0.015). In the PSM population, median OS remained significantly better in the repeat‐hepatectomy group (60.1 vs. 33.1 months; p = 0.0023); median RFS was 12.4 months for the repeat‐hepatectomy group, versus 9.8 months in the single‐hepatectomy group (p = 0.0050). Repeat hepatectomy was associated with lower risk of death (hazard ratio: 0.283; p = 0.000012). Obesity, tobacco use, and high intraoperative blood loss were associated with significant risk of death (p < 0.05).
Conclusion
In CLM with hepatic recurrence, second hepatectomy was beneficial for OS. With PSM, the OS benefit of performing a second hepatectomy remained significant.
Keywords: adenocarcinoma, colon cancer, hepatic resection, propensity score matching, rectal cancer
1. INTRODUCTION
When colorectal cancer metastasizes, the liver is the most frequently affected site, occurring in approximately 20%–30% of cases. 1 , 2 Curative‐intent surgical resection of single‐site colorectal liver metastasis (CLM) began in the 1980s, 3 and has since become the standard of care when feasible, shown to prolong survival. 4 , 5 Yet, despite optimal oncologic resections and recent advances in operative approaches, subsequent isolated hepatic recurrences are not unusual, and are found to occur in over 50% of cases. 6 , 7 , 8 , 9 , 10 In these instances, performing a repeat (i.e., second) hepatectomy for CLM appears to be safe and effective in recurrent hepatic metastasis, and likely contributes to improved survival. 6 , 9 , 11 , 12 , 13 , 14 , 15 However, the long‐term outcomes of repeat hepatectomy in posthepatectomy hepatic recurrences remain unclear, since most published reports are from single‐center series, and to date all published data are retrospective. 10
The first descriptive studies of repeat hepatectomy for liver‐recurrence in CLM emerged in 1993, in two articles published in the same volume of the British Journal of Surgery: Vaillant et al. 16 with a case series of 18 patients who underwent repeat hepatectomy; followed by Elias et al. 17 in 28 similar patients. These initial reports supported the safety of performing repeat hepatectomy in the setting of CLM. The following year, the Vaillant group went on to publish the first large multicenter series of repeat hepatectomies in recurrent CLM. 18 Since then, there have been a total of at least 29 studies that examined outcomes in repeat hepatectomy for CLM. 10 Recently, a large Korean single‐center retrospective study found that patients who received repeat hepatectomy in CLM had significantly longer overall survival (OS), compared to patients who received only one hepatectomy (83 vs. 25 months). 19 Additionally, several meta analyses have reinforced the consensus that repeat hepatectomy in the setting of recurrent CLM is likely beneficial for outcomes. 8 , 10 , 13
However, a common critique of these studies is the strong potential for selection bias, since they are all retrospective. A clinician will tend to select healthier patients for whom to offer a potentially highly‐morbid, repeat operation. Additionally, single‐center studies, which comprise the majority of reports on this subject, may be more vulnerable to potential bias compared to multicenter studies. 20 Until prospective studies emerge, a powerful way to counterbalance selection bias is with propensity score matching (PSM). 21 Yet, to date there have been no reports to our knowledge on outcomes of repeat hepatectomy for recurrent CLM that incorporate PSM into the study design.
Here, we present our experience with repeat hepatectomy for CLM from a large international multicenter collaborative. From our database of 1004 consecutive CLM hepatectomy procedures in five major hepatobiliary institutions, 218 patients developed isolated hepatic recurrences, and 51 of these patients underwent a second hepatectomy. We report an improvement in OS for this group, as well as in our propensity‐matched cohort. Our findings add to the literature supporting that performance of repeat hepatectomy in the setting of recurrent CLM is associated with improved outcomes.
2. METHODS
The Colorectal Liver Operative Metastasis International Collaborative (COLOMIC), is an international collaborative association of five major hepatobiliary institutions, consisting of: Wake Forest University, Mayo Clinic Jacksonville, University of California San Francisco, Yale University, and University of Hong Kong. Institutional Review Board approval was obtained at each participating institution before data collection. This database retrospectively compiled cases of CLM treated with hepatectomy, performed at these five institutions between 2000 and 2018. The hepatectomies must have been curative‐intent, and included major and minor cases, anatomic and nonanatomic segmental resections, laparoscopic or open, and all technical methods of liver transection (crush/clamp, energy device, or hybrid technique). Wedge resections for diagnostic biopsy, or core tissue biopsies per se were excluded. Patients treated with resection plus ablation were included but ablation only cases were excluded.
A total of 1004 consecutive cases were collected from all participating institutions. Collected variables included: patient baseline demographics, medical comorbidities (including body mass index [BMI] and all components of the Charlson‐Deyo score 22 ), global functional status, 23 , 24 operative characteristics including estimated blood loss (EBL), tumor pathologic characteristics (gross and microscopic), complications (in terms of Clavien‐Dindo classification 25 ), neoadjuvant and adjuvant therapies, recurrences including date detected and anatomic site(s), follow‐up, and survival.
For this study, we selected the subset of patients from the database who developed single‐site tumor recurrence limited to the liver, after initial hepatectomy (n = 218 patients). Of these, 51 patients went on to receive a repeat (i.e., second) hepatectomy operation for hepatic recurrence. For both groups (single‐hepatectomy and repeat‐hepatectomy patients), outcomes were measured from the time of first hepatectomy, to the time of first recurrence or death. The Student's t test and Chi‐square analysis were performed for basic comparisons of patient baseline characteristics. For outcomes analysis of OS and recurrence‐free survival (RFS), the Kaplan–Meier method was performed to determine significant differences between groups. 26 RFS refers to recurrence time after first hepatectomy, for all patients including those who received two hepatectomy operations. Median follow‐up time was calculated using the method of Schemper and Smith. 27
To counterbalance the inherent selection bias present in retrospective observational analyses, 28 we performed PSM using all covariates listed in Table 1, with a 1:2 ratio (i.e., 1—repeat‐hepatectomy: 2—single‐hepatectomy patients) using a nearest neighbor algorithm. 21 , 29 , 30 This yielded PSM groups with n = 51 in the repeat‐hepatectomy group and n = 102 in the single‐hepatectomy group. Matching was followed by analysis via conventional univariate log‐rank testing and multivariate Cox proportional hazards regression modeling, 30 with all covariates included. Kaplan–Meier survival analyses with log‐rank testing were performed on both the PSM in addition to non‐PSM patient populations. Statistical significance was set at p < 0.05 (bolded in tables).
Table 1.
Patient and operative characteristics in the unmatched COLOMIC database population that had hepatic recurrence of disease at any point after initial hepatectomy
| Characteristic | Patients with hepatic recurrence receiving repeat hepatectomy | Patients with hepatic recurrence receiving one hepatectomy | Statistical test | ||
|---|---|---|---|---|---|
| Patients with hepatic recurrences n = 218 | 51 | 167 | |||
| Age at operation, median years ± SD | 55.5 | ±10.7 | 60.6 | ±11.7 | (t test, p < 0.01) |
| Sex, n = (%) | (χ 2, p = 0.83) | ||||
| Male | 30 | (58.8%) | 101 | (60.5%) | |
| Female | 21 | (41.2%) | 66 | (39.5%) | |
| Race | (χ 2, p = 0.59) | ||||
| White | 39 | (43.3%) | 118 | (41.4%) | |
| Black | 5 | (8.9%) | 17 | (9.2%) | |
| Asian | 4 | (7.3%) | 25 | (13.0%) | |
| Hispanic/other | 3 | (5.6%) | 7 | (4.0%) | |
| Body mass index, mean ± SD | 28.0 | ±6.6 | 27.5 | ±6.3 | (t test, p = 0.67) |
| Cardiac disease (MI, CHF) | 1 | (2.0%) | 10 | (6.0%) | |
| Peripheral vascular disease (PVD) | 0 | (0.0%) | 2 | (1.2%) | |
| Chronic obstructive pulmonary disease | 3 | (5.9%) | 8 | (4.8%) | |
| Diabetes | 7 | (13.7%) | 20 | (12.0%) | |
| Renal disease (CKD II or higher) | 0 | (0.0%) | 8 | (4.8%) | |
| Smoking history | 20 | (39.2%) | 53 | (31.7%) | |
| Cerebrovascular disease | 0 | (0.0%) | 4 | (2.4%) | |
| Charlson‐Deyo Score, mean ± SD | 8.6 | ±1.06 | 8.5 | ±0.99 | (t test, p = 0.88) |
| Functional status, n = (%) | (χ 2, p = 0.043) | ||||
| Independent | 49 | (96.1%) | 137 | (82.0%) | |
| Partially‐dependent | 2 | (3.9%) | 25 | (15.0%) | |
| Totally‐dependent | 0 | (0.0%) | 5 | (3.0%) | |
| ASA physical status, n = (%) | (χ 2, p = 0.25) | ||||
| I | 1 | (2.0%) | 5 | (3.0%) | |
| II | 7 | (13.7%) | 44 | (26.3%) | |
| III | 41 | (80.4%) | 110 | (65.9%) | |
| IV | 2 | (3.9%) | 8 | (4.8%) | |
| Received neoadjuvant chemotherapy | 38 | (74.5%) | 96 | (57.5%) | (χ 2, p = 0.029) |
| Received adjuvant chemotherapy | 37 | (72.5%) | 126 | (75.4%) | (χ 2, p = 0.676) |
| Operative approach: | (χ 2, p = 0.030) | ||||
| Open | 25 | (49.0%) | 110 | (65.9%) | |
| Laparoscopic | 26 | (51.0%) | 57 | (34.1%) | |
| Resection Anatomical Type: | (χ 2, p < 0.01) | ||||
| Anatomic | 18 | (35.3%) | 95 | (56.9%) | |
| Nonanatomic | 33 | (64.7%) | 72 | (43.1%) | |
| Resection Segmental Type: | (χ 2, p = 0.038) | ||||
| Minor | 37 | (72.5%) | 94 | (56.3%) | |
| Major | 14 | (27.5%) | 73 | (43.7%) | |
| Average estimated blood loss (ml) ±SD | 374 | ±421 | 677 | ±903 | (t test, p = 0.031) |
| Mean number of resected lesions | 2.33 | 2.84 | (t test, p = 0.18) | ||
| Largest lesion size, mean (mm) | 15.2 | 20.5 | (t test, p = 0.15) | ||
| Pathologic margin status | (χ 2, p = 0.98) | ||||
| Negative (R0) | 43 | (84.3%) | 141 | (84.4%) | |
| Positive (R1 or R2) | 8 | (15.7%) | 26 | (15.6%) | |
| Tumor grade | (χ 2, p = 0.022) | ||||
| G1 (well‐differentiated) | 2 | (3.9%) | 13 | (7.8%) | |
| G2 (moderately‐differentiated) | 24 | (47.1%) | 103 | (61.7%) | |
| G3 (poorly‐differentiated) | 8 | (15.7%) | 9 | (5.4%) | |
| Unknown/not reported | 17 | (33.3%) | 42 | (25.1%) | |
Note: χ 2 = Chi‐square.
Abbreviations: ASA, American Society of Anesthesiologists; CHF, congestive heart failure; CKD, chronic kidney disease; COLOMIC, Colorectal Liver Operative Metastasis International Collaborative; MI, myocardial infarction; PVD, peripheral vascular disease; SD, standard deviation; SEM, standard error of the mean.
Data management was performed with Microsoft Excel version 2016 (Microsoft); and statistical analysis was performed with IBM SPSS software version 26 (International Business Machines). Data available on request due to privacy/ethical restrictions.
3. RESULTS
From a total of 218 patients in the COLOMIC database with isolated hepatic metastatic recurrences, approximately 23% (n = 51) went on to receive a second hepatectomy, whereas n = 167 received only one hepatectomy operation (Table 1). Compared with the single‐hepatectomy population, the repeat‐hepatectomy population was younger on average by 5.1 years (p < 0.01), had a greater proportion of functionally‐independent patients (96.1% vs. 82.0%, p = 0.043). In terms of operative and pathologic characteristics, the repeat‐hepatectomy population was more likely to have received a laparoscopic versus open approach (p = 0.030), more likely to have received a nonanatomic type resection (p < 0.01), more likely to have received a minor versus major segmental type resection (p = 0.038), and had significantly lower EBL, by approximately 300 ml (p = 0.031). These two populations did not significantly differ in terms of sex, racial composition, BMI, or Charlson‐Deyo score. The American Society of Anesthesiologists (ASA) physical status classification scores were not significantly different between the two groups. A greater proportion of the repeat patients received neoadjuvant chemotherapy before the first hepatectomy operation, compared with the single‐hepatectomy patient group (74.5% vs. 57.5%, p = 0.029), but the two groups were equivalent in terms of receiving adjuvant chemotherapy after first hepatectomy (74.5% vs. 75.4%; p = 0.676).
Median follow‐up was 62.6 months for the overall population from time of first hepatectomy (75.5 months in the repeat hepatectomy group, and 55.8 months in the single‐hepatectomy group). In the unmatched database population, OS and RFS were significantly better in the repeat hepatectomy group compared to the single hepatectomy group (Table 2). Median OS was significantly longer in the repeat hepatectomy group compared to single‐hepatectomy group (60.1 vs. 38.3 months, p = 0.015). Median RFS was also significantly better in the repeat hepatectomy group (p = 0.0073). Median lengths of stay (LOS) were equivalent between groups (5.0 days), and complication rates by Clavien‐Dindo classification were not significantly different between groups.
Table 2.
Survival and outcomes (OS and RFS) in the unmatched COLOMIC database population that had hepatic recurrence after initial hepatectomy
| Patients with hepatic recurrence receiving repeat hepatectomy | Patients with hepatic recurrence receiving one hepatectomy | ||||
|---|---|---|---|---|---|
| Characteristic | Statistical test | ||||
| Number of patients, n= | 51 | 167 | |||
| Median overall survival, months ± SEM | 60.1 | ±2.30 | 38.3 | ±5.58 | (Log‐rank, p = 0.015) |
| Median recurrence‐free survival, months ± SEM | 12.4 | ±1.07 | 10.1 | ±1.15 | (Log‐rank, p = 0.0073) |
| Median length of stay, days ± SEM | 5.0 | ±0.60 | 5.0 | ±1.24 | (t test, p = 0.53) |
| Complication rate, Clavien‐Dindo Grades I–V, (proportion, %) | (23/51) | 45.1% | (68/167) | 40.7% | (χ 2, p = 0.58) |
| Serious complication rate: Clavien‐Dindo Grades IIIa–V, (proportion, %) | (4/51) | 7.8% | (19/167) | 11.4% | (χ 2, p = 0.47) |
| 90‐Day mortality rate, Clavien‐Dindo Grade V, (proportion, %) | (0/51) | 0.0% | (2/167) | 1.2% | (χ 2, p = 0.43) |
Note: Additional secondary outcomes of hospital postoperative length of stay and postoperative complication rates are shown. χ 2 = Chi‐square.
Abbreviations: COLOMIC, Colorectal Liver Operative Metastasis International Collaborative; OS, overall survival; RFS, recurrence‐free survival; SEM, standard error of the mean.
Since we found significant differences between our two patient groups in several key baseline characteristics as noted above, we went on to perform PSM to counterbalance selection bias. After performing PSM in a 1:2 ratio, all of our observed baseline variable differences between the two population groups became equalized (Table 3). As in the overall population analysis, after PSM the repeat‐hepatectomy patients continued to have significantly longer OS and RFS (p = 0.0023 and p = 0.005, respectively), compared to the single‐hepatectomy patients. After PSM, median LOS and complication rates remained similar between groups.
Table 3.
Patient and operative characteristics in the PSM matched COLOMIC database population with hepatic recurrence after initial hepatectomy, showing equivalence of measured variables between the compared groups
| Characteristic | Patients with hepatic recurrence receiving repeat hepatectomy | Patients with hepatic recurrence receiving one hepatectomy | Statistical test | ||
|---|---|---|---|---|---|
| Patients with hepatic recurrences n = 153 | 51 | 102 | |||
| Age at operation, median years ± SD | 55.5 | ±10.7 | 57.6 | ±11.3 | (t test, p < 0.26) |
| Sex, n = (%) | (χ 2, p = 0.73) | ||||
| Male | 30 | (58.8%) | 57 | (55.9%) | |
| Female | 21 | (41.2%) | 45 | (44.1%) | |
| Race | (χ 2, p = 0.73) | ||||
| White | 39 | (43.3%) | 78 | (43.3%) | |
| Black | 5 | (8.9%) | 9 | (8.1%) | |
| Asian | 4 | (7.3%) | 12 | (10.5%) | |
| hispanic/other | 3 | (5.6%) | 3 | (2.9%) | |
| Body mass index, mean ± SD | 28.0 | ±5.9 | 27.7 | ±6.4 | (t test, p = 0.80) |
| Cardiac disease (MI, CHF) | 2 | (3.9%) | 1 | (1.0%) | |
| Peripheral vascular disease (PVD) | 0 | (0.0%) | 0 | (0.0%) | |
| Chronic obstructive pulmonary disease | 3 | (5.9%) | 5 | (4.9%) | |
| Diabetes | 7 | (13.7%) | 13 | (12.7%) | |
| Renal disease (CKD II or higher) | 0 | (0.0%) | 1 | (1.0%) | |
| Smoking history | 20 | (39.2%) | 35 | (34.3%) | |
| Cerebrovascular disease | 0 | (0.0%) | 0 | (0.0%) | |
| Charlson‐Deyo Score, mean ± SD | 8.6 | ±1.17 | 8.5 | ±1.14 | (t test, p = 0.84) |
| Functional status, n = (%) | (χ 2, p = 0.590) | ||||
| Independent | 49 | (96.1%) | 94 | (92.2%) | |
| Partially‐dependent | 2 | (3.9%) | 7 | (6.9%) | |
| Totally‐dependent | 0 | (0.0%) | 1 | (1.0%) | |
| ASA physical status, n = (%) | (χ 2, p = 0.75) | ||||
| I | 1 | (2.0%) | 2 | (2.0%) | |
| II | 7 | (13.7%) | 20 | (19.6%) | |
| III | 41 | (80.4%) | 74 | (72.5%) | |
| IV | 2 | (3.9%) | 6 | (5.9%) | |
| Received neoadjuvant chemotherapy | 38 | (74.5%) | 72 | (70.6%) | (χ 2, p = 0.611) |
| Received adjuvant chemotherapy | 37 | (72.5%) | 80 | (78.4%) | (χ 2, p = 0.419) |
| Operative approach: | (χ 2, p = 0.205) | ||||
| Open | 25 | (49.0%) | 61 | (59.8%) | |
| Laparoscopic | 26 | (51.0%) | 41 | (40.2%) | |
| Reection anatomical type: | (χ 2, p = 0.13) | ||||
| Anatomic | 18 | (35.3%) | 49 | (48.0%) | |
| Nonanatomic | 33 | (64.7%) | 53 | (52.0%) | |
| Reection segmental type: | (χ 2, p = 0.151) | ||||
| Minor | 37 | (72.5%) | 62 | (60.8%) | |
| Major | 14 | (27.5%) | 40 | (39.2%) | |
| Average estimated blood loss (ml) ±SD | 374 | ±395.23 | 417 | ±373.58 | (t test, p = 0.51) |
| Mean number of resected lesions | 2.33 | 2.79 | (t test, p = 0.169) | ||
| Largest lesion size, mean (mm) | 15.2 | 17.0 | (t test, p = 0.52) | ||
| Pathologic margin status | (χ 2, p = 0.88) | ||||
| Negative (R0) | 43 | (84.3%) | 85 | (83.3%) | |
| Positive (R1 or R2) | 8 | (15.7%) | 17 | (16.7%) | |
| Tumor grade | (χ 2, p = 0.240) | ||||
| G1 (well‐differentiated) | 2 | (3.9%) | 6 | (5.9%) | |
| G2 (moderately‐differentiated) | 23 | (45.1%) | 56 | (54.9%) | |
| G3 (poorly‐differentiated) | 8 | (15.7%) | 8 | (7.8%) | |
| Unknown/not reported | 17 | (33.3%) | 32 | (31.4%) | |
| PSM median OS, months ± SEM | 60.1 | ±2.30 | 33.1 | ±4.43 | (Log‐rank, p = 0.0023) |
| PSM median RFS, months ± SEM | 12.4 | ±1.07 | 9.8 | ±1.31 | (Log‐rank, p = 0.0050) |
| PSM median length of stay, days ± SEM | 6 | ±2.18 | 6 | ±1.51 | (t test, p = 0.86) |
| PSM complication rate, C–D Grades I–V (n, %) | (23/51) | 6.1% | (45/102) | 10.8% | (χ 2, p = 0.91) |
| PSM serious complication rate: C–D Grades IIIa–V (n, %) | (4/51) | 1.1% | (11/102) | 2.6% | (χ 2, p = 0.56) |
| PSM 90‐day mortality rate, C–D Grade V (n, %) | (0/51) | 0.0% | (1/102) | 0.2% | (χ 2, p = 0.48) |
Note: Outcomes from the PSM matched cohort are also shown (OS, RFS, average postoperative length of stay, and complication rates). χ 2 = Chi‐Square.
Abbreviations: ASA, American Society of Anesthesiologists; C‐D, Clavien‐Dindo; CHF, congestive heart failure; CKD, chronic kidney disease; MI, myocardial infarction; PVD, peripheral vascular disease; PSM, propensity score matched; SD, standard deviation; SEM, standard error of the mean.
Using Kaplan–Meier survival analysis on the PSM populations, we observed the repeat‐hepatectomy group had significantly longer median OS (Figure 1A) and median RFS (Figure 1B) versus the single‐hepatectomy group (Log‐rank χ 2 = 9.298, p < 0.005 and χ 2 = 7.874, p < 0.01, respectively). The results were similar in Kaplan–Meier survival analysis of the database population without PSM in which the repeat‐hepatectomy group also had significantly longer median OS and RFS (Figure S1A,B), Log‐rank χ 2 = 5.937, p < 0.02 and χ 2 = 7.20, p < 0.01, respectively. This additional analysis demonstrated that outcomes remained significantly better in the repeat‐hepatectomy group (OS and RFS), regardless of whether or not PSM is performed.
Figure 1.
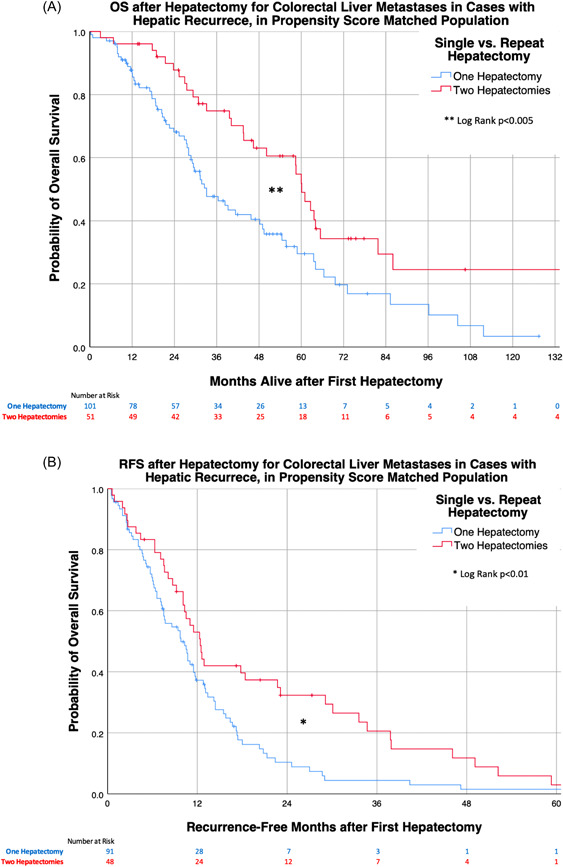
Kaplan–Meier survival curves in the PSM matched cohort patients with hepatic recurrence after initial hepatectomy, comparing those who received repeat‐hepatectomy versus single‐hepatectomy, (A) OS and (B) RFS. Time zero is from initial hepatectomy, for all groups. OS, overall survival; PSM, propensity score matching; RFS, recurrence‐free survival
We went on to perform univariate log‐rank tests and multivariate Cox proportional hazards regression analysis on the PSM groups, using OS as the primary outcome (Table 4). In the univariate and multivariate analyses, receiving a second hepatectomy was significantly protective compared to receiving one hepatectomy—hazard ratio (HR): 0.509, 95% confidence interval (CI: 0.327–0.792, p = 0.003 in univariate analysis, and HR: 0.283, 95% CI: 0.161–0.498, p = 0.000012 in multivariate analysis. Several variables found with statistically significant hazard ratios in the univariate analyses were no longer significant in the multivariate analysis: location (institution), Asian race, functional status, ASA physical status, and lesion size. Additional variables found independently‐associated with significantly higher hazard ratios in the multivariate analysis were: high BMI, positive smoking history, and high EBL (p = 0.010, p = 0.032, p = 0.002, respectively).
Table 4.
Univariate log‐rank tests and multivariate Cox proportional hazards regression analyses of the PSM matched COLOMIC database population
| Univariable (n = 153) | Multivariable (n = 153) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Crude HR | 95% CI | p value | Adjusted HR | 95% CI | p value | ||
| Received second hepatectomy (Yes vs. No) | 0.509 | 0.327–0.792 | 0.003 | 0.283 | 0.161–0.498 | 0.000012 | ||
| Institution (comparison of 5 participating sites) | [Reference] | 0.044 | [Reference] | 0.072 | ||||
| Age at operation | 1.008 | 0.990–1.027 | 0.395 | 0.992 | 0.968–1.016 | 0.515 | ||
| Sex (male vs. female) | 0.776 | 0.519–1.160 | 0.216 | 0.981 | 0.561–1.716 | 0.947 | ||
| Race | ||||||||
| White | [Reference] | 0.106 | [Reference] | 0.492 | ||||
| Black | 0.939 | 0.494–1.786 | 0.848 | 1.098 | 0.469–2.571 | 0.830 | ||
| Asian | 0.083 | 0.012–0.597 | 0.013 | 0.440 | 0.050–3.851 | 0.459 | ||
| Hispanic/other | 0.946 | 0.410–2.186 | 0.897 | 0.418 | 0.125–1.398 | 0.157 | ||
| Body mass index, mean ± SD | 1.039 | 1.009–1.071 | 0.011 | 1.057 | 1.013–1.102 | 0.010 | ||
| Cardiac disease (MI, CHF) | 2.700 | 0.660–11.04 | 0.167 | 7.946 | 0.890–70.92 | 0.063 | ||
| Chronic obstructive pulmonary disease | 1.303 | 0.527–3.222 | 0.567 | 1.418 | 0.375–5.368 | 0.607 | ||
| Diabetes | 1.436 | 0.835–2.472 | 0.191 | 1.103 | 0.451–2.696 | 0.830 | ||
| Renal disease (CKD II or higher) | 2.189 | 0.796–6.022 | 0.129 | 0.432 | 0.047–3.947 | 0.457 | ||
| Smoking history | 1.001 | 0.660–1.518 | 0.996 | 1.964 | 1.058–3.645 | 0.032 | ||
| Charlson‐Deyo score | 1.116 | 0.960–1.298 | 0.151 | 1.031 | 0.667–1.593 | 0.892 | ||
| Functional status (independent vs. partially/totally‐dependent) | 0.132 | 0.018–0.949 | 0.044 | 0.422 | 0.019–9.363 | 0.585 | ||
| ASA physical status (I/II vs. III/IV) | 2.119 | 1.178–3.812 | 0.012 | 2.100 | 0.988–4.465 | 0.054 | ||
| Received neoadjuvant chemotherapy (yes vs. no) | 1.076 | 0.687–1.685 | 0.749 | 1.329 | 0.754–2.342 | 0.325 | ||
| Received adjuvant chemotherapy (yes vs. no) | 1.030 | 0.659–1.611 | 0.896 | 1.551 | 0.796–3.023 | 0.197 | ||
| Operative approach (laparoscopic vs. open) | 0.989 | 0.642–1.524 | 0.961 | 1.119 | 0.604–2.073 | 0.721 | ||
| Resection anatomical type (nonanatomic v.s anatomic) | 1.262 | 0.842–1.891 | 0.260 | 0.689 | 0.336–1.411 | 0.308 | ||
| Resection segmental type (major vs. minor) | 0.884 | 0.580–1.348 | 0.567 | 1.616 | 0.716–3.648 | 0.248 | ||
| Average estimated blood loss (ml) ±SD | 1.000 | 0.999–1.000 | 0.640 | 0.999 | 0.998–0.999 | 0.002 | ||
| Number of resected lesions | 0.985 | 0.903–1.075 | 0.739 | 0.964 | 0.862–1.078 | 0.518 | ||
| Largest lesion size | 0.978 | 0.963–0.994 | 0.006 | 0.991 | 0.967–1.015 | 0.464 | ||
| Pathologic margin status (R1/R2 vs. R0) | 1.341 | 0.801–2.244 | 0.264 | 1.981 | 0.948–4.142 | 0.069 | ||
| Tumor Grade | ||||||||
| G1 (well‐differentiated) | [Reference] | 0.923 | [Reference] | 0.997 | ||||
| G2 (moderately‐differentiated) | 0.967 | 0.430–2.175 | 0.935 | 1.058 | 0.255–4.386 | 0.938 | ||
| G3 (poorly‐differentiated) | 1.150 | 0.445–2.974 | 0.773 | 1.136 | 0.303–4.261 | 0.850 | ||
Abbreviations: ASA, American Society of Anesthesiologists; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; COLOMIC, Colorectal Liver Operative Metastasis International Collaborative; HR, hazard ratio; MI, myocardial infarction; PSM, propensity score matching; SD, standard deviation.
Examining the population that received two hepatectomies, we went on to compare operative and postoperative characteristics of those patients at the time of first versus second hepatectomy (Table S1). There were no statistically significant differences in age, median length of stay, serious complication rate, or 90‐day mortality at second hepatectomy compared to first hepatectomy (p > 0.05). A similar proportion of cases were performed laparoscopically (p = 0.55). There were similar proportions of anatomic resections (vs. nonanatomic, p = 0.68) and major segmentectomy (vs. minor, p = 0.52) at the time of second versus first hepatectomy. The mean number of resected lesions, largest lesion size, and pathologic margin statuses were all similar and noncontributory (p > 0.7). The only significantly different variable found was the average EBL, being approximately double at the time of second hepatectomy (678 ml) compared to first hepatectomy (374 ml), p = 0.023.
4. DISCUSSION
Using our unmatched cohort, we corroborated here the existing consensus that repeat hepatectomies for hepatic recurrence after initial hepatectomy for CLM are generally associated with better survival outcomes, compared to those who do not receive a second hepatectomy. The median OS was significantly longer in the repeat‐hepatectomy population, but the complication rates and LOS were similar, compared to the single hepatectomy group. As expected, we noted that younger, healthier patients tended to receive repeat hepatectomies, and this was likely related to selection bias. It was also not unexpected that a higher percentage of repeat‐hepatectomy patients received neoadjuvant chemotherapy compared to the single‐hepatectomy population, since systemic chemotherapy is often not offered to frail or sick patients who are not expected to tolerate it well, using the same rationale for not offering a potentially morbid second hepatectomy. It is interesting to note that for first‐hepatectomy, the laparoscopic approach was more often performed in the population that went on to receive a second hepatectomy, which may be more feasible to perform when the first operation was done with a minimally invasive approach. Within the population that went on to receive a second hepatectomy operation, operative and pathologic characteristics were all comparable except for a higher EBL during the second operation, and most importantly the complication rates and 90‐day mortality rate were comparable at the time of second operation. This confirms the safety of performing a second hepatectomy in appropriate operative candidates with isolated liver recurrence.
The median RFS was longer in the repeat hepatectomy group compared to single hepatectomy group. Since RFS in our study refers to recurrence in relation to the first hepatectomy in both populations, the performance of a second hepatectomy will have no direct effect on RFS. Thus the difference in RFS between groups likely represents a correlation between favorable tumor biology and the performance of a second hepatectomy. Rapid hepatic recurrence after initial hepatectomy would be indicative of an aggressive metastatic phenotype, and would be correlated with additional tumor‐factors (size, number and grade) that would make a second hepatectomy less feasible or beneficial. This is supported by a recent study by Wong et al. 31 at OHSU, which found poor outcomes after second hepatectomy for CLM if recurrence after first resection occurred sooner than 12 months, or recurrent tumors were greater than 3 cm or three or greater in number. Conversely, a less aggressive tumor phenotype would lead to longer RFS and more favorable tumor‐factors that increase the feasibility and safety of a second hepatic resection. Thus, a repeat hepatectomy can be considered and performed for all potential operative candidates in the setting of second hepatic recurrence in CLM, but our findings suggest a second hepatectomy would most likely benefit patients with a longer time interval to first‐recurrence, in light of generally better tumor biology.
These numerous differences in baseline characteristics between single‐hepatectomy and repeat‐hepatectomy populations were precisely the reason we went on to perform PSM combined with multivariate regression, to counterbalance the effect of selection bias. We also aimed to mitigate selection bias by including the wide variety of hospitals, in distinct geographic regions, in our collaborative. Notably, there have been two studies that used PSM to specifically compare laparoscopic versus open operative approaches in patients for repeat hepatectomies in CLM. 32 , 33 But these studies did not compare single‐hepatectomy versus repeat‐hepatectomy patients. Here, we have confirmed that PSM is feasible for this comparison, and appears to be effective in counterbalancing selection bias. We utilized a 1:2 PSM ratio, which was guided by our initial cohort numbers, but alternative ratios may potentially be equally‐valid in datasets from other investigators. More important than ratio was the selection of variables for inclusion in the PSM algorithm: For any study it is based on selection of known significant prognostic variables. Here, we tried to use as many relevant baseline clinical patient variables, operative variables, and tumor/pathologic variables as feasible. The end result was a matched population that no longer carried any significant differences in measured variables between patient populations.
We went on to demonstrate that despite using PSM followed by multivariate regression analysis in this subset, there was still a strong OS benefit for the group that received two hepatectomies, as was also noted in our unmatched cohort. Our findings are important because they corroborate the current consensus in the field, using statistical techniques that had not been utilized before to compare these patient groups in this clinical scenario. Given these findings, we speculate a third hepatectomy for isolated liver recurrence after second hepatectomy may also be potentially beneficial in selected patients. In 1997, the French group of Dr. Henri Bismuth published a case series with up to four serial hepatectomies for CLM, showing a median OS of approximately 33 months from time of first hepatectomy, for patients who underwent three hepatectomies (n = 15). 11 More recently, in a retrospective study from 2019, a Japanese group found good survival rates after third hepatectomy for CLM, from time of first hepatectomy (median OS 54 months, n = 13). 34 Since a majority of patients in both studies received systemic chemotherapy at some point in their operative courses, the improved numbers may be explained by improved chemotherapy regimens that have been developed since the 1990s. Although we did not include third hepatectomy patients in our study, the improved survival benefit of repeat hepatectomy in CLM, especially in combination with chemotherapy, may continue well past hepatectomy number two in the appropriately selected patient.
In our study, systemic chemotherapy was given to a large subset of patients after initial hepatectomy, between hepatectomies, and/or after second hepatectomy if applicable. This is usually an individualized decision—taking into consideration patient comorbidities, general state of health, and patient preferences. We recently found that receiving neoadjuvant‐only chemotherapy before liver resection was not correlated with improved survival compared to surgery alone. 35 In our unmatched populations in this study, a comparable proportion of patients in both groups received chemotherapy after initial hepatectomy, but a significantly greater proportion of patients in the repeat‐hepatectomy group received chemotherapy before initial hepatectomy. However, in our PSM analysis, neoadjuvant chemotherapy before first hepatectomy was not correlated with improved survival, suggesting the treatment effect, if any, is modest at best.
A related finding in our PSM analysis is that high body mass index, positive smoking history, and high EBL during initial hepatectomy, were identified as hazard factors that significantly correlated with decreased OS in this patient population. The other two hazards identified, positive smoking history and high BMI, would generally not be contraindications to performing a second‐hepatectomy in most cases, but our data suggests these factors need to be taken into account during multidisciplinary discussion for management of isolated recurrent CLM.
One limitation of our study is PSM and regression analyses are imperfect methods to counterbalance selection bias, because it is not possible to take every potentially‐biased patient baseline variable into account. We used known significant prognostic factors to select what we thought were the most relevant variables in this correction. We did include participating institution as a variable in our regression analysis. We believe this is an important factor to include in multicenter studies, because doing so will take many additional incalculable variables into account which may otherwise bias the group selections. This includes regional differences in care as well as differences in patient populations. Our OS results are in accordance with survival data of published studies in the repeat hepatectomy population. 8 , 10 Although this was a retrospective study, our large multicenter database with a diverse patient population allows for generalizability within this class of study design.
In conclusion, patients in our diverse study population who received a second hepatectomy for isolated liver recurrence after initial hepatectomy, had significantly longer OS and recurrence‐free survival. Thus, in properly‐selected patients with isolated hepatic recurrence after initial hepatectomy for CLM, a second hepatectomy will likely be beneficial.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SYNOPSIS
We report long‐term survival outcomes for patients with colorectal liver metastases initially treated with hepatectomy, followed by a repeat hepatectomy after recurrence. Analysis of our penta‐institutional international cohort database of colorectal liver metastasis patients treated with hepatectomy revealed improved overall survival in those who received a second hepatectomy in a propensity score matched model. Tobacco use, obesity, and high intraoperative blood loss were identified as clinical factors independently associated with significantly higher hazard of death.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENT
Wake Forest University Comprehensive Cancer Center Biostatistics shared resource funded via the NCI grant award P30CA012197.
Valenzuela CD, Moaven O, Gawdi R, et al. Outcomes after repeat hepatectomy for colorectal liver metastases from the colorectal liver operative metastasis international collaborative (COLOMIC). J Surg Oncol. 2022;126:1242‐1252. 10.1002/jso.27056
This work was presented at the Americas Hepato‐Pancreato‐Biliary Association 2021 Annual Meeting in Miami, FL.
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Finlay IG, McArdle CS. Occult hepatic metastases in colorectal carcinoma. Br J Surg. 1986;73:732‐735. [DOI] [PubMed] [Google Scholar]
- 2. Ohlsson B, Tranberg KG, Lundstedt C, Ekberg H, Hederstrom E. Detection of hepatic metastases in colorectal cancer: a prospective study of laboratory and imaging methods. Eur J Surg. 1993;159:275‐281. [PubMed] [Google Scholar]
- 3. Steele G, Jr. , Bleday R, Mayer RJ, Lindblad A, Petrelli N, Weaver D. A prospective evaluation of hepatic resection for colorectal carcinoma metastases to the liver: gastrointestinal tumor study group protocol 6584. J Clin Oncol. 1991;9:1105‐1112. [DOI] [PubMed] [Google Scholar]
- 4. Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yan TD, Sim J, Black D, Niu R, Morris DL. Systematic review on safety and efficacy of repeat hepatectomy for recurrent liver metastases from colorectal carcinoma. Ann Surg Oncol. 2007;14:2069‐2077. [DOI] [PubMed] [Google Scholar]
- 6. Kin T, Nakajima Y, Kanehiro H, et al. Repeat hepatectomy for recurrent colorectal metastases. World J Surg. 1998;22:1087‐1091. [DOI] [PubMed] [Google Scholar]
- 7. Yamada H, Kondo S, Okushiba S, Morikawa T, Katoh H. Analysis of predictive factors for recurrence after hepatectomy for colorectal liver metastases. World J Surg. 2001;25:1129‐1133. [DOI] [PubMed] [Google Scholar]
- 8. Lopez P, Marzano E, Piardi T, Pessaux P. Repeat hepatectomy for liver metastases from colorectal primary cancer: a review of the literature. J Visc Surg. 2012;149:e97‐e103. [DOI] [PubMed] [Google Scholar]
- 9. Wicherts DA, de Haas RJ, Salloum C, et al. Repeat hepatectomy for recurrent colorectal metastases. Br J Surg. 2013;100:808‐818. [DOI] [PubMed] [Google Scholar]
- 10. Hellingman T, de Swart ME, Heymans MW, Jansma EP, van der Vliet HJ, Kazemier G. Repeat hepatectomy justified in patients with early recurrence of colorectal cancer liver metastases: A systematic review and meta‐analysis. Cancer Epidemiol. 2021;74: 101977. [DOI] [PubMed] [Google Scholar]
- 11. Adam R, Bismuth H, Castaing D, et al. Repeat hepatectomy for colorectal liver metastases. Ann Surg. 1997;225:51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu QD, Vezeridis MP, Avradopoulos KA, Wanebo HJ. Repeat hepatic resection for recurrent colorectal cancer. World J Surg. 1997;21:292‐296. [DOI] [PubMed] [Google Scholar]
- 13. Antoniou A, Lovegrove RE, Tilney HS, et al. Meta‐analysis of clinical outcome after first and second liver resection for colorectal metastases. Surgery. 2007;141:9‐18. [DOI] [PubMed] [Google Scholar]
- 14. de Jong MC, Mayo SC, Pulitano C, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: Results from an international multi‐institutional analysis. J Gastrointest Surg. 2009;13:2141‐2151. [DOI] [PubMed] [Google Scholar]
- 15. Shaw IM, Rees M, Welsh FK, Bygrave S, John TG. Repeat hepatic resection for recurrent colorectal liver metastases is associated with favourable long‐term survival. Br J Surg. 2006;93:457‐464. [DOI] [PubMed] [Google Scholar]
- 16. Vaillant JC, Balladur P, Nordlinger B, et al. Repeat liver resection for recurrent colorectal metastases. Br J Surg. 1993;80:340‐344. [DOI] [PubMed] [Google Scholar]
- 17. Elias D, Lasser P, Hoang JM, et al. Repeat hepatectomy for cancer. Br J Surg. 1993;80:1557‐1562. [DOI] [PubMed] [Google Scholar]
- 18. Nordlinger B, Vaillant JC, Guiguet M, et al. Survival benefit of repeat liver resections for recurrent colorectal metastases: 143 cases. Association Francaise de Chirurgie. J Clin Oncol. 1994;12:1491‐1496. [DOI] [PubMed] [Google Scholar]
- 19. Park J, Lee SD, Han SS, et al. Repeat hepatectomy for recurred colorectal liver metastasis: is it justified? Ann Surg Treat Res. 2019;97:7‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Unverzagt S, Prondzinsky R, Peinemann F. Single‐center trials tend to provide larger treatment effects than multicenter trials: a systematic review. J Clin Epidemiol. 2013;66:1271‐1280. [DOI] [PubMed] [Google Scholar]
- 21. Newgard CD, Hedges JR, Arthur M, Mullins RJ. Advanced statistics: the propensity score—a method for estimating treatment effect in observational research. Acad Emerg Med. 2004;11:953‐961. [DOI] [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 23.User Guide for the 2014 ACS‐NSQIP Participant Use Data File. Available: https://www.facs.org/-/media/files/quality-programs/nsqip/nsqip_puf_userguide_2014.ashx [accessed June 24, 2021].
- 24. Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281‐284. [Google Scholar]
- 25. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457‐481. [Google Scholar]
- 27. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials. 1996;17:343‐346. [DOI] [PubMed] [Google Scholar]
- 28. Brazauskas R, Logan BR. Observational studies: matching or regression? Biol Blood Marrow Transplant. 2016;22:557‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Performing a 1:N Case‐Control Match on Propensity Score. Available: https://support.sas.com/resources/papers/proceedings/proceedings/sugi29/165-29.pdf [accessed 12/28, 2021].
- 30. Austin PC, Thomas N, Rubin DB. Covariate‐adjusted survival analyses in propensity‐score matched samples: imputing potential time‐to‐event outcomes. Stat Methods Med Res. 2020;29:728‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong LH, Sutton TL, Walker BS, et al. Surgical and oncologic outcomes following repeat hepatic resection of colorectal liver metastasis: who benefits? Am J Surg. 2021;221:1114‐1118. [DOI] [PubMed] [Google Scholar]
- 32. Hallet J, Sa Cunha A, Cherqui D, et al. Laparoscopic compared to open repeat hepatectomy for colorectal liver metastases: a multi‐institutional propensity‐matched analysis of short‐ and long‐term outcomes. World J Surg. 2017;41:3189‐3198. [DOI] [PubMed] [Google Scholar]
- 33. van der Poel MJ, Barkhatov L, Fuks D, et al. Multicentre propensity score‐matched study of laparoscopic versus open repeat liver resection for colorectal liver metastases. Br J Surg. 2019;106:783‐789. [DOI] [PubMed] [Google Scholar]
- 34. Matsuoka H, Morise Z, Tanaka C, et al. Repeat hepatectomy with systemic chemotherapy might improve survival of recurrent liver metastasis from colorectal cancer‐a retrospective observational study. World J Surg Oncol. 2019;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gawdi R, Valenzuela CD, Moaven O, et al. Perioperative chemotherapy for resectable colorectal liver metastases: analysis from the colorectal operative liver metastases international collaborative (COLOMIC). J Surg Oncol. 2022;126:339‐347. 10.1002/jso.26893 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


