Abstract
The septum is a key structure at the core of the forebrain that integrates inputs and relays information to other brain areas to support cognition and behaviours such as feeding and locomotion. Underlying these functions is a rich diversity of neuronal types and an intricate complexity of wiring across and within the septal region. We currently have very little understanding of how septal neuronal diversity emerges during development. Using transgenic mice expressing Cre in different subsets of telencephalic precursors we explored the origins of the three main neuronal types of the septal complex: GABAergic, cholinergic and glutamatergic neurons. We find that septal neurons originate from distinct neuroepithelial domains of the developing septum and are born at different embryonic time points. An exception to this is the GABAergic medial septal Parvalbumin-expressing population which is generated outside the septum from surrounding germinal zones. We identify the transcription factor BSX as being expressed in the developing glutamatergic neuron population. Embryonic elimination of BSX in the septum results in a reduction of septal glutamatergic cell numbers and a consequent deficit in locomotion. Further refinement of septal neuron diversity is needed to understand the multiple roles of septal neurons and their contribution to distinct behaviours.
Subject terms: Cell fate and cell lineage, Developmental neurogenesis
Cre transgene-based fate mapping in mice reveals that most septal neurons originate from progenitor domains in the septum and identifies Bsx as an embryonic marker and essential regulator of glutamatergic neuron development and locomotion behaviour.
Introduction
The septal complex is an expanding structure in primate evolution that forms an integral part of the limbic system, connecting the telencephalon with the hypothalamus and brain stem1–3. Although largely considered a relay centre, its reciprocal connections with the hippocampus and rhythmic drive of hippocampal theta demonstrate a prominent role in cognitive processing and coordination across different nuclei. In addition, the progressive deterioration of septal and extra-septal cholinergic neurons of the basal forebrain in early Alzheimer’s disease patients and during normal aging further implicates this structure in cognition and memory4–6.
The septo-hippocampal projection system is the largest component of the medial septum (MS) and the ventral limb of the diagonal band (vDB) region. It consists of theta pacemaker GABAergic, and modulatory cholinergic ascending projections7–12, as well as a more recently-identified glutamatergic MS projection to the hippocampus13–15. Back-projections from the hippocampus targeting MS and lateral septal (LS) components also participate in theta rhythmicity16,17. In addition to hippocampal and cortical projections, which convey cognitive information, the LS receives affective information from the amygdala, hypothalamus and bed nucleus of the stria terminalis, acting as a nodal point for information relay to diencephalic, mesencephalic and rhomboencephalic regions3,18. The LS is almost entirely GABAergic but is characterised by expression of a wide range of markers, including calcium-binding proteins, peptides and hormones19.
In addition to cognitive processing, the septum participates in the organisation and execution of voluntary motor behaviours through a process of sensorimotor integration. MS lesions and inactivation of the MS both result in reduced locomotion and motor activity20,21. More recent studies further support the involvement of hippocampal theta in voluntary locomotion in rodents and humans22–24. In particular, MSvDB glutamatergic neurons projecting to the hippocampus control the initiation, speed and duration of locomotion, as well as the entrainment of hippocampal theta oscillations before locomotion onset24. The LS is thought to mediate the regularity of theta oscillations and locomotion and integrate locomotion and reinforcement behaviour through its descending feedback from the hippocampus and connections to the lateral hypothalamus and ventral tegmental area25.
We know little about how different septal neurons are specified and how they assemble into circuits. A few studies that addressed the development of this region provide evidence for a temporal and spatial bias in the generation of the various neuronal types populating the different septal nuclei26–30. The septal embryonic progenitor domain has been broadly subdivided into pallial and subpallial regions31,32 and other smaller subdomains according to the expression of molecular markers33. The contribution of these regions to different septal neurons and the importance of the molecular subdivisions of the septal neuroepithelium remain poorly characterised.
We used a series of transgenic mice expressing Cre recombinase in different forebrain progenitor regions to identify the origin and lineage of neuronal populations in the adult septum. We focused on nuclei of the MSvDB and subpopulations of LS neurons. We find that most septal neurons examined and, in particular, most LS neurons, are generated from different focal zones within the septal neuroepithelium. On the other hand, Parvalbumin (PV)-expressing neurons of the MSvDB originate from surrounding neuroepithelial zones. MSvDB glutamatergic neurons have a largely septal origin and can be identified by expression of unique genetic markers. Using mice expressing Cre under control of the homeobox-encoding gene Bsx, as well as Bsx conditional loss-of-function mice, we demonstrate that this transcription factor is required for the development of MSvDB glutamatergic neurons and their contribution to mouse locomotor activity.
Results
Neurons of the septum
At a gross anatomical level, the boundaries of the MSvDB and the LS can be identified by their different cytoarchitecture; the LS has a complex organisation of subnuclei while the MS is arranged in layers or lamellae of neurons around an axis of symmetry34–36. We examined the LS and MSvDB for expression of neurotransmitter markers as well as the three calcium-binding proteins Calbindin (CB), Calretinin (CR) and Parvalbumin (PV), all of which had been reported in the septum in previous studies (Fig. 1). We focused on three different Bregma levels that we refer to as Rostral (R), Intermediate (I) and Caudal (C) (Fig. 1f, see also Methods section). Quantification of absolute and average number of marker+ve cells per level is shown in Table 1. Where possible, we birth-dated these neurons in order to determine their temporal emergence during embryogenesis (Supplementary Fig. 1).
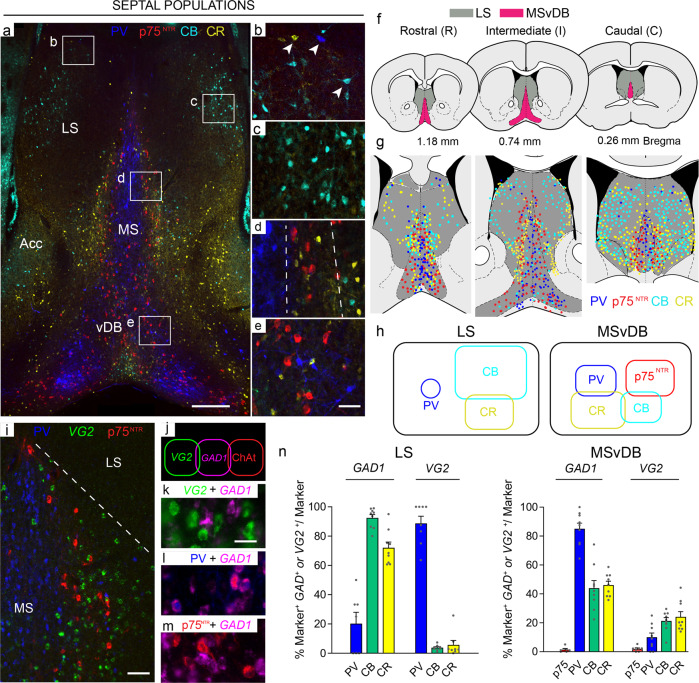
Fig. 1. Chemoarchitecture of the septal complex.
a Representative coronal sections through the adult mouse brain showing the organisation of septal nuclei. The four main neuronal subpopulations examined in this study are: PV (blue), p75NTR (red), CR (yellow) and CB (cyan). The picture was obtained by merging images from two consecutive sections. b–e Boxed areas in a are shown at higher magnification: b, c LS; d MS; e vDB. b Immunoreactivity for the three calcium-binding proteins in non-overlapping neuronal populations throughout the LS (arrowheads). c CB-expressing neurons in the LS show the typical arrangement of a nuclear structure. d The laminar organisation of the MS is shown, dashed lines indicate the borders of the layers. e p75NTR -, CB- and PV-immunoreactive neuronal populations are interspersed at the border of the MS and vDB. f Diagrams showing the three rostro-caudal levels analysed in this study. MSvDB and LS boundaries used in counts are shown (modified from ref. 71) and distribution of septal populations at rostral (R), intermediate (I) and caudal (C) levels. g Diagram showing relative abundance and overlap among the different populations of septal neurons at the three different levels examined in this study h Summary of the neuronal markers examined in this study and their overlap in the LS and the MSvDB. All overlaps indicated are <15%. i GABAergic (PV-expressing), glutamatergic and cholinergic neurons in the MSvDB. j MSvDB neurons expressing different neurotransmitters represent largely distinct populations. k–m Representative overlap between GABAergic, glutamatergic and cholinergic populations. n Quantification of co-localisation between GAD1 or VG2 cells and markers in the LS and MSvDB. n = 3 independent mice used for each marker. LS: GAD1 PV n = 7 sections; CB, CR n = 9 sections. VG2 PV, CB, CR n = 8 sections. MSvDB GAD1 p75 n = 6 sections; PV, CB, CR n = 9 sections. VG2 p75NTR n = 7 sections; PV, CB, CR n = 9 sections. Histograms show mean + SEM. Source data are provided in Supplementary Data 1. LS lateral septum, MS medial septum, Acc shell of the nucleus accumbens, vDB vertical limb of the diagonal band. Scale bars: a 200 μm, e, i 50 μm, k 10 μm.
Table 1.
Cells counted in the MSvDB and the LS.
| Marker | Level | Mouse lines | Animals | Total cells counted | Average number of cells | Std | SEM |
|---|---|---|---|---|---|---|---|
| MSvDB | |||||||
| p75NTR | R | 8 | 24 (n = 3 each line) | 1649 | 69 | 25.41 | 5.19 |
| I | 8 | 24 (n = 3 each line) | 3691 | 153.79 | 21.06 | 4.30 | |
| C | 8 | 24 (n = 3 each line) | 1446 | 60.25 | 10.91 | 2.23 | |
| CB | R | 8 | 24 (n=3 each line) | 1836 | 77 | 18.23 | 3.72 |
| I | 8 | 24 (n = 3 each line) | 2627 | 109 | 25.62 | 5.23 | |
| C | 8 | 24 (n = 3 each line) | 2500 | 105 | 19.41 | 3.96 | |
| PV | R | 8 | 24 (n = 3 each line) | 850 | 39 | 31.57 | 6.44 |
| I | 8 | 24 (n = 3 each line) | 2429 | 101 | 22.92 | 4.68 | |
| C | 8 | 24 (n = 3 each line) | 1350 | 56 | 16.03 | 3.27 | |
| CR | R | 8 | 24 (n = 3 each line) | 1859 | 81 | 23.21 | 4.74 |
| I | 8 | 24 (n = 3 each line) | 3729 | 155 | 24.62 | 5.03 | |
| C | 8 | 24 (n = 3 each line) | 2198 | 96 | 20.48 | 4.18 | |
| VG2 | R | 7 | 21 (n = 3 each line) | 2444 | 116 | 29.75 | 6.49 |
| I | 7 | 21 (n = 3 each line) | 3982 | 182 | 51.26 | 11.19 | |
| C | 7 | 21 (n = 3 each line) | 5121 | 254 | 79.25 | 17.29 | |
| LS | |||||||
| CB | R | 8 | 24 (n = 3 each line) | 1070 | 24 | 11.64 | 2.38 |
| I | 8 | 24 (n = 3 each line) | 4124 | 172 | 98 | 20.04 | |
| C | 8 | 24 (n = 3 each line) | 17,550 | 366 | 158 | 32.22 | |
| PV | R | 8 | 24 (n = 3 each line) | 239 | 6 | 5 | 1.03 |
| I | 8 | 24 (n = 3 each line) | 290 | 12 | 5 | 1.02 | |
| C | 8 | 24 (n = 3 each line) | 335 | 8 | 10 | 2.08 | |
| CR | R | 8 | 24 (n = 3 each line) | 1421 | 32 | 30 | 6.06 |
| I | 8 | 24 (n = 3 each line) | 2417 | 101 | 37.01 | 7.55 | |
| C | 8 | 24 (n = 3 each line) | 7242 | 161 | 182 | 37.18 | |
| VG2 | R | 7 | 21 (n = 3 each line) | 540 | 26 | 15 | 3.27 |
| I | 7 | 21 (n = 3 each line) | 695 | 35 | 23 | 5.05 | |
| C | 7 | 21 (n = 3 each line) | 991 | 52 | 30 | 6.46 | |
Cells expressing p75NTR, CB, PV, CR and VG2 were counted at three different rostro-caudal levels (R, I, C) of the MSvDB and the LS for lineage tracing purposes. The number of mouse lines as well as the total number of animals used for each marker are shown. The total number of marker+ve cells counted for each of these markers as well as the average number of cells counted per animal are also indicated for each level.
CB-, CR- and PV-expressing GABAergic neurons have previously been identified in the LS37,38. We detected CB- and CR-expressing cells at all levels of the LS and these formed largely non-overlapping populations (<5% of CB+ve cells are double-labelled for CR and <8% of CR+ve cells co-label with CB - tested using two different antibodies per marker (see Methods section; Fig. 1a–c, g, h). Small numbers of PV-expressing neurons are found scattered throughout the LS, and these do not overlap with either CB or CR (Fig. 1a, b, g, h). Birth-dating LS populations at 2-day intervals from E10.5 to E18.5 using EdU incorporation every other day and postnatal analysis at P30, showed a peak of PV neuron generation at E10.5, whereas all other populations examined peaked at later stages (Supplementary Fig. 1a, b).
The MS is continuous ventrally with the vDB and, for the purpose of this study, we analysed the entire MSvDB complex. GABAergic PV-expressing projection neurons constitute the core of the MS laminar structure, while cholinergic neurons - identified by expression of p75NTR - occupy a more lateral position (Fig. 1a, d, g, i). PV and p75NTR neurons form entirely distinct populations (Fig. 1g, h)34. CB and CR-immunoreactive neurons also constitute a large portion of this region, partially intermingling with cholinergic neurons, but also extending to a more lateral position, bordering with the lateral septum35 (Fig. 1a, d, g). A small overlap between CB and CR expression exists (<15% of CB are double-labelled for CR and <15% of CR co-label with CB) (Fig. 1a, d, e, h). <10% overlap exists between PV and CR and between CB and p75NTR. Birth-dating these neurons during embryogenesis showed early emergence of PV+ve and CR+ve populations and a later generation of p75NTR and CB+ve neurons (Supplementary Fig. 1a, c).
In addition to the GABAergic and cholinergic cells in the MSvDB and the LS, a population of neurons that uses glutamate as a neurotransmitter has been identified13–15,39. These express the vesicular glutamate transporter 2 (VG2)40. VG2+ve MSvDB neurons display a heterogeneous firing pattern. They provide local excitatory input to cholinergic and GABAergic septal neurons41, as well as long-range input to the hippocampus via the fornix. VG2+ve neurons are found mainly within the MSvDB, intermingled among cholinergic neurons (Fig. 1i). A small population of VG2+ve neurons is also scattered within the LS. GABAergic, cholinergic and glutamatergic neurons of the MSvDB are largely non-overlapping populations with only 1.4 ± 0.9% of the cholinergic and 1.6 ± 0.2% of the glutamatergic neurons co-labelling with GAD1 (Fig. 1i–m).
We examined the neurotransmitter phenotype of septal p75NTR, PV, CB and CR neurons by quantifying their co-expression of GAD1 (Gad67) and VG2, markers that detect inhibitory and excitatory neuronal phenotypes, respectively. In the LS, the majority of CB+ve and CR+ve cells co-express GAD1 (92.5 ± 2.3%, and 70.3 ± 4.7%, respectively), whereas nearly all LS PV+ve neurons express VG2 (94.2 ± 3.3%; Fig. 1n). In contrast, PV+ve neurons in the MSvDB are GABAergic (95.8 ± 4.2%), as are some CB+ve and CR cells+ve (43.9 % ± 5.2%, and 46.12 ± 2.6%, respectively). Small numbers of PV+ve (7.6 ± 3.3%), CB+ve (21.3 ± 2.3%) and CR+ve (26.17 ± 3.8) MSvDB neurons co-express VG2 (Fig. 1n). Only 1.1 ± 0.5% of the p75NTR immunolabelled neurons co-express VG2 and these constitute 0.6 ± 0.3% of the VG2 MSvDB population.
In summary, the septal region is populated by a large variety of neuronal subtypes, which show characteristic marker expression and distribution. The distribution of EdU-incorporating cells within the septum shows an outside-in generation of septal nuclei with respect to the ventricular zones and the telencephalic lumen: early-born cells are located medially within the septum whereas late-born cells occupy lateral positions close to the lumen. MSvDB PV neurons, which occupy the most medial position of the septum, constitute one of the first populations of neurons to be generated.
Septal and extra-septal neuroepithelial zones generate LS and MSvDB neurons
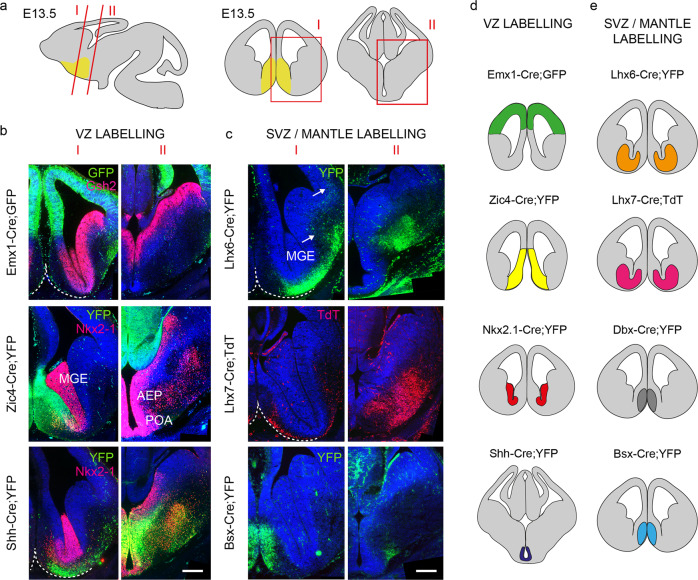
In order to identify the embryonic origin of septal neurons we made use of a series of Cre-expressing transgenic mouse lines crossed to suitable reporters to label septal and extra-septal neuroepithelial cells (Fig. 2). Emx1-Cre;GFP labels the pallial neuroepithelium42 (Fig. 2a, b). Zic4-Cre;YFP labels the entire septal neuroepithelium at all dorso-ventral and anterior-posterior levels and is mutually exclusive with the pallial Emx1-Cre43,44 (Fig. 2a, b). Nkx2.1-Cre;YFP labels a caudo-ventral domain of the septal neuroepithelium, as well as the medial ganglionic eminence (MGE) and the preoptic area (POA) and overlaps partly with Zic4-Cre42,44,45. Shh-Cre;YFP labels a subdivision of the POA and a small region of the ventral MGE46,47 (Fig. 2a, b). We also labelled embryonic neurons within the SVZ/mantle of the telencephalon using mice expressing Cre under control of Lhx645 (Fig. 2a, c), Dbx148, and two newly-generated mouse lines expressing Cre under control of Lhx7 and Bsx, respectively (Fig. 2a, c and Supplementary Fig. 2). Lhx7 is known to be expressed in forebrain cholinergic neurons, whereas the expression of Bsx in the septum is uncharacterised49. A summary of the Cre-expressing lines used in our study is shown in Fig. 2d, e).
Fig. 2. Transgenic mice used to fate-map the embryonic septum.
a Schematic representation of a sagittal E13.5 mouse brain section and the corresponding coronal cuts shown in I and II. Boxed areas in red are shown for rostral (I) and caudal (II) levels. The position of the septum is indicated in yellow. b YFP/GFP expression in forebrain germinal regions (ventricular zone, VZ) of three lines used for fate mapping. Cre recombination can be detected in the neocortex (Emx1-Cre;GFP), the septum (Zic4-Cre;YFP) and the AEP/POA (Shh-Cre;YFP). Immunolabelling for Gsh2 and Nkx-2-1 delineates the subpallium and the MGE/POA regions, respectively. Scale bar: 200 μm. c Transgenic lines with expression in the subventricular (SVZ) and mantle zones. In Lhx6-Cre;YFP, YFP labelling spans the MGE SVZ and mantle. YFP+ve neurons initiating their migration toward the cortex are indicated with white arrows. TdT expression is detected in presumptive immature cholinergic forebrain neurons in Lhx7-Cre;TdT. YFP immunoreactivity can be detected in the mantle zone of the septum in Bsx-Cre;YFP mice. Ventral boundaries are indicated by a dashed white line. AEP anterior entopeduncular region, MGE medial ganglionic eminence, POA preoptic area. Scale bar: 200 μm. d, e Summary of VZ- and SVZ/mantle-expressing Cre lines used in this study. The area of expression for each transgenic line is identified by a different colour. The same colour coding is used in subsequent fate-mapping Figs. 3–5.
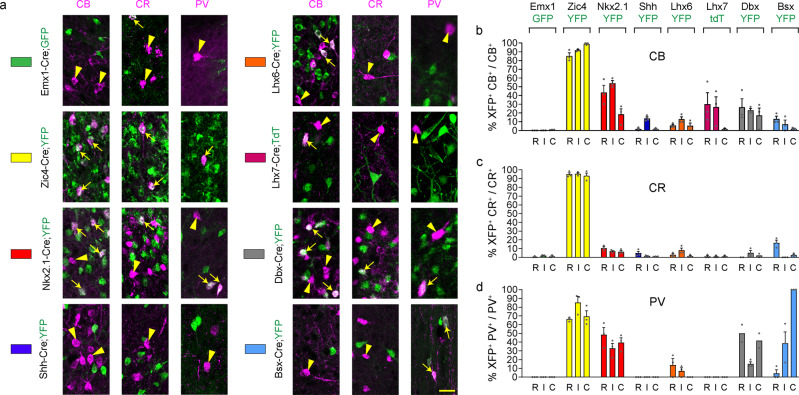
In order to fate-map the embryonic origin of septal neurons, we quantified the extent of co-localisation between the reporter gene (GFP, YFP or tdTomato) and the various septal neuronal markers in Cre-expressing mice at P30 at the three rostro-caudal levels shown in Fig. 1f. We found little or no contribution of the pallial neuroepithelium to LS and MSvDB neurons using Emx1-Cre;GFP mice (Figs. 3 and 4), with the exception of small numbers of LS CR neurons where <5% of the population expressed GFP (Fig. 3a, c). The vast majority of LS CR-expressing cells have a dorsal septal origin (as evidenced by reduced labelling in Nkx2-1Cre;YFP mice), while CB and PV LS populations have a mixed dorsal and ventral septal origin (Fig. 3a–d). Only small subsets of CB+ve, CR+ve and PV+ve neurons were labelled with Lhx6-Cre, whereas Dbx1-Cre and Bsx-Cre made a bigger contribution to these populations (Fig. 3a–d). Lhx7-Cre labelled subsets of CB+ve cells and was excluded from CR+ve and PV+ve populations. Given the absence of p75NTR+ve cholinergic neurons in the LS and the known role for LHX7 in the determination of cholinergic versus GABAergic cell fate50, these CB cells in the LS may represent non-cholinergic cells that may have expressed low levels of the Cre transgene but never became cholinergic. Overall, all LS neurons examined have a predominantly septal origin, with the majority of neurons being labelled with YFP in Zic4-Cre;YFP mice (Fig. 3a–d).
Fig. 3. Septal embryonic origins of lateral septal neurons.
a Contribution of different forebrain domains to LS populations. Double-labelling for CB, CR or PV and the fluorescent reporter protein GFP/YFP/TdT in the various transgenic lines at P30. White arrows and arrowheads indicate double and single labelled cells, respectively. b–d Histograms showing the percentage of neurons double labelled for GFP/YFP/TdT over the total population. Each transgenic mouse line is identified by a different colour as shown in a and as summarised in Fig. 2d, e. n = 3 brains per transgenic line per marker except the following where n = 2 for Dbx-Cre;YFP mice: CR R and C, PV R and C. Histograms show mean + SEM. Source data are provided in Supplementary Data 1. Scale bar: 25 μm.
Fig. 4. Septal and extra-septal embryonic origins of medial septal neurons.
a Contribution of different forebrain domains to MS populations. Double-labelling for p75NTR, CB, CR or PV and the fluorescent reporter protein GFP/YFP/TdT in the various transgenic lines at P30. White arrows and arrowheads indicate double and single labelled cells, respectively. b–e Histograms showing the percentage of neurons double labelled for GFP/YFP/TdT over the total population. Each transgenic line is identified by a different colour as shown in a and as summarised in Fig. 2d, e. n = 3 brains per transgenic line per marker except the following where n = 2 for Dbx-Cre;YFP mice: PV R, CR R and C. Histograms show Mean + SEM. Source data are provided in Supplementary Data 1. Scale bar: 25 μm.
p75NTR+ve cholinergic MSvDB neurons at all three rostro-caudal levels examined originate exclusively from Nkx2.1-expressing neuroepithelial cells in the caudo-ventral embryonic septum (Fig. 4a, b), as recently shown43. In contrast, PV-expressing cells of the MSvDB have a mixed origin that lies outside the septum, as evidenced by minimal labelling of this population in Zic4-Cre;YFP mice (Fig. 4a, c). Partial labelling of this population in Nkx2.1-Cre;YFP and Shh-Cre;YFP mice suggests that these cells may have a dual MGE/POA origin (Fig. 4c). Successful labelling of nearly all MSvDB PV neurons in Nestin-Cre;YFP mice - which should label all neuroepithelial forebrain regions early during development – indicates that partial labelling in other Cre mice is not caused by a failure of reporter expression (Supplementary Fig. 3a–c). CB+ve and CR+ve MSvDB neurons originate from dorsal and ventral neuroepithelial septal precursors, as they showed almost complete co-localisation with YFP in Zic4-Cre;YFP mice, and partial co-localisation with YFP in Nkx2-1-Cre;YFP mice (Fig. 4a, d, e).
Altogether, our data show that all neurons examined, with the exception of MSvDB PV+ve cells, originate from septal neuroepithelial precursors. LS CR+ve cells have an exclusive dorsal-septal origin and MSvDB cholinergic neurons have an exclusive ventral septal origin. All other neuronal populations examined are generated from both dorsal and ventral septal precursors. MSvDB PV+ve cells originate outside the septum from surrounding precursors. A summary of the contribution of the various germinal regions and precursors to the septal populations analysed in this study is shown in Table 2.
Table 2.
Fate-mapping summary.
| Emx1-Cre;GFP | Zic4-Cre:YFP | Nkx2.1-Cre;YFP | Shh-Cre;YFP | |||||
|---|---|---|---|---|---|---|---|---|
| LS (%) | MS (%) | LS (%) | MS (%) | LS (%) | MS (%) | LS (%) | MS (%) | |
| p75 | – | 0 | – | 94 | – | 94 | – | 11 |
| PV | 0 | 0 | 73 | 8 | 40 | 42 | 0 | 30 |
| CB | 0.3 | 0.1 | 92 | 79 | 38 | 34 | 6 | 7 |
| CR | 1.2 | 0.8 | 94 | 78 | 8 | 27 | 3 | 11 |
| Lhx6-Cre;YFP | Lhx7-Cre;YFP/tdT | Dbx-Cre;YFP | Bsx-Cre;YFP | |||||
|---|---|---|---|---|---|---|---|---|
| LS (%) | MS (%) | LS (%) | MS (%) | LS (%) | MS (%) | LS (%) | MS (%) | |
| p75 | – | 79 | – | 91 | – | 36 | – | 0 |
| PV | 5 | 43 | 6 | 4 | 35 | 14 | 48 | 2 |
| CB | 8 | 9 | 19 | 8 | 22 | 32 | 7 | 49 |
| CR | 4 | 14 | 1 | 3 | 2 | 25 | 6 | 27 |
Summary of the septal contribution of different forebrain domains to LS and MS neuronal populations identified by expression of p75NTR, PV, CB and CR. The different forebrain domains were labelled using the indicated Cre-expressing driver lines crossed to fluorescent reporters. Their spatial distribution is summarised in Fig. 2d, e.
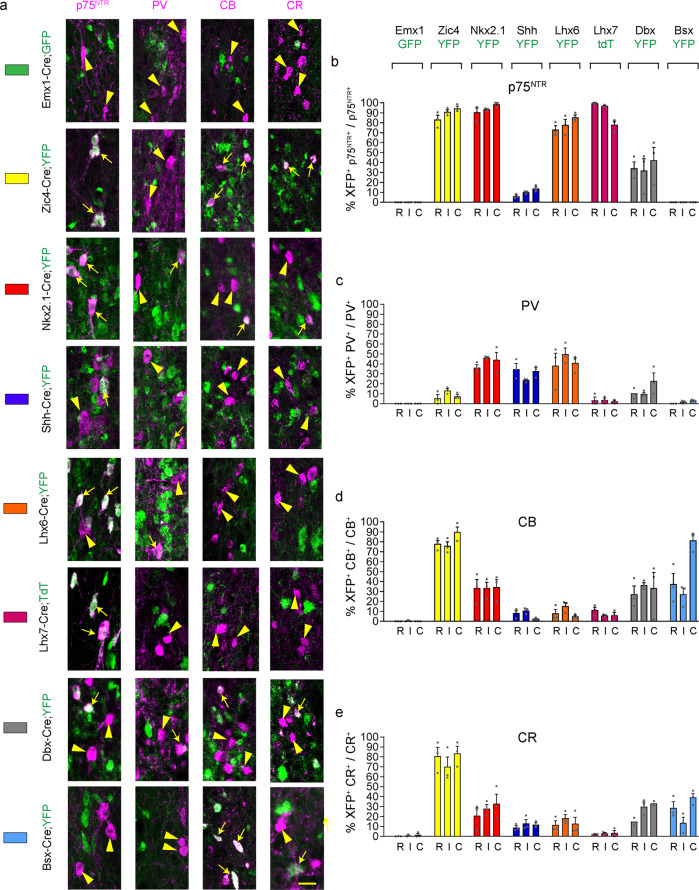
Labelling of septal neurons with mouse lines expressing Cre in the SVZ/mantle showed that p75NTR-immunoreactive cholinergic neurons in the MSvDB originate from neural precursors/immature neurons expressing Lhx6 and Lhx7 (Fig. 4a, b), as previously shown for other forebrain cholinergic neurons50. Comparable numbers of PV+ve neurons in the MSvDB co-expressed YFP in Lhx6-Cre;YFP and Nkx2.1-Cre;YFP mice (Fig. 4a, c), consistent with the notion that LHX6 is activated downstream of NKX2.151. Variable proportions of p75NTR+ve, PV+ve, CB+ve and CR+ve MSvDB cells were labelled with YFP in Dbx1-Cre mice, suggesting heterogeneity within these populations (Fig. 4a–e). There was no co-labelling between PV or p75NTR with YFP in Bsx-Cre;YFP mice, indicating that Bsx-Cre labels a distinct population of cells within the septum (Fig. 4b, c).
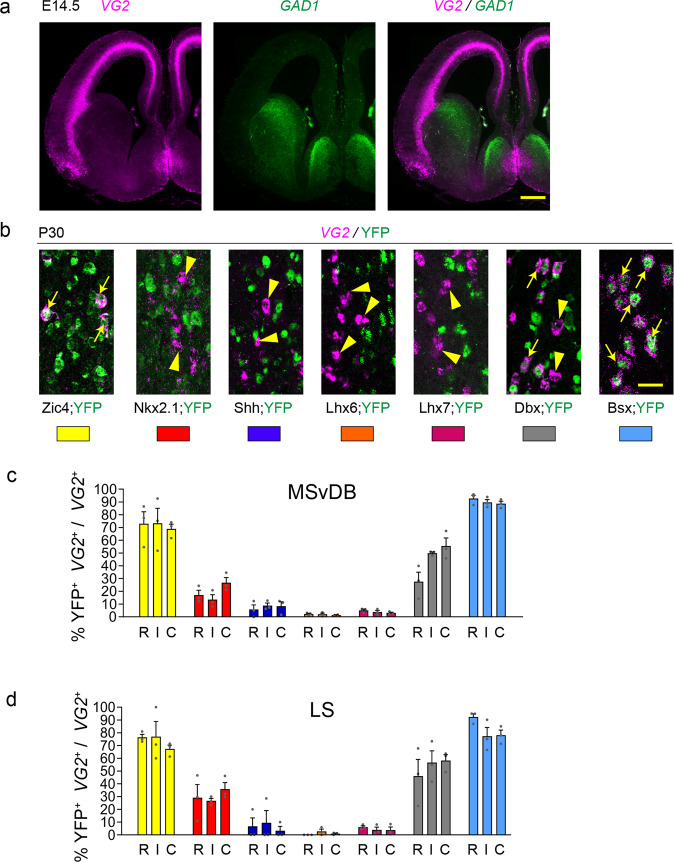
Septal origins and unique identifiers of septal glutamatergic neurons
VG2 transcripts can be detected in the developing medial septum at early developmental stages in a mutually exclusive pattern to GAD1 (Fig. 5a). The location and pattern of VG2 expression is reminiscent of Bsx in the embryonic septum (Fig. 2c, e). We therefore used our Bsx-Cre;YFP mice and the rest of the panel of Cre mice, to examine the origin of septal glutamatergic neurons. In the MSvDB nearly all VG2-expressing neurons were labelled with YFP in Bsx-Cre;YFP mice at the three Bregma levels examined (R, 92.6 ± 2.5%; I, 89.6 ± 2.3%; C, 88.7 ± 1.9%; Fig. 5b, c). The vast majority of these were also labelled in Zic4-Cre;YFP mice (R, 72.9 ± 9.4%; I, 73.2 ± 11.8%; C, 68.9 ± 3.7%; Fig. 5b, c). Dbx1, a transcription factor-encoding gene known for its essential role in the generation of transient forebrain glutamatergic Cajal-Retzius cells, shows a history of expression within subsets of septal glutamatergic neurons, suggesting a heterogeneity within the population and a possible role for this gene in their development (Fig. 5b, c). Small numbers of scattered glutamatergic neurons are also found in the lateral septum and these show similar origins as those in the MSvDB (Figs. 1n and 5d). In conclusion, our data show that septal VG2 neurons originate largely from septal progenitors. Nearly all septal glutamatergic neurons are generated from precursors/immature neurons expressing Bsx, identifying Bsx as an early embryonic marker for these cells.
Fig. 5. Septal origins of septal glutamatergic neurons with unique developmental codes.
a VG2 and GAD1 expression in the forebrain of a WT E14.5 mouse. b Contribution of different forebrain domains to VG2 MS populations. Double-labelling for VG2 and YFP in the various transgenic lines at P30. White arrows and arrowheads indicate double and single labelled cells, respectively. c, d Histograms showing the percentage of VG2 neurons double labelled for YFP over the total VG2 population in the MSvDB (c) and LS (d). Each transgenic line in c, d is identified by a different colour as shown in b and as summarised in Fig. 2d, e. n = 3 brains per transgenic line per marker. Histograms show mean + SEM. Source data are provided in Supplementary Data 1. Scale bars: a 200 μm; b 25 μm.
A role for Bsx in septal glutamatergic neurons and locomotor behaviour
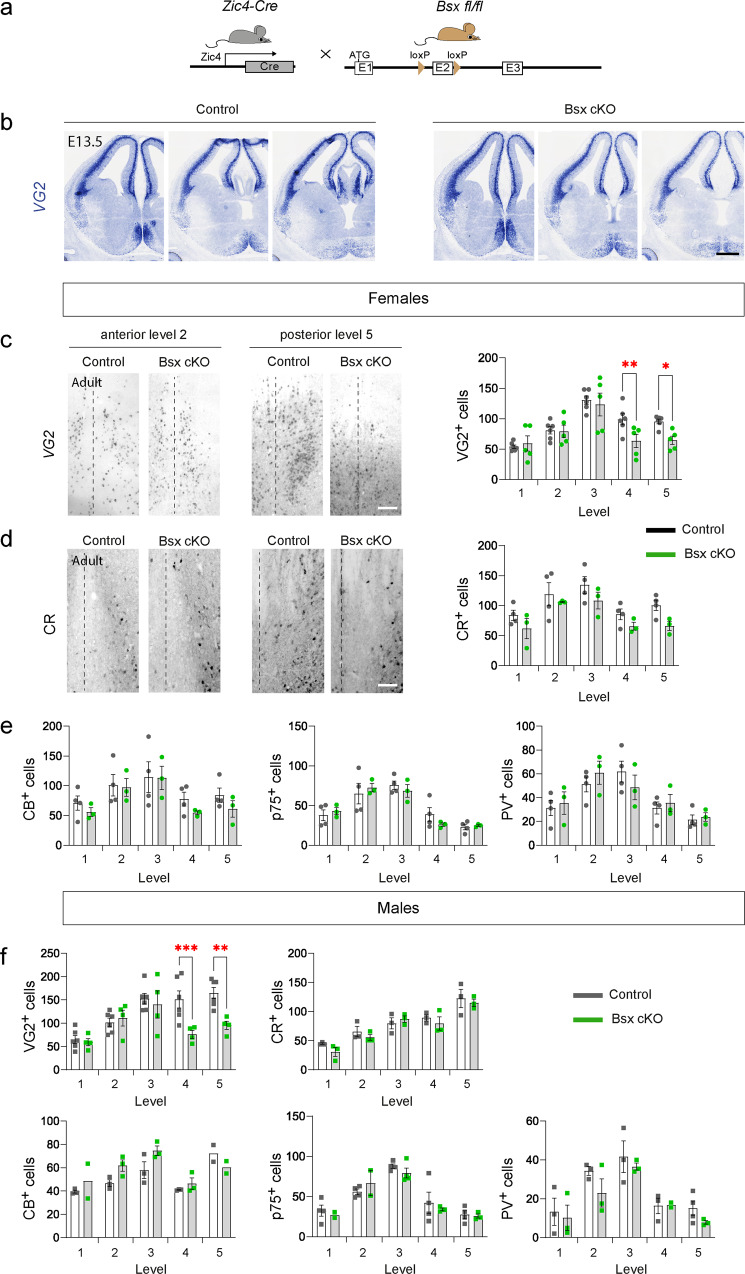
BSX is an evolutionarily conserved homeobox-encoding gene expressed in the septum, epiphysis, mammillary bodies and arcuate nucleus49. In the absence of BSX, in Bsx germline loss-of-function mice, hypothalamic neurons display abnormal maturation and reduction of NPY52. To assess the requirement for BSX in septal neurons without affecting the hypothalamus, we generated mice carrying a conditional Bsx allele and crossed these to Zic4-Cre to obtain Zic4-Cre;Bsxfl/fl mice (referred to as septal Bsx cKO; Fig. 6a and Supplementary Fig. 4). Within the septum, we could not directly identify the cells undergoing recombination of the Bsx allele due to lack of antibodies that detect the BSX protein. However, given that ~100% of VG2+ve MSvDB neurons are labelled in Bsx-Cre mice and ~70% of the VG2+ve population is labelled in Zic4-Cre mice (Fig. 5c), we can infer that the majority of BSX-expressing VG2+ve cells in this region will undergo recombination in septal BsxcKO mice. Outside the septum, we detected an overlap between YFP and Bsx in the epiphysis in Zic4-Cre;YFP embryos but virtually no overlap in the developing hypothalamus (Supplementary Fig. 5a). The absence of overlap between Zic4-Cre and Bsx in the hypothalamus was also confirmed by normal expression of Npy in the arcuate nucleus of adult Bsx cKO mice (Supplementary Fig. 5b).
Fig. 6. Essential role for BSX in medial septal glutamatergic neurons.
a Strategy for conditional deletion of Bsx in septal neurons. b Representative in situ hybridisation for VG2 in control and Bsx cKO embryos at E13.5. c Representative expression of VG2 at two anterior-posterior levels of the septum (levels 2 and 5) in female adult control and Bsx cKO mice and quantification of VG2 neurons at five different anterior-posterior levels (anterior level 1 - posterior level 5). Control n = 6, Bsx cKO n = 5 mice at each level. 2-way RM ANOVA, Fisher’s LSD test, level 4 P = 0.009, level 5 P = 0.023. d Representative expression of CR at two rostro-caudal levels of the septum (levels 2 and 5) in female adult control and Bsx cKO mice and quantification of CR neurons at five different anterior-posterior levels. Control n = 4, Bsx cKO n = 3. 2-way RM ANOVA, Fisher’s LSD test. e Quantification of CB, p75 and PV neurons at five different rostro-caudal levels in female adult control and Bsx cKO mice. Control n = 4, Bsx cKO n = 3. 2-way RM ANOVA, Fisher’s LSD test. f Quantification of VG2, CR, CB, p75 and PV neurons at five different rostro-caudal levels in male adult control and Bsx cKO mice. VG2: n = 6 control, n = 4 Bsx cKO. CR, CB and PV, n = 3 mice of each genotype at each level except the following where n = 2: CB level 1 Bsx cKO, level 5 control and Bsx cKO and PV level 4 Bsx cKO. p75, control n = 4, Bsx cKO n = 3 except levels 1 and 2 Bsx cKO where n = 2. 2-way RM ANOVA, Fisher’s LSD test. VG2 level 4 P = 0.0006, level 5 P = 0.0013. All data show mean ± SEM. Source data are provided in Supplementary Data 1. Level 1: Bregma 1.18 mm; Level 2: Bregma 0.98 mm; Level 3: Bregma 0.74 mm; Level 4: Bregma 0.50 mm; Level 5: Bregma 0.26 mm. Scale bars: b 150 μm; c, d 100 μm.
Embryonic deletion of Bsx using Zic4-Cre did not result in noticeable loss of VG2+ve cells in the embryonic septum (Fig. 6b). We quantified VG2+ve neurons of the MSvDB in male and female adult control and Bsx cKO mice at five different anterior-posterior Bregma levels (levels 1 – 5 in anterior-posterior order; see Methods section). Significant loss of VG2 expression was detected in both sexes in Bsx cKO mice at posterior septal levels (Fig. 6c, f). There was no significant loss of CR+ve, PV+ve, CB+ve or p75NTR+ve neurons in the MS upon deletion of Bsx with Zic4-Cre (Fig. 6d–f). Our data suggest that Bsx is required for differentiation/survival of subsets of glutamatergic neurons at posterior septal levels. The maturation status and integrity of remaining VG2+ve neurons upon BSX loss remains unknown.
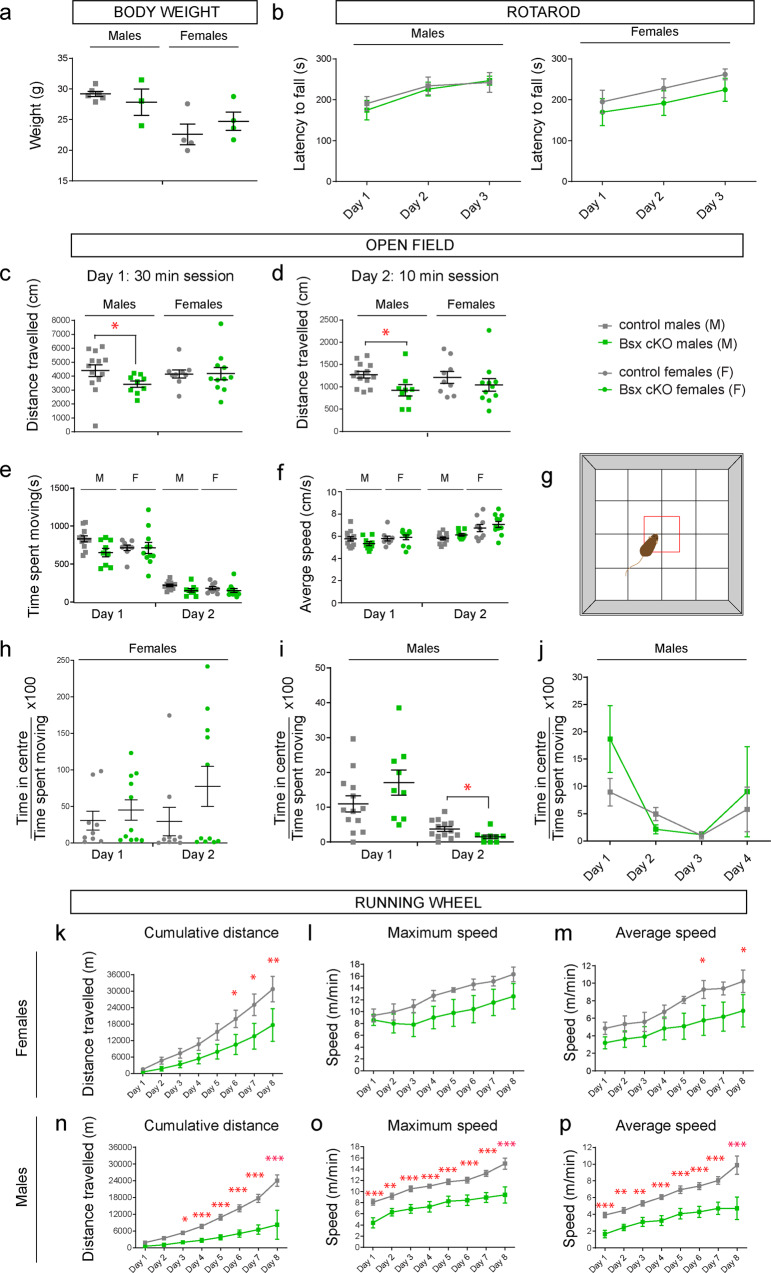
Studies using mice that lack BSX in the germline suggested a role for BSX in the hypothalamus and linked hypothalamic defects to locomotion deficits52. Our Bsx cKO mice allowed us to assess behaviours upon deletion of Bsx in septal neurons when hypothalamic expression of Bsx is intact. Before the initiation of behavioural testing, all mice (males and females) appeared normal with no visible impairments such as lacrimation, tremor, convulsions, piloerection, barbering, or other unusual behaviours. Bsx cKO mutant male and female mice had comparable weights to their littermate controls (Fig. 7a). Motor coordination and balance were assessed on a Rotarod apparatus. After one day of training, mice received three days of testing on accelerating rods. Both male and female Bsx cKO mice showed significant improvement throughout the three-day period, with no difference in latency to fall compared to controls (Fig. 7b). To test general locomotion, animals were exposed to an open field task over 2–4 consecutive days (30 mins on day 1 and 10 mins on subsequent days). Compared to controls, mutant males travelled shorter distances on both session days 1 and 2 (Fig. 7c, d). However, there were no significant differences in average time spent moving or the average speed between control and mutant male or female mice (Fig. 7e, f). We examined anxiety by calculating the time spent in the centre of the open field arena over the entire time spent moving (Fig. 7g). Male and female cKO mice spent a similar amount of time in the centre of the arena as their littermate controls (Fig. 7h–j), suggesting lack of anxiety defects. Although a statistically significant reduction in the time spent in the centre was observed in males on day 2, this did not prove to be significant over a 4-day period (Fig. 7j). Altogether the data suggest that deletion of Bsx in the septum does not lead to neurophysiological impairments, weight gain or anxiety, and does not affect coordination, balance or the ability to learn certain motor tasks. However, the reduced distance travelled in the open field test suggests reduced locomotion and voluntary movement.
Fig. 7. Behavioural assessment of septal Bsx cKO mice: essential role for Bsx in locomotion.
a Body weight: no differences in body weight values found between control and mutant mice. Males: controls n = 6, Bsx cKO n = 3. Females: controls n = 4, Bsx cKO n = 4. Unpaired t-test with Welch’s correction. P > 0.05. b Rotarod: latency to fall from the Rotarod throughout a 3-day test is shown. Males: controls n = 8, Bsx cKO n = 6. Females: controls n = 7, Bsx cKO n = 6. 2-way RM ANOVA. No significant effect of genotype. Gradual improvement was detected for all mice indicating that motor learning has occurred (Effect of Day, 2-way RM ANOVA, Males, P = 0.0003; Females, P < 0.0001). c–j Open field task. c–d distance travelled over a 2-day test. Only mutant males show reduced distance travelled on both days compared to controls. Mann Whitney U test, Day 1, P = 0.025; Day 2, P = 0.014). e, f similar time spent moving and average speed between control and mutant mice over a 2-day test. g diagram showing the open field arena. The centre of the arena is shown as a red box. h similar time spent in centre between female control and mutant mice. i, male mutants showed reduced time in centre on day 2 (Mann Whitney U test, Day 2, P = 0.0215) but this was not significant over a 4-day test period (j). c–i Males: controls n = 13, Bsx cKO n = 9. Females: controls n = 9, Bsx cKO n = 11. j: controls n = 6, Bsx cKO n = 5. k–p Running wheel task: female and male BSX cKO mice show reduced cumulative distance run on the wheel. Females: n = 4 mice for each genotype. 2-way RM ANOVA, Fisher’s LSD test *P < 0.05, **P < 0.01, ***P < 0.001. Distance: effect of Day P < 0.0001. Max speed: effect of Day P < 0.0001. Average speed: effect of Day P < 0.0001. Males: controls n = 11; Bsx cKO n = 9 except day 8 where n = 3 for both groups. Distance: effect of Genotype P < 0.0001, Day P < 0.0001 and Interaction P < 0.0001. Max speed: effect of Genotype P = 0.0004 and Day P < 0.0001. Average speed: effect of Genotype P = 0.0002 and Day P < 0.0001. All graphs show mean ± SEM. Source data are provided in Supplementary Data 1.
To further explore the possibility that loss of Bsx in septal neurons affects voluntary locomotion, we chose a task that allows behavioural assessment in a more natural environment compared to the open field arena. The running wheel task is carried out in home cages containing a wheel and a detection system to automatically measure locomotion over 24 h during 8 consecutive days (see Methods section). Mice were single-housed in cages with running wheels in separate sessions for 8 consecutive days. All four groups, male and female control and mutants, significantly improved their daily wheel-running skills, reaching the maximal values on the 8th day (Fig. 7k–p). This indicates a normal ability to learn a certain motor task. However, both male and female mutants exhibited reduced performance, measured as cumulative distance travelled and average speed (Fig. 7k, m, n, p). Maximum speed was also significantly reduced for male Bsx cKO mice (Fig. 7o). These effects were more pronounced in male mice compared to females. Taken together, our behavioural analysis allows us to conclude that loss of BSX in septal glutamatergic neurons negatively impacts voluntary locomotion.
Discussion
In the present study, we examined the embryonic origin and birthdate of septal neurons using newly-generated and existing genetic tools and distinguishing between LS and MSvDB populations. Most neurons originate from local septal precursors and are generated during early embryogenesis. An exception to this is the GABAergic MSvDB PV-expressing population which originates from surrounding extra-septal germinal zones. Glutamatergic septal neurons are also generated from local septal precursors and express the homeobox-encoding gene Bsx at embryonic stages. Loss of Bsx from septal glutamatergic neurons during embryogenesis causes selective loss of glutamatergic neuron subsets and deficits in voluntary locomotion.
Our data are consistent with previous birth-dating studies showing that the MSvDB has the earliest and shortest time of neurogenesis, while LS cells are generated at later time points over a prolonged time span26,27,30. This temporal bias results in a medio-lateral order of neuronal deposition and may lead to the emergence of the structural lamellar organisation of the adult MSvDB. Most septal neurons are generated after E10.5 and originate from resident precursors. PV MSvDB neurons are generated outside the septum at early embryonic stages (<E10 possibly) and reside in the most medial position of the MS. They are entirely distinct from PV LS neurons which have a septal origin and a glutamatergic phenotype. The external origin of MS PV neurons implies migration into the septum during embryogenesis. Such long-distance migration into the septum has also been identified for posterior septal glutamatergic neurons that populate the triangular septal nucleus and the bed nuclei of the anterior commissure. These neurons originate from diencephalic precursors in the thalamic eminence and migrate rostrally to enter the telencephalon53. GnrH-producing neurons also migrate through the septum from the nasal placode on their way towards the POA54. Thus, distinct birthdates, along with spatially distinct embryonic origins and long-distance migration, contribute to septal neuronal diversity.
Our study is limited in terms of lateral septal cell types examined, and this is largely due to technical limitations [neuropeptides such as somatostatin, enkephalin and oestrogen receptors previously shown to be expressed in the septum19 have high turnover making their histological detection difficult, if not impossible, without a pre-treatment with toxic doses of colchicine], scarce availability of unique identifiers for different cells types, and lack of precise anatomical boundaries that define LS subnuclei. Transcriptomic analysis of septal neurons through single cell sequencing, combined with knowledge of anatomical projections and connectivity, will provide more refined molecular handles that will enable further dissection of the embryonic origin and function of LS neurons.
In addition to PV MSvDB neurons, the other prominent projections of the septo-hippocampal system originate from cholinergic and glutamatergic MS populations. We have previously explored the origins of the cholinergic MSvDB neurons43. The origin of glutamatergic neurons has not been examined. Their common neurotransmitter phenotype with cortical pyramidal neurons led to the suggestion that these neurons may have a pallial origin55. However, we demonstrate that these neurons have a partially overlapping lineage with transient glutamatergic migratory Cajal-Retzius cells48 and originate from resident septal precursors.
Our findings indicate that, in contrast to the pallium and the subpallium, which generate neurons with district neurotransmitter phenotypes, the septum can generate glutamatergic, GABAergic and cholinergic neuronal cell types. It is unknown how this is orchestrated. It is possible that precursors are segregated within the septum. Alternatively, similar to cholinergic and GABAergic pallidal and striatal neurons, there may exist bipotential precursors whose differentiation is dependent on intrinsic factors50,56. A better molecular dissection of the embryonic septum will shed light on such questions of lineage and fate.
Bsx is a conserved homeobox gene expressed in different regions of the brain49. It has been implicated in mouse feeding and locomotion behaviour52, mouse pup growth57 and zebrafish pineal gland development58,59. Bsx does not regulate precursor patterning, but rather terminal differentiation and expression of genes such as Npy and Agrp in the hypothalamus52,60 through direct binding to promoter regions52,61. Mice lacking BSX in the germline exhibit reduced locomotor activity and attenuated response to fasting, implicating BSX in locomotion and the control of energy balance52. In that study, locomotor deficits were attributed to hypothalamic defects, particularly in the NPY/AgRP neurons of the arcuate nucleus52. However, the possibility that BSX-expressing neurons outside the hypothalamus may contribute to the phenotype had not been explored.
Our fate-mapping analysis identified BSX as a marker for septal glutamatergic neurons and our conditional deletion approach allowed us to evaluate its role in the septum and related behaviours in the absence of hypothalamic defects. The integrity of the hypothalamus in our septal Bsx cKO mice is supported by a number of observations: (1) Zic4-Cre and Bsx are expressed in largely non-overlapping regions of the developing hypothalamus, (2) Npy expression in the arcuate nucleus is unaltered in adult cKO and (3) body weight is unaffected in conditional mutant mice. By deleting septal Bsx while preserving hypothalamic expression, we find loss of septal glutamatergic neurons and associated reduced voluntary locomotion with preserved motor skills (see Rotarod). The loss of neurons and behavioural alterations are present in both male and female mice but are more pronounced in males. The reason for this sex-specific phenotype severity is unknown. Nevertheless, our findings, together with the prominent role of glutamatergic MS neurons in voluntary movement and locomotion24,62, allow us to propose that septal loss of BSX in glutamatergic neurons is sufficient to mediate locomotion deficits. Given the known interaction of the hypothalamus with the glutamatergic component of the MS24,62,63, hypothalamic loss of BSX may contribute additional locomotion deficits in germline Bsx mutant mice. Finally, given the role of septal glutamatergic neurons in hippocampal theta oscillations24, analysis of hippocampal network activity, and further behavioural testing would be needed to assess the impact of BSX loss on hippocampal dynamics and cognitive processing.
The overlap between Zic4-YFP expression and Bsx in the epiphysis suggests that there is additional deletion of Bsx in the pineal gland in our Bsx cKO mice. BSX regulates the development of the pineal complex in zebrafish60. Therefore, it is possible that the effect in locomotion may be partially mediated by alterations in circadian rhythms. BSX is also required for the expression of the corticotropin-releasing hormone ligand gene, uts1, in the zebrafish septum60. Whether the orthologue of this neuromodulator gene (Ucn) is also expressed in the mammalian medial septum and maps to glutamatergic neurons needs to be determined. As the function of BSX in mammalian septal neurons is entirely unexplored, further experiments are required to characterise the molecular pathways regulated by this transcription factor and which contribute to the regulation of locomotion.
In conclusion, our findings demonstrate a predominant septal origin of septal neurons with a substantial contribution from neighbouring precursor regions. Successive neuronal temporal specification together with migration of neuron subsets into the septum contribute to the generation of septal neuronal diversity. The transcription factor BSX is an early marker for septal glutamatergic neurons and is essential for their development/maturation and contribution to locomotion. Further dissection of septal neuronal diversity and function is needed to understand the multiple roles of septal neurons and their contribution to diverse behaviours.
Methods
Transgenic mice
Emx1-Cre (MGI:3761167)42, Zic4-Cre (MGI:4840322)43, Nkx2.1-Cre (MGI:3761164)42, Shh-Cre (JAX 005622)64, Lhx6-Cre (JAX 026555)45, Dbx1-Cre (MGI:3757955)48, Nestin-Cre (JAX 003771) and three reporter mice, Rosa26R-GFP (JAX 004077)65, R26R-YFP (JAX 006148)66 and Rosa26R-tdTomato (JAX 007914)67 used in this study have been described previously. Crosses between Cre-expressing mice (e.g. Zic4-Cre) and fluorescent reporters (e.g. Rosa26R-YFP) are abbreviated in the manuscript (e.g. Zic4-Cre;YFP or Zic4;YFP). Mice expressing Cre under control of the Lhx7 or Bsx were generated using bacterial artificial chromosome (BAC) transgenic technology68. Details on the generation of the previously reported Lhx6-Cre mice are also provided in this study. The codon-improved Cre recombinase (iCre)69 containing a nuclear localisation signal was fused to the initiation codon of each of the genes using a PCR-based approach. This was followed by a Simian Virus 40 polyadenylation signal and a Kanamycin resistance cassette that was flanked by FRT sites for selection of recombinant BACs. BAC recombination was designed to delete the remaining ATG-encoding exon together with 50-200 bp from the downstream intron. The following BACs were used: Lhx7-Cre: 214 Kb BAC RP23-379E17 containing 130 Kb upstream and 61 Kb downstream of the Lhx7 gene; Lhx6-Cre: 235 Kb BAC RP24-384G1 containing 137 Kb upstream and 80 Kb downstream; Bsx-Cre: 180 Kb BAC RP24-255N16 containing 70 Kb upstream and 110 Kb downstream. Kanamycin resistance was removed in vitro, and the BACs were linearised and purified prior to microinjection into fertilised eggs. Bsx conditional knock-out mice were obtained as Knock-out first, promoter driven (tm1a) frozen embryos from UCDavies KOMP Repository. They can now be obtained from MMRRC (Bsxtm1a(KOMP)Wtsi MMRRC:052869-UCD, MGI:4363233). ‘Knock-out first’ mice were converted to conditional deletion mutants (tm1c) using germline recombination with a FLP-expressing mouse. This results in loxP sites inserted in intron 1 and intron 2. Loss of exon 2 through Cre excision (and generation of the tm1d allele) was confirmed using a PCR-based approach. Primer sequences used for iCre and Bsx allele detection are as follows: iCreF (iCre250S): 5′ GAG GGA CTA CCT CCT GTA CC 3′; iCreR (iCre 880AS): 5′ TGC CCA GAG TCA TCC TTG GC 3′; Int1F1 (BsxInt1F1): 5′ CAA CCC TGC TAC TGA CAA GG 3′; Int1R1 (BsxInt1R1): 5′ CTT CCA GTT ATC TGT TAG GCC 3′; Int2R1 (BsxInt2R1): 5′ GGT TCT GGG CCA GCC CTG GGC 3′. Loss of function in tm1a mice was confirmed by loss of hypothalamic NPY expression in the ‘knock-out first’ mice52. All mice used in this study were maintained on a C57BL/6/CBA background. Mouse colonies were maintained at the Wolfson Institute for Biomedical Research, University College London, following UCL ethical approval and in accordance with United Kingdom legislation (ASPA 1986).
Tissue preparation
The day of the vaginal plug was considered E0.5, and the day of birth was considered day 0. Whole embryo heads (for embryos E13.5 and younger) were fixed overnight in 4% (w/v) paraformaldehyde (PFA) in PBS. Postnatal animals were anesthetized and perfused first with saline (0.9 % NaCl) followed by 4% (w/v) PFA through the left ventricle of the heart. Adult brains were dissected out, sliced into 2 or 3 mm-slices using a mouse brain coronal matrix (PlasticsOne), and post-fixed in 4% PFA overnight. Fixed samples were cryoprotected overnight by immersion in 20% (w/v) sucrose in PBS. All samples were embedded in Tissue-Tek OCT compound (R. A. Lamb Medical Supplies, Eastbourne, UK), frozen on dry ice, and stored at −80 °C.
Immunohistochemistry
Embryonic brains were cut on a cryostat into 20-μm-thick coronal or horizontal sections (E13.5) and collected directly on Superfrost plus slides (BDH Laboratory Supplies, Poole, UK). Adult animals were anaesthetized and transcardially perfused with 4% PFA in PBS, the brains were extracted and post-fixed overnight in 4% PFA at 4 degrees, and then transferred in PBS. Adult sections were cut coronally (30 μm thickness) and were serially collected in PBS for free floating procedure. All sections were blocked in PBS containing 10% heat-inactivated sheep serum (Sigma, St. Louis, MO) and 0.1% Triton X-100 (Sigma) at room temperature for 1h. Immunohistochemistry was performed with the following primary antibodies: rat anti-GFP IgG2a (1:1000; Nacalai Tesque, Kyoto,Japan, #04404-84); mouse anti-calbindin (#300), rabbit anti-calbindin (#CB38a), rabbit anti-calretinin (#7697), mouse anti-calretinin (#6B3), (all 1:1000 from Swant, Bellizona, Switzerland); mouse anti-parvalbumin (1:1000, Chemicon Millipore #MAB1572), rabbit anti-p75NTR (1:1000; Promega, Southampton, UK, #G3231), rabbit anti-TTF-1 (NKX2-1) (1:100 Santa Cruz Biotechnology, CA, #sc-13040) and rabbit anti-Gsx2 (1:500 Millipore #ABN162). Primary antibodies were applied overnight at 4 °C. Secondary antibodies used were AlexaFluor 488- conjugated, AlexaFluor 568-conjugated, and AlexaFluor 647-conjugated donkey anti-rabbit IgG or donkey anti-rat IgG or donkey anti-mouse IgG (all used at 1:1000; Invitrogen, Carlsbad, CA) and were applied for 60 min at room temperature together with Hoescht 33258 (1:10 4; Sigma) to detect cell nuclei. All secondary antibodies were diluted in block solution (1:1000). Floating sections were transferred onto Superfrost plus slides (BDH Laboratory Supplies) and air dried. All sections were coverslipped with Dako fluorescent mounting medium. For detection of markers on Bsx cKO and control sections, endogenous peroxidase activity was quenched with 0.6% H2O2 for 20 minutes and antibodies were applied overnight as described above. A biotin-conjugated secondary antibody (donkey anti-rabbit IgG, 1:500; Millipore) was used to detect the primary antibodies followed by the Avidin/Biotinylated enzyme Complex (ABC) reaction (Vectastain ABC kit, Vector Laboratories) prepared according to manufacturer’s instructions, and addition of DAB reagent (Vector Laboratories). After DAB reaction, all slides were dehydrated in progressively increasing concentration of ethanol and xylene, and were mounted with DPX mounting media.
RNA In situ hybridization (ISH)
Fixed samples were cryoprotected for >12 hours by immersion in 20%(w/v) sucrose/PBS pre-treated with diethyl pyrocarbonate (DEPC) (Sigma), embedded in OCT (Tissue Tek; Raymond Lamb Ltd Medical Supplies) and frozen on dry ice by immersion in isopentane. All samples were stored at −80 °C until needed. Sections were collected on Superfrost Plus slides (VWR International) and allowed to air dry before hybridising overnight at 65 °C (in a chamber humidified with 50% v/v deionized formamide and containing 1x SSC buffer) in a buffer containing digoxigenin (DIG)- labelled antisense RNA probe (prepared according to manufacturer’s instructions and diluted 1:1000 in hybridisation buffer). Hybridisation buffer consisted of 50% v/v deionized formamide (Sigma), 1x “Salts” [2 M NaCl, 50 mM EDTA, 100 mM Tris-HCl, pH 7.5, 50 mM NaH2PO42H2O, 50mM Na2HPO4, 0.1 mg/ml tRNA from baker’s yeast (phenol chloroform extracted, Roche Diagnostics)], 1x Denhardt’s solution (Sigma), and 10% w/v dextran sulphate (pre-dissolved in DEPC-treated water and maintained at 4 °C as 50% stock). Sections were washed three times at 65 °C for 30 min each in pre-warmed wash solution (50% v/v formamide, 1x SSC, 0.1% Tween 20), followed by two washes in 1x MABT (100 mM maleic acid, 150 mM NaCl, pH 7.5, 0.1% Tween 20) at room temperature also for 30 min each. Blocking was performed using 2% w/v Blocking Reagent (Roche Diagnostics), 10% v/v heat-inactivated sheep serum (Sigma) in 1x MABT for 1 hour at room temperature and anti-DIG antibody conjugated with alkaline phosphatase (AP) (Roche Diagnostics) diluted 1:1500 in blocking solution was applied overnight at 4 °C. Following washes in 1x MABT, sections were equilibrated in 100 mM NaCl, 100 mM Tris-HCl, pH 9.5, 0.1% Tween 20 for 10 min. Development was performed at 37 °C for 4–8 h with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate in freshly prepared staining solution containing 100 mM NaCl, 50 mM MgCl2, 100 mM Tris-HCl, pH 9.5, and 0.1% Tween 20. To increase sensitivity, 5% (w/v) polyvinyl alcohol was included during staining. For fluorescent detection of ISH signal, DIG-labelled (or FITC-labelled) RNA probes were detected with an anti-DIG (or anti-FITC) POD-conjugated antibody followed by incubation with Tyramide-Cy3 (or Tyramide Cy5) (1:100, TSA-Plus, PerkinElmer) for 10 min – 3 hours at room temperature. FISH was sometimes followed by IHC as follows: FISH detection was stopped with 3% H2O2 before sections were used for immunodetection as described in the previous section. The following plasmids were used to generate RNA probes: IMAGE clone 374236 for Gad1 (Gad67) (linearised with XhoI and transcribed with T3), a 0.7 kb DNA fragment corresponding to the 3’ UTR sequence of the mouse Bsx gene and cloned into pCRIITopo (linearised with BamHI and transcribed with T7), a 2.5 kb fragment from mouse vGlut2 (Slc17a6) cDNA cloned into pT7T3-PacI (a kind gift from T.Jessell, Columbia University, USA) (linearised with EcoRI and transcribed with T3) and a 1 kb cDNA for iCre cloned into pBluescriptII (linearised with EcoRV and transcribed with T7).
EdU birthdating
5-ethynyl-2′-deoxyuridine (EdU, Molecular Probes) was dissolved in sterile PBS at 2 mg/ml. Pregnant females were administered five intraperitoneal injections of EdU (10mg/Kg body weight) at two-hour intervals starting at 10:00 am. The pups were perfused at P30 and the tissue processed as described above with the exception of tissue fixation that was performed for 45 min at room temperature in 4% PFA. EdU detection was carried out after immunohistochemistry for the various markers using the Click-iT EdU Alexa Fluor 647 Imaging Kit (Molecular Probes) according to manufacturer’s instructions.
Image processing
Images were captured with a Zeiss fluorescent microscope or with a Leica confocal microscope. Images were further processed with Adobe Photoshop (Adobe Systems Inc., San Jose, CA) for general contrast and brightness enhancements. The final compositing of the figures was performed with Adobe Illustrator (Adobe Systems Inc., San Jose, CA). Images of RNA ISH were taken using a ZEISS Axio Scan.Z1 and processed using ZEISS ZEN lite software.
Mouse behaviour
Two cohorts of male and female mice (from the age of 3 months) have been assessed on a battery of behavioural tests. All experiments took place between 09:00 to 17:00 in a room where external sounds were masked by white noise. The mice were left in the room for 30 minutes before each test session in order to minimise distress caused by the transportation. The tests were performed in the following sequence: neurophysiological assessment, open field, rotarod, running wheel.
An initial neurophysiological screening consisting of very short tests to assess broadly sensory and motor function, and general health was performed on all animals two weeks before the start of the tests.
Open field
Mice were exposed to open field (OF) over two days. Day 1: 30-min session in the non-transparent arena (dimensions: 30 × 30 × 40 cm); Day 2: animals were exposed to the open field for 10 min. For a subgroup of male mice, the OF task was extended for 2 extra days with one 10 min-sessions each day. Four main parameters: distance travelled, time spent moving (s), mean speed (cm/s), time spent in central area (s) were obtained for further analysis. Tracking of the mice was carried out with ActualTrack software (Actual Analytics, Edinburgh, UK).
Rotarod test
Evaluation of fine motor coordination and balance was assessed via Rotarod apparatus over four days. On the first day, mice underwent one training session consisting of 3 trials of 120 seconds each. Rotating rod spinning speed was kept constant (4 revolutions per minute). During the subsequent 3 test days the rotating rod was set to accelerate from 4 to 40 revolutions per minute over 300 s. Mice were given 15 min of rest between the trials for them to fully recover. Latency to fall was registered once the mouse landed on a lever. No differences in weight were observed between the groups indicating that this factor did not have an effect on the task.
Running wheels
As a further measure of motor performance, mice were selectively assigned to a running wheel test according to their weight and Rotarod performance. For this, animals were exposed to the housing cages with a voluntary access to the attached running wheels and 24 hours access to food and water. Running wheels were equipped with irregularly spaced crossbars70. One wheel revolution corresponds to a running distance of 0.38 m. A rotation sensor was connected to the wheel axis and the wheels were connected to the automated recording systems that allowed calculating average speed, maximum speed and total distance run. Locomotion of the animals was assessed throughout 8-day period. Recordings were collected at a 1-min-interval between 6 p.m. and 7 a.m. (dark cycle), and 1-h-interval between 7 a.m. and 6 p.m. (light cycle).
Statistics and reproducibility
Experiments presented in this study were repeated independently a minimum of two times. For all quantification experiments a minimum of three mice was used for each genotype. For all experiments animals are considered as biological replicates and for cell counts, sections and levels are technical replicates. Precise n numbers used in each experiment is specified in the figure legends. The extent of co-localisation between GFP/YFP/tdTomato and the various markers in each of the transgenic mice was determined as follows. Counts were performed at three different rostro-caudal levels corresponding approximately to Bregma 1.18 (rostral section), Bregma 0.74 (intermediate section) and Bregma 0.26 (caudal section) for each brain. On each section the midline was drawn, and counts were performed on both sides separately for each nucleus (MSvDB and LS) and averaged per slice. Absolute numbers of cells counted per level is shown in Table 1. For comparison of Bsx cKO and controls, counts were performed at five different rostro-caudal levels corresponding approximately to Bregma 1.18 mm Level 1; Bregma 0.98 mm Level 2; Bregma 0.74 mm Level 3; Bregma 0.50 mm Level 4; Bregma 0.26 mm, Level 5. Where possible, cell counts and behavioural experiments were performed by investigators blind to the genotype. Statistical analysis was performed in GraphPad Prism (GraphPad Software, La Jolla, CA, USA). All data were tested for normality using a Kolmogorov-Smirnov or Shapiro-Wilk tests and subsequently analysed using an appropriate statistical test: Unpaired t-test with Welch’s correction or two-way ANOVA with post hoc uncorrected Fisher’s Least Significant Difference (LSD) test for normally distributed data; and the nonparametric two-tailed Mann–Whitney U test for non-normally distributed data.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank our colleagues at the Wolfson Institute for Biomedical Research (University College London) for helpful comments and discussions. We thank A. Pierani for providing mouse Dbx-Cre tissue for fate-mapping. A.N.R. was supported by a PhD studentship from the Wellcome Trust. Financial support for the work was provided by the European Research Council (Grant agreement 207807), the UK Biotechnology and Biological Sciences Research Council (BB/N009061/1) and the UK Wellcome Trust (108726/Z/15/Z).
Author contributions
Conceptualisation by L.M. and N.K.; Investigation by L.M., Z.A., M.A., Y.M., N.B-V., A.N.R. and N.K. Writing by L.M. and N.K. Funding acquisition by N.K.
Peer review
Peer review information
Communications Biology thanks Thomas Theil and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: George Inglis. Peer reviewer reports are available.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files. Source data for all the figures are provided in Supplementary Data 1.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lorenza Magno, Email: l.magno@ucl.ac.uk.
Nicoletta Kessaris, Email: n.kessaris@ucl.ac.uk.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-022-04066-5.
References
- 1.Andy OJ, Stephan H. The septum in the human brain. J. Comp. Neurol. 1968;133:383–410. doi: 10.1002/cne.901330308. [DOI] [PubMed] [Google Scholar]
- 2.Stephan H, Andy OJ. Quantitative comparisons of brain structures from insectivores to primates. Am. Zool. 1964;4:59–74. doi: 10.1093/icb/4.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Swanson LW, Cowan WM. The connections of the septal region in the rat. J. Comp. Neurol. 1979;186:621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- 4.Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 5.Whitehouse PJ, et al. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 6.Schliebs R, Arendt T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J. Neural Transm. 2006;113:1625–1644. doi: 10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- 7.Apartis E, Poindessous-Jazat FR, Lamour YA, Bassant MH. Loss of rhythmically bursting neurons in rat medial septum following selective lesion of septohippocampal cholinergic system. J. Neurophysiol. 1998;79:1633–1642. doi: 10.1152/jn.1998.79.4.1633. [DOI] [PubMed] [Google Scholar]
- 8.Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- 9.Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J. Comp. Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- 10.Kohler C, Chan-Palay V, Wu JY. Septal neurons containing glutamic acid decarboxylase immunoreactivity project to the hippocampal region in the rat brain. Anat. Embryol. 1984;169:41–44. doi: 10.1007/BF00300585. [DOI] [PubMed] [Google Scholar]
- 11.Toth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. J. Physiol. 1997;500:463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoder RM, Pang KC. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15:381–392. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]
- 13.Huh CY, Goutagny R, Williams S. Glutamatergic neurons of the mouse medial septum and diagonal band of Broca synaptically drive hippocampal pyramidal cells: relevance for hippocampal theta rhythm. J. Neurosci. 2010;30:15951–15961. doi: 10.1523/JNEUROSCI.3663-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colom LV, Castaneda MT, Reyna T, Hernandez S, Garrido-Sanabria E. Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse. 2005;58:151–164. doi: 10.1002/syn.20184. [DOI] [PubMed] [Google Scholar]
- 15.Sotty F, et al. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J. Physiol. 2003;551:927–943. doi: 10.1113/jphysiol.2003.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toth K, Borhegyi Z, Freund TF. Postsynaptic targets of GABAergic hippocampal neurons in the medial septum-diagonal band of broca complex. J. Neurosci. 1993;13:3712–3724. doi: 10.1523/JNEUROSCI.13-09-03712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leranth C, Frotscher M. Organization of the septal region in the rat brain: cholinergic-GABAergic interconnections and the termination of hippocampo-septal fibers. J. Comp. Neurol. 1989;289:304–314. doi: 10.1002/cne.902890210. [DOI] [PubMed] [Google Scholar]
- 18.Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res. Brain Res. Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Risold PY, Swanson LW. Chemoarchitecture of the rat lateral septal nucleus. Brain Res. Brain Res. Rev. 1997;24:91–113. doi: 10.1016/s0165-0173(97)00008-8. [DOI] [PubMed] [Google Scholar]
- 20.Lee EH, Lin YP, Yin TH. Effects of lateral and medial septal lesions on various activity and reactivity measures in rats. Physiol. Behav. 1988;42:97–102. doi: 10.1016/0031-9384(88)90267-3. [DOI] [PubMed] [Google Scholar]
- 21.Oddie SD, Bland BH. Hippocampal formation theta activity and movement selection. Neurosci. Biobehav. Rev. 1998;22:221–231. doi: 10.1016/s0149-7634(97)00003-1. [DOI] [PubMed] [Google Scholar]
- 22.Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav. Brain Res. 2001;127:119–136. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 23.Caplan JB, et al. Human theta oscillations related to sensorimotor integration and spatial learning. J. Neurosci. 2003;23:4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuhrmann F, et al. Locomotion, theta oscillations, and the speed-correlated firing of hippocampal neurons are controlled by a medial septal glutamatergic circuit. Neuron. 2015;86:1253–1264. doi: 10.1016/j.neuron.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Tsanov M. Differential and complementary roles of medial and lateral septum in the orchestration of limbic oscillations and signal integration. Eur. J. Neurosci. 2018;48:2783–2794. doi: 10.1111/ejn.13746. [DOI] [PubMed] [Google Scholar]
- 26.Bayer SA. The development of the septal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J. Comp. Neurol. 1979;183:89–106. doi: 10.1002/cne.901830108. [DOI] [PubMed] [Google Scholar]
- 27.Creps ES. Time of neuron origin in preoptic and septal areas of the mouse: an autoradiographic study. J. Comp. Neurol. 1974;157:161–243. doi: 10.1002/cne.901570205. [DOI] [PubMed] [Google Scholar]
- 28.Horvath S, Szabo K, Gulyas M, Palkovits M. Ontogenetic development of septal nuclei in the rat. Anat. Embryol. 1988;177:267–275. doi: 10.1007/BF00321137. [DOI] [PubMed] [Google Scholar]
- 29.Schambra UB, Sulik KK, Petrusz P, Lauder JM. Ontogeny of cholinergic neurons in the mouse forebrain. J. Comp. Neurol. 1989;288:101–122. doi: 10.1002/cne.902880109. [DOI] [PubMed] [Google Scholar]
- 30.Wei B, et al. The onion skin-like organization of the septum arises from multiple embryonic origins to form multiple adult neuronal fates. Neuroscience. 2012;222:110–123. doi: 10.1016/j.neuroscience.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Lopez M, et al. Histogenetic compartments of the mouse centromedial and extended amygdala based on gene expression patterns during development. J. Comp. Neurol. 2008;506:46–74. doi: 10.1002/cne.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puelles L, et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J. Comp. Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Flames N, et al. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J. Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiss J, Patel AJ, Baimbridge KG, Freund TF. Topographical localization of neurons containing parvalbumin and choline acetyltransferase in the medial septum-diagonal band region of the rat. Neuroscience. 1990;36:61–72. doi: 10.1016/0306-4522(90)90351-4. [DOI] [PubMed] [Google Scholar]
- 35.Kiss J, Magloczky Z, Somogyi J, Freund TF. Distribution of calretinin-containing neurons relative to other neurochemically identified cell types in the medial septum of the rat. Neuroscience. 1997;78:399–410. doi: 10.1016/s0306-4522(96)00508-8. [DOI] [PubMed] [Google Scholar]
- 36.Kiss J, Patel AJ, Freund TF. Distribution of septohippocampal neurons containing parvalbumin or choline acetyltransferase in the rat brain. J. Comp. Neurol. 1990;298:362–372. doi: 10.1002/cne.902980308. [DOI] [PubMed] [Google Scholar]
- 37.Zhao C, Eisinger B, Gammie SC. Characterization of GABAergic neurons in the mouse lateral septum: a double fluorescence in situ hybridization and immunohistochemical study using tyramide signal amplification. PLoS ONE. 2013;8:e73750. doi: 10.1371/journal.pone.0073750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- 39.Lin W, McKinney K, Liu L, Lakhlani S, Jennes L. Distribution of vesicular glutamate transporter-2 messenger ribonucleic Acid and protein in the septum-hypothalamus of the rat. Endocrinology. 2003;144:662–670. doi: 10.1210/en.2002-220908. [DOI] [PubMed] [Google Scholar]
- 40.Hajszan T, Alreja M, Leranth C. Intrinsic vesicular glutamate transporter 2-immunoreactive input to septohippocampal parvalbumin-containing neurons: novel glutamatergic local circuit cells. Hippocampus. 2004;14:499–509. doi: 10.1002/hipo.10195. [DOI] [PubMed] [Google Scholar]
- 41.Manseau F, Danik M, Williams S. A functional glutamatergic neurone network in the medial septum and diagonal band area. J. Physiol. 2005;566:865–884. doi: 10.1113/jphysiol.2005.089664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kessaris N, et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magno L, et al. NKX2-1 is required in the embryonic septum for cholinergic system development, learning, and memory. Cell Rep. 2017;20:1572–1584. doi: 10.1016/j.celrep.2017.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin AN, et al. The germinal zones of the basal ganglia but not the septum generate GABAergic interneurons for the cortex. J. Neurosci. 2010;30:12050–12062. doi: 10.1523/JNEUROSCI.6178-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fogarty M, et al. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flandin P, Kimura S, Rubenstein JL. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J. Neurosci. 2010;30:2812–2823. doi: 10.1523/JNEUROSCI.4228-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelman DM, et al. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J. Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bielle F, et al. Multiple origins of Cajal-Retzius cells at the borders of the developing pallium. Nat. Neurosci. 2005;8:1002–1012. doi: 10.1038/nn1511. [DOI] [PubMed] [Google Scholar]
- 49.Cremona M, Colombo E, Andreazzoli M, Cossu G, Broccoli V. Bsx, an evolutionary conserved Brain Specific homeoboX gene expressed in the septum, epiphysis, mammillary bodies and arcuate nucleus. Gene Expr. Patterns. 2004;4:47–51. doi: 10.1016/s1567-133x(03)00151-0. [DOI] [PubMed] [Google Scholar]
- 50.Fragkouli A, van Wijk NV, Lopes R, Kessaris N, Pachnis V. LIM homeodomain transcription factor-dependent specification of bipotential MGE progenitors into cholinergic and GABAergic striatal interneurons. Development. 2009;136:3841–3851. doi: 10.1242/dev.038083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du T, Xu Q, Ocbina PJ, Anderson SA. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- 52.Sakkou M, et al. A role for brain-specific homeobox factor Bsx in the control of hyperphagia and locomotory behavior. Cell Metab. 2007;5:450–463. doi: 10.1016/j.cmet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe K, Irie K, Hanashima C, Takebayashi H, Sato N. Diencephalic progenitors contribute to the posterior septum through rostral migration along the hippocampal axonal pathway. Sci. Rep. 2018;8:11728. doi: 10.1038/s41598-018-30020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giacobini P, et al. Hepatocyte growth factor acts as a motogen and guidance signal for gonadotropin hormone-releasing hormone-1 neuronal migration. J. Neurosci. 2007;27:431–445. doi: 10.1523/JNEUROSCI.4979-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iyer A, Tole S. Neuronal diversity and reciprocal connectivity between the vertebrate hippocampus and septum. Wiley Interdiscip. Rev. Dev. Biol. 2020;9:e370. doi: 10.1002/wdev.370. [DOI] [PubMed] [Google Scholar]
- 56.Lopes R, Verhey van Wijk N, Neves G, Pachnis V. Transcription factor LIM homeobox 7 (Lhx7) maintains subtype identity of cholinergic interneurons in the mammalian striatum. Proc. Natl Acad. Sci. USA. 2012;109:3119–3124. doi: 10.1073/pnas.1109251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McArthur T, Ohtoshi A. A brain-specific homeobox gene, Bsx, is essential for proper postnatal growth and nursing. Mol. Cell Biol. 2007;27:5120–5127. doi: 10.1128/MCB.00215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mano H, Asaoka Y, Kojima D, Fukada Y. Brain-specific homeobox Bsx specifies identity of pineal gland between serially homologous photoreceptive organs in zebrafish. Commun. Biol. 2019;2:364. doi: 10.1038/s42003-019-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schredelseker, T. & Driever, W. Bsx controls pineal complex development. Development10.1242/dev.163477 (2018). [DOI] [PubMed]
- 60.Schredelseker T, Veit F, Dorsky RI, Driever W. Bsx is essential for differentiation of multiple neuromodulatory cell populations in the secondary prosencephalon. Front. Neurosci. 2020;14:525. doi: 10.3389/fnins.2020.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee B, et al. Brain-specific homeobox factor as a target selector for glucocorticoid receptor in energy balance. Mol. Cell. Biol. 2013;33:2650–2658. doi: 10.1128/MCB.00094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oddie SD, Stefanek W, Kirk IJ, Bland BH. Intraseptal procaine abolishes hypothalamic stimulation-induced wheel-running and hippocampal theta field activity in rats. J. Neurosci. 1996;16:1948–1956. doi: 10.1523/JNEUROSCI.16-05-01948.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leranth C, Kiss J. A population of supramammillary area calretinin neurons terminating on medial septal area cholinergic and lateral septal area calbindin-containing cells are aspartate/glutamatergic. J. Neurosci. 1996;16:7699–7710. doi: 10.1523/JNEUROSCI.16-23-07699.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harfe BD, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 65.Mao X, Fujiwara Y, Chapdelaine A, Yang H, Orkin SH. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 2001;97:324–326. doi: 10.1182/blood.v97.1.324. [DOI] [PubMed] [Google Scholar]
- 66.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee EC, et al. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73:56–65. doi: 10.1006/geno.2000.6451. [DOI] [PubMed] [Google Scholar]
- 69.Shimshek DR, et al. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 2002;32:19–26. doi: 10.1002/gene.10023. [DOI] [PubMed] [Google Scholar]
- 70.McKenzie IA, et al. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paxinos, G., Franklin, K. B. J. & Franklin, K. B. J. The Mouse Brain In Stereotaxic Coordinates. 2nd edn. (Academic Press, 2001).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files. Source data for all the figures are provided in Supplementary Data 1.