Abstract
Background
Neutralizing monoclonal antibodies (mAbs) were authorized for the treatment of COVID-19 outpatients based on clinical trials completed early in the pandemic, which were underpowered for mortality and subgroup analyses. Real-world data studies are promising for further assessing rapidly deployed therapeutics.
Research Question
Did mAb treatment prevent progression to severe disease and death across pandemic phases and based on risk factors, including prior vaccination status?
Study Design and Methods
This observational cohort study included nonhospitalized adult patients with SARS-CoV-2 infection from November 2020 to October 2021 using electronic health records from a statewide health system plus state-level vaccine and mortality data. Using propensity matching, we selected approximately 2.5 patients not receiving mAbs for each patient who received mAb treatment under emergency use authorization. The primary outcome was 28-day hospitalization; secondary outcomes included mortality and hospitalization severity.
Results
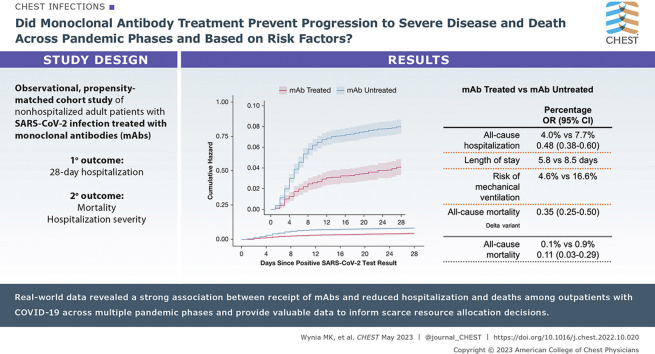
Of 36,077 patients with SARS-CoV-2 infection, 2,675 receiving mAbs were matched to 6,677 patients not receiving mAbs. Compared with mAb-untreated patients, mAb-treated patients had lower all-cause hospitalization (4.0% vs 7.7%; adjusted OR, 0.48; 95% CI, 0.38-0.60) and all-cause mortality (0.1% vs 0.9%; adjusted OR, 0.11; 95% CI, 0.03-0.29) to day 28; differences persisted to day 90. Among hospitalized patients, mAb-treated patients had shorter hospital length of stay (5.8 vs 8.5 days) and lower risk of mechanical ventilation (4.6% vs 16.6%). Results were similar for preventing hospitalizations during the Delta variant phase (adjusted OR, 0.35; 95% CI, 0.25-0.50) and across subgroups. Number-needed-to-treat (NNT) to prevent hospitalization was lower for subgroups with higher baseline risk of hospitalization; for example, multiple comorbidities (NNT = 17) and not fully vaccinated (NNT = 24) vs no comorbidities (NNT = 88) and fully vaccinated (NNT = 81).
Interpretation
Real-world data revealed a strong association between receipt of mAbs and reduced hospitalization and deaths among COVID-19 outpatients across pandemic phases. Real-world data studies should be used to guide practice and policy decisions, including allocation of scarce resources.
Key Words: COVID-19, Delta variant, hospitalization, mechanical ventilation, monoclonal antibody, outpatient
Graphical Abstract
Take-home Points.
StudyQuestion: Does real-world evidence show that treatment with neutralizing mAbs was correlated with lower progression to severe disease and death during the Delta, Alpha, and pre-Alpha variant phases of the pandemic, adjusting for risk factors, including vaccination status?
Results: We examined outcomes of 36,077 patients with COVID-19 between November 2020 and October 2021 using EHR data combined with state-level vaccine and mortality data. Following adjustments made for multiple other factors, the odds of 28-day hospitalization were reduced by more than one-half (OR, 0.48; 95% CI, 0.38-0.60) and odds of death by 89% (OR, 0.11; 95% CI, 0.03-0.29) among patients receiving mAbs. Results were similar across pandemic phases and multiple clinical subgroups, but the NNT to prevent hospitalization was much lower for subgroups with elevated baseline risk of hospitalization.
Interpretation: Real-world data revealed a strong association between receipt of mAbs and reduced hospitalization and death among COVID-19 outpatients across multiple pandemic phases and provided valuable data to inform scarce resource allocation decisions.
High rates of COVID-19 transmission and illness persist, especially among unvaccinated individuals, as well as those with waning vaccine or infection-related immunity, such as older adults or those with certain chronic medical conditions.1 , 2 Neutralizing monoclonal antibody (mAb) treatment provides immediate passive immunity against SARS-CoV-2, the virus that causes COVID-19. Several mAb products have received emergency use authorization (EUA) from the US Food and Drug Administration.3 These authorizations were based on early phase II/III randomized controlled trials that showed a reduction in a combined end point of hospitalization or death among high-risk outpatients with early symptomatic infection. However, these trials were small in size, with few deaths and conducted prior to the emergence of the Delta variant or widespread availability of vaccines against SARS-CoV-2.4, 5, 6
Once a promising therapeutic agent has been authorized for emergency use, it becomes more challenging to recruit patients into randomized controlled trials, as patients may seek active therapy and clinicians may view randomization to a placebo group as unethical.7 Consequently, studies of mAbs following EUA have primarily been small observational trials, confirming reduced hospitalization rates but not large enough to detect a mortality benefit nor to assess any potential heterogeneity of mAb treatment effects according to comorbid conditions or vaccination status.8, 9, 10 The latter information could be especially useful in policymaking about how best to allocate limited access to mAb treatment during shortages.11 , 12 Furthermore, no published studies have yet directly evaluated the effectiveness of currently available mAbs against the Delta variant of SARS-CoV-2, which arose in summer 2021 in the United States.
The rapidly evolving nature of the COVID-19 pandemic, including both the emergence of new variants of the virus and use of EUAs allowing early access to novel therapeutics, makes it critical to build robust research platforms for real-world evidence generation.13 , 14 In early 2021, we created a real-world evidence platform to assess the ongoing clinical impacts of mAb therapy on high-risk outpatients with early symptomatic COVID-19.
The study objective was to evaluate the effectiveness of mAb treatment and progression to severe disease, including hospitalization, severity of hospitalization, and mortality. The goal of the overall platform was to include changes in the pandemic, including emergence of new variants, in near real-time with sufficient power to assess potential mortality benefits and effectiveness among patients with various risk factors for progression to severe disease, including vaccination status.
Study Design and Methods
Study Oversight and Data Sources
We conducted a propensity-matched observational cohort study, as part of a statewide implementation/effectiveness pragmatic trial, in a collaboration between University of Colorado researchers, University of Colorado Health leaders, and the Colorado Department of Public Health and Environment. The study was approved by the Colorado Multiple Institutional Review Board with a waiver of informed consent (#21-2935). Data were obtained from the electronic health record (EHR; Epic) of the University of Colorado Health, the largest health system in Colorado with 13 hospitals around the state and 141,000 annual hospital admissions. EHR data were merged with statewide data on vaccination status from the Colorado Comprehensive Immunization Information System and mortality from Colorado Vital Records.
Patient Population Studied
We included patients diagnosed with SARS-CoV-2 infection between November 20, 2020, and October 7, 2021, allowing for at least 28 days of follow-up as of November 4, 2021 (N = 36,077). They were identified by using EHR-based date of SARS-CoV-2-positive testing (by polymerase chain reaction or antigen tests) or date of administration of mAb treatment (if no SARS-CoV-2 test result date were available). The decision to seek mAb treatment was made by patients and clinicians, and a statewide referral system was established by the Colorado Department of Public Health and Environment to facilitate patient referrals to facilities for mAb infusion.15 We did not exclude patients solely for lack of EUA eligibility based on EHR data, because not all eligibility criteria were consistently available in the EHR (additional Methods are provided in e-Appendix 1). Patients were excluded who received mAb treatment on the same day of or during hospitalization, as these patients already had the primary outcome. Logistic regression was used for propensity score estimation16 with nearest neighbor matching17 applied to select an approximate 2.5:1 mAb-untreated to mAb-treated matched cohort. Matching factors included baseline demographic characteristics, clinical variables, and time. The primary analysis cohort included patients with a documented mAb administration date (n = 2,675) and propensity-matched control subjects who did not receive mAb treatment (n = 6,677). The effectiveness of matching using standardized mean differences was assessed.18
Outcomes
The primary outcome was all-cause hospitalization within 28 days of a positive SARS-CoV-2 test result, obtained from EHR data. Secondary outcomes included all-cause hospitalization to day 90, all-cause mortality to days 28 and 90, and ED visits to day 28. Among those hospitalized, outcomes included disease severity based on maximum level of respiratory support, hospital and ICU length of stay (LOS), and rates of ICU admission, mechanical ventilation, and in-hospital mortality. Subgroups examined for the primary outcome included age, sex, combined race/ethnicity, insurance status, immunocompromised status, total number of other comorbidities, specific comorbidities, vaccination status, pandemic phase, and type of mAb treatment.
Variable Definitions
The treatment variable was mAb administration, and the primary starting point (time zero) was the date of any SARS-CoV-2-positive test result. We imputed missing test dates based on the distribution of observed mAb administration dates (additional Methods are provided in e-Appendix 1). Hospitalization was defined as any inpatient or observation encounter documented in the EHR. ED visits were defined as any visit to the ED, with or without an associated inpatient or observation encounter. Presence of comorbid conditions was determined by using a 90-day look-back period in the EHR using established algorithms, and immunosuppressed status was further validated by manual chart reviews. Severity of COVID-19 disease was estimated by using ordinal categories of respiratory support requirements at an encounter level, based on the highest level of support received among the following types (in increasing order): no oxygen, standard (nasal cannula/face mask) oxygen, high-flow nasal cannula or noninvasive ventilation, and invasive mechanical ventilation.19 In-hospital mortality was the highest level of disease severity.
Pandemic phase was categorized according to SARS-CoV-2-positive date based on the prevalent variant in Colorado as pre-Alpha (November 2020-February 2021), Alpha (March 2021-June 2021), and Delta (July 2021-December 2021). No virus sequencing results were available on an individual patient basis. Vaccination status at the time of SARS-CoV-2-positive date was categorized as fully vaccinated (at least 14 days following the primary vaccine series) or not fully vaccinated, which included partially vaccinated (receipt of at least one vaccine dose but primary series either not completed or completed within 14 days of SARS-CoV-2-positive test date) or not known to be vaccinated. mAb treatments included bamlanivimab (Eli Lilly), casirivimab + imdevimab (Regeneron), bamlanivimab + etesevimab (Eli Lilly), and sotrovimab (GlaxoSmithKline) Additional Methods are provided in e-Appendix 1.
Statistical Analysis
We present results descriptively and adjusted for potential confounders. All regression models for outcomes were adjusted for age, sex, race/ethnicity, insurance status, BMI, immunocompromised status, number of comorbidities, pandemic phase, and vaccination status. For binary outcomes such as hospitalization, logistic regression was used to determine odds of the outcome. For count outcomes such as LOS, Poisson regression was used to estimate incidence rates. Disease severity was analyzed by using ordinal logistic regression to estimate the proportional odds. Cumulative incidence curves were constructed by using Kaplan-Meier estimates to visually assess temporal trends according to treatment status.
Subgroup analyses were conducted to estimate heterogeneity of treatment effect for the primary outcome of all-cause hospitalization to day 28. For each subgroup, we calculated unadjusted rates of hospitalization, number-needed-to-treat (NNT) to prevent one hospitalization (based on absolute risk reduction in unadjusted hospitalization rates), and adjusted relative odds of hospitalization. Results are presented as effect sizes, with 95% CIs, and were not adjusted for multiple comparisons.
Three sensitivity analyses were performed (additional Methods are provided in e-Appendix 1). Briefly, the first evaluated a full imputation approach for missingness in key variables, including BMI, immunocompromised status, race/ethnicity, and number of comorbid conditions. The second included only EUA-eligible subjects (e-Table 9) as verified by using available EHR data. The third used a more conservative imputation method for missing SARS-CoV-2-positive test dates by assuming all missing positive test dates were 10 days prior to the mAb administration date (the maximum time difference allowed by the EUA). All outcome models were repeated for these two cohorts and results compared with primary analyses. All statistical analyses were performed by using R Statistical Software version 3.6.0 (R Foundation for Statistical Computing).
Results
Characteristics of mAb-Treated and mAb-Untreated Cohorts
Of 36,077 patients with SARS-CoV-2 infection, 2,675 receiving mAbs were matched to 6,677 patients not receiving mAbs (e-Fig 1). The characteristics of mAb-treated and mAb-untreated patients in the primary cohort are presented in Table 1 . The mAb-treated cohort generally reflects EUA criteria for use of mAbs, with many being older (40.7% were aged ≥ 65 years), having higher BMI (50.1% with BMI > 25 kg/m2), and/or having one or more comorbidities (73.6%). Although there were clinically important differences between mAb-treated and mAb-untreated patents in the full cohort (e-Table 1), propensity matching eliminated clinically meaningful differences between groups on matching variables (e-Table 2, Table 1). The mean ± SD time from positive SARS-CoV-2 test result to receipt of mAb treatment was 3.7 ± 2.5 days.
Table 1.
Baseline Characteristics According to mAb Treatment Status for Primary Matched Cohort
| Characteristic | mAb Treated (n = 2,675) | mAb Untreated (n = 6,677) |
|---|---|---|
| Age, ya | ||
| 18-54 | 1,018 (38.1) | 3,025 (45.3) |
| 55-64 | 569 (21.3) | 1,635 (24.5) |
| ≥ 65 | 1,088 (40.7) | 2,017 (30.2) |
| Femalea | 1,453 (54.3) | 3,705 (55.5) |
| Race/ethnicitya | ||
| Non-Hispanic White | 2,215 (82.8) | 5,323 (79.7) |
| Hispanic | 264 (9.9) | 775 (11.6) |
| Non-Hispanic Black | 64 (2.4) | 189 (2.8) |
| Other | 132 (4.9) | 390 (5.8) |
| Insurance statusa | ||
| Private/commercial | 1,355 (50.7) | 3,840 (57.5) |
| Medicare | 1,052 (39.3) | 1,989 (29.8) |
| Medicaid | 164 (6.1) | 543 (8.1) |
| None/uninsured | 44 (1.6) | 118 (1.8) |
| Other/unknown | 60 (2.2) | 187 (2.8) |
| BMI, kg/m2a | ||
| < 18.5 | 23 (0.9) | 60 (0.9) |
| 18.5-24.9 | 362 (13.5) | 875 (13.1) |
| 25.0-29.9 | 571 (21.3) | 1,374 (20.6) |
| ≥ 30.0 | 770 (28.8) | 2,013 (30.1) |
| Missing | 949 (35.5) | 2,355 (35.3) |
| Immunocompromiseda | 809 (30.2) | 1,677 (25.1) |
| No. of other comorbid conditionsa | ||
| 0 | 708 (26.5) | 1,837 (27.5) |
| 1 | 681 (25.5) | 1,967 (29.5) |
| ≥ 2 | 1,286 (48.1) | 2,873 (43.0) |
| Diabetes | 561 (21.0) | 1,173 (17.6) |
| Cardiovascular disease | 557 (20.8) | 1,290 (19.3) |
| Pulmonary disease | 891 (33.3) | 2,109 (31.6) |
| Renal disease | 344 (12.9) | 607 (9.1) |
| Hypertension | 1,293 (48.3) | 2,881 (43.1) |
| Obesity | 808 (30.2) | 2,073 (31.0) |
| Vaccination status | ||
| Not known to be vaccinated | 1,620 (60.6) | 4,394 (65.8) |
| Partially vaccinated | 148 (5.5) | 485 (7.3) |
| Fully vaccinated | 907 (33.9) | 1,798 (26.9) |
| Pandemic phase | ||
| Pre-Alpha: November 2020-February 2021 | 388 (14.5) | 984 (14.7) |
| Alpha: March 2021-June 2021 | 615 (23.0) | 1,794 (26.9) |
| Delta: July 2021-September 2021 | 1,672 (62.5) | 3,899 (58.4) |
| Type of monoclonal antibody | ||
| Bamlanivimab | 413 (15.4) | … |
| Bamlanivimab + etesevimab | 87 (3.3) | … |
| Casirivimab + imdevimab | 2,157 (80.6) | … |
| Sotrovimab | 18 (0.7) | … |
Data are presented as No. (%).
Variables used in the propensity matching. mAb = monoclonal antibody.
Hospitalization and Mortality
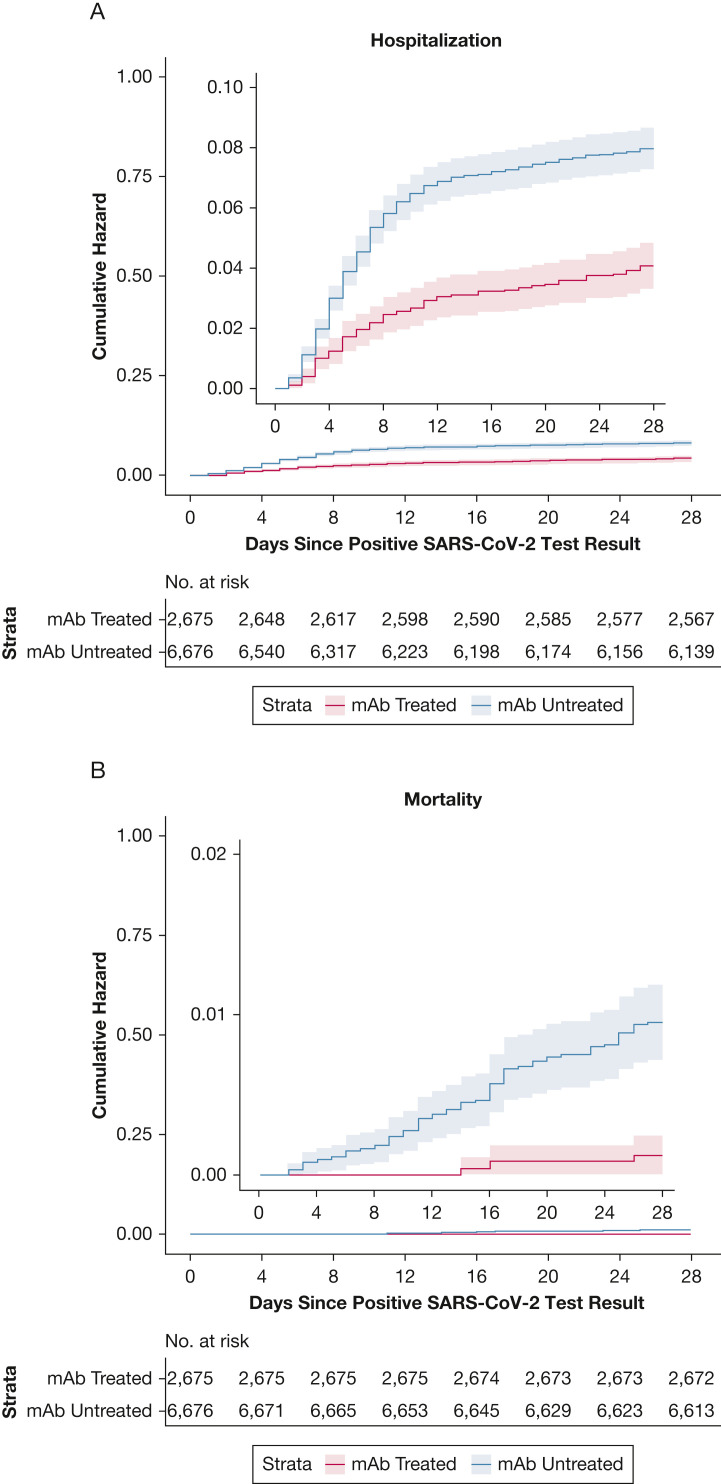
The rate of 28-day all-cause hospitalization was lower among mAb-treated patients compared with matched mAb-untreated control subjects (4.0% v 7.7%; adjusted OR, 0.48; 95% CI, 0.38-0.60) (Table 2 ); the full model results are provided in e-Table 3. All-cause 28-day mortality in the mAb-treated group was 0.1% compared with 0.9% among the mAb-untreated group (adjusted OR, 0.11; 95% CI, 0.03-0.29). These differences persisted to day 90 (90-day hospitalization adjusted OR of 0.53 [95% CI, 0.44-0.65] and 90-day mortality adjusted OR of 0.17 [95% CI, 0.06-0.35]). Overall, ED visit rates were higher for mAb-treated patients compared with mAb-untreated patients (18.7% vs 16.9%; adjusted OR, 1.24; 95% CI, 1.09-1.40); however, mAb-treated patients had fewer ED visits resulting in hospitalization (16.0% vs 37.6%; adjusted OR, 0.29; 95% CI, 0.21-0.38).
Table 2.
Primary and Secondary Outcomes According to mAb Treatment Status
| Outcome | mAb Treated | mAb Untreated | Adjusted OR | 95% CI |
|---|---|---|---|---|
| Overall sample size | n = 2,675 | n = 6,677 | … | … |
| All-cause hospitalization | ||||
| 28-day (primary outcome) | 108 (4.0) | 511 (7.7) | 0.48 | 0.38-0.60 |
| 90-day | 138 (5.2) | 590 (8.8) | 0.53 | 0.44-0.65 |
| All-cause mortality | ||||
| 28-day | 3 (0.1) | 63 (0.9) | 0.11 | 0.03-0.29 |
| 90-day | 6 (0.2) | 84 (1.3) | 0.17 | 0.06-0.35 |
| Any ED visit to day 28 | 501 (18.7) | 1,128 (16.9) | 1.24 | 1.09-1.40 |
| ED visit leading to hospitalization | 80/501 (16.0) | 424/1,128 (37.6) | 0.29 | 0.21-0.38 |
| Hospitalized sample size | n = 108 | n = 511 | … | … |
| Hospital LOS, mean ± SD, da | 5.8 ± 6.5 | 8.5 ± 9.8 | 0.64 | 0.51-0.82 |
| IMV or death | 5 (4.6) | 85 (16.6) | 0.22 | 0.07-0.52 |
| ICU admission | 13 (12.0) | 100 (19.6) | 0.52 | 0.26-0.97 |
| ICU LOS, mean ± SD, da | 3.5 ± 2.8 | 8.6 ± 9.9 | 0.22 | 0.10-0.48 |
Data are presented as No. (%) unless otherwise indicated. All regression models adjusted for age, sex, race/ethnicity, BMI, immunocompromised status, number of other comorbidities, insurance status, pandemic phase, and vaccination status. IMV = invasive mechanical ventilation; LOS = length of stay; mAb = monoclonal antibody.
Poisson regressions presented as adjusted incidence rate ratios with 95% CIs.
Based on a time-to-event analysis, the benefits associated with reduced hospitalization are largely accrued within 10 days of the positive test date, while the mortality benefit of mAb treatment continues to accrue over 28 days (Fig 1 ). Treatment benefits persisted to day 90 for both hospitalization and death (e-Fig 2).
Figure 1.
Cumulative incidence plots for all-cause hospitalization (A) and mortality (B) to day 28 according to mAb treatment status. mAb = monoclonal antibody.
Severity of Hospitalization
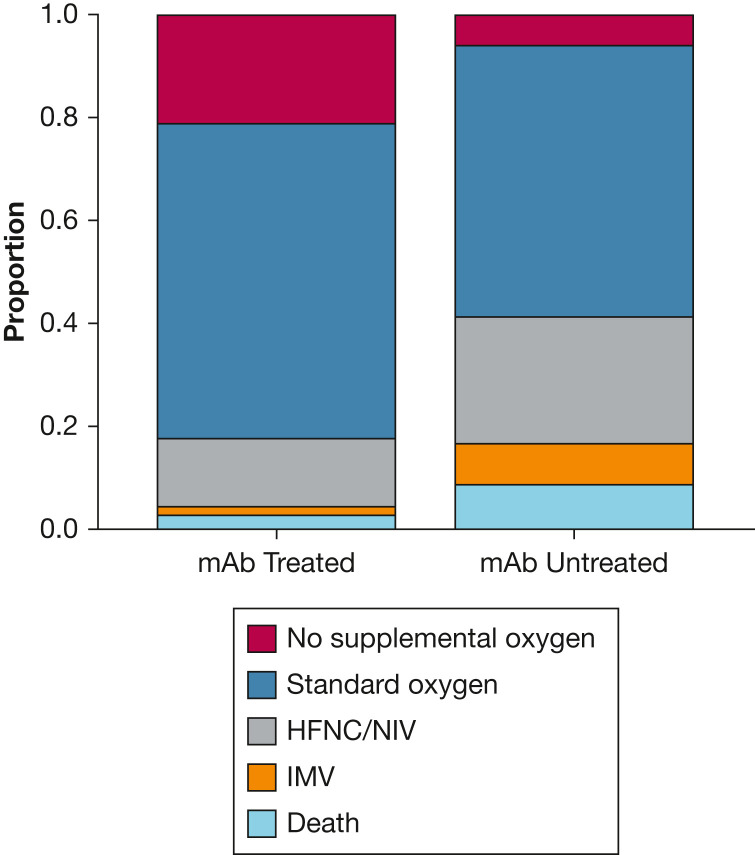
For patients requiring hospitalization, prior receipt of mAbs was associated with lower hospital LOS among survivors (5.8 vs 8.5 days; adjusted incidence rate ratio, 0.64; 95% CI, 0.51-0.82) and a lower rate of ICU admission (12.0% vs 19.6%; adjusted OR, 0.52; 95% CI, 0.26-0.97), and mechanical ventilation or death (4.6% vs 16.6%; adjusted OR, 0.22; 95% CI, 0.07-0.52) (Table 2). For those requiring ICU care, prior receipt of mAbs was associated with shorter ICU LOS (3.5 vs 8.6 days; adjusted incidence rate ratio, 0.22; 95% CI, 0.10-0.48). Overall, severity of hospitalization was lower across the illness continuum for mAb-treated patients (Fig 2 ).
Figure 2.
Maximum respiratory support according to mAb treatment status among patients hospitalized within 28 days. Comparing severity of hospitalizations for 108 mAb-treated and 511 mAb-untreated patients, the maximum level of respiratory support was lower for mAb-treated patients (adjusted proportional OR, 0.25; 95% CI, 0.16-0.38). HFNC = high-flow nasal cannula; IMV = invasive mechanical ventilation; mAb, monoclonal antibody; NIV = noninvasive ventilation.
Subgroup Analyses
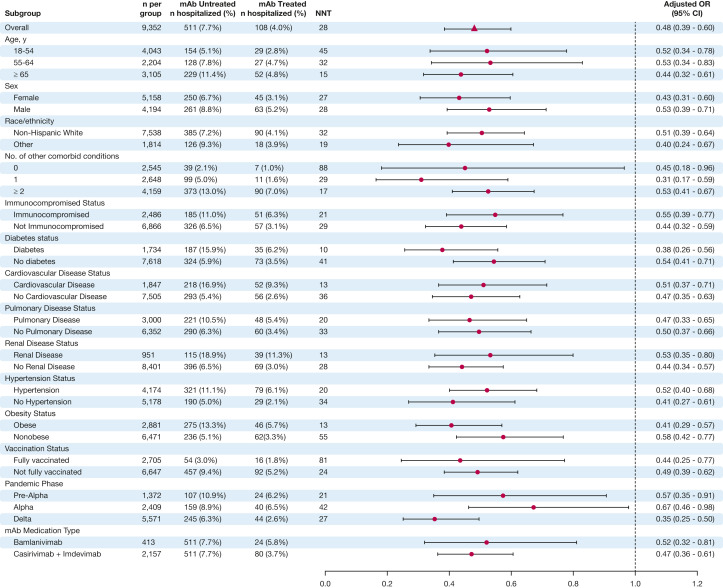
The relative benefit of mAb therapy on reducing 28-day hospital admissions among key demographic and clinical subgroups was broadly similar across all subgroups (Fig 3 ). Of note, the association between mAb treatment and prevention of hospitalizations was at least as high during the Delta phase (OR, 0.35; 95% CI, 0.25-0.50), compared with the Alpha phase (OR, 0.67; 95% CI, 0.46-0.98). In addition, there was similar relative effectiveness for fully vaccinated (OR, 0.44; 95% CI, 0.25-0.77) and not fully vaccinated (OR, 0.49; 95% CI, 0.39-0.62) patients. However, the absolute treatment effect was higher for subgroups with higher baseline risk of hospitalization. For example, the number-needed-to-treat (NNT) to prevent one hospitalization was 15 for patients aged ≥ 65 years, 17 for those with at least two comorbid conditions, and 24 for those not fully vaccinated against SARS-CoV-2, compared with NNT of 45 for age 18 to 45 years, 88 for those without comorbidities, and 81 for fully vaccinated patients. Notably, only a small proportion of patients who were fully vaccinated against SARS-CoV-2 were hospitalized (1.8% of mAb-treated and 3.0% of mAb-untreated patients), and no patients died who were fully vaccinated and received mAb treatment.
Figure 3.
Subgroup analysis of mAb effect on 28-day hospitalization. For each subgroup, we calculated unadjusted rates of hospitalization, NNT to prevent one hospitalization (based on absolute risk reduction in unadjusted hospitalization rates), and adjusted relative odds of hospitalization. Each adjusted OR represents a separate model. All regression models adjusted for age, sex, race/ethnicity, BMI, immunocompromised status, number of comorbidities, insurance status, pandemic phase, and vaccination status. Results were not adjusted for multiple comparisons. mAb = monoclonal antibody; NNT = number-needed-to-treat.
Sensitivity Analyses
Three sensitivity analyses were performed, the first evaluating a full multiple imputation approach to key missing variables, the second restricting the cohort to only patients meeting EUA eligibility criteria based on available EHR data, and the third using a more conservative imputation method when the date of a positive SARS-CoV-2 test result was missing. None of these analyses materially changed the main results (e-Tables 4-8).
Discussion
We report real-world evidence that presents novel results on both a high effectiveness of mAb treatment in reducing hospitalization during the Delta variant phase and a remarkable overall mortality benefit with an 89% lower mortality at 28 days. Neutralizing mAbs are widely seen as important tools for managing surging cases of COVID-19; however, prior studies could not evaluate effectiveness of mAbs against Delta variant infections and have been underpowered to evaluate the impact of mAbs on the most clinically important outcome, patient mortality. The current study fills these key knowledge gaps.
There have also been critical gaps in understanding the effects of mAbs on important subgroups of patients, such as those with older age, comorbid conditions, and prior SARS-CoV-2 vaccination. With the large sample size, we describe the clinical benefits of mAb administration among virtually all subgroups examined, with similar relative benefits in terms of reduced odds of hospitalizations across all subgroups. These subgroup findings highlight the need to interpret relative benefits in light of highly variable absolute hospitalization rates, because the NNT to avert one hospitalization depends on both mAb effectiveness and baseline rates of hospitalization. For example, we found a similar relative effect size for vaccinated and unvaccinated patients, but the NNT to avert one hospitalization among unvaccinated patients is 24, whereas the NNT for vaccinated patients is 81. These results are of practical importance for policymakers and clinicians because there have been shortages of mAb supplies and infusion capacity.11 , 12 Specifically, our findings suggest that the most efficient use of limited mAb infusion capacity to alleviate strain on hospitals is to preferentially administer mAbs to patients at highest baseline risk for hospitalization, including those who are older, not fully vaccinated, or with multiple comorbid conditions. Notably, 28-day hospitalization among mAb-treated but not fully vaccinated patients was almost threefold higher (5.2%) than for mAb-treated patients who were fully vaccinated (1.8%) and higher even than mAb-untreated patients who were fully vaccinated (3.0%). These data support that SARS-CoV-2 vaccination remains the first-line intervention to prevent COVID-19 hospitalizations, with mAb treatment best used as supplemental therapy for high-risk patients.
The current study has several limitations. The setting was a single health system; although large and representing both urban and rural settings and community and academic hospitals, it is geographically limited to one US state. The study sample had relatively low racial and ethnic minority representation, limiting our ability to detect differences across these key subgroups. Although we used statewide data for mortality and vaccination status, hospitalizations were collected only within this single health system. If mAb-untreated patients were also less likely to be seen in the health system for other services (hence, more likely to be hospitalized elsewhere), this may bias our results toward the null. We also relied on EHR data, including manual chart reviews, which may have missing or inaccurate information about the presence of chronic conditions.20 These factors might have limited our ability to detect the impact of mAb treatment, especially between subgroups. These EHR data do not contain information on SARS-CoV-2 variants at the patient level, and thus variant phases are presented chronologically. However, during Colorado’s Delta phase, > 99% of sequenced SARS-CoV-2 was Delta variant.21 The study’s large sample size allowed the detection of meaningful benefits of mAb therapy for most subgroups, but the study could not detect potentially relevant differences between subgroups. Our propensity scoring method achieved excellent matching between mAb-treated and mAb-untreated patient groups across multiple variables, but unmeasured confounders may remain. Finally, our study was conducted prior to the emergence of the Omicron variant, and there is in vitro evidence of reduced SARS-CoV-2 neutralization by some authorized mAbs.22 , 23 Forthcoming studies will evaluate the effectiveness of each available mAb treatment during the Omicron phase of the pandemic.
Interpretation
Real-world evidence in this study showed that mAb treatment was associated with lower hospitalizations and deaths among COVID-19 outpatients across multiple pandemic phases, compared with matched mAb-untreated patients. For hospitalized patients, prior mAb treatment was associated with notably lower disease severity, including reduced hospital length of stay, ICU length of stay, mechanical ventilation, and death. When access to mAbs is limited, prioritizing patients at highest risk for hospitalization has the most potential to reduce health system strain during the COVID-19 pandemic.
Funding/Support
This study was funded by National Institutes of Health/National Center for Advancing Translational Sciences [grants UL1TR002525, UL1TR002535-03S3, and UL1TR002535-04S2].
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: M. K. W. received research funding from the Patient-Centered Outcomes Research Institute and the Office of the Assistant Secretary for Preparedness and Response; and is an unpaid advisor to National Academies of Sciences, Engineering, and Medicine, including on crisis standards of care during the COVID-19 pandemic, and to the Defense Advanced Research Projects Agency, the Hastings Center, and the Lancet on projects unrelated to mAb treatment. T. D. B. and N. E. C. have received research grants from the National Institutes of Health outside the current work. A. A. G. has received other COVID-19 research grants from the National Institutes of Health, Department of Defense, Centers for Disease Control and Prevention, AbbVie, and Faron Pharmaceuticals, outside the current work. None declared (L. E. B., C. B. D., B. M. K., D. A. M., T. C. O., S. R., J. D. S., H. R. S., A. F. W., R. D. Z., R. J. S.).
Acknowledgments
Author contributions: A. A. G. had full access to all the data in the study and takes responsibility for the integrity of the data, accuracy of the data analysis, and study as a whole. M. K. W., L. E. B., T. D. B., N. E. C., B. M. K., A. F. W., R. J. S., and A. A. G contributed substantially to the study design; T. D. B., C. B. D., D. A. M., T. C. O., S. R., J. D. S., H. R. S., and R. D. Z. contributed substantively to the data collection; L. E. B., N. E. C., and A. F. W. conducted the data analysis; and M. K. W. and A. A. G. wrote the first draft of the manuscript. All authors contributed to the interpretation of results and revising the manuscript.
Collaborators: A full list of the Monoclonal Antibody (mAb) Colorado Research Team and Collaborators is given in e-Appendix 1.
Role ofsponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables are available online under “Supplementary Data”.
Supplementary Data
References
- 1.Johns Hopkins Coronavirus Resource Center United States cases by county. Johns Hopkins University & Medicine. 2020. https://coronavirus.jhu.edu/us-map
- 2.Centers for Disease Control and Prevention Science Brief: SARS-CoV-2 infection-induced and vaccine-induced immunity. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html [PubMed]
- 3.COVID-19 Treatment Guidelines Panel Coronavirus Disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov
- 4.Dougan M., Nirula A., Azizad M., et al. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med. 2021;385(15):1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinreich D.M., Sivapalasingam S., Norton T., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A., Gonzalez-Rojas Y., Juarez E., et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Lynch H., Caplan A., Furlong P., Bateman-House A. Helpful lessons and cautionary tales: how should COVID-19 drug development and access inform approaches to non-pandemic diseases. Am J Bioeth. 2021;21(12):14–19. doi: 10.1080/15265161.2021.1974975. [DOI] [PubMed] [Google Scholar]
- 8.McCreary E.K., Bariola J.R., Minnier T., et al. A learning health system randomized trial of monoclonal antibodies for COVID-19. https://www.medrxiv.org/content/10.1101/2021.09.03.21262551v1
- 9.Webb B.J., Buckel W., Vento T., et al. Real-world effectiveness and tolerability of monoclonal antibody therapy for ambulatory patients with early COVID-19. Open Forum Infect Dis. 2021;8(7) doi: 10.1093/ofid/ofab331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bariola J.R., McCreary E.K., Wadas R.J., et al. Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among non-hospitalized adults with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infect Dis. 2021;8(7) doi: 10.1093/ofid/ofab254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein L. Biden Administration moves to stave off shortages of monoclonal antibodies. Washington Post. 2021. https://www.washingtonpost.com/health/2021/09/14/monoclonal-antibodies-shortage
- 12.National Academies of Sciences Engineering, and Medicine News Release. Strategies to allocate scarce COVID-19 monoclonal antibody treatments to eligible patients examined in new rapid response to government. 2021. https://www.nationalacademies.org/news/2021/01/strategies-to-allocate-scarce-covid-19-monoclonal-antibody-treatments-to-eligible-patients-examined-in-new-rapid-response-to-government
- 13.Angus D.C. Optimizing the trade-off between learning and doing in a pandemic. JAMA. 2020;323(19):1895–1896. doi: 10.1001/jama.2020.4984. [DOI] [PubMed] [Google Scholar]
- 14.ISPOR About real-world evidence. 2021. https://www.ispor.org/strategic-initiatives/real-world-evidence/about-real-world-evidence
- 15.Colorado Department of Public Health and Environment Treatments for COVID-19. 2021. https://covid19.colorado.gov/for-coloradans/covid-19-treatments#collapse-accordion-40911-4
- 16.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho D., Imai K., King G., Stuart E. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Political Analysis. 2007;15:199–236. [Google Scholar]
- 18.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health COVID treatment guidelines Clinical spectrum of SARS-CoV-2 infection. 2021. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum [PubMed]
- 20.Bennett T.D., Moffitt R.A., Hajagos J.G., et al. Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US National COVID Cohort Collaborative. JAMA Netw Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colorado Department of Public Health and Environment COVID-19 data. 2021. https://covid19.colorado.gov/data
- 22.Takashita E., Kinoshita N., Yamayoshi S., et al. Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant. N Engl J Med. 2022;386(10):995–998. doi: 10.1056/NEJMc2119407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanBlargan L.A., Errico J.M., Halfmann P.J., et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies. Nat Med. 2022;28(3):490–495. doi: 10.1038/s41591-021-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.