Abstract
Timely and reliable detection of acute primary human cytomegalovirus (HCMV) infection is important in prenatal screening programs and for differential diagnosis of infectious mononucleosis-like disease. Enzyme-linked immunosorbent assays (ELISAs) based on HCMV proteins enable the sensitive detection of immunoglobulin M (IgM) antibodies during primary infection. However, concerns have been raised about possible cross-reactivities of the HCMV antigens used for the design of such ELISAs with IgM antibodies induced by Epstein-Barr Virus (EBV). In this study we investigated whether IgM antibodies generated during acute EBV infection reacted with recombinant HCMV antigens. Serum samples from patients with primary EBV infection frequently scored positive when tested in different HCMV IgM ELISAs, irrespective of whether conventional or recombinant antigens were used for the design of the HCMV IgM assays. Such cross-reactive IgM antibodies were found to be directed against short glycine-rich motifs contained within the nonstructural HCMV proteins pUL44 and pUL57. Further analyses revealed that these glycine-rich motifs were major antigenic domains for IgM antibodies induced during HCMV infection. Their deletion from recombinant proteins abrogated reactivity with IgM synthesized during HCMV infection. EBV-induced IgM antibodies that reacted with HCMV antigens showed similar kinetics of reactivity in HCMV- or EBV-specific assays in the course of primary EBV infection, indicating that the two populations of antibodies were highly overlapping. The results demonstrate that primary EBV infection leads to the induction of IgM antibodies that specifically bind to widely used diagnostic antigens of HCMV. This has to be considered in the interpretation of HCMV-specific IgM assays.
Human cytomegalovirus (HCMV), a betaherpesvirus, has been widely recognized as a major health care problem in immunosuppressed individuals, such as AIDS patients or transplant recipients (21, 33, 53). It is also the most frequent cause of congenital disease in the western hemisphere (5, 13). In contrast, infection in immunocompetent adults may remain asymptomatic. Occasionally, however, patients with primary HCMV infection will present with lymphocytosis, fever, lymphadenopathy, and other symptoms resembling those of infectious mononucleosis (IM) caused by Epstein-Barr Virus (EBV) (6, 38). In these cases, differentiation between infections with either virus cannot be established on the basis of clinical signs alone and laboratory testing is required.
Nucleic acid and antigen detection protocols have been established for HCMV infection and are widely used for monitoring immunosuppressed patients (4, 12, 14, 16, 44). In contrast, measurement of virus-specific immunoglobulin M (IgM) antibodies is performed mainly to detect acute HCMV infection in normal individuals and to screen women during pregnancy (6, 43). IgM-specific enzyme-linked immunosorbent assays (ELISAs) with cell culture-derived conventional HCMV antigens have been developed. However, some of these assays have proven unsatisfactory with respect to sensitivity and specificity (25). Therefore, considerable effort has focused on defining viral antigens to be used in recombinant HCMV IgM assays (17, 23, 24, 26, 31, 34, 46, 48, 49, 51). Of the over 200 HCMV proteins, only the structural proteins pp150 and pp65 and the nonstructural proteins pUL80a, pUL44 (p52), and pUL57 have been identified as being sufficient and necessary for sensitive and specific detection of antiviral IgM during acute infection (17, 23, 26, 31, 48, 49). However, one major concern about using these proteins as recombinant ELISA antigens was that they might react with IgM antibodies raised against other herpesviruses, thus rendering the results obtained by such assays equivocal.
In this respect, infection with EBV is of major concern. Very specific IgM reactivity with repetitive, glycine-alanine-rich elements (Gly-Ala repeats) contained in EBV nuclear antigen 1 (EBNA-1) has been observed with sera from patients with acute HCMV infection (36). These Gly-Ala repeats are also major antigenic determinants of EBNA-1 for the induction of IgM (40). The IgM antibodies against Gly-Ala repeats correlate well with the acute phase of IM, and assays based on peptides from these repeats have been suggested to be sensitive diagnostic antigens (42). The potential for reactivity of these antigens with sera from patients with acute HCMV infection has been acknowledged (36). However, no detailed analysis of a possible reactivity of sera from IM patients with particular antigens used for HCMV serodiagnostics has been reported.
An apparent feature of the primary structure of pUL44 and pUL57 is glycine-rich stretches of 8 to 13 amino acids (aa). Although these motifs are much shorter than the Gly-Ala repeats of EBNA-1 and consist mainly of glycines, they could potentially react with IgM antibodies induced by Gly-Ala repeats.
In this study we show that primary infection with EBV leads to the synthesis of IgM antibodies that react with antigenic fragments of pUL44 and pUL57 of HCMV. The glycine-rich domains of these proteins were identified as targets of EBV-induced IgM. These antibodies show the same kinetics of reactivity as IgM directed against Gly-Ala repeats. It is therefore suggested that during EBV infection, IgM antibodies are induced that react with the N-terminal half of EBNA-1 as well as with pUL44 and pUL57 of HCMV.
MATERIALS AND METHODS
Cloning and expression of recombinant proteins.
DNA fragments encoding EBV and HCMV antigens were expressed as glutathione S-transferase (GST) fusions by using the pGEX3x vector system (Pharmacia, Freiburg, Germany).
Clone UL57/3, containing aa 545 to 601 of pUL57 of HCMV strain Ad169 fused to GST (see Fig. 1 and 2) has been described previously (49).
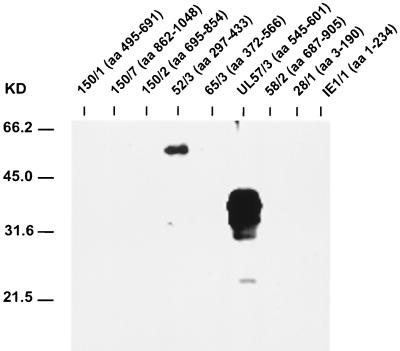
FIG. 1.
IgM-specific immunoblot analysis of different HCMV recombinant antigens with a selected serum sample from an HCMV-seronegative patient with acute EBV-induced IM (serum sample 8 [Table 1]). Amino acid numbering with respect to HCMV proteins in recombinant antigens is given in brackets. The positions of molecular mass markers are shown on the left.
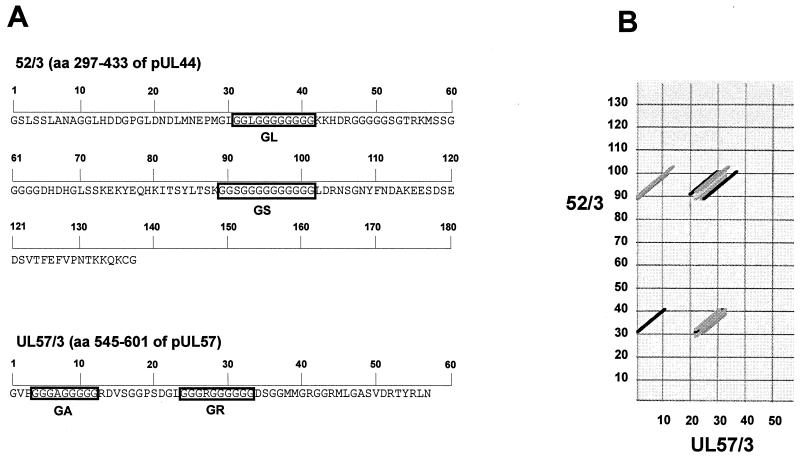
FIG. 2.
Amino acid sequence of antigenic fragments 52/3 and UL57/3 of pUL44 and pUL57, respectively, and sequence comparison by dot plot analysis. Amino acids with respect to pUL44 (p52) and pUL57 contained in recombinant antigens 52/3 and UL57/3 are given in brackets. Numbers indicate amino acid positions with respect to recombinant proteins. (A) Amino acid sequence of the carboxy-terminal part of p52 (pUL44) and the internal portion of pUL57 in HCMV recombinant antigens. Glycine-rich motifs GL, GS, GA, and GR are depicted by boxes. (B) Dot-plot comparison of 52/3 and UL57/3 using the program DNASTAR Lasergene (similarity, 70%; window, 10). Shading of graphs indicates the level of homology.
The EBNA-1 Gly-Ala construct was generated as a GST fusion protein by cloning the DNA fragment of the Gly-Ala region of the EBV EBNA-1 polypeptide (see Fig. 3). The DNA fragment comprised aa 90 to 109 of EBNA-1 with the sequence GAGAGAGGAGAGGAGAGGGA. To extend the Gly-Ala stretch, a second copy of the DNA fragment was inserted in frame by repeating the cloning step.
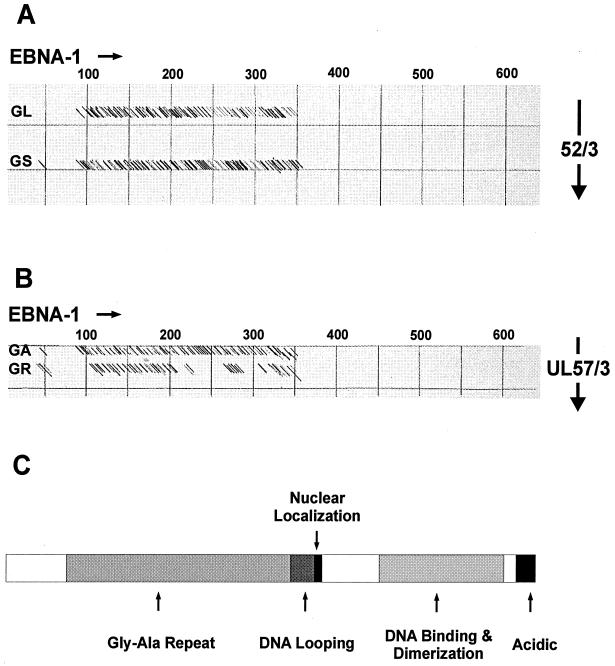
FIG. 3.
Dot plot comparison of EBNA-1 with recombinant antigens 52/3 (aa 297 to 433 of pUL44) and UL57/3 (aa 545 to 601 of pUL57) of HCMV, containing glycine-rich motifs GL and GS or GA and GR, respectively. (A and B) Dot plot comparisons of EBNA-1 with recombinant antigens 52/3 (A) and UL57/3 (B) (similarity, 65%; window, 11). Numbers indicate amino acids of EBNA-1. (C) Schematic representation of the EBNA-1 protein with the positions of the different functional domains indicated.
Clone UL57/3 mut (see Fig. 4) was generated by oligonucleotide ligation. In a first step, the oligonucleotide pair gatcctgGGTGTTCCGGGTCGTGACGTTTCTGGTGGTCCGTCTGACGGTCTGGGTctcgagg and aattcctcgagACCCAG ACCGTCAGACGGACCACCAGAAACGTCACGACCCGGAACACCcag was annealed at 55°C and the resulting double-stranded DNA fragment was inserted into the EcoRI- and BamHI-cleaved pGEX3x vector. In a second step, oligonucleotides tcgagGACTCTGGTGGTATGATGGGTCGTGGTGGT CGTATGCTGGGTGCTTCTGTTGACCGTACCTACCGTCTGAACgagatc tagg and aattccctagatctcGTTCAGACGGTAGGTACGGTCAACAGAAGCACCCAGCATACGACCACCACGACCCATCATACCACCAGAGTCc were annealed and the resulting fragment was inserted by using an internal XhoI restriction site and EcoRI. The clone GST-UL57/3 mut expressed a GST fusion protein that shared sequence with UL57/3 (aa 545 to 601) exept for the internal glycine repeat sequence (see Fig. 4A). The expression constructs 150/1, 150/2, 150/7, 52/3, 65/3, 58/2, 28/1, and IE1 have been described previously (47, 51).
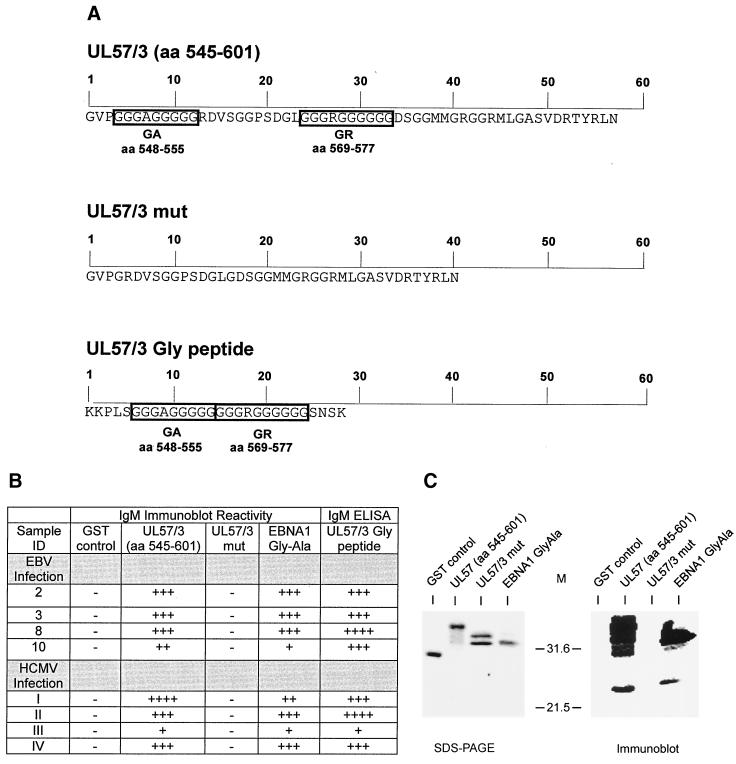
FIG. 4.
Deletion analysis of glycine-rich motifs in UL57/3 (aa 545 to 601). (A) Amino acid sequences of recombinant proteins UL57/3 and UL57/3 mut and the peptide UL57/3-Gly. The glycine-rich motifs are indicated by boxes. Numbers at the top of the sequences indicate amino acids contained in the recombinant proteins and in the peptide. (B) Immunoblot and ELISA reactivity of sera from patients with acute EBV infection (serum samples 2, 3, 8, and 10 correspond to the serum samples in Table 2) or from patients with acute HCMV infection (I to IV). Reactivity in immunoblots and ELISAs is depicted by − (negative), + (moderately reactive), ++ (reactive), +++ (highly reactive), and ++++ (very highly reactive). (C) Coomassie brillant blue-stained polyacrylamide gel and immunoblot of recombinant proteins. Recombinant antigens were subjected to analysis in about equal amounts, as verified by the Coomassie stain. The blot was probed with serum sample II (HCMV infection), providing an example of reactivity. The antigens used are indicated above the gel and the blot, respectively. Positions of molecular weight markers (M) are shown between the gel and blot. SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
The EBNA-1-Gly-Ala, UL57/3 (aa 545 to 601), and GST-UL57 mut recombinant proteins were purified nearly to homogeneity as described previously (20) and were used for ELISA with GST as a control. For immunoblot analyses, GST fusion proteins and GST as negative control were obtained as crude bacterial lysates.
Synthetic peptides.
Peptide UL57/3 Gly (KKPLSGGGAGGGGGGGGRGGGGGSNSK), comprising both glycine-rich amino acid stretches of UL57/3 (see Fig. 4A), was chemically synthesized by S. Modrow (University of Regensburg, Regensburg, Germany) and used in ELISA. Flanking amino acids were added to increase the solubility and binding capacity of the peptide for coating onto ELISA microwell plates.
Immunoblot analysis.
Immunoblot analysis was performed with crude bacterial lysates. Equal amounts of recombinant proteins, as deduced from Coomassie blue staining, were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15% polyacrylamide). After separation, the polypeptides were transferred onto Immobilon P membranes (Millipore, Bedford, Mass.) under semidry conditions. Membranes were blocked for 1 h with 1% bovine serum albumin (Sigma, Deisenhofen, Germany) and incubated overnight with serum samples, which were diluted 1:200 in 1× phosphate-buffered saline–0.1% bovine serum albumin. After the membranes were washed, specific antibody binding was visualized by incubation with horseradish peroxidase-conjugated polyclonal rabbit anti-human immunoglobulin (Dako, Glostrup, Denmark) using diaminobenzidine as a substrate.
ELISA analysis.
Purified UL57/3 (aa 545 to 601), UL57/3 mut, EBNA-1-Gly-Ala fusion proteins, and the peptide UL57/3 Gly (see Fig. 4A) were coated onto 96-well polystyrene microtiter plates at a concentration of 1.0 μg/ml (recombinant proteins) or 5.0 μg/ml (peptide), as specified previously (51). Serum samples were diluted 1:21 and were incubated for 60 min at 37°C. Bound IgM antibodies were detected by incubation for 30 min 37°C with a specific mouse monoclonal antibody (Janssen, Beerse, Belgium) conjugated with horseradish peroxidase. The ELISA was developed with a ready-to-use 3,3′,5,5′-tetramethylbenzidine reagent (Sigma, Deisenhofen, Germany) for 30 min at room temperature. The reaction was stopped with 1 N sulfuric acid, and absorbance was read at 450 nm, using a reference wavelength of 620 nm. Cutoff values were set for each antigen at 300 milli-optical densities (mOD). All other reagents were standard components from commercially available ELISA kits (Biotest, Dreieich, Germany). For the determination of routine parameters of HCMV and EBV serology, serum samples were tested with anti-HCMV IgM (Biotest RecELISA 1, Abbott Laboratories [Wiesbaden, Germany] RecELISA 2, and Diamedix [Miami, Fla.] ConvELISA) and IgG ELISA kits (Biotest) and with anti-EBV VCA-IgM, anti-EBV VCA-IgG, and the anti-EBV EBNA-IgG ELISA kits, respectively (Biotest). Heterophile antibodies were detected by a commercially available Paul Bunnell assay (Biokit, Barcelona, Spain).
Serum samples.
Serum samples, obtained from patients with IM or acute HCMV infection, were included in the analyses. Primary EBV infection was suspected when IgG and IgM antibodies against viral capsid antigen (VCA) were detectable in the serum samples in the absence of IgG antibodies against EBNA-1. A total of 11 serum samples, matching these criteria, were obtained from 11 otherwise healthy individuals with clinical signs of IM. In addition, seven and eight serial serum samples were obtained from two otherwise healthy individuals with IM. Serum samples I and II (see Fig. 4) were obtained from patients with detectable HCMV IgM. Serum sample III was obtained from a liver transplant recipient with acute HCMV infection. Serum sample IV was obtained in the course of a primary infection during pregnancy (HCMV IgG seroconversion). EBNA-1-specific IgG antibodies and IgG antibodies against VCA were detectable in serum samples I, II, and IV, in the absence of VCA-specific IgM, thus indicating past EBV infection. EBV serology could not be performed on serum sample III, since no serum was available for further testing.
RESULTS
EBV-induced IgM antibodies react in HCMV ELISAs.
IgM antibodies synthesized in the course of primary HCMV infection cross-react with EBV antigens (36). In a first set of experiments, we investigated whether, conversely, EBV induced IgM antibodies would react with HCMV antigens in standard diagnostic assays. A panel of 11 serum samples obtained from patients with clinical and serological evidence of acute IM was selected for these analyses. All sera contained IgM antibodies against EBV VCA but lacked IgG antibody reactivity against EBNA-1 (Table 1). Heterophile antibodies were detected in all serum samples tested. IgG antibodies against HCMV were found by ELISA in 4 of 11 patients, indicating past infection. The serum samples were analyzed by using three different, commercially available HCMV IgM ELISA kits. One of these assays was a sandwich IgM test designed with cell culture-derived conventional HCMV antigen preparations (HCMV-IgM ConvELISA). The two other assays involved recombinant fragments of immunoreactive HCMV proteins (31, 48). Of the 11 serum samples, 8 and 10 scored positive in the two recombinant-antigen assays. Surprisingly, 7 of 11 serum samples were also found to be reactive in the conventional test.
TABLE 1.
EBV- and HCMV-specific ELISA reactivity of serum samples from patients with primary EBV infection
| Serum IDa | Heterophile antibodies | Reactivityb in:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Anti-EBV VCA IgM ELISA | Anti-EBV VCA IgG ELISA | Anti-EBV EBNA IgG ELISA | HCMV-IgG ELISA | HCMV-IgM RecELISA 1 | HCMV-IgM RecELISA 2 | HCMV-IgM ConvELISA | ||
| 1 | Positive | R | R | NR | R | NR | R | R |
| 2 | Positive | R | R | NR | R | R | R | R |
| 3 | Positive | R | R | NR | R | R | R | R |
| 4 | Positive | R | R | NR | R | R | R | R |
| 5 | Positive | R | R | NR | NR | R | R | R |
| 6 | Positive | R | R | NR | NR | NR | NR | NR |
| 7 | Positive | R | R | NR | NR | R | R | NR |
| 8 | Positive | R | R | NR | NR | R | R | R |
| 9 | Positive | R | R | NR | NR | R | R | NR |
| 10 | Positive | R | R | NR | NR | R | R | R |
| 11 | Positive | R | R | NR | NR | NR | R | NR |
All serum samples were obtained from nonimmunosuppressed individuals with clinical signs of acute IM.
All ELISAs were performed with commercially available test kits. Scores were determined according to the instructions of the manufacturers. R, reactive; NR, not reactive.
In seven of the serum samples, no IgG antibodies against HCMV could be found, indicating the absence of past or recent HCMV infection in these patients. Of these sera, five and six scored reactive in the two recombinant HCMV IgM assays. Three of these serum samples were also reactive in the conventional IgM test. These results indicated that in the course of primary EBV infection, IgM antibodies were synthesized that reacted with HCMV proteins used as antigens for the design of both conventional and recombinant IgM assays.
The nonstructural proteins pUL44 and pUL57 are major targets of EBV-induced IgM.
ELISA experiments had indicated that EBV-induced IgM antibodies reacted with HCMV antigens widely used for serodiagnostic assays. To further determine the nature of this reactivity, immunoblot analyses were carried out. The serum samples were tested against a panel of bacterially expressed HCMV proteins and protein fragments which comprised antigens considered to be important for HCMV IgM serodiagnostics (27, 31, 51) (Table 2).
TABLE 2.
IgM immunoblot reactivity between serum samples from patients with primary EBV infection and recombinant HCMV antigens
| Serum IDa | Reactivity of serum withb:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 150/1 (aa 495–691) | 150/7 (aa 862–1048) | 150/2 (aa 695–854) | 52/3 (aa 297–433) | 65/3 (aa 372–546) | UL57/3 (aa 545–601) | 58/2 (aa 687–905) | 28/1 (aa 3–190) | IE1/1 (aa 1–234) | |
| 1 | + | ++ | + | (+) | − | ++ | − | − | − |
| 2 | + | − | ++ | ++ | − | − | − | − | − |
| 3 | + | − | − | ++ | − | ++ | − | − | − |
| 4 | + | − | + | ++ | − | ++ | − | − | − |
| 5 | − | − | − | ++ | − | ++ | − | − | − |
| 6 | − | − | − | − | − | ++ | − | − | − |
| 7 | − | − | − | (+) | − | ++ | − | − | − |
| 8 | − | − | − | ++ | − | +++ | − | − | − |
| 9 | − | − | − | (+) | − | ++ | − | − | − |
| 10 | − | − | − | + | − | +++ | − | − | − |
| 11 | − | − | − | − | − | ++ | − | − | − |
All serum samples were obtained from nonimmunosuppressed individuals with clinical signs of acute IM.
(+), borderline; +, moderately reactive; ++, reactive; +++, highly reactive.
The sera from four patients with IM that contained HCMV-specific IgG reacted with recombinant antigen fragments from the nonstructural proteins pUL44 (52/3) or pUL57 (UL57/3) or both in IgM-specific immunoblots. In addition, each of these four sera contained detectable IgM antibodies binding to at least one recombinant fragment of the pp150/pUL32 protein of HCMV. In contrast, all seven sera from patients with primary EBV infections without an indication of previous HCMV infection reacted with one or both of the antigens 52/3 and UL57/3 but not with any of the other HCMV antigens tested. The example of an immunoblot probed with serum sample 8 (sample numbers from Table 1) is shown in Fig. 1. In all cases where both p52/3 and UL57/3 were detected, the latter antigen appeared to react more intensively. Two of seven serum samples reacted with UL57/3 only, whereas one sample was reactive exclusively with p52/3. These results indicated that in the absence of past HCMV infection, EBV primary infection leads to the synthesis of IgM antibodies that show focused reactivity against p52/3 and UL57/3 of HCMV.
Glycine-rich motifs in pUL44 and pUL57 are targets of EBV-induced IgM.
Primary HCMV infection is known to induce IgM antibodies that react with long glycine-alanine (Gly-Ala) repeats found within the N-terminal half of the EBV EBNA-1 protein (19, 36). These repetitive sequences of EBNA-1, which can contain up to 200 residues, are also a major target of IgM antibodies during primary EBV infection (40, 41). Although no large Gly-Ala repeats were contained within the primary structure of p52/3 or UL57/3 of HCMV, short stretches of 9 to 13 aa, consisting mainly of glycine residues, were found on inspection of the primary structures of both antigen fragments (Fig. 2A). A dot plot comparison of the primary structures of these two polypeptides displayed homology only within the glyine-rich motifs (Fig. 2B). A similar dot plot analysis comparing 52/3 or UL57/3 with EBNA-1 revealed significant homology between the Gly-Ala repeat of EBNA-1 and the glycine-rich motifs of the HCMV polypeptides (Fig. 3). We therefore hypothesized that Gly-Ala-specific IgM antibodies, which are synthesized during primary EBV infection, react with glycine-rich motifs in diagnostic antigens from HCMV, thereby resulting in false-positive reactivity in HCMV IgM tests. To prove this experimentally, the glycine-rich motifs were deleted from UL57/3. UL57/3 was chosen for further analysis because of its strong reactivity in IgM blots compared to 52/3 (Fig. 1 and Table 2). A mutant of UL57/3 was constructed and expressed in bacteria that lacked the glycine-rich motifs (Fig. 4A). In addition, a recombinant GST fusion protein containing the Gly-Ala repeat region from EBNA-1 was cloned and analyzed for reactivity in parallel. The antigens were subjected to IgM-specific immunoblot analysis with four of the sera from EBV primary infections. As expected, all four serum samples showed distict reactivity with the polypeptide containing the Gly-Ala repeat (Fig. 4B). The UL57/3 protein was also readily detected by these sera, and the reaction was comparable to that with EBNA-1 Gly-Ala. However, the UL57-mut polypeptide was no longer a target of EBV-induced as well as HCMV-induced IgM. An example of an immunoblot is shown in Fig. 4C. To analyze whether reactivity could be restored by the glycine-rich motifs, a peptide of 28 aa which contained both glycine-rich motifs (GA and GR) from UL57/3 in the central part (UL57/3 Gly) was synthesized and used for ELISA. All four sera from patients with EBV primary infection scored positive in this assay (Fig. 4B). These results demonstrated that during EBV infection, IgM antibodies are induced that specifically react with glycine-rich domains contained in HCMV diagnostic antigens.
CMV-specific IgM antibodies react with glycine-rich motifs.
Both pUL44 (p52) and pUL57 are pronounced target antigens of IgM antibodies synthesized during acute HCMV infection (17, 23, 26, 30, 31, 48, 49). To analyze whether glycine-rich domains contained in these viral proteins were also the targets of IgM antibodies induced during acute HCMV infection, immunoblot analysis was performed. Four sera from patients with acute HCMV infection were tested against UL57/3 and UL57/3 mut (Fig. 4B). As expected, all the sera reacted with UL57/3. These sera also reacted with the EBNA-1 Gly-Ala protein from EBV, as described previously by others for sera from patients with acute HCMV infections (36). However, no reaction with UL57/3 mut was detected, indicating that the major epitopes of UL57/3 are confined to the glycine-rich motifs. This was further substantiated by analyzing the sera in the UL57/3 Gly peptide ELISA, where all serum samples scored moderately to highly positive (Fig. 4B). Thus, the glycine-rich domains are the major epitopes in UL57/3 reactive with IgM induced after both EBV and HCMV infections.
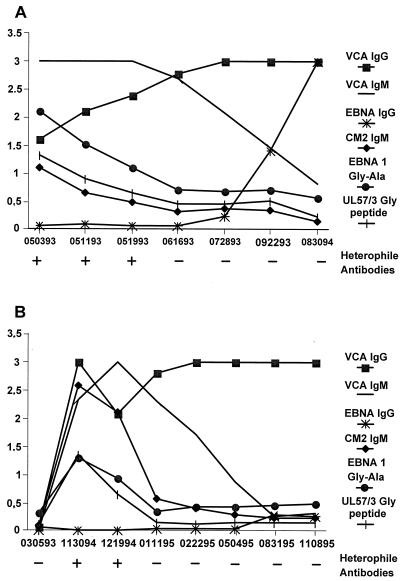
EBV-induced IgM shows parallel kinetics of reactivity against EBV and HCMV antigens.
IgM antibodies directed against epitopes in the Gly-Ala repeat region of EBNA-1 are detectable during the acute phase of IM and titers against this antigen decline thereafter (36). To investigate whether IgM against the glycine-rich regions from HCMV p52 and pUL57 show similar kinetics, sequential sera from two patients with EBV-induced IM were analyzed. The patients were adolescent males with symptoms characteristic of IM. The sera from the first patient contained detectable IgM and IgG antibodies against VCA in the absence of IgG antibodies against EBNA-1 in early serum samples (Fig. 5A). At that time, he presented with symptoms of IM. As expected, IgM antibodies directed against the Gly-Ala repeat of EBNA-1 could be detected early in infection, and the levels of these antibodies declined in the course of convalescence. Using an ELISA with the CM2 antigen, which consists of two copies of 52/3 and one copy of UL57/3, IgM reactivity was observed with kinetics paralleling that of Gly-Ala-specific IgM. Strikingly, IgM against the UL57/3 Gly peptide, consisting only of the glycine repeats of UL57/3, showed almost identical kinetics to that of IgM directed against the Gly-Ala repeat protein, suggesting that overlapping populations of antibodies react with both antigens.
FIG. 5.
Kinetics of serological parameters in sequential serum samples from two individuals with EBV-induced IM as detected by ELISA. The recombinant antigens used for the ELISAs are shown on the right. Serum samples were obtained at the dates indicated below the figures. Optical density values are shown on the left. The detectability of heterophile antibodies in each serum sample is shown below each plot and is indicated by + or −.
A similar result was obtained in the analysis of serial serum samples from the second patient (Fig. 5B). No reactivity in any of the parameters tested was found in a serum sample drawn 7 months before the onset of symptoms. When the patient presented with symptoms characteristic of IM, IgM and IgG antibodies against VCA and heterophile antibodies, but no IgG antibodies against EBNA-1, were detectable in serum. Again, IgM antibodies against the Gly-Ala repeat of EBNA-1 could be detected in early serum samples, but the titers declined over time. The IgM against CM2 of HCMV showed parallel kinetics, although higher levels of antibodies appeared to be present in the early serum samples compared to the IgM levels against the Gly-Ala protein. Again, the kinetics of IgM against the UL57/3 Gly peptide showed remarkable similarity to the kinetics of IgM against the Gly-Ala recombinant protein. These results suggest that overlapping populations of IgM antibodies react with both the Gly-Ala repeats of EBNA-1 and the glycine-rich domains of p52 and UL57 or, alternatively, that different populations with identical kinetics are present in the sera of patients with IM.
DISCUSSION
Detection of virus-specific IgM by ELISA, microagglutination, or immunoblotting is a widely used strategy to define HCMV infection as the etiology of disease in immunocompetent patients (15, 17, 23, 26, 31, 32, 48, 49, 51). In addition, screening for HCMV-specific IgM against defined viral antigens has been suggested as a method of improving the detection of maternal infection during pregnancy (8, 28). A major issue in the development of reliable assays is the identification of viral proteins that react with antibodies induced by other herpesviruses. Since EBV and HCMV show considerable DNA and protein sequence homologies in conserved gene blocks, particular proteins or protein fragments have been excluded from serological assays (2, 7, 39, 51). Pursuing this strategy has allowed the development of recombinant tests for both viruses (15, 26, 31, 45, 48, 50, 51). However, even with these assays, reactivity of antibodies directed against conserved small epitopes in nonhomologous proteins of EBV and HCMV cannot be totally excluded. Accordingly, Rhodes et al. have reported that IgM antibodies recognizing epitopes within the Gly-Ala repeat of EBNA-1 are induced during acute infection with both EBV and HCMV (36).
We have observed frequent reactivity of sera obtained from patients with confirmed EBV IM in HCMV IgM ELISA, irrespective of the source of the antigen used for the design of the test. This is in accordance with results of other studies (9, 31). While this work was in progress, Deyi et al. reported false-positive IgM antibody testing for HCMV in patients with acute EBV infection (9). Such reactivity was found in samples from both HCMV IgG-seronegative and -seropositive individuals without evidence of acute HCMV infection. False-positive results were confined mainly to the one recombinant HCMV IgM assay tested. This test was also used in our study (HCMV-IgM RecELISA 2). However, reactivity was also found in some cases when a conventional IgM enzyme immunoassay was used (9).
Four of our patients with acute EBV infection were HCMV IgG seropositive. Concomitant HCMV and EBV primary infections cannot be excluded in these patients, although such coinfections would have to be considered rare. A recent study found no evidence for reactivation of latent HCMV by acute EBV infection, thus rendering it highly unlikely that the patients analyzed in our study encountered acute EBV and HCMV infection concomitantly (1). Polyclonal B-cell activation, frequently seen in EBV infection, could be a more likely explanation for the development of antibodies reacting with heterologous antigens (18, 22). In the seven patients without detectable HCMV IgG antibodies, however, specific stimulation of B- cells by EBV proteins was assumed to be the reason for such reactivity.
Consequently, a more detailed study was initiated to elucidate the target structures of EBV-induced IgM within HCMV antigens. In particular, we investigated whether IgM antibodies specific for the Gly-Ala repeat sequences of EBNA-1 were reactive with the proteins of HCMV widely used as antigens for IgM serodiagnostics. Neither an EBNA-1 homologous protein nor a polymeric structure comparable to the Gly-Ala repeat has been identified in HCMV (7). It was suggested that Gly-Ala-specific IgM reacted with a small glycine-rich motif contained within the UL112-113 family of proteins of HCMV, but no experimental data have been presented to support that (36). Inspection of the amino acid sequences of the HCMV proteins pp150, pp65, pUL80a, pUL44, and pUL57 revealed short glycine-rich motifs of 5 to 13 aa in the carboxy-terminal part of pUL44 and the central parts of pUL57. pUL44 and pUL57 are contained in antigen preparations widely used for the design of commercially available HCMV IgM ELISAs and agglutination assays, as well as for the design of immunoblots assays (8, 23, 31, 32, 48).
With sera from patients with EBV-induced IM which indicated no evidence of past HCMV infection but showed strong IgM reactivity with a recombinant Gly-Ala repeat protein, reactivity was found exclusively with fragments derived from pUL44 and pUL57 but not with the other HCMV antigens tested so far. This was suggestive of a highly focused IgM reactivity induced by EBV antigens. Deletion of the glycine-rich motifs from the recombinant pUL57 protein completely abrogated reactivity in IgM-specific blots with EBV-induced IgM, indicating that the major target epitopes were contained in these domains. This was proven by using the glycine domains from pUL57 as the antigen, which could fully restore reactivity, showing that EBV infection leads to the induction of a population of IgM antibodies with strong reactivity to glycine stretches.
The glycine-rich motifs had significant sequence homology. They were also found by computer-based analysis to be homologous to the Gly-Ala sequences of EBNA-1, although they consist mainly of glycine homopolymers with other amino acids interspersed. Despite these apparent structural deviations from the Gly-Ala repeat elements, the glycine-rich motifs apparently induced IgM antibodies during HCMV infection, and these antibodies could very specifically react with epitopes contained in the Gly-Ala repeat (36). We could confirm and extent the results of this study by showing that sera from patients with acute HCMV infection react with both the Gly-Ala repeat and the glycine-rich motifs from pUL57. In this setting, the glycine-rich motif appears to be the major target of the HCMV-specific IgM response since its deletion from the recombinant proteins abolished reactivity whereas use of the glycine peptide in ELISA restored that reactivity.
The kinetics of IgM reactivity against EBV and HCMV recombinant proteins during EBV-induced IM were strikingly similar. The levels of IgM antibodies against the Gly-Ala repeats paralleled the levels of IgM reactivity with the CM2 fusion antigen and the UL57 glycine peptide. This indicated that either a highly overlapping population of antibodies reacted with both HCMV and EBV antigens or two separate populations with highly concordant kinetics were induced during EBV infection. More detailed competition experiments using a set of synthetic peptides will be necessary to resolve this issue.
Some of the ELISAs for the detection of HCMV-specific IgM using cell culture-derived antigen have been compromised by their poor performance with respect to sensitivity and specificity and by the low concordance of results (25, 52). In addition, antigen preparations used for these assays are poorly defined and contain different amounts of proteins conserved between different herpesviruses. Therefore, the results of these assays may be difficult to interpret. Recombinant tests involving the dominant IgM target antigens of HCMV have been designed (26, 31, 32, 48, 49). pUL44 and pUL57 have been identified as major IgM antigens for such assays, and, consequently, most commercially available recombinant HCMV IgM assays contain fragments of one or both antigens. We have shown here that these two antigen components may react with IgM induced during EBV infection. This may result in false interpretation if EBV serological testing is not performed in parallel with HCMV diagnostics. Removal of pUL44 and pUL57 from antigen preparations of recombinant assays is very likely to result in significantly reduced sensitivity and, probably, in concomitantly reduced specificity, since other antigens may have to be added. This would be particularly unacceptable for prenatal screening of HCMV infection, where a high sensitivity of IgM detection is crucial. In addition, other HCMV proteins may contain glycine-rich domains that would potentially react (36).
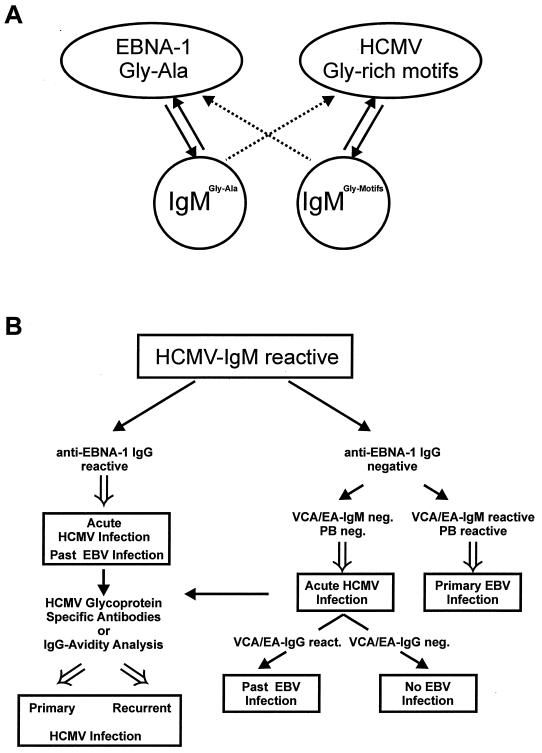
A possible algorithm to resolve this obvious dilemma is shown in Fig. 6. Due to the reactivity of IgM antibodies induced both by EBNA-1 and by the glycine-rich motifs of HCMV antigens (Fig. 6A), an HCMV IgM-reactive serum sample needs to be controlled (Fig. 6B). Analysis of such a serum sample for IgG antibodies specific for EBNA-1 provides a dichotomy for further testing. Detectability of EBNA-1 IgG indicates past EBV infection and argues for acute HCMV infection. Recent developments to identify primary HCMV infection by measuring glycoprotein-specific antibodies or IgG avidity may help futher substantiate the diagnosis of acute HCMV infection (10, 11, 29; M. Rothe, D. Lang, R. Vornhagen, W. Hinderer, H. H. Sonneborn, and B. Plachter, unpublished data). Lack of IgG reactivity against EBNA-1 in combination with IgM reactivity against VCA or early antigen suggests acute EBV infection. Absence of such EBV-specific IgM and negative Paul Bunnell analysis speaks against acute EBV infection, and, consequently, acute HCMV infection needs to be verified in this setting (Fig. 6B). An algorithm as suggested here can help avoid false results for patients suspected of having acute EBV or HCMV infection. Additional testing may be necessary, depending on each individual patient, to discriminate between the two pathogens.
FIG. 6.
Schematic representation of the reactivities of IgM antibodies induced against Gly-Ala repeats of EBNA-1 and against glycine-rich motifs of HCMV antigens, and algorithm for the processing of HCMV IgM-reactive serum samples. (A) EBNA-1 Gly-Ala repeats and HCMV glycine-rich motifs both induce IgM during acute infection with EBV and HCMV. Both populations of IgM antibodies show highly specific reactivity with the antigens from both viruses. (B) HCMV IgM-reactive serum samples should be tested for anti-EBNA-1 IgG antibodies. Detectability of such antibodies indicates past EBV infection and suggests acute HCMV infection, represented by the positive HCMV IgM ELISA (left). Confirmation and differentiation between acute primary and recurrent HCMV infection can be achieved by testing for HCMV glycoprotein-specific antibodies of by antibody avidity testing. Lack of detectability of anti-EBNA-1 IgG requires further EBV IgM serodiagnosis to distinguish between acute EBV and acute HCMV infection. PB, Paul Bunnell test.
Gly-Ala-reactive IgM antibodies induced during EBV infection and HCMV infection cross-react with autoantigens (35–37). The biological role of such antibodies in antiviral defense against EBV and HCMV infections remains elusive. The presence of antibodies capable of reacting with both viruses would provide a means for broader protection. It remains to be determined whether the primary repertoire of antibodies which arise early during EBV infection are then selected to enter the pool of memory cells and can be activated by HCMV infection (3).
In summary, we have shown here that EBV infection induces IgM antibodies that react specifically with short glycine-rich sequences in HCMV proteins. Since such glycine-rich sequences may occur in antigens from other viruses as well, further analysis will have to be performed to evaluate the potential of EBV-induced IgM to react with other pathogens, thereby compromising the serodiagnosis.
ACKNOWLEDGMENTS
We thank Christine Rhode, Bernd Deißler, Petra Volland, and Marianne Nashir-Heyer for excellent technical assistance.
This work was supported by Deutsches Bundesministerium für Forschung und Technologie, Verbund Komplikationen der Organtransplantation durch Herpesviren.
REFERENCES
- 1.Aalto S M, Linnavuori K, Peltola H, Vuori E, Weissbrich B, Schubert J, Hedman L, Hedman K. Immunoreactivation of Epstein-Barr virus due to cytomegalovirus primary infection. J Med Virol. 1998;56:186–191. [PubMed] [Google Scholar]
- 2.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, et al. DNA sequence and expression of the B95–8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 3.Berek C, Ziegner M. The maturation of the immune response. Immunol Today. 1993;14:400–404. doi: 10.1016/0167-5699(93)90143-9. [DOI] [PubMed] [Google Scholar]
- 4.Boeckh M, Boivin G. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin Microbiol Rev. 1998;11:533–554. doi: 10.1128/cmr.11.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boppana S B, Fowler K B, Vaid Y, Hedlund G, Stagno S, Britt W J, Pass R F. Neuroradiographic findings in the newborn period and long-term outcome in children with symptomatic congenital cytomegalovirus infection. Pediatrics. 1997;99:409–414. doi: 10.1542/peds.99.3.409. [DOI] [PubMed] [Google Scholar]
- 6.Britt W J, Alford C A. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2493–2524. [Google Scholar]
- 7.Chee M S, Bankler A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchison C A, Kouzarides T, Martignetti J A, Preddie E, Satchwell S C, Tomlinson P, Weston K M, Barrell B G. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 8.Daiminger A, Bader U, Eggers M, Lazzarotto T, Enders G. Evaluation of two novel enzyme immunoassays using recombinant antigens to detect cytomegalovirus-specific immunoglobulin M in sera from pregnant women. J Clin Virol. 1999;13:161–171. doi: 10.1016/s1386-6532(99)00028-1. [DOI] [PubMed] [Google Scholar]
- 9.Deyi Y M, Goubau P, Bodeus M. False-positive IgM antibody tests for cytomegalovirus in patients with acute Epstein-Barr virus infection. Eur J Clin Microbiol Infect Dis. 2000;19:557–560. doi: 10.1007/s100960000317. [DOI] [PubMed] [Google Scholar]
- 10.Eggers M, Bader U, Enders G. Combination of microneutralization and avidity assays: improved diagnosis of recent primary human cytomegalovirus infection in single serum sample of second trimester pregnancy. J Med Virol. 2000;60:324–330. doi: 10.1002/(sici)1096-9071(200003)60:3<324::aid-jmv11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Eggers M, Metzger C, Enders G. Differentiation between acute primary and recurrent human cytomegalovirus infection in pregnancy, using a microneutralization assay. J Med Virol. 1998;56:351–358. [PubMed] [Google Scholar]
- 12.Einsele H, Ehninger G, Steidle M, Vallbracht A, Müller M, Schmidt H, Saal J G, Waller H D, Müller C A. Polymerase chain reaction to evaluate antiviral therapy for cytomegalovirus disease. Lancet. 1991;338:1170–1172. doi: 10.1016/0140-6736(91)92032-w. [DOI] [PubMed] [Google Scholar]
- 13.Fowler K B, McCollister F P, Dahle A J, Boppana S, Britt W J, Pass R F. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130:624–630. doi: 10.1016/s0022-3476(97)70248-8. [DOI] [PubMed] [Google Scholar]
- 14.Gerna G, Baldanti F, Middeldorp J M, Furione M, Zavattoni M, Lilleri D, Revello M G. Clinical significance of expression of human cytomegalovirus pp67 late transcript in heart, lung, and bone marrow transplant recipients as determined by nucleic acid sequence-based amplification. J Clin Microbiol. 1999;37:902–911. doi: 10.1128/jcm.37.4.902-911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorgievski-Hrisoho M, Hinderer W, Nebel-Schickel H, Horn J, Vornhagen R, Sonneborn H H, Wolf H, Siegl G. Serodiagnosis of infectious mononucleosis by using recombinant Epstein-Barr virus antigens and enzyme-linked immunosorbent assay technology. J Clin Microbiol. 1990;28:2305–2311. doi: 10.1128/jcm.28.10.2305-2311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grefte J M, van der Gun B T, Schmolke S, van der Giessen M, van Son W J, Plachter B, Jahn G, The T H. The lower matrix protein pp65 is the principal viral antigen present in peripheral blood leukocytes during an active cytomegalovirus infection. J Gen Virol. 1992;73:2923–2932. doi: 10.1099/0022-1317-73-11-2923. [DOI] [PubMed] [Google Scholar]
- 17.Greijer A E, van de Crommert J M, Stevens S J, Middeldorp J M. Molecular fine-specificity analysis of antibody responses to human cytomegalovirus and design of novel synthetic-peptide-based serodiagnostic assays. J Clin Microbiol. 1999;37:179–188. doi: 10.1128/jcm.37.1.179-188.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haukenes G, Viggen B, Boye B, Kalvenes M B, Flo R, Kalland K H. Viral antibodies in infectious mononucleosis. FEMS Immunol Med Microbiol. 1994;8:219–224. doi: 10.1111/j.1574-695X.1994.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 19.Hennessy K, Kieff E. One of two Epstein-Barr virus nuclear antigens contains a glycine-alanine copolymer domain. Proc Natl Acad Sci USA. 1983;80:5665–5669. doi: 10.1073/pnas.80.18.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinderer W, Plachter B, Vornhagen R. Identification of immunoreactive viral proteins. In: Sinclair J, editor. Cytomegal ovirus. Totowa, N.J: Humana Press Inc.; 2000. pp. 21–37. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson M A, Mills J. Serious cytomegalovirus disease in the acquired immunodeficiency syndrome (AIDS). Clinical findings, diagnosis, and treatment. Ann Intern Med. 1988;108:585–594. doi: 10.7326/0003-4819-108-4-585. [DOI] [PubMed] [Google Scholar]
- 22.Karner W, Bauer G. Activation of a varicella-zoster virus-specific IgA response during acute Epstein-Barr virus infection. J Med Virol. 1994;44:258–262. doi: 10.1002/jmv.1890440308. [DOI] [PubMed] [Google Scholar]
- 23.Landini M P, Lazzarotto T, Maine G T, Ripalti A, Flanders R. Recombinant mono- and polyantigens to detect cytomegalovirus-specific immunoglobulin M in human sera by enzyme immunoassay. J Clin Microbiol. 1995;33:2535–2542. doi: 10.1128/jcm.33.10.2535-2542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landini M P, Rossier E, Schmitz H. Antibodies to human cytomegalovirus structural polypeptides during primary infection. J Virol Methods. 1988;22:309–317. doi: 10.1016/0166-0934(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 25.Lazzarotto T, Dal-Monte P, Boccuni M C, Ripalti A, Landini M P. Lack of correlation between virus detection and serologic tests for diagnosis of active cytomegalovirus infection in patients with AIDS. J Clin Microbiol. 1992;30:1027–1029. doi: 10.1128/jcm.30.4.1027-1029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazzarotto T, Ripalti A, Bergamini G, Battista M C, Spezzacatena P, Campanini F, Pradelli P, Varani S, Gabrielli L, Maine G T, Landini M P. Development of a new cytomegalovirus (CMV) immunoglobulin M (IgM) immunoblot for detection of CMV-specific IgM. J Clin Microbiol. 1998;36:3337–3341. doi: 10.1128/jcm.36.11.3337-3341.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazzarotto T, Varani S, Gabrielli L, Spezzacatena P, Landini M P. New advances in the diagnosis of congenital cytomegalovirus infection. Intervirology. 1999;42:390–397. doi: 10.1159/000053976. [DOI] [PubMed] [Google Scholar]
- 28.Lazzarotto T, Varani S, Guerra B, Nicolosi A, Lanari M, Landini M P. Prenatal indicators of congenital cytomegalovirus infection. J Pediatr. 2000;137:90–95. doi: 10.1067/mpd.2000.107110. [DOI] [PubMed] [Google Scholar]
- 29.Lazzarotto T, Varani S, Spezzacatena P, Gabrielli L, Pradelli P, Guerra B, Landini M P. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Viral Immunol. 2000;13:137–141. doi: 10.1089/vim.2000.13.137. [DOI] [PubMed] [Google Scholar]
- 30.Maine G T, Lazzarotto T, Chovan L E, Flanders R, Landini M P. The DNA-binding protein pUL57 of human cytomegalovirus: comparison of specific immunoglobulin M (IgM) reactivity with IgM reactivity to other major target antigens. Clin Diagn Lab Immunol. 1996;3:358–360. doi: 10.1128/cdli.3.3.358-360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maine G T, Stricker R, Schuler M, Spesard J, Brojanac S, Iriarte B, Herwig K, Gramins T, Combs B, Wise J, Simmons H, Gram T, Lonze J, Ruzicki D, Byrne B, Clifton J D, Chovan L E, Wachta D, Holas C, Wang D, Wilson T, Tomazic-Allen S, Clements M A, Wright G L, Jr, Lazzarotto T, Ripalti A, Landini M P. Development and clinical evaluation of a recombinant-antigen-based cytomegalovirus immunoglobulin M automated immunoassay using the Abbott AxSYM analyzer. J Clin Microbiol. 2000;38:1476–1481. doi: 10.1128/jcm.38.4.1476-1481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nulens E, Bodeus M, Bonelli F, Soleti A, Goubau P. Reactivity to p52 and CM2 recombinant proteins in primary human cytomegalovirus infection with a microparticle agglutination assay. Clin Diagn Lab Immunol. 2000;7:536–539. doi: 10.1128/cdli.7.4.536-539.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel R, Paya C V. Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997;10:86–124. doi: 10.1128/cmr.10.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Re M C, Landini M P. IgM to human cytomegalovirus: comparison of two enzyme immunoassays and IgM reactivity to viral polypeptides detected by immunoblotting. J Clin Lab Anal. 1989;3:169–173. doi: 10.1002/jcla.1860030307. [DOI] [PubMed] [Google Scholar]
- 35.Rhodes G, Rumpold H, Kurki P, Patrick K M, Carson D A, Vaughan J H. Autoantibodies in infectious mononucleosis have specificity for the glycine-alanine repeating region of the Epstein-Barr virus nuclear antigen. J Exp Med. 1987;165:1026–1040. doi: 10.1084/jem.165.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhodes G, Smith R S, Rubin R E, Vaughan J, Horwitz C A. Identical IgM antibodies recognizing a glycine-alanine epitope are induced during acute infection with Epstein-Barr virus and cytomegalovirus. J Clin Lab Anal. 1990;4:456–464. doi: 10.1002/jcla.1860040613. [DOI] [PubMed] [Google Scholar]
- 37.Rhodes G H, Valbracht J R, Nguyen M D, Vaughan J H. The p542 gene encodes an autoantigen that cross-reacts with EBNA-1 of the Epstein Barr virus and which may be a heterogeneous nuclear ribonucleoprotein. J Autoimmun. 1997;10:447–454. doi: 10.1006/jaut.1997.9996. [DOI] [PubMed] [Google Scholar]
- 38.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 39.Rudolph S A, Kühn J E, Korn K, Braun R W, Jahn G. Prokaryotic expression of the major capsid protein of human cytomegalovirus and antigenic cross-reactions with herpes simplex virus type 1. J Gen Virol. 1990;71:2023–2031. doi: 10.1099/0022-1317-71-9-2023. [DOI] [PubMed] [Google Scholar]
- 40.Rumpold H, Rhodes G H, Bloch P L, Carson D A, Vaughan J H. The glycine-alanine repeating region is the major epitope of the Epstein-Barr nuclear antigen-1 (EBNA-1) J Immunol. 1987;138:593–599. [PubMed] [Google Scholar]
- 41.Smith R S, Rhodes G, Vaughan J H, Horwitz C A, Geltosky J E, Whalley A S. A synthetic peptide for detecting antibodies to Epstein-Barr virus nuclear antigen in sera from patients with infectious mononucleosis. J Infect Dis. 1986;154:885–889. doi: 10.1093/infdis/154.5.885. [DOI] [PubMed] [Google Scholar]
- 42.Smith R S, Rhodes G, Vaughan J H, Horwitz C A, Geltosky J E, Whalley A S. A synthetic peptide for detecting antibodies to Epstein-Barr virus nuclear antigen in sera from patients with infectious mononucleosis. J Infect Dis. 1986;154:885–889. doi: 10.1093/infdis/154.5.885. [DOI] [PubMed] [Google Scholar]
- 43.Stagno S, Tinker M K, Elrod C, Fuccillo D A, Cloud G, O'Beirne A J. Immunoglobulin M antibodies detected by enzyme-linked immunosorbent assay and radioimmunoassay in the diagnosis of cytomegalovirus infections in pregnant women and newborn infants. J Clin Microbiol. 1985;21:930–935. doi: 10.1128/jcm.21.6.930-935.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Bij W, van Son W J, van der Berg A P, Tegzess A M, Torensma R, The T H. Cytomegalovirus (CMV) antigenemia: rapid diagnosis and relationship with CMV-associated clinical syndromes in renal allograft recipients. Transplant Proc. 1989;21:2061–2064. [PubMed] [Google Scholar]
- 45.van Grunsven W M, Spaan W J, Middeldorp J M. Localization and diagnostic application of immunodominant domains of the BFRF3-encoded Epstein-Barr virus capsid protein. J Infect Dis. 1994;170:13–19. doi: 10.1093/infdis/170.1.13. [DOI] [PubMed] [Google Scholar]
- 46.Van Zanten J, Lazzarotto T, Campisi B, Vornhagen R, Jahn G, Landini M P, The T H. Comparative immunoblot analysis with ten different, partially overlapping recombinant fusion proteins derived from five different cytomegalovirus proteins. Microbiologica. 1995;18:223–228. [PubMed] [Google Scholar]
- 47.Vornhagen R, Hinderer W, Plachter B, Jahn G, The T H, Sonneborn H H. Construction of recombinant autologous fusion proteins which enables IgG-specific serodiagnosis of past HCMV-infection. Biotest Bull. 1995;5:221–227. [Google Scholar]
- 48.Vornhagen R, Hinderer W, Sonneborn H H, Bein G, Matter L, The T H, Enders G, Jahn G, Plachter B. IgM-specific serodiagnosis of acute HCMV-infection using recombinant autologous fusion proteins. J Virol Methods. 1996;60:73–80. doi: 10.1016/0166-0934(96)02047-2. [DOI] [PubMed] [Google Scholar]
- 49.Vornhagen R, Hinderer W, Sonneborn H H, Bein G, Matter L, The T H, Jahn G, Plachter B. The DNA-binding protein pUL57 of human cytomegalovirus is a major target antigen for the immunoglobulin M antibody response during active infection. J Clin Microbiol. 1995;33:1927–1930. doi: 10.1128/jcm.33.7.1927-1930.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vornhagen R, Hinderer W, Sonneborn H H, Bein G, Matter L, The T H, Jahn G, Plachter B. Immunoglobulin A-specific serodiagnosis of acute human cytomegalovirus infection by using recombinant viral antigens. J Clin Microbiol. 1996;34:1020–1023. doi: 10.1128/jcm.34.4.1020-1023.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vornhagen R, Plachter B, Hinderer W, The T H, Van Zanten J, Matter L, Schmidt C A, Sonneborn H H, Jahn G. Early serodiagnosis of acute human cytomegalovirus infection by enzyme-linked immunosorbent assay using recombinant antigens. J Clin Microbiol. 1994;32:981–986. doi: 10.1128/jcm.32.4.981-986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber B, Prosser F, Munkwitz A, Doerr H W. Serological diagnosis of cytomegalovirus infection: comparison of 8 enzyme immunoassays for detection of HCMV-specific IgM antibody. J Clin Virol. 1994;2:245–259. doi: 10.1016/0928-0197(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 53.Zaia J A. Cytomegalovirus infections. In: Thomas E D, Forman S J, Blume K G, editors. Hematopoetic cell transplantation. Oxford, United Kingdom: Blackwell Science, Ltd.; 1999. p. 560. [Google Scholar]