Abstract
Nijmegen breakage syndrome (NBS) is a genetic disorder characterized by immunodeficiency, microcephaly, and “bird-like” facies. NBS shares some clinical features with ataxia telangiectasia (AT), including increased sensitivity to ionizing radiation, increased spontaneous and induced chromosome fragility, and strong predisposition to lymphoid cancers. The mutated gene that results in NBS codes for a novel double-stranded DNA break repair protein, named nibrin. In the present work, a Spanish NBS patient was extensively characterized at the immunological and the molecular DNA levels. He showed low CD3+-cell numbers and an abnormal low CD4+ naive cell/CD4+ memory cell ratio, previously described in AT patients and also described in the present report in the NBS patient. The proliferative response of peripheral blood lymphocytes in vitro to mitogens is deficient in NBS patients, but the possible link among NBS mutations and the abnormal immune response is still unknown.
Nijmegen breakage syndrome (NBS) is a rare, autosomal recessive disorder characterized by microcephaly, immunodeficiency, and a predisposition to cancer (27). It shares some striking clinical and cellular similarities to the genetic disease ataxia telangiectasia (AT), and for this reason, NBS has been classified as a variant of AT (12). However, NBS patients have neither ataxia nor telangiectasia, and microcephaly is absent from AT patients (25, 27). The serum α-fetoprotein concentration is within the normal range in NBS patients, in contrast to AT patients, about 90% of whom are found to have elevated serum α-fetoprotein concentrations (31). In addition, different defective genes in patients with AT and NBS have been identified (3, 23, 28) and have been mapped in chromosomes 11q23 (8) and 8q21–24, respectively (22), which demonstrates that NBS is a genetic entity distinct from AT.
Patients with both NBS and AT display chromosome instability, hypersensitivity to ionizing radiation, and a lack of DNA replication delay in response to radiation, which is governed, in normal cells, by the protein kinase C (PKC)-mediated upregulation of tumor suppresor protein p53 (9, 13, 14, 15, 18). These similarities suggest that ATM and nibrin, the proteins responsible for AT and NBS, respectively, may play a role in common functions, which appear to be defective in both diseases.
Both ATM and nibrin participate in the processing of double-stranded breaks in DNA (3, 25). It has recently been shown that nibrin, in particular, forms a trimolecular complex, together with Rad50 (a protein similar to those required for the structural maintenance of chromosomes) and Mre11 (with both structural and catalytic activities, including single-stranded DNA endonuclease and double-stranded DNA exonuclease activities). The complex participates in the repair of double-stranded DNA breaks induced by radiation, and the Mre11 hyperphosphorylation observed after DNA damage is dependent on the presence of intact nibrin (6, 7). Recently, it has been shown that the phosphorylation of nibrin induced by ionizing radiation requires catalytically active ATM (29, 32, 33), demonstrating that both proteins may participate in common cellular activation pathways.
The immune deficiency is also severe in patients with NBS and concerns the humoral and cellular immune systems. Given the similarities between NBS and AT, an extensive analysis of the immune system was carried out in an NBS patient. Cellular, humoral, and innate immunities were studied by determining variations in lymphocyte subpopulations, peripheral blood mononuclear cell (PBMC) responses to a complete panel of mitogens that analyze the different lymphocyte activation pathways (T-cell function, B-cell function, and T- and B-cell cooperation), immunoglobulin values, and circulating levels of complement. In addition, the molecular characterization of our NBS patient's mutation has also been carried out.
MATERIALS AND METHODS
Patient.
Our patient is a 5-year-old Spanish boy (born in July 1995) from nonconsanguineous parents. The patient has microcephaly, “bird-like” facies, short height, and normal levels of α-fetoprotein. A brother, probably falsely diagnosed as having lymphoma with Bloom syndrome, died after a bone marrow transplantation. The patient's immunity was monitored for 3 years. He showed persistent fever and symptoms compatible with an acute Epstein-Barr virus (EBV) infection; anti-EBV immunoglobulins (anti-VCA-immunoglobulin G [IgG], 141 [normal value, <11]; anti-VCA-IgM; 25 [normal value, <11]; anti-EBNA, 12 [normal value <11]) were detected in July 1998. Two monoclonal IgM kappa paraproteins were also detected by immunofixation-electrophoresis, and B-cell lymphocytosis was observed in the periphery (see Table 1).
TABLE 1.
Humoral immunity and lymphocyte phenotype in the patient
| Ig or phenotype | Value ina:
|
Reference valuesb | ||
|---|---|---|---|---|
| July 1996 | April 1997 | August 1998 | ||
| Humoral immunity | ||||
| IgG concn (mg/dl) | 188 | 218 | 234 | 518–1447 |
| IgA concn (mg/dl) | 7 | 27 | 23 | 23–137 |
| IgM concn (mg/dl) | 152 | 172 | 222 | 42–212 |
| IgE concn (IU/ml) | — | <8 | <8 | 2–600 |
| IgD concn (mg/dl) | — | 0.9 | <0.85 | 0.94–4.6 |
| IgG1 concn (mg/dl) | — | 168 | 196 | 381–884 |
| IgG2 concn (mg/dl) | — | <4 | <4 | 70–443 |
| IgG3 concn (mg/dl) | — | 15 | <2 | 17–90 |
| IgG4 concn (mg/dl) | — | 3 | 10 | 1–116 |
| Absolute no. of lymphocytes/μl | 1,347 | 2,394 | 2,708 | 1,600–4,000 |
| T cells (%) | ||||
| CD2 | — | 46 | 36 | 62–87 |
| CD5 | — | 30 | 30 | 61–82 |
| CD28 | — | 17 | 20 | 40–65 |
| CD3 | 50 | 29 | 31 | 59–77 |
| CD3+ CD4+ | 20 | 8 | 13 | 29–49 |
| CD4 naive | — | 1 | 2 | 16–39 |
| CD4 memory | — | 7 | 11 | 5–15 |
| CD3+ CD8+ | 30 | 18 | 15 | 11–37 |
| NK cells, CD16+ CD3− (%) | 45 | 52 | 14 | 3–18 |
| B cells, CD19 (%) | 8 | 13 | 50 | 8–23 |
Relevant data are shown in boldface. —, not determined.
Reference values for humoral immunity and PBMC phenotypes were obtained from 100 unrelated healthy children under age 14 years.
Immunochemistry and biochemical assays.
Total serum immunoglobulin (IgG, IgA, and IgM) levels, complement factor (C3 and C4) levels, and α-fetoprotein concentrations were measured by nephelometry (Array 360 system; Beckman, Brea, Calif.). Serum hemolytic capacity (CH100) and serum IgE, IgD, and IgG subclass levels were measured with radial immunodiffusion kits (The Binding Site, Birmingham, United Kingdom) and commercial reagents.
Proliferation assays with PBMCs.
A total of 8 × 104 PBMCs were placed in round-bottom microtiter plates (Nunc, Roskilde, Denmark) in 170 μl of AIM-V culture medium (Gibco BRL, Paisley, United Kingdom) supplemented with 1% penicillin-streptomycin (Difco) and 1% glutamine (20 mM; Whittaker, Walkersville, Md.) (1). The optimal concentrations of each stimulus were calculated from the dose-response curves used in our laboratory after standardization with control samples. The stimuli or their combinations were used in triplicate, and the results were taken into account if data were altered in several follow-up tests (see Table 2) (1). The wells were individually pulsed with 1 μCi of [3H]thymidine after 3 days of culture, and uptake of the [3H]thymidine was measured in a liquid scintillation counter (1205-Betaplate; Pharmacia LKB-Wallac, Turku, Finland). To facilitate the interpretation of the results, the data were normalized as the net percentage of the counts per minute obtained with a given stimulus relative to the maximum stimulus in the same assay (the maximum stimulus or 100% response is equal to the stimulus obtained with phytohemagglutinin A [PHA] plus interleukin-2 [IL-2], done in parallel for each experiment): (the proliferative response to one mitogen [in counts per minute] × 100)/maximum stimulus in the same assay (in counts per minute). The counts per minute corresponding to background proliferation (calculated with cells in AIM-V medium) were always 0 to 1% of the mean counts per minute for all the stimuli for the controls and the patient.
TABLE 2.
Lymphocyte function in the patient
| Stimulus | Value ina:
|
Reference valuesb | |
|---|---|---|---|
| April 1997 | July 1998 | ||
| Antigens | |||
| Enterotoxin A | 28 | 11 | 43–81 |
| Protein A of Staphylococcus aureus | 10 | 4 | 9–14 |
| Protein A + PMA | 26 | 22 | 22–28 |
| Monoclonal antibodies | |||
| α-CD2 + PMA | 54 | 31 | 33–77 |
| α-CD3 | 33 | 21 | 21–32 |
| α-CD3 + PMA | 25 | 31 | 56–78 |
| α-CD26 + PMA | 29 | 56 | 30–98 |
| α-CD28 + PMA | 23 | 38 | 15–100 |
| α-CD69 + PMA | 6 | 14 | 11–36 |
| Lectins | |||
| PHA | 51 | 25 | 29–34 |
| PHA + PMA | 40 | 48 | 59–72 |
| ConA | 18 | 30 | 34–44 |
| ConA + PMA | 5 | 10 | 48–87 |
| PWM | 17 | 10 | 27–37 |
| PWM + PMA | 11 | 11 | 32–54 |
PBMC function is expressed as the net relative percentage of each stimulus to the maximum stimulus. The maximum stimulus or 100% response is equal to the stimulus obtained with PHA plus IL-2, done in parallel for each experiment. Relevant data are shown in boldface.
Reference values were obtained from healthy children under age 14 years assayed in parallel.
Cytogenetic studies.
Chromosome preparations and Giemsa-stained metaphases were obtained by standard methods from PHA-stimulated PBMCs (24). Chromosome instability, with 16% of peripheral cells showing spontaneous chromosome breaks, was observed in the patient at the age of 1 year. The following rearrangements were found: 5% t(7;14)(p13;q11.2); 1% 47,XY,der(7)t(7;14) (p13;q11.2),der(7)t(7;7)(pter→q35::p13→pter),del(14)(q11.2),+f;4% inv(7)(p13;q35); 1% t(1;8)(p13;q24.1),inv(7)(p13q35); 3% t(7;14)(q35;q11.2); 1% t(1;7)(p13;q35); and 1% t(14;20)(q11.2;q13.3). Analysis of 25 unbanded, stained metaphase cells from PHA-stimulated PBMCs showed a spontaneous chromosome breakage rate of 0.08 breaks/cell (control range, 0 to 0.05 breaks/cell), and analysis of 50 unbanded, stained metaphase cells exposed to diepoxybutane (0.1 μg/ml) showed a chromosome breakage rate of 0.26 breaks/cell (control range, 0 to 0.1 breaks/cell).
Cytofluorographic analysis.
For direct immunofluorescence, 105 cells were incubated for 30 min with monoclonal antibodies for detection of the different lymphocyte populations (T, B, and NK cells) and subpopulations (see Table 1) (1). Cells were washed twice with phosphate-buffered saline plus 0.01% NaN3, and three-color and quantitative analysis for two-color fluorescence was carried out in an EPICS-XL flow cytometer (20).
Scanning for mutations.
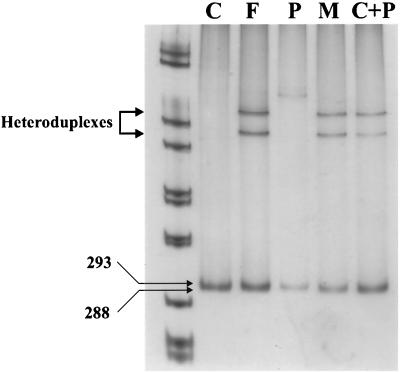
Cytoplasmic RNA was extracted by using the Nonidet P-40 lysis method (24). DNA was obtained from the nuclear pellet by standard methods (4). Reverse transcription was done with 0.5 μg of cytoplasmic RNA by a one-step reverse transcription-PCR method (Gibco BRL) by using for the reaction specific, partly overlapping primers that cover all of the nibrin-coding sequence. The primers used were NBS[1] (a) (5′-AGCCCCGGTTACGCGGTTGC-3′) and (b) (5′-GGCTTTACAATTGGACGTCC-3′), NBS[2] (a) (5′-ATGCACTCACCTTGTCATGG-3′) and (b) 5′-CGCCAATCCAATTTCTGC-3′), NBS[3] (a) 5′-AATGGATATGCTCCAAAGGC-3′) and (b) (5′-TTATACTTGGCAATTTAGTTGG-3′), NBS[4] (a) (5′-TTTGGCTAAGATGAGAATCC-3′) and (b) (5′-TTGCTACTTTCTGGTACTGC-3′), and NBS[5] (a) (5′-AAGGCCAAGGATGGATATAG-3′) and (b) (5′-GCTTACTAGGAAGTTTTTCCATGG-3′). Additional primers used for sequencing of the mutation in exon 6 were NBS(EX6D) (5′-CACTCCGTTTACAATTTAATAGC-3′) and NBS(EX6I) (5′-CACAAAATCCCAAAATGAAATACG-3′), which rendered a product of 293 bp. DNA amplification was done as described previously (4). Scanning for mutations was done by restriction endonuclease fingerprinting assay (16). One microgram of the PCR product was digested separately with five restriction endonucleases. Denatured and nondenatured PCR digestion products were electrophoresed in 6 to 10% polyacrylamide gels with the Protean II vertical electrophoresis system (gel size; 20 by 20 cm; Bio-Rad Laboratories, Hercules, Calif.) and silver stained (Bio-Rad). The heteroduplex analysis was developed to determine the carrier status of the patient's family. Briefly, an aliquot of the genomic 293-bp PCR fragment was heat denatured and then renaturalized and electrophoresed in a nondenaturing polyacrylamide gel.
For DNA sequencing, PCR products were purified by using the QIAquick PCR purification kit (QIAGEN, Hilden, Germany). Double-stranded DNA was directly sequenced by Sanger's dideoxy chain terminator method (2) with dye-labeled dideoxy terminators by PCR (Applied Biosystems, Warrington, United Kingdom).
RESULTS
Mutation analysis and karyotype.
Nibrin cDNA was scanned for mutations; the analysis was based on reverse transcription-PCR followed by a modification of the restriction endonuclease fingerprinting assay (16). The coding sequence of the nibrin mRNA was divided into five partially overlapping fragments, and each fragment was analyzed separately. The analysis showed a change in the NBS[3] cDNA fragment with respect to that for a healthy control. Direct sequencing revealed a homozygous 5-bp deletion (AAAAC) at nucleotide 657 (exon 6), which shifts the normal reading frame from residue 238 and which produces a truncation of the protein at residue 254 due to generation of a premature stop codon, therefore, the normal nibrin protein is not synthesized, causing the complete loss of function of this protein.
A heteroduplex assay of exon 6 was designed to test the carrier status of the patient's parents. Figure 1 shows a band of 293 bp corresponding to the nonmutated NBS gene fragment for the normal control. For the lanes for the patient's father and mother, this band was indistinguishable from the one representing the mutated allele, since the latter one is only 5 bp smaller. However, the lanes for the parents showed two more bands which did not appear in those for the control or the patient. In order to demonstrate that the two bands could correspond to heteroduplexes formed among PCR products coming from both mutated and nonmutated alleles, individual PCR products from the control and the patient were mixed, heat denatured, renatured again, and run in the electrophoresis (lane C+P). Figure 1 (lane C+P) shows a two-band pattern similar to that described above, supporting the presence of the heteroduplexes in the parents. This demonstrated the carrier status of both parents for the same mutation.
FIG. 1.
Heteroduplex assay for the patient's family. Lanes: C, control; F, father; P, patient; M, mother. In lane C+P, parts of the control and patient amplifications were mixed and treated in the same way. As can be seen, a heteroduplex is formed, as in the mutation carriers. The homoduplex migrates at 293 bp in the control.
Karyotype analysis of the patient's PBMCs showed three different clonal populations of cells (16% of the cells examined) with rearrangements involving chromosomes 7 and 14 at breakpoints 7p13, 7q35, and 14q11.2. The rearrangements corresponded to chromosome bands containing immunologically relevant genes (T-cell-receptor α, β, and γ chains and the immunoglobulin heavy chain) (19).
Humoral immunity and lymphocyte subpopulations.
Humoral immunity was altered in the patient: a total absence of IgG2, low IgG1 and IgG3 levels, and a severe IgG reduction were the main findings (Table 1). The cellular peripheral phenotype (Table 1) and the lymphocyte function (Table 2) were also dramatically altered. The patient showed a progressive impairment in T-cell development, as shown by the small number of CD3+ cells. Low CD4+ cell levels accounted for the CD3+-cell reduction, while the CD8+ T-cell number was normal (Table 1). The patient had a severe disruption of the CD4+ naive cell/CD4+ memory cell ratio, with there being “memory” cells almost exclusively (contrary to what is expected in a healthy boy). A B-cell (CD19+) increase was recorded in the third study, which, together with a biclonal IgM kappa paraprotein detection, reflected the EBV infection recorded in the patient by that time. In addition, a significant NK-cell (CD16+) increase was recorded in the first two studies; the NK-cell number was normal in August 1998 (Table 1). The following parameters analyzed were unaltered in the patient compared to those for the controls: −C3, C4, and CH100 concentrations or activity in peripheral blood and −CD18, CD7, CD43, CD57, T-cell receptor γδ, and CD14 PBMC subpopulations (data not shown).
Impairment of PMA plus lectin activating responses.
NBS deficiency disrupts several lymphocyte activation pathways. The patient's PBMCs presented decreased proliferative responses to some of the stimuli assayed in the in vitro functional evaluation. The normalization of the data and the use of a serum-free medium allowed comparison of results obtained at different times (in long-term follow-ups) (Table 2). The patient's proliferative responses to IL-2, protein A, CD2, CD28, PHA, or CD3 alone were within the normal range of values. The levels of enterotoxin A-, concanavalin A (ConA)-, and pokeweed (PWM)-induced proliferation were reduced in two studies. Moreover, when phorbol myristate acetate (PMA) was used as a costimulus together with lectins (PHA [first study], ConA, and PWM) no additional induction or a mild inhibition of the responses was obtained. The following parameters were analyzed (using PKC- and non-PKC-dependent stimuli) and were found to be unaltered in the patient compared to those in the controls: proliferation mediated by PMA, recombinant IL-2 (rIL-2), enterotoxin C1, α-CD2, α-CD2 plus rIL-2, α-CD3 plus rIL-2, α-CD2 plus α-CD28, α-CD3 plus α-CD28, PHA plus rIL-2, ConA plus rIL-2, and ionomycin plus PMA (data not shown). Also, a set of recall antigens produced no reaction in vivo (30). Similar results were obtained for three different patients with AT (6a).
DISCUSSION
The present work describes a genetic, phenotypic, and functional characterization of a Spanish NBS patient. The 5-bp deletion in exon 6 has been described previously and represents the founder mutation in most populations of NBS patients; this deletion is present in 90% of the patients with NBS (11, 28). The alterations in chromosomes 7 and 14 recorded in the patient are also commonly found in patients with NBS (27). The chromosome alterations affect the T-cell receptor α-, β-, and γ-chain genes. This could explain the rearrangement failures in T-cell receptors, the probable accumulation of unstable hybrid T-cell receptor molecules and the abortion of its surface expression, and the small number of CD3+ cells. A low proportion of the CD4+ T-cell subset and a decreased CD4+/CD8+ ratio were also found. The prevalence of memory CD4+ cells (and the practical absence of naive CD4+ cells) is described in patients with NBS. Thus, it is possible that a reduced output of T cells from the thymus leads to the accumulation of memory T cells in the periphery. This also occurs in AT patients, in whom dysplastic changes or the absence of the thymus is constantly found (19). The reasons for the large numbers of NK cells observed throughout the first set of studies (Table 1) remain unknown, but a relatively large number of NK cells was noted in other NBS patients (5). On the other hand, the IgG2 deficiency has also been observed in patients with other primary immunodeficiencies that affect T-cell receptor expression and/or function, like those with the CD3γ deficiency (21).
B-cell function was deregulated in the patient and protein A (B-cell antigen)-induced responses were normal in the patient only in the first study, with the deregulation confirmed by the observed reduction in IgG levels in the periphery. Moreover, T-cell–B-cell cooperation, as measured by PWM-induced mitogenesis (which acts by contact of T cells and B cells) was also affected. Naive T cells (CD4+ CD45RA+) are supposed to proliferate better with classical mitogens (PHA, ConA, PWM), and these response patterns (low-level proliferative responses to PHA, Con A and PWM) strongly correlate with the very low proportion of CD4+ CD45RA+ T cells in the patient (19).
The immune consequences of a deficiency of the NBS gene product in many ways resemble the abnormalities seen in AT: hypogammaglobulinemia, alteration of the proportions of lymphocyte subpopulations, and similar defective blastogeneses.
The results obtained from the functional assays with PBMCs show an impairment in some of the lymphocyte proliferative responses induced by PMA, which is an analogue of diacylglycerol and a specific direct activator of the PKC pathway. We have previously reported that PBMCs from AT patients show a general impaired response to different mitogens, especially when phorbol esters (like PMA) are used as costimuli. In patients with AT, PMA inhibits in particular T-lymphocyte proliferative responses to CD3, CD28, and PHA, as well as, mainly, to ConA, PWM, anti-CD26, and anti-CD69.
Interestingly, some evidence for an interplay of the NBS gene product with the ATM protein has been derived from complementation studies based on radiation-induced chromosome breakage in heterodikaryons made from cells from patients with NBS and AT (26, 29, 31, 32). The data obtained from AT patients suggest a differential dependence of PKC isoforms (10) in differential transduction pathways. Moreover, as in AT, the protein coded by the NBS gene, nibrin, may also be involved in cell cycle control, with nibrin acting on p53 modulating genome stability, tumor susceptibility, and apoptosis (17).
Finally, further biochemical assays are needed to find out whether there is more than one PKC activation pathway.
ACKNOWLEDGMENTS
The contributions of M. A. García-Pérez and L. M. Allende were equal, and the order of the authors is arbitrary.
This work was supported in part by grants from the Ministerio de Educación y Ciencia (PM95-57, PM96-21, and PM99-23) and Comunidad de Madrid (06/70/97 and 83/14/98).
REFERENCES
- 1.Allende L M, Corell A, Manzanares J, Madruga D, Marcos A, Madroño A, López-Goyanes A, García-Pérez M A, Moreno J M, Rodrigo M, Sanz F, Arnaiz-Villena A. Immunodeficiency associated with anorexia nervosa is secondary and improves after refeeding. Immunology. 1998;94:543–551. doi: 10.1046/j.1365-2567.1998.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnaiz-Villena A, Timón M, Corell A, Pérez-Aciego P, Martín-Villa J M, Regueiro J R. Primary immunodeficiency caused by mutations in the gene encoding the CD3-γ subunit of the T-lymphocyte receptor. N Engl J Med. 1992;327:529–533. doi: 10.1056/NEJM199208203270805. [DOI] [PubMed] [Google Scholar]
- 3.Carney J P, Maser R S, Olivares H, Davis E M, Le Beau M, Yates J R, Hays L, Morgan W F, Petrini J H. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 4.Corell A, Martin-Villa J M, Morales P, de Juan M D, Varela P, Vicario J L, Martínez-Laso J, Arnaiz-Villena A. Exon-2 nucleotide sequences, polymorphism and haplotype distribution of a new HLA-DRB gene: HLA-DRBς. Mol Immunol. 1991;28:533–543. doi: 10.1016/0161-5890(91)90168-j. [DOI] [PubMed] [Google Scholar]
- 5.Der Kaloustian V M, Kleijer W, Booth A, Auerbach A D, Mazer B, Elliott A M, Abish S, Usher R, Watters G, Vekemans M, Eydoux P. Possible new variants of the Nijmegen breakage syndrome. Am J Hum Genet. 1996;65:21–26. doi: 10.1002/(SICI)1096-8628(19961002)65:1<21::AID-AJMG3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Dong Z, Zhong Q, Chen P L. The Nijmegen breakage syndrome protein is essential for Mre11 phosphorylation uponDNA damage. J Biol Chem. 1999;274:19153–19156. doi: 10.1074/jbc.274.28.19513. [DOI] [PubMed] [Google Scholar]
- 6a.García-Pérez M A, Allende L M, Corell A, Varela P, Moreno A A, Sotoca A, Moreno A, Paz-Artal E, Barreiro E, Arnaiz-Villena A. Novel mutations and defective protein kinase C activation of T-lymphocytes in ataxia-telangiectasia. Clin Exp Immunol. 2001;123:472–480. doi: 10.1046/j.1365-2249.2001.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gatei M, Young D, Cerosaletti K M, Desai-Mehta A, Spring K, Kozlov S, Lavin M F, Gatti R A, Concannon P, Khanna K. ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nat Genet. 2000;25:115–119. doi: 10.1038/75508. [DOI] [PubMed] [Google Scholar]
- 8.Gatti R A, Berkel I, Boder E, Braedt G, Charmley P, Concannon P, Ersoy F, Foround T, Jaspers N G, Lange K, et al. Localization of an ataxia-telangiectasia gene to chromosome 11q22–23. Nature. 1988;336:577–580. doi: 10.1038/336577a0. [DOI] [PubMed] [Google Scholar]
- 9.Gennery A R, Cant A J, Jeggo P A. Immunodeficiency associated with DNA repair defects. Clin Exp Immunol. 2000;121:1–7. doi: 10.1046/j.1365-2249.2000.01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann J. The potential for isoenzyme-selective modulation of protein kinase C. FASEB J. 1997;11:649–669. doi: 10.1096/fasebj.11.8.9240967. [DOI] [PubMed] [Google Scholar]
- 11.The International Nijmegen Syndrome Study Group. Nijmegen breakage syndrome. Arch Dis Child. 2000;82:400–406. doi: 10.1136/adc.82.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaspers N G, Taalman R D, Baan C. Patients with an inherited syndrome characterized by immunodeficiency, microcephaly, and chromosomal instabilty; genetic relationship to ataxia-telangiectasia. Am J Hum Genet. 1988;42:66–73. [PMC free article] [PubMed] [Google Scholar]
- 13.Jongmans W, Vuillaume M, Chrzarowska K, Smeets D, Sperling K, Hall J. Nijmegen breakage syndrome cells fail to induce the p53-mediated DNA damage response following exposure to ionizing radiation. Mol Cell Biol. 1997;17:5016–5022. doi: 10.1128/mcb.17.9.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastan M B, Zhan Q, el-Deiry W S, Carrier F, Jacks T, Walsh W V, Plunkett B S, Vogelstein B, Fornace A J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 15.Khanna K K, Lavin M F. Ionizing radiation and UV induction of p53 protein by different pathways in ataxia-telangiectasia cells. Oncogene. 1993;8:3307–3312. [PubMed] [Google Scholar]
- 16.Liu Q, Sommen S S. Restriction endonuclease fingerprinting (REF): a sensitive method for screening mutations in long, contiguous segments of DNA. BioTechniques. 1995;18:470–477. [PubMed] [Google Scholar]
- 17.Meyn M S. Ataxia-telangiectasia and cellular responses to DNA damage. Cancer Res. 1995;55:5991–6001. [PubMed] [Google Scholar]
- 18.Mirzayans R, Famulski K S, Enns L, Fraser M, Paterson M C. Characterization of the signal transduction pathway mediating gamma ray-induced inhibition of DNA synthesis in human cells: indirect evidence for involvement of calmodulin but not protein kinase C nor p53. Oncogene. 1995;11:1597–1605. [PubMed] [Google Scholar]
- 19.Paganelli R, Scala E, Scarselli E, Ortolani C, Cossarizza A, Carmini D, Aiuti F, Fiorilli M. Selective deficiency of CD4+/CD45RA+ lymphocytes in patients with ataxia-telangiectasia. J Clin Immunol. 1992;12:84–91. doi: 10.1007/BF00918137. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Aciego P, Alarcón B, Arnaiz-Villena A, Terhorst C, Timon M, Segurado O G, Regueiro J R. Expression and function of a variant T cell receptor complex lacking CD3-γ. J Exp Med. 1991;174:319–326. doi: 10.1084/jem.174.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regueiro J R, Pérez-Aciego P, Aparicio P, Martínez C, Morales P, Arnaiz-Villena A. Low IgG2 and polysaccharide response in a T cell receptor expression defect. Eur J Immunol. 1990;20:2411–2416. doi: 10.1002/eji.1830201108. [DOI] [PubMed] [Google Scholar]
- 22.Saar K, Chrzanowska K H, Stumm M, Jung M, Nurnberg G, Wienker T F, Seemanova E, Wegner R D, Reis A, Sperling K. The gene for the ataxia-telangiectasia variant, Nijmegen breakage syndrome, maps to a 1 cM interval on chromosome 8q21. Am J Hum Genet. 1997;60:605–610. [PMC free article] [PubMed] [Google Scholar]
- 23.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle D A, Smith S, Uziel T, Sfez S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 Kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 24.Seabright M A. A rapid banding technique for human cytogenetics. Lancet. 1997;ii:971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- 25.Shiloh Y. Ataxia-telangiectasia and the Nijmegen breakage syndrome: related disorders but genes apart. Annu Rev Genet. 1997;31:635–662. doi: 10.1146/annurev.genet.31.1.635. [DOI] [PubMed] [Google Scholar]
- 26.Stumm M, Sperling K, Wegner R D. Noncomplementation of radiation-induced chromosome aberrations in ataxia-telangiectasia/ataxia-telangiectasia-variant heterodikaryons. Am J Hum Genet. 1997;60:1246–1251. [PMC free article] [PubMed] [Google Scholar]
- 27.van der Burgt I, Chrzanowska K H, Smeets D, Weemaes C. Nijmegen breakage syndrome. J Med Genet. 1996;33:153–156. doi: 10.1136/jmg.33.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varon R, Vissinga C, Platzer M, Cerosaletti K M, Chrzanowska K H, Saar K, Beckmann G, Seemanova E, Cooper P R, Nowak N J, Stumm M, Weemaes C M, Gatti R A, Wilson R K, Digweed M, Rosenthal A, Sperling K, Cancannon P, Reis A. Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell. 1998;93:467–476. doi: 10.1016/s0092-8674(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 29.Wang J Y J. New link via a web of human genes. Nature. 2000;405:404–405. doi: 10.1038/35013171. [DOI] [PubMed] [Google Scholar]
- 30.Wegner R D, Metzger M, Hanefeld F, Jaspers N G J, Baan C, Magdorf K, Kunze J, Sperling K. A new chromosomal instability disorder confirmed by complementation studies. Clin Genet. 1988;33:20–32. [PubMed] [Google Scholar]
- 31.Woods C G, Taylor A M R. Ataxia-telangiectasia in the British Isles. The clinical and laboratory features of 70 affected individuals. Q J Med New Ser. 1992;82:169–179. [PubMed] [Google Scholar]
- 32.Wu X, Ranganathan V, Weisman D S, Heine W F, Ciccone D N, O'Neill T B, Crick K E, Pierce K A, Lane W S, Rathbun G, Livingston D M, Weaver D T. ATM phosphorylation of Nijmegen breakage syndrome protein is required in a DNA damage response. Nature. 2000;405:477–482. doi: 10.1038/35013089. [DOI] [PubMed] [Google Scholar]
- 33.Zhao S, Weng Y C, Yuan S S, Lin Y T, Hsu H C, Lin S C, Gerbino E, Song M H, Zdzienicka M Z, Gatti R A, Shay J W, Ziv Y, Shiloh Y, Lee E Y. Functional link between ataxia-telangiectasia and Nijmegen breakage syndrome gene products. Nature. 2000;405:473–477. doi: 10.1038/35013083. [DOI] [PubMed] [Google Scholar]