Abstract
With more than 5 million fatalities and close to 300 million reported cases, COVID-19 is the first documented pandemic due to a coronavirus that continues to be a major health challenge. Despite being rapid, uncontrollable, and highly infectious in its spread, it also created incentives for technology development and redefined public health needs and research agendas to fast-track innovations to be translated. Breakthroughs in computational biology peaked during the pandemic with renewed attention to making all cutting-edge technology deliver agents to combat the disease. The demand to develop effective treatments yielded surprising collaborations from previously segregated fields of science and technology. The long-standing pharmaceutical industry's aversion to repurposing existing drugs due to a lack of exponential financial gain was overrun by the health crisis and pressures created by front-line researchers and providers. Effective vaccine development even at an unprecedented pace took more than a year to develop and commence trials. Now the emergence of variants and waning protections during the booster shots is resulting in breakthrough infections that continue to strain health care systems. As of now, every protein of SARS-CoV-2 has been structurally characterized and related host pathways have been extensively mapped out. The research community has addressed the druggability of a multitude of possible targets. This has been made possible due to existing technology for virtual computer-assisted drug development as well as new tools and technologies such as artificial intelligence to deliver new leads. Here in this article, we are discussing advances in the drug discovery field related to target-based drug discovery and exploring the implications of known target-specific agents on COVID-19 therapeutic management. The current scenario calls for more personalized medicine efforts and stratifying patient populations early on for their need for different combinations of prognosis-specific therapeutics. We intend to highlight target hotspots and their potential agents, with the ultimate goal of using rational design of new therapeutics to not only end this pandemic but also uncover a generalizable platform for use in future pandemics.
Keywords: SARS-CoV-2, COVID-19, Drug targeting, Rational improvement, Artificial intelligence, Target-based drug discovery, Mathematical modeling
1. Introduction
Since the beginning of the COVID-19 pandemic, which is caused by SARS-CoV-2, there has been an impending question ‘what can be the standard course of therapy, and which agents need to be trialed. The first year of the pandemic followed Murphy's Law (Bloch, 2003) with the ensuing chaos causing severe mortality rates due to a lack of population immunity and the use of ineffective interventions. The rapid global spread of the disease overwhelmed medical care systems due to exponential regional surges. As of July 4th, 2022, the pandemic has claimed 6.35 million lives worldwide and caused over 0.5 billion cases of infection (“WHO Coronavirus Disease (COVID-19) Dashboard,” n.d.). The USA has been the worst hit with more than a million deaths out of 87.5 million cases (Dong et al., 2020; Ruhm, 2022). The surge in cases is often at an intensity that its severity is made worse by a shortage of medical resources. This has stymied trials conducted for several agents (Robinson et al., 2022). Many promising initial reports of therapeutic approaches became proven failures, and yet they often were needlessly trialed repeatedly by different groups. Hampering effective therapeutic development, the rush to trials often fell short in the number of patients recruited. This under empowerment and the varying degree of symptom sets leads to prognosis and therapeutic response variability which makes it difficult to stratify patient populations. This was further exacerbated by the changing pathophysiology caused by newer variants, which combined with the evolving self-medication landscape, resulted in inconsistent trial data for some agents and ultimately unreliable outcome results (Watson, 2022). Prohibitive costs of newer drugs, as well as antibody therapies, have generated worldwide interest in trying a variety of agents to reduce the severity of COVID-19 infection. For instance, preliminary evidence suggested that hydroxychloroquine (HQ) therapy can reduce viral load (Gautret et al., 2020). However, a recent meta-analysis of multiple trial data has now concluded that although HQ therapy is safe at the trial doses used, it remains ineffective in reducing mortality and severity of disease (T. Gupta et al., 2022). Conversely, other trials have shown more promising results, such as the use of Oseltamivir (Theraflu), which statistically demonstrated to reduce mortality in COVID-19 patients (Zendehdel et al., 2022). Additionally, various comorbidities like old age, diabetes, obesity, hypertension, and the immunocompromised state contribute to COVID-19 mortality, their associations are still not enough to stratify patients and take universal prophylactic measures (Gentile and Schiano Moriello, 2022) and as a result, new therapeutic interventions remain in high demand.
Computational structural biology is a interdisciplinary field performed on computer or via computer simulation that encompasses the theory and application of approaches to model, predict, and explain biological function at the molecular level, well-known as in silico experiment. Proteins are flexible molecules that undergo conformational changes (such as folding and unfolding or domain motions) as part of their interactions with other biopolymers as partners or drug molecules. Conformational changes of the proteins might reflect a closed, open, or intermediate states and this dynamical aspect plays a critical role in drug discovery. Nowadays, molecular dynamics makes it possible to simulate these conformational changes with a timescale ranging from nanoseconds to microseconds of time. Molecular dynamics simulations is a computer (in silico) technique that makes it possible to predict how a system will evolve over time and, consequently, to predict the movement of the molecules in the system. In silico methods (molecular modeling, molecular docking or screening, molecular dynamics, etc) could be used to efficiently identify and design drug candidates, to study their interactions with their targets. The Nobel Prize in Chemistry 2013 has been awarded to Martin Karplus, Michael Levitt and Arieh Warshel for development of multiscale models of complex chemical systems as computational techniques for structural biology (https://www.nobelprize.org).
In silico drug discovery has proved to be instrumental in suggesting numerous agents and many of the predicted agents have been used to manage COVID-19. It has been a long-standing principle that the fixed 3D structure of protein dictated by amino acid composition is the basis for assigning function. There have been exceptions to this principle in multiple instances when proteins have multiple structures owing to disordered regions (Anjum et al., 2022; Prateek Kumar et al., 2022a; J. Zhang et al., 2022). This is more evident in RNA viral proteomes due to a higher rate of mutations and a protein often has more than one function. For instance, PLpro is a protease and a deubiquitinase, all of which are important for viral envelope formation, and their functional activities are associated with inflammasome formation in infected cells (Lewis et al., 2022; J. Zhang et al., 2022). Such redundancy, size limitations, and genetic instabilities call for highly flexible proteins which are generally seen in the experimentally solved crystal structure, their variabilities in viral proteins in the form of multiple ‘states’ and confirmations (Fornasier et al., 2022; Siragusa et al., 2022). As starting crystal structure is the bottleneck of any virtual screening effort, this variability led to numerous ‘false’ hits that had no agreement between binding prediction and biological activity (Martin et al., 2020). Like all the other fields, the field of computational biology methods also had multiple breakthroughs which now have more applications than just COVID-19 drug discovery research. Additionally, we now have AI predictions for the shape of nearly every known protein, which can be structurally complementary to drug discovery (Callaway, 2022). Many laboratories have been pioneering novel technologies in the machine learning, AI, and conformational dynamics space (Caulfield and Medina-Franco, 2011; Coban et al., 2020, 2021a, 2021b; Hines et al., 2019a, 2019b; Kayode et al., 2016; Puschmann et al., 2017; Savytskyi et al., 2013).
Recently, the anti-cancer drug Pralatrexate was discovered to have in vitro EC50 values of 0.008 μM. While being a strong immunosuppressant it's usability in COVID-19 is highly debatable the pipeline that delivered this compound comprised of deep learning models and force field dynamics simulations (Zhang et al., 2020, Zhang et al., 2020). With newer and faster methods made available there are multiple methods producing a similar pipeline (Rapicavoli et al., 2022; Zhang et al., 2022, Zhang et al., 2022, Zhang et al., 2022, Zhang et al., 2022). Free energy perturbation calculations enabled Zhang et al., in 2022 to improve main protease Triarylpyridinone inhibitors to have EC50 values as low as 0.080 μM (Ramos et al. 1987).
In this review, we try to boil down protein-inhibitor relationships that have been exploited as anti-COVID-19 therapeutics or have a high validated potential for the same. Such information should be used to steer the computational learning approaches through AI to understand why these work and others don't despite having positive classic predicted interactions. Additionally, we provide a comprehensive analysis of existing, approved, and experimental therapeutics with their mechanism of action against either the viral or host protein targets.
2. Drugging COVID-19: what constitutes a “good” drug?
There have been some controversial agents that have undergone trials against SARS-CoV-2 due to some in vitro reports or proposed mechanisms of action (Ivanova et al., 2022). Many of these agents did not have a consistent effect and had surprising side effects such as QT prolongations (abnormal heart rhythms and sudden cardiac arrest) e.g Chloroquine and Hydroxychloroquine (Table 4) (Deng et al., 2022). While others were not fully effective at tolerable doses e.g. Ivermectin (Hariyanto et al., 2022), some were mildly effective even though they had no interaction with SARS-CoV-2 targets, e.g. oseltamivir (Zendehdel et al., 2022). Some were highly dangerous, especially with the misinformation inspired panicked patient self-medications e.g. Chlorine Dioxide (Chejfec-Ciociano et al., 2022). Since the beginning of the pandemic, Ibuprofen was contraindicated as it is known to increase ACE2 receptor expression in the cells exacerbating viral infectiousness. However, there was widespread use of nebulized ibuprofen (NaIHS) as a wonder cure and reported to be highly effective, had negative correlations and so-called positive effects were probably due to concomitant aggressive corticosteroid therapy (Calonico et al., 2022). As a result, there is a need to understand both classical drug targets and other modalities that may be therapeutic.
Table 4.
Descriptions of anti-COVID-19 agents (non-virus-specific) with data from clinical trials.
| # | Name of the Agent | Total no of patients and trials | No of days of treatment | Outcome (Negative SARS-CoV-2 test conversions (NSTC)) | Contraindications | Refs |
|---|---|---|---|---|---|---|
| 01 | Chloroquine and Hydroxychloroquine | 50 trials 61991 patients |
dosing was usually 400 mg orally BID on day 1 and 200 mg BID on days 2–5. | Found no reductions in mortality, hospitalization, symptoms, and ICU dependency incidence of mechanical ventilation, and NSTC OR = 0.97. | Significant increased odds of QT prolongations (rates 0.39 vs 0.29 treated vs. 0.13 vs 0.09 control) | (Barratt-Due et al., 2021; Deng et al., 2022; Kalantari et al., 2021; Taccone et al., 2020) |
| 02 | Ivermectin | 19 studies 4328 Patients |
Dosing was usually 400 μg per kilogram for 3 days or placebo | Found no reductions in mortality, hospitalization, symptoms and ICU dependency, incidence of mechanical ventilation, and NSTC OR = 0.25 | (Hariyanto et al., 2022; Reis et al., 2022; Shafiee et al., 2022) | |
| 03 | Steroids (Methylprednisolone and Glucocorticoid) | 62 studies,5 trials,7 works of literature 23597 patients |
Dosing was usually (1–2 mg/kg/day for ≤7 days). | Found great reductions in mortality up to 20% (RR = 73 TO 77), hospitalization, symptoms and ICU dependency, the incidence of mechanical ventilation (RR 0.77, increased 28-day ventilator-free days (MD = 0.5 TO 2.81) low-dose (≤2 mg/kg/day) methylprednisolone treatment for ≤7 days was associated with relatively better clinical outcomes, without increasing the duration of viral shedding | could slightly prolong the duration of viral shedding (MD 1.03) | (Ebrahimi Chaharom et al., 2022; Hong et al., 2022; Salvarani et al., 2022; J. Tu et al., 2022; J.G. Zein et al., 2022) |
| 04 | Clevudine | 1 study 61 patients |
Dosing was usually 120 mg orally per day for 14 days |
Found no reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC | Song et al. (2021) | |
| 05 | Methylene Blue | 1 study 63 patients |
Dosing was usually Methylene blue 0.5 mg via nebulization TID |
Found no reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC | (Alemany et al., 2022; Patidar et al., 2022) | |
| 06 | Nitazoxanide | 4 studies 1926 patients |
Dosing was usually 500–600 mg TID for 5–7 days |
Found improvement in the inflammatory outcome but no reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC | (Blum et al., 2021; Mendieta Zerón et al., 2021; Miorin et al., n.d.; Rocco et al., 2021; Rossignol et al., 2022) | |
| 07 | C21 | 1 phase 2 trial 106 patients |
Dosing was usually 100 mg C21 BID 7 days in addition to standard of care |
Found marked reduction of requirement for O2 on day 14. along with no reductions in mortality, hospitalization, symptoms, and ICU dependency incidence of mechanical ventilation, and NSTC | Tornling et al. (2021) | |
| 08 | Niclosamide | 1 phase 2 trial 73 patients |
Dosing was usually 2 g orally daily for 7 days | Found no reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC | Cairns et al. (2022) | |
| 09 | Nafamostat (Nafabelltan) | 1 Pase 2 trial 104 patients |
Dosing was usually 4.8 mg/kg/day plus standard-of-care | Found a shorter median time to clinical improvement in a small group of high-risk patients requiring O2 treatment and no reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC in other patient groups | Zhuravel et al. (2021) | |
| 10 | Indomethacin | 1 study 210 patients (N = 103) |
Dosing was usually 75 mg (OD for BMI <30 and BID for BMI >30) For 5 days |
Found significant symptomatic relief and improved oxygen saturation level, none in the indomethacin group was desaturated. The median days for the resolution of fever is less than 7 days, and cough and myalgia are significantly reduced | Ravichandran et al. (2022) |
3. Techniques for elucidation of drug-target interaction and efficacy
One of the foundations of drug design is to utilize a molecular model of druggable targets. Today's drug discovery labs can draw from a multitude of techniques for determining experimental structures, yet the different techniques have their strengths and weaknesses. For example, membrane proteins are notoriously difficult to crystallize, so the gold standard x-ray crystallography is generally not successful. Typically, cryo-EM is utilized for large proteins/complexes, such as membrane proteins. The caveat here is that cryo-EM is in general a lower resolution technique and may bias conformations because of the air-water interface. A relatively new structural technique is x-ray free-electron laser (XFEL), coupled with lipid-cubic phase crystallization (Ono et al., 2022). Essentially, this consists of growing small crystals in a lipidic environment that is more amenable for membrane proteins, which are then injected at random orientations and illuminated with extremely brilliant x-ray photons to generate diffraction patterns. This has been successfully applied to a variety of membrane proteins recently, though not as yet any COVID-19-related target; however, this technique has potential application in the field as shown with other viruses (Townsend et al., 2021). Proteases and kinase inhibitors have traditionally held roles as drugs of choice for inhibiting virion production (Bain et al., 2003; Mahdi et al., 2020; Pearlman, 2012; “Protein Kinase Inhibitors,” 2012; Zhou et al., 2015), however, in recent times the shift to virus centric proteins has made progress (Chakraborty et al., 2021; Dai et al., 2020; Narayanan et al., 2022; Prajapat et al., 2020; Y.-X. Zheng et al., 2021). Added to these new targets has been the implementation of new computational tools to more quickly address the urgency of the need (Callaway, 2022; Coban et al., 2021b).
4. Enter the era of the machine: learning to use algorithm-guided drug design
The complex multivariate approaches to drug modeling on a molecular structure are well suited to the application of machine learning (ML) techniques. Generative chemistry is at the forefront of new medicinal chemistry design workflows, where the implementation of layered data with context to various data sources allows us to integrate complex datasets into the framework of a deep learning or machine-based intelligence that can find associations otherwise not possible. Both ML and artificial intelligence (AI) are being applied to many areas of biological research. With respect to COVID-19, ML has been used to help screen drug targets, druggable sites on the targets, drugs, and drug-target interactions (El-Behery et al., 2021). This has led to the repurposing of drugs that are already FDA-approved for COVID-19 therapy, the discovery of novel molecules as potential drugs, and the identification of cryptic binding pockets introduced by virus/host protein-protein interaction (Dang and Song, 2022). In addition, ML has been used to mine bioinformatics data and analyze biological pathways to identify novel pathways that can lead to a greater understanding of the disease mechanism, as well as detect additional points of intervention (Auwul et al., 2021). AI has assisted in the analysis of samples to help make rapid diagnoses with a less expensive assay that is highly sensitive, selective, and accurate (Jaroenram et al., 2022; Lai et al., 2022). The method works by employing two pH-dependent dyes and a reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay; the colorimetric readout data was used to train an algorithm for classification i.e. diagnosis of positive or negative infection status. Other uses of ML related to COVID-19 are the large-scale screening for anti-COVID-19 biomolecules in foods (Laponogov et al., 2021). The study used a similar approach to standard drug screening but started with a database of food-based bioactive molecules; they identified 52 molecules predicted to disrupt the COVID-19-host interactome. Engaging in multiple treatment paradigms is beneficial in that it increases the likelihood of therapeutic benefit to the patient, decreases the chance of the virus developing resistance, and can reduce dosing to limit adverse side effects. Interruption of COVID-19 progression with multi-drug therapy looking for synergetic effect with computational biology for high-throughput screening has been successful (Coban et al., 2021b), which has the capability of using mixed algorithms to examine the impact of structural changes. As a result, the application of ML and AI techniques is expected to yield rapid progress in the discovery of new candidates for antiviral use.
5. In silico deduced target-specific leads that reached clinical trials
Favipiravir is a purine analog that is a potent RNA-dependent RNA polymerase (RdRp) inhibitor initially selected on basis of similarities with known target EBOLA RdRp (da Silva et al., 2022; Mashayekhi-Sardoo and Hosseinjani, 2022). Favipiravir showed a 62.8% viral clearance in 4 days compared to untreated (Ivashchenko et al., 2021). While favipiravir has little effect on nonhospitalized patients, its use among hospitalized patients has led to faster viral clearance and better radiological imaging endpoints in multiple trials (Hung et al., 2022). With upcoming reports of long-term lung damage in both hospitalized and nonhospitalized patients (C. Wang et al., 2022; J. Yu et al., 2022), there is a need for a retrospective follow-up trial needed to assess favipiravir's long-term benefits. Icatibant is a known bradykinin type 2 receptor antagonist that was computationally predicted to target the SARS-CoV-2 main protease (Liu and Wang, 2020). However, the clinical trial (NCT04978051) results were inconclusive (Malchair et al., 2022) and there is no target-specific inhibition data available. Lopinavir & Ritonavir are other predicted inhibitors of 3CLpro (Reina and Iglesias, 2022), however, numerous clinical trials have failed to establish their clinical usefulness as anti-COVID-19 medications (Cao et al., 2020; Sheahan et al., 2020). PF-07321332 (nirmatrelvir) a rationally improved second-generation frontrunning drug from Pfizer is in the Phase3 clinical trial, It targets 3CLpro and thereby inhibits viral replication (Vandyck and Deval, 2021). Ciclosporin/Cyclosporine immunomodulatory drug is a calcineurin inhibitor that was discovered through computational host interactome modeling for the SARS virus (SARS-CoV) (Pfefferle et al., 2011) and was predicted to have a positive effect on COVID-19 through immunosuppression (Ellinger et al., 2021). Further, it was found to have antiviral activity in vitro (Dittmar et al., 2021). Later HR (hazard ratio) improvement value of 2.15 was observed in a combination trial with a low dose of steroid (Galvez-Romero et al., 2021) and was an efficacious treatment option in the COQUIMA cohort (Schuurmans and Hage, 2021) and multiple variants (Fenizia et al., 2022). Another 3CLpro inhibitor, found through in silico screenings was Cepharanthine (CEP), a small phyto-alkaloid obtained from the Stephania cepharantha. CEP had IC50 of 1.90 μm (Hijikata et al., 2022) against the Wuhan strain (wild type) and consistent activity against three other VOCs (Prabhakaran Kumar et al., 2022). It's a promising anti-COVID-19 candidate in animal testing offering significant protection from lung fibrosis in bleomycin (BLM)-challenged rats (Li et al., 2022).
6. Cytotherapy
Cellular therapies have been proven to protect immunosuppressed patients (>20% mortality rate) by providing anti-viral cellular immunity and immune modulation for vulnerable patient populations (Farhangnia et al., 2022; Verma et al., 2022). Different trials with SARS-CoV-2 specific T-cell trials (allogeneic CSTs familial or HLA matched), Natural killer (NK) cell (e.g. FT516 cells), Tregs (T regulatory cell), and Mesenchymal Stem Cell Infusion or Stem Cell Products have shown therapeutic potential comparable to available antiviral therapies (Conway et al., 2022). With a longer lifespan of T-cells, there is longer-lasting protection than humoral immunity.
7. Biological activities of SARS-CoV-2 components as potential therapeutic targets
A wide variety of targets are addressable for attenuating the infection progression of SARS-CoV-2 as depicted in Fig. 1 . As previously mentioned, therapeutics active on some of these targets are now in clinical trials. Yet many more Non-Structural Protein (NSP) targets have been identified and are in various stages of development (Table 1 ). In this review, we will address both classical drug targets (enzymatic vs non-enzymatic) and new modalities for possible use as COVID therapies.
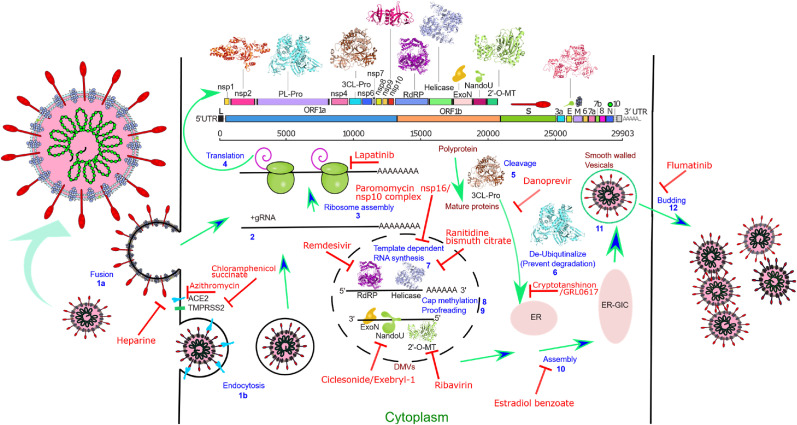
Fig. 1.
Schematic depiction of different SARS-CoV-2 proteome (ORF map) coded targets(3D ribbons or cartoons) involved in different steps of viral replication (labeled blue) and various example inhibitors (labeled red). The infection cycle starts when the SARS-CoV-2 Spike protein binds to a Human receptor followed by either viral-host cell fusion (1a) or endocytosis (1b). Fusion directly allows the viral RNA to enter the host cell (2), The large viral script is known to encode 29 viral proteins (3), A viral-specific translation yields two replicase polyproteins, pp1a and pp1ab, and many small ORFs(4). The two major polyproteins are processed by two proteases, PLpro and 3CLpro(5), generating 16 NSPs. ExoN possesses a viral exoribonuclease activity (9). Viral Helicase plays a critical role in viral replication by unwinding dsRNA formed during replication as well as tertiary structures of genomic RNA. (7). The enzyme 2′-O-MT methylates the viral 2′ end which is important for selective translation and protection from host RNA degradation (8). RdRP along with different NSPs is involved in viral-host cell replication through catalyzing template synthesis of polynucleotides in the 5′–3′ direction (7). NendoU is an Mn2+ dependent hexamer (dimer of trimer) enzyme responsible for protein interference with the innate immune system. For viral assembly of structural proteins (S, E, and M) in the endoplasmic reticulum, along with the N protein is combined with the (+) gRNA to become a compact helical nucleoprotein complex(10). They assemble to form a virus particle in the endoplasmic reticulum-Golgi apparatus compartment and are then excreted from the cell through budding mediated by the fusion of smooth-walled vesicles to the plasma membrane (11–12).
Table 1.
In vitro validated anti-SARS-CoV-2 agents reported with a known target.
| Agent name & SARS-CoV-2 target | Kind of agent | Assay/validation with SARS-CoV-2 | IC50 (μM) If available |
Previously known target? | Mechanism of action of approved use | Reference |
|---|---|---|---|---|---|---|
| Darunavir NSP enzymatic -Main peptidase |
Protease inhibitors (synthetic compound) | It was done using the High-performance liquid chromatography (HPLC) method. | 5.55 | Target decreasing the risk of HIV transmission to other people. | Works by decreasing HIV amount in the blood. | Costanzo et al. (2020) |
| Teicoplanin NSP enzymatic -Main peptidase |
Glycopeptide antibiotic | It was done using the ultra-high performance liquid chromatography–high-resolution mass spectrometry method. | 8.78 | Target various infections caused by gram-positive bacteria. | It inhibits peptidoglycan polymerization, leading to the inhibition of bacterial cell wall synthesis and cell death. | (F. Yu et al., 2022) |
| Nelfinavir NSP enzymatic -Main peptidase |
A viral protease inhibitor | Done using in vitro and in vivo genetic toxicology assays. | 37 | Targets HIV in adults and children. | Works by preventing HIV virion from fully maturing and becoming infective. | (Foo et al., 2021; Ohashi et al., 2021) |
| Bortezomib NSP enzymatic -Main peptidase |
A proteasome inhibitor | Done using HPLC-UV Method | 1.39 | Targets multiple myeloma, or mantle cell lymphoma in patients. | Works by preventing uncontrolled degradation of IκB, an inhibitory protein of NF-κB. | Shen et al. (2022) |
| α-ketoamide inhibitor compound 13b NSP enzymatic -Main peptidase |
Protease inhibitor | It was done using MD simulation. | 0.67 ± 0.18 | Targets M pro of α-and β-coronaviruses in addition to 3C proteases of enterovirus. | Works by inhibiting the replication of SARS-CoV-2 in human Calu3 lung cells. | Zhang et al. (2020) |
| Telaprevir NSP enzy matic -Main peptidase |
An NS3/4A viral protease inhibitor | It was done using in-vitro analysis. | 11.54 | Targets chronic Hepatitis C Virus infections. | Works by inhibiting viral HCV genotype 1 replication. | Mahmoud et al. (2021). |
| Boceprevir NSP enzymatic -Main peptidase |
Protease inhibitor. | It was done through molecular docking and subsequent experimental validation. | 1.95 ± 1.62 (EC50) | Targets chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV). | Works by binding the serine (S139) residue in the active site via an (α)-ketoamide functional group, inhibiting the proteolytic activity of the HCV 1a and 1b encoded enzyme. | Ma et al. (2020b) |
| Ebselen NSP enzymatic -Main peptidase |
Antioxidant drug | It was done using in vitro and in vivo studies. | 4.67 | Targets Meniere's Disease, Type 2 Diabetes Mellitus, and Type 1 Diabetes Mellitus. | Works by modulating metalloproteins, enzymatic cofactors, gene expression, epigenetics, antioxidant defenses, and immune systems. | Jin et al. (2020) |
| Dactolisib NSP enzymatic -Main peptidase |
An imidazoquinoline derivative. | It was done using in vitro and in vivo studies. | 0.225 | Targets Cancer, Solid Tumor, Renal Cancer, Breast Cancer, and Cowden Syndrome, among others. | Works by inhibiting PI3K kinase and mTOR kinase in the PI3K/AKT/mTOR kinase signaling pathway, which may result in tumor cell apoptosis and growth inhibition in PI3K/mTOR- overexpressing tumor cells. | Garcia et al. (2021) |
| Alvocidib NSP enzymatic -Main peptidase |
A synthetic flavonoid | It was done using bioanalytical methods. | Targets cancer. | Works by inhibiting cyclin-dependent kinases, arresting cell division, and causing apoptosis in non-small lung cancer cells. | Fong (2020) | |
| Methotrexate NSP enzymatic -Main peptidase |
Antimetabolites | It was done using the HPLC-SRM-MS plasma analysis. | It targets severe psoriasis, certain types of cancer including uterine, breast, and lung cancer, certain types of lymphoma, certain cancers of the head and neck, and leukemia. | Works by slowing the growth of cancer cells. Equally, it decreases the activity of the immune systems to treat rheumatoid arthritis. | Stegmann et al. (2021) | |
| Carmofur NSP enzymatic -Main peptidase |
Antineoplastic drug or chemotherapeutic agent. | It was done using In Vitro and in Vivo biological evaluations. | 28.2 ± 9.5 | Targets colorectal and breast cancer. | Works by controlling cancer cell proliferation, suppressing N-acylethanolamine acid amidase (NAAA) activity. | Ma et al. (2020a) |
| Conivaptan NSP enzymatic -Main peptidase |
An antidiuretic hormone inhibitor. | Done using bio-analytical HPLC-MS/MS method | 12.2 ± 4.20 | Target euvolemic or hypervolemic hyponatremia in hospitalized patients. | Works by raising serum levels. | Yang et al. (2020) |
| Atovaquone NSP enzymatic -Main peptidase |
An antiprotozoal agent. | It was done using a spectrophotometric method. | 6.78 ± 0.73 | Targets Pneumocystis pneumonia in adults and teenagers. | Works by stopping specific protozoa from causing pneumonia. | Yang et al. (2020) |
| Vilazodone NSP enzymatic -Main peptidase |
An antidepressant | It was done using the Spectrofluorimetric Detection method. | below 15 | Targets depression in adults. | Works by raising the serotonin activity in the brain. | Ghasemiyeh et al. (2021) |
| Michael acceptor inhibitor N3 NSP enzymatic -Main peptidase |
Protease inhibitor. | It was done using QM/MM simulations. | 16.77 (EC50) | Targets SARS-CoV-2. | Works by inhibiting SARS-CoV-2 3CLpro. | Jin et al. (2020) |
| Raloxifene NSP enzymatic -Main peptidase |
A selective estrogen receptor modulator. | It was done using competitive binding assays. | 4.50–7.99 | Targets osteoporosis and breast cancer in high-risk postmenopausal women. | Works by promoting estrogen-like effects on lipid metabolism. | Imamura et al. (2021) |
| Ouabain NSP enzymatic -Main peptidase |
A cardioactive glycoside. | It was done through cell biological studies. | 0.030 μM - 0.075 | It targets atrial fibrillation and flutter, and heart failure. | Works by inhibiting the Na–K-ATPase membrane pump. | Farag et al. (2020) |
| GC373 NSP enzymatic -Main peptidase |
Feline drug | It was done using downstream biochemical assays. | 0.40 0.05 | Targets SARS-CoV-2. | Works by inhibiting SARS-CoV-2 M pro. | Vuong et al. (2020) |
| GC376 NSP enzymatic -Main peptidase |
Prodrug | It was done using a fluorescence resonance energy transfer (FRET)–based cleavage assay. | 0.19 0.04 | Targets SARS-CoV-2. | Works by inhibiting SARS-CoV-2 M pro. | Vuong et al. (2020) |
| Imatinib NSP enzymatic -Main peptidase |
A tyrosine kinase inhibitor | It was done using UPLC-MS/MS assay and ultrafiltration method. | 0.17 | Targets gastrointestinal stromal tumors, leukemias, systemic mastocytosis, myelodysplastic/myeloproliferative disease, dermatofibrosarcoma protuberans, and hypereosinophilic syndrome. | Works by inhibiting the Bcr-Abl tyrosine kinase and proliferation of cells and induces apoptosis in fresh leukemia cells and Bcr-Abl positive cell lines. | Han et al. (2021) |
| Triclabendazole NSP enzymatic -Main peptidase |
An anthelmintic drug. | It was done using in Vitro and animal studies. | 70 | Targets fascioliasis in livestock and humans. | Works by reducing resting membraned and inhibiting tubulin function and enzyme and protein necessary for Fasciola species survival. | Gao et al. (2020) |
| Emedastine NSP enzymatic -Main peptidase |
A selective H1-receptor antagonist. | It was done using an in Vitro study. | 82 ± 7 | Targets allergic conjunctivitis. | Works by managing symptoms of allergic conjunctivitis. | Gao et al. (2020) |
| Bendamustine NSP enzymatic |
An antineoplastic agent | It was done using an in-vitro study. | 26 ± 1 | Targets chronic lymphocytic leukemia (CLL) and indolent B-cell non-Hodgkin lymphoma. | Working by causing intra- and inter-strand crosslinks between DNA bases resulting in cell death. | Gao et al. (2020) |
| Mebendazole NSP enzymatic -Main peptidase |
An anthelmintic or anti-worm medication. | It was done using a spectrophotometric method in the UV region. | 0.25–1.2 | Targets infections caused by hookworm, pinworm, whipworm, and roundworm infections. | Works by preventing newly hatched insect larvae (worms) from growing or multiplying in your body. | Ahmed et al. (2021) |
| Carprofen NSP enzymatic -Main peptidase |
A nonsteroidal anti-inflammatory drug | It was done using an in vitro Study. | 3.97 ± 0.60% | Targets arthritic symptoms in geriatric dogs. | Works by inhibiting cyclooxygenase activity. | Gimeno et al. (2020) |
| Lapatinib NSP enzymatic -Main peptidase |
An anti-cancer drug | It was done using in Vitro and animal studies. | 31.1 | Targets solid tumors such as breast and lung cancer. | Works by binding to the intracellular phosphorylation domain to prevent receptor autophosphorylation upon ligand binding. | Lau et al. (2021) |
| Celecoxib NSP enzymatic -Main peptidase |
A nonsteroidal anti-inflammatory drug. | It was done using a validated HPLC analytical method. | 13.02 | Targets mild to moderate pain and symptoms of arthritis. | Works by suppressing hormones causing inflammation and pain. | Mostafa et al. (2020) |
| Retapamulin NSP enzymatic -Main peptidase |
A topical antibiotic agent. | It was done through In Vitro studies. | Targets impetigo. | Works by inhibiting the initiation of protein synthesis by binding to a specific site on the 50S subunit of the bacterial ribosome. | ||
| Bafetinib NSP enzymatic -Main peptidase |
Antineoplastic drug | It was done through a quantitative readout performed by mass spectrometry. | 0.79 | Targets Adult Gliosarcoma, Adult Mixed Glioma, Adult Glioblastoma, Chronic Myeloid Leukemia, and Acute Lymphocytic Leukemia, among others. | Works by inhibiting the Bcr/Abl fusion protein tyrosine kinase. | Meyer et al. (2021) |
| Masitinib NSP enzymatic -Main peptidase |
Antineoplastic and immunomodulating agents. | It was done using randomized, placebo-controlled phase trial studies. | 3.8 | Targets cell tumors in dogs. | Works by inhibiting tyrosine-kinase. | Drayman et al. (2021) |
| Simeprevir NSP enzymatic -Main peptidase |
A direct-acting antiviral agent | It was done using HPLC with Fluorescence Detection. | 9.6 ± 2.3 | Targets chronic hepatitis C viral infection in adults with HCV genotype 1 or 4. | Works by inhibiting HCV NS3/4A protease. | Lo et al. (2021) |
| Grazoprevir NSP enzymatic -Main peptidase |
An antiviral and NS3/4A protease inhibitor | It was done using the RP-HPLC method. | Targets hepatitis C infections. | Works by inhibiting viral HCV replication. | Abidi et al. (2021) | |
| Ciluprevir NSP enzymatic -Main peptidase |
An orally active inhibitor of the HCV NS3 protease. | It was done using a randomized, multiple-dose, double-blind, placebo-controlled pilot study. | 20.77 | Targets hepatitis treatment. | Works by blocking NS3 protease-dependent polyprotein processing in HCV replicon-containing cells. | Baker et al. (2021) |
| Narlaprevir NSP enzymatic -Main peptidase |
An antiviral drug and protease and proteinase inhibitor. | It was done using In Vivo and In Vitro studies. | 1.10 | Targets chronic hepatitis. | Works by inhibiting hepatitis C protease, SARS coronavirus main proteinase, and coronavirus. | (Bai et al., 2021; Baker et al., 2021) |
| Silibinin NSP enzymatic -Main peptidase |
An antioxidant and antineoplastic agent. | It was done using in Vitro and anima research studies. | Targets toxic liver damage and cancer. | Works by altering cell proliferation, metastasis, invasion, apoptosis, and angiogenesis. | Hamdy et al. (2022) | |
| Suramin & Quinacrine NSP enzymatic -Main peptidase |
Protease inhibitor | It was done using in Vitro studies. | 6.3 ± 1.4 | Targets SARS-CoV-2. | Works by inhibiting ARS-CoV-2 main protease (3CLpro). | Eberle et al. (2021) |
| Bisindolmaleimide-IX NSP enzymatic -Main peptidase |
An enzyme inhibitor. | It was done using a virtual screening pipeline and in-vitro validation assays. | 113.7 ± 5.2 | Targets chronic lymphocytic leukemia. | Works by inhibiting protein kinase C and inducing apoptosis. | Gupta et al. (2021b) |
8. NSP enzymatic targets
8.1. Main Peptidase
The 3CLpro/Mpro gene is the Main Peptidase of SARS coronavirus and is responsible for ∼11 cleavage sites in viral propeptide. As a result, it is an essential target for both viral replicase as well as structural assembly for completing the viral cycle (Gupta et al., 2021b). This 306 amino acid long protease has a catalytic core with C145 and H41 and is highly conserved among variants to preserve essential function (Gupta et al., 2021a) but also has multiple conformation states making drug targeting difficult (Savytskyi and Kornelyuk, 2022). The most recent PF-07321332 (nirmatrelvir) is a Pfizer anti-SARS-CoV-2 compound targeting 3CLpro (Reina and Iglesias, 2022). In combination with ritonavir, a xenobiotic degradation reducing agent for PF-07321332 (Lamb, 2022), the drug combination has shown a strong efficacy across multiple SARS-CoV-2 variants (Ullrich et al., 2022). Additional research on combinations with other antiviral agents targeting different components (e.g. Monupiravir/remdesivir for RdRp) is ongoing (Table 1). Earlier, in silico predictions discovered a 3CLpro inhibitor, Atazanavir, that was later shown to block viral replication (Fintelman-Rodrigues et al., 2020) and showed positive outcomes in various trials (Kalantari et al., 2021). However, due to many side effects such as hepatotoxicity, Atazanavir failed to be a drug of choice in the long run (Mazaherpour et al., 2021). Daclatasvir is a well-accepted HCV therapeutic and its combination with sofosbuvir is well tolerated and efficacious (Merat, 2020). While both Daclatasvir and sofosbuvir had anti-SARS-CoV-2 activity, the combination showed inconsistent results in different trials but had an overall positive effect (Chan et al., 2021, p. 2). Another anti-HCV protease inhibitor Danoprevir showed some efficacy in initial trials (H. Chen et al., 2020) but was abandoned in Phase 4 trials (NCT04345276).
8.2. Papain-like proteinases
Papain-like viral protease (Plpro) is named NSP 3 and is a versatile enzyme that processes the viral polypeptide into functional proteins similar to 3CLpro but has Catalytic triad C111, H272, and D286 which is also highly conserved (Fu et al., 2021). While activating it also protects viral peptides being attacked by host proteasome machinery and de-ubiqutinylase Lys-linked polyUb chains (Lewis et al., 2022). Although a potential therapeutic target, drugs blocking Plpro have yet to be identified.
8.3. RNA-dependent RNA polymerase
Viral RNA-dependent RNA polymerase (RdRp) is identified in the SARS-CoV-2 genome as the NSP 12, It's part of a large replicase complex carrying out RNA replication. This protein class has been a highly exploited target in several RNA viruses and the resulting inhibitors have served as a rich pool for many repurposable antivirals (Abolhassani et al., 2021). While all the natural variants in SARS-CoV-2 are highly susceptible to remdesivir (Pitts et al., 2022), studies have shown the possibility of mutational resistance which is contraindicated for monotherapy (Stevens et al., 2022). Azvudine is a 4′-Modified Nucleoside and a potent anti-HIV drug candidate (Chang, 2022). Early trials showed Azvudine as a promising anti-COVID-19 agent with evident shortening of nucleic acid negative conversion (Ren et al., 2020), but it has only been regionally approved as an anti-HIV therapeutic in China and has not been trialed elsewhere. AT527 (RO-7496998) a.k.a. bemnifosbuvir is an oral purine nucleotide prodrug that has potent in vitro antiviral activity SARS-CoV-2 (Shannon et al., 2022) and has also shown a shortening of disease tenure in early trials (Good et al., 2021). Clevudine a pyrimidine analog is an anti-HBV drug that underwent a trial in the Korean republic but was grossly ineffective (Song et al., 2021). Sofosbuvir (PSI-7977), an approved anti-HCV phosphoramidite prodrug (Messina et al., 2022), is a treatment that has been shown to reduce mortality and improve associated clinical outcomes in patients with COVID-19 (Hsu et al., 2022). Molnupiravir is a prodrug and it is hydrolyzed by esterases to form intermediate ribonucleoside N-hydroxycytidine (NHC) which is further phosphorylated intracellularly yielding active agent NHC triphosphate (NHC-TP) (Cox et al., 2021; Wahl et al., 2021). It is a well-tolerated and highly effective anti-COVID-19 treatment owing to its high bioavailability (Table .2 ) (Caraco et al., 2022; Jayk Bernal et al., 2022; Whitley, 2022).
Table 2.
Descriptions of anti-viral agents from clinical trials.
| # | Name of the agent | The total no of patients and trials | No of days of treatment | Outcome (Negative SARS-CoV-2 test conversions (NSTC)) | Contraindications | Reference(s) |
|---|---|---|---|---|---|---|
| 01 | Remdesivir | 13 studies >100000 patients |
Dosing was usually 200 mg on day 1 followed by 100 mg for 5 days or up to 10 days | Found significant greater improvement in mortality, hospitalization, symptoms, and ICU dependency incidence of mechanical ventilation, in patients with no oxygen or low oxygen (efficacy 74%–87%) however, did not lower any kind of risk in patients receiving high-flow oxygen | (Angamo et al., 2022; Barratt-Due et al., 2021; Beckerman et al., 2022; Beigel et al., 2020; Costanzo et al., 2020) | |
| 02 | Lopinavir/Ritonavir | 38 studies 12352 patients |
Dosing was usually Lopinavir/ritonavir 400mg/100 mg BID for 5–10days. Along with standard-of-care | Found no reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC (MD: 1.09) | (Bahman Amani et al., 2021; Costanzo et al., 2020; Kalantari et al., 2021; Mazaherpour et al., 2021) | |
| 03 | Oseltamivir | 5 studies >100000 patients |
Dosing was usually 30 mg,45 mg,75 mg, and BID for 5 days. Along with standard-of-care | Found no reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC (SMD of 1.65 days) | increased severity of disease and risk of mortality OR = 4.20, | Zendehdel et al. (2022) |
| 04 | Umifenovir(arbidol) | 16 studies and 1 phase 3 trails | Dosing was usually Umifenovir 800 mg BID, maximum 14 days, along with standard-of-care | Found no reductions in mortality, hospitalization, symptoms, and ICU dependency incidence of mechanical ventilation, and NSTC RR = 1.1 | associated with higher adverse events RR: 2.24 | (Behnam Amani et al., 2021; Y. Lin et al., 2021; Ramachandran et al., 2022) |
| 05 | Sofosbuvir-based (Daclatasvir, ledipasvir, velpatasvir, ravidasvir) | 8 articles and 11 trials and 4 studies 3079 patients |
Dosing was usually 400 mg Sofosbuvir and 60 mg Daclatasvir |
Found lower mortality OR = 0.49 to 0.59 RR = 0.31, ICU dependency incidence of mechanical ventilation (RR = 1.20, P = 0.011), and some certainty of the evidence for clinical recovery with combination with Sofosbuvir/Daclatasvir. | (Chan et al., 2021; Hsu et al., 2022; Kow et al., 2022; Merat, 2020; Messina et al., 2022; A.F.M.Z. Zein et al., 2022) | |
| 06 | Molnupiravir | 1 Phase 3 trial 1433 patients |
Dosing was usually 800 mg orally BID daily for 5 days only |
initiated within 5 days after the onset of symptoms found reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC | there is a theoretical risk that molnupiravir will be metabolized by the human host cell and incorporated into the host DNA, leading to mutations | (Caraco et al., 2022; Jayk Bernal et al., 2022; Wong et al., 2022) |
| 07 | Nirmatrelvir based | 1 Phase 2–3 trial 2246 patients |
Dosing was usually 300 mg of nirmatrelvir plus 100 mg of ritonavir BID for 5 days |
Found reductions in progression to severe RR reduction 88.9%, along with reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and viral load was lower at day 5 of treatment | (Hammond et al., 2022; Lamb, 2022; Reina and Iglesias, 2022; Wong et al., 2022) | |
| 08 | GIAPREZA (Angiotensin II receptor blocker) | 1 study 132 patients |
Dosing was usually One-time inclusion | Found faster decrease in Fio2 and positive effect on BP in the first 12H of infusion and later no reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC | Serpa Neto et al. (2022) | |
| 09 | Losartan (Angiotensin II receptor blocker) | 2 studies 1683 patients |
Dosing was usually Max 50 mg orally twice daily for 10 days |
Found losartan has a protective role against COVID-19 mortality in hypertensive patients only. No reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC in non-hypertensive patients | Puskarich et al. (2022) | |
| 10 | Famotidine (Selective histamine H2-receptor (H2R) antagonist) | 9 studies 39745 patients |
Dosing was usually 20 or 40 mg oral or IV median of 5–6 days | Found no reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC | Freedberg et al. (2020) | |
| 11 | Plitidepsin | 1 phase 1 trial 46 patients | Dosing was usually 1.5 mg (n = 15), 2.0 mg (n = 16), or 2.5 mg (n = 15) OD for 3 days. | Found reductions in viral load concerning their baseline value, and improvement of biomarkers associated with inflammatory processes. There were reports of prompt clearance of pneumonia infiltrates in some participants with available chest imaging performed for medical reasons | Varona et al. (2022) | |
| 12 | Heparin (Standard heparin, and low molecular weight heparin) | 3 Trials, 33 studies 25768 patients |
No fixed does was used | Found significant reduction in mortality, invasive mechanical ventilation, and any thrombotic event in moderately ill patients and found no reductions in mortality, hospitalization, symptoms and ICU dependency incidence of mechanical ventilation, and NSTC | (Giossi et al., 2021; Thachil, 2020) |
8.4. Helicase
NSP 13 is an ATP-dependent Helicase with a 5'to 3′ polarity acting on either double-stranded RNA/DNA (Shu et al., 2020). Among all SARS-CoV-2 NSPs, Helicase is the most conserved among different beta coronavirus species (Jang et al., 2020). There are reports of helicase forming a complex with RdRp/replicase complex suggesting a role in proofreading during RNA replication (J. Chen et al., 2020). Also, there are isolated reports of helicase affecting infected cell interferon (IFN) signaling to neighboring healthy cells by altering JAK1 phosphorylation of SAT1 (Fung et al., 2022). While there were multiple helicase inhibitors discovered against SARS and MERS there were not many interesting leads for SARS-CoV-2 despite high sequence similarity (Cimolai, 2020) except amantadine or memantine that have been shown in isolated reports to be effective in COVID-19 with neurological symptoms (Rejdak and Grieb, 2020). Ranitidine bismuth citrate also targets helicases and was initially shown to be highly effective in protecting Syrian hamster COVID-19 animal models (Yuan et al., 2020). While SARS-CoV-2 helicase is highly susceptible to bismuth salts, which are accepted to be the primary mechanism (Shu et al., 2020), zinc chelation (Zamai, 2021, p. 20) and allosteric main protease inhibition (Tao et al., 2021) additional mechanisms in play. A pilot study showed that 50% of patients receiving Bismuth Subsalicylate (BSS) became RT-PCR negative, however, authors state issues with dosage and bioavailability.
8.5. NendoU
NSP 15 is a uridylate-specific endoribonuclease (NendoU) that exists as a homo hexamer (Tran et al., 2022). While NendoU is highly conserved among most of the nidoviruses, especially vertebrates infecting coronaviruses, its knockouts are known to replicate at par with wild types (Grellet et al., 2022). The role of NendoU is to protect viral RNA from host intracellular defenses (Boodhoo et al., 2022). A few of the known corticosteroids can inhibit SARS/MERS in vitro and were also reported to have potent activity against SARS-CoV-2 with IC50s niclosamide (0.28 μM), ciclesonide (4.33 μM), and tilorone (4 μM) (Ko et al., 2021). Ciclesonide has been shown to lose antiviral activity on MERS-Nendou mutants (Matsuyama et al., 2020). While Ciclesonide has been part of many therapeutic combinations, there have been a few focused monotherapy randomized trials with inhaled formulations that have resulted in lower hospitalizations and reduced respiratory symptoms in treated patients (Clemency et al., 2022; Ezer et al., 2021). Ciclesonide is of particular interest for long-haul patient management for preventing severe lung damage (Ruggiero et al., 2022). Exebryl-1 a known β-amyloid anti-aggregation molecule (Alzheimer's therapy) was shown to have consistent antiviral activity between 10 and 66 μM, in various cell lines and was discovered through high throughput screens (Choi et al., 2021). Exebryl-1 has been shown to disturb hexamerization of NendoU critical for its activity (Tran et al., 2022). So far there are no trials with Exebryl-1 against COVID-19, but negative drug interactions with COVID-19 medications with Alzheimer's disease does suggest a utility for this repurposable agent (Balli et al., 2020).
8.6. Other targets
ADP ribose phosphatase (NSP3) is another interesting target playing a role in cellular immune evasion by SARS-CoV-2 by resisting ADP-ribosylation of host proteins induced by IFN (Russo et al., 2021). Exoribonuclease (ExoN, NSP14) is a 5‘-to-3‘ exonuclease and has been the focus of many computational drug screening pipelines (Castillo-Garit et al., 2021; Gupta et al., 2021b). ExoN is inhibited by S-adenosylhomocysteine (Riccio et al., 2022) which is a marker for severe COVID-19 (Ponti et al., 2021) and its abundance may have been protecting liver cholangiocytes expressing ACE-2. NSP16 is another critical target which is an Mn2+ dependant putative 2'-O-methyl transferase that forms a heterodimer with NSP10 (Minasov et al., 2021).
9. Non-enzymatic targets
9.1. 3a Ion channel
ORF3a encodes an accessory protein that forms K+ channels that trigger NLRP3 activation resulting in the maturation of IL-1β and cleavage/activation of Gasdermin via NFκB (Kern et al., n.d.; J. Zhang et al., 2022). ORF3a is susceptible to amantadine (Toft-Bertelsen et al., 2021) which has been shown to improve patient conditions suffering from COVID-19-Related Diffuse Leukoencephalopathy (Lam et al., 2022). In a larger trial with co-morbidities in Parkinson's and multiple sclerosis patients already receiving amantadine, there was significant prevention of COVID-19 infection (Kamel et al., 2021). A larger trial is in progress and its results are awaited (Rejdak and Grieb, 2020). Tomar et al., 2021 reported many more FDA-approved drugs with significant in vitro activity against heterologously expressed 3a Ion channel; Plerixafor, Kasugamycin, Capreomycin, Pentamidine, Spectinomycin, Flumatinib, Darapladib, Floxuridine, and Fludarabine (Tomar et al., 2021, 2021).
9.2. Non-structural protein 1
NSP-1 is the host shutoff factor that halts the translational machinery of SARS-CoV-2 infected cells by binding with the mRNA channel within the ribosome (Simeoni et al., 2021). The main c-terminal domain playing a role in the ribosome binding can be blocked by Mitoxantrone hydrochloride (Novantrone) (Prateek Kumar et al., 2022b). Notably, Mitoxantrone HCL also blocks viral entry through perturbing spike-heparan sulfate interactions (Q. Zhang et al., 2022).
9.3. Other SARS-CoV-2 targets
NTD-N-protein or N terminal domain of Nucleocapsid protein is responsible for binding and thereby assembling the RNA genome of SARS-CoV-2 (Ye et al., 2020). Recently multiple in vitro anti-SARS-CoV-2 molecules were discovered as interacting with the NTD-N-protein through isothermal titration calorimetry with EC50s: Telmisartan (1.02 μM), Bictegravir (8.11 μM), Bisdemethoxycurcumin (1.64 μM), and MCC-555 (4.26 μM) (Dhaka et al., 2022). Additional targets have been proposed and investigated as drug targets in silico. NSP2 is involved in host signaling interferences, NSP3 mediates a bipartite shift of host translational machinery to translate viral RNA only, NSP4 plays a role in the replicase complex assembly, and NSP18 is critical for replication (Yan et al., 2022).
10. Structural protein targets
10.1. Envelope protein
The E protein is a transmembrane cation-selective viroporin with Ca2+ and/or K+ selectivity (Hong et al., 2020; Mandala et al., 2020). Similar to previous reports with SARS/MERS, SARS-CoV-2, the E protein also forms an inflammasome by TLR2 or NRLP5 activation through NF-kB due to K+ influx (Yalcinkaya et al., 2021; M. Zheng et al., 2021). β-boswellic acid and glycyrrhizic acid natural product combinations have been shown to shorten the recovery time (Gomaa et al., 2022), and in a suggestion of a possible mechanism, they have shown positive binding with the E protein in vitro (Fatima et al., 2022). There are a few phytochemicals i.e. proanthocyanidins (PAC), wortmannin, and veliparib reported to block E protein in vitro (Y. Wang et al., 2022).
10.2. Spike glycoprotein
Spike protein, (S1, S2, S3) is the largest protein coded by the SARS-CoV-2 genome. It has various domains including transmembrane, S1 & S2 domains. S1 binds to different receptors (ACE2, CD147, B0AT1, and NRP1) and interacts with heparan sulfate and the S2 domain is a viral fusion domain. S1 domain has open and closed states to maintain the receptor-binding domain (RBD) specificity (Gupta et al., 2021c; Jackson et al., 2022). The fusion inhibitors are discussed in detail in later RBD-ACE-2 interaction inhibition. S2 activation requires cleavage of spike protein mediated by furin and TMPRSS2 (Y. Gupta et al., 2022). Itraconazole and Estradiol Benzoate were found to be interacting with the S2 domain of spike protein and had in vitro activities of IC50 0.45 (μM) and 1.02 (μM) respectively (Yang et al., 2021). Itraconazole synergistically improved the remdesivir efficacy in vitro (Schloer et al., 2021). Pan-CoV fusion inhibitor EK1 (fusion domain S2) is efficacious against all variants suggesting high target conservancy despite the high degree of amino acid mutations in SARS-CoV-2 variants (Lan et al., 2021). Further, a designer peptide mimicking the HR2 sub-domain of the S2 fusion domain (VVNIQKEIDRLNEVAKNLNESLID) was designed in silico and validated both by MD simulations and in vitro testing (Kandeel et al., 2021; Manna et al., 2020).
10.3. Homo sapiens (Host) COVID-19 therapeutic targets
In addition to targeting SARS-CoV-2 proteins, another therapeutic approach is to target host proteins that enable viral infection, replication, and spread (Fig. 2 ). The interventions range from interfering with the host receptors for SARS-CoV-2 (e.g. ACE2), to blocking the proteolytic processing needed for viral particle internalization (e.g. Cathepsin L).
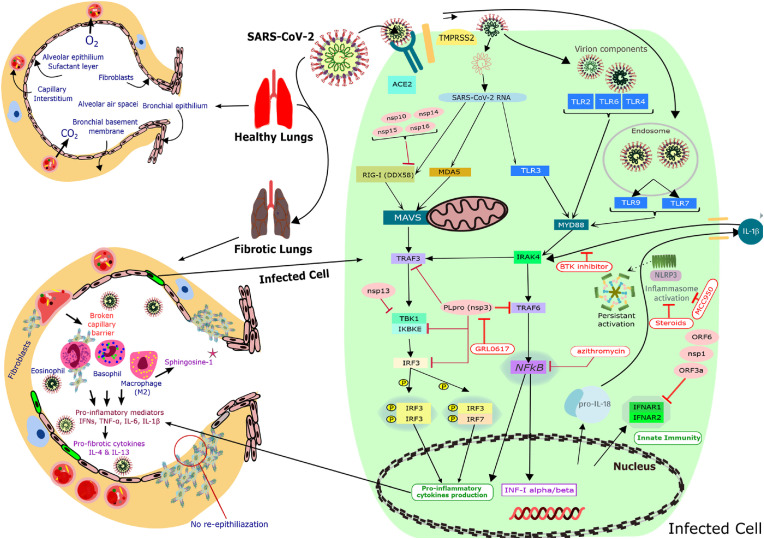
Fig. 2.
Host proteome targets involved in COVID-19 hyperimmune and their inhibitors. Cartoon representation of molecular components involved in hyperimmune reaction leading to the severe clinical presentation (ARDS) among COVID-19 patients. The lung fibrosis observed in COVID-19 patients and resulting hypoxia is the main reason for mortality in severe cases along with immunosuppressed conditions and concomitant infections. Classic pathways are hijacked in COVID-19-associated lung fibrosis by various proteins coded by SARS-CoV-2. In COVID-19 patients due to inflammation mediators such as IL-6 and cytokine storm or increased release from damaged/dying cells, there is a loss of lung surface area to fibrosis. There is an aggravation of the infection cycle due to hypoxia-induced ACE2, TMPRSS2 overexpression, and furin cell surface localization. Multiple immune suppressants and modulators have been effective in reducing the severity and mortality as seen in large trials. However, the mechanism for which is still not well established. There are many other agents known to modulate many members of this cascade, especially the NLRP3 pathway responsible for characteristic COVID-19 storm but not yet exploited due to a rather recent elucidation.
10.4. Viral receptors targets of human host
Table 3.
Drugs targeting different viral/host proteins with in vitro validations.
| Name & discovered target | Type | Indication | Mechanism of Action (General) | IC50 | Refs | |
|---|---|---|---|---|---|---|
| Haloperidol Peptides |
Conventional antipsychotic agent: Haloperidol works by inhibiting the SARS-CoV-2. | Psychotic patients | It is administered in the treatment of mental disorders such as schizophrenia. | It works by inducing a high potency suppression of undesired mental reactions in schizophrenia patients. | 6.5 μM | (Daniel et al., 2015; Pandey et al., 2020) |
| PD-144418 Peptides |
Sigma 1 agent works by exerting antiviral effects on SARS-CoV-2 protein. | Psychotic activities of patients | It is the highest selective sigma receptor ligand. | It works through selecting the stimuli that are insignificant to ion channels or enzyme actions in patients. | 0.08Μm | Vela (2020) |
| Clemastine Peptides |
Antihistamine agent Works by blocking interactions between SARS-CoV-2 nonstructural protein NSP6 and host sigma-1 receptor. | Rhinitis, allergic skin, or pruritus patients. | It is a significant histamine H1 remedy for treating rhinitis, skin allergy, and pruritus. | It works by inducing sedative and anticholinergic reactions in patients. | 8.32 μM | Reznikov et al. (2021) |
| Cloperastine Peptides |
Antitussive agent Works by blocking interactions between SARS-CoV-2 nonstructural protein NSP6 and host sigma-1 receptor | Bronchus infections | It cures coughs associated with bronchus infection. | It acts through an antihistaminic activity that causes mild broncho relaxant. effect on patients | 0.08 μM | (Reznikov et al., 2021; Vela, 2020) |
| Progesterone Peptides |
Steroid hormone Works by inhibiting the SARS-CoV-2 priming protease TMPRSS2. | Ovaries and adrenal cortices. | It is produced by the corpus luteum in the second half of the menstrual cycle. | It works by regulating the inner lining of the uterus. | 173–196 μM. | (Shah, 2021; Vela, 2020) |
| Aprotinin Spike processing enzymatic -Serine protease 10 |
It is a fibrinolytic agent that Works by controlling SARS-CoV-2 replication | It occurs in the bovine lung | It is a naturally occurring inhibitor which is a polypeptide of 58 amino acids. | It functions by inhibiting the action of certain serine proteases such as trypsin, plasmin, and chymotrypsin. | 20 μM | (Bojkova et al., 2020; da Silva et al., 2022) |
| MI-1900 Spike processing enzymatic -Serine protease 10 |
It is an antithrombin agent that works by reducing 25-fold virus titer in SARS-CoV-2 Calu-3 infected cells. | Myocardial patients | It is applied to restore coronary patency in myocardial patients | This drug acts by reducing the size of the infarct on patients’ heart structure. | 10 μM | (Lin et al., 2022; Russo et al., 2021) |
| MI-432 Spike processing enzymatic -Serine protease 10 |
It is an antiviral agent Works by inhibiting the protease TMPRSS2. |
Used by patients suffering from herpes simplex virus | It reduces the rate of virus growth and development and therefore suppresses any potential survival of the virus. | It is applied as a cream on herpes simplex patients to relieve pain and irritations that cause sores. | 1.30 ± 0.14 μM | Lin et al. (2022) |
| Nafamostat Spike processing enzymatic -Serine protease 10 |
Synthetic serine protease Works by blocking SARS-CoV-2 infection of Calu-3 cells. |
Patients with inflammatory reactions. | It acts alongside anticoagulant and anti-inflammatory effects. | It performs by inhibiting the activities of proteases such as plasmin, kallikrein, and trypsin. | 0.010 μM | Yamamoto et al. (2020) |
| E-64d Spike processing enzymatic -Serine protease 10 |
Prodrug ethyl ester Works by inhibiting coronaviral entry in certain cell types. | Patients with inflammatory reactions. | It is only active in its acidic form (E64c). | It hydrolyzed from E64d to E64c in the gut to inhibit cysteine proteases. | Mellott et al. (2021) | |
| PCI-27483 Spike processing enzymatic -Serine protease 10 |
Selective inhibitor Works by inhibiting TMPRSS2 in biochemical and cell infection assays. |
It is administered on TF-expressive cancer patients | It inhibits tumor invasiveness in cancer patients | It performs by inhibiting cell migration and angiogenesis reactions that cause tumor invasiveness. | 1.41 ± 0.04 μM | (Sun et al., 2021, p. 2) |
| Otamixaban Spike processing enzymatic -Serine protease 10 |
FXa agent Works by suppressing TMPRSS2 activity and SARS-CoV-2 infection. |
Patients with acute coronary diseases | This is an activated factor X (FXa) inhibitor involved applied in acute coronary syndrome patients | It acts through a high selection of FXa compounds to inhibit the generation of thrombin. | Hempel et al. (2021) | |
| MI-1851 Spike processing enzymatic -Furin |
Novel furin inhibitor agent Works by inhibiting furin to prevent the spread of SARS-CoV-2 |
SARS-Cov-2 patients | It prevents proteolytic processing of S-protein | It acts by inhibiting the conversion of furin in HEK293 cells to S protein. | 10 μM | Devi et al. (2022) |
| Terifumide Spike processing enzymatic - Cathepsin |
Malononitilamide agent Works by inhibiting SARS-CoV-2 replication. |
Beta-1a patients | It inhibits the proliferation of both T and B cells. | It acts by blocking the mitochondrial enzyme hydro-orotate dehydrogenase | 67 μM | Rabie (2021) |
| Leflunomide Spike processing enzymatic - Cathepsin |
Immunomodulatory agent Works by inhibiting SARS-CoV-2 replication. |
Rheumatoid arthritis. | It decreases inflammation and slows the rate of arthritis inflammation. | It performs by inhibiting the action of pyrimidines in synthesis. | 200 μM | Rabie (2021) |
| Favipiravir Spike processing enzymatic - Cathepsin |
Therapeutic agent Works by inhibiting SARS-CoV-2 infections. |
Influenza patients | It is used to cure influenza. | It works as a chain terminator during the incorporation of viral RNA and hence reducing the viral load. | 200 μM | Costanzo et al. (2020) |
| Amantadine Spike processing enzymatic - Cathepsin |
Antiviral agent Works by inhibiting SARS-CoV-2 replication. |
Influenza patients | It is used to treat patients with advanced influenza symptoms. | It works by reducing dopamine release and blocking dopamine reuptake. | 83–119 μM. | (Fink et al., 2021; Rejdak and Grieb, 2020) |
| Sulfated polysaccharides Homo Sapien Targets |
Sulfate agent Works by binding to the viral spike glycoprotein, preventing virus entry into the host cell |
adipocytes | Induces the extraction of algae type called sargassum Hymenophyllum | It acts by reducing inflammatory reactions. | 512–289 μM | Yim et al. (2021) |
| Teicoplanin Structural protein targets |
Bacteriostatic agent Works by preventing entry of SARS-CoV-2 into the cellular cytoplasm. | Bacterial infection | It inhibits the synthesis of bacterial peptidoglycan | It acts by binding to the d-alanyl-d-alanine moiety. | 2.038 μM | (F. Yu et al., 2022) |
| Nelfinavir Structural protein targets |
Anticancer agent Works by inhibiting SARS-CoV-2 replication |
Cancer patients | It induces stress in the endoplasmic. | It acts through HIV protease inhibition. | 3.3 μM | Foo et al. (2021) |
| Cepharanthine Structural protein targets |
Antiviral agent Works by inhibiting SARS-CoV-2 entry into the host cell. |
Covid-19 patients | It is used to derail the entry of the COVID-19 virus into a host | It acts by blocking target cells of viral binding. | 2.8 μM | Hijikata et al. (2022) |
| Trimipramine Structural protein targets |
Antiviral agent Works to inhibit SARS-CoV-2 by targeting viral proteins. |
Influenza patients | It is used to treat patients with advanced influenza symptoms. | It works by reducing dopamine release and blocking dopamine reuptake. | 1.5 μM | Xiang et al. (2022) |
| Osimertinib Structural protein targets |
Selective inhibitor Works by preventing SARS-CoV-2 entry into host cells. |
It is administered to patients | It inhibits tumor invasiveness in cancer patients | It performs by inhibiting cell migration and angiogenesis reactions that cause tumor invasiveness. | 3.98 μM | Xiang et al. (2022) |
| Abemaciclib\ Structural protein targets |
Sensitizing agent Works by preventing SARS-CoV-2 entry into host cells. |
Cancer patients | It inhibits the conversions of 2-anilino-2, 4-pyrimidine from palbociclib | The drug blocks the spread of cancer infections by inhibiting the replication of associated cells. | 3.16 μM | Xiang et al. (2022) |
| Ingenol Structural protein targets |
Mebutate agent Works by preventing SARS-CoV-2 entry into host cells. |
Keratosis patients | It cures skin conditions. | It is applied to the skin to kill cells causing scaly skin patches. | 0.06 μM | Xiang et al. (2022) |
| Imatinib Structural protein targets |
Fusion agent Works by preventing SARS-CoV-2 entry into host cells. |
Leukemia patient | It is an inhibitor of the fusion process | It functions by inhibiting protein fusion of Bcr-Abl | 5.32 μM | Xiang et al. (2022) |
| Itraconazole Structural protein targets |
Antiviral agent Works by preventing SARS-CoV-2 S protein-mediated intercellular fusion |
Covid-19 patients | It is used to derail the entry of the COVID-19 virus into a host | It acts by blocking target cells of viral binding. | 0.45 μM | Yang et al. (2021) |
| Estradiol benzoate Structural protein targets |
Ester agent Works by preventing SARS-CoV-2 protein-mediated intercellular fusion. |
Adult human | It is a steroid sex hormone | It acts by maintaining fertility and secondary behaviors. | 1.02 μM | Yang et al. (2021) |
| Fluoxetine Structural protein targets |
Serotonin agent Works by inhibiting cytokine release to prevent SARS-CoV-2 in human lung tissue. |
Mentally disorder patients | It is used to treat depression | It acts by preventing serotonin reuptake. | 0.8 μM | Zimniak et al. (2021) |
| Citalopram Structural protein targets |
Serotonin agent Work by reducing viral infection by SARS-CoV-2. |
Mentally disorder patients | It helps to maintain mental balance. | Its acts by increasing the amount of serotonin. | 27.51 μM | Fred et al. (2022) |
| Reboxetine Structural protein targets |
Antidepressant agent Work by reducing viral infection by SARS-CoV-2. |
Mentally disorder patients | It reduces panic disorder and attention deficit hyperactivity. | It acts by reducing norepinephrine reuptake inhibitor | 17.69 μM | Fred et al. (2022) |
| Chlorpromazine Structural protein targets |
Antitussive agent Work by reducing viral infection by SARS-CoV-2. |
Bronchus infections | It cures coughs associated with a bronchial infection. | It works by reducing dopamine release and blocking dopamine reuptake. | 0.972 μM | Fred et al. (2022) |
| Flupenthixol Structural protein targets |
Antiviral agent Work by reducing viral infection by SARS-CoV-2. |
Covid-19 patients | It is used to treat patients with advanced influenza symptoms. | It works by reducing dopamine release and blocking dopamine reuptake. | 1.072 μM | Fred et al. (2022) |
| Pimozide Structural protein targets |
Oral diphenylbutylpiperidine antipsychotic agent Work by reducing viral infection by SARS-CoV-2. |
Butyrophenones patients | It induces stress in the endoplasmic. | It acts through HIV protease inhibition | 4.539 μM | Fred et al. (2022) |
| Mitoxantrone hydrochloride non-enzymatic targets |
Bacteriostatic agent Works by inhibiting ROS1 fusion protein and its downstream signaling minimizing cell apoptosis. |
Bacterial infection | It inhibits the synthesis of bacterial peptidoglycan | It acts by binding to the d-alanyl-d-alanine moiety. | 2.99 ± .608 μM | |
| Capreomycin non-enzymatic targets |
Selective inhibitor Works by inhibiting SARS-CoV2 protease. |
It is administered to patients | It inhibits tumor invasiveness in cancer patients | It performs through inhibiting cell migration and angiogenesis reactions that cause tumor invasiveness | 1 μM | Kumar et al. (2021) |
| Pentamidine non-enzymatic targets |
Anti-infective agent Works by blocking the SARS-CoV-2 3a-channel. | Pneumonia patients | It treats pneumonia caused by organisms. | It acts by blocking the spread of cold in the host body. | 7.5 μM. | Andreana et al. (2022) |
| Spectinomycin non-enzymatic targets |
Ester agent Works by blocking the SARS-CoV-2 3a-channel. |
Adult human | It is a steroid sex hormone | It acts by maintaining fertility and secondary behaviors. | 50 μM. | (Tomar et al., 2021, 2021, 2021) |
| Kasugamycin non-enzymatic targets |
Serotonin agent Works by blocking the SARS-CoV-2 3a-channel. |
Mentally disorder patients | It is used to treat depression | It acts by preventing serotonin reuptake. | 50 μM. | (Tomar et al., 2021, 2021, 2021) |
| Plerixafor non-enzymatic targets |
Mebutate agent Works by blocking the SARS-CoV-2 3a-channel. |
Keratosis patients | It cures skin conditions. | It is applied to the skin to kill cells causing scaly skin patches. | 50 μM. | (Tomar et al., 2021, 2021, 2021) |
| Flumatinib non-enzymatic targets |
Antiviral agent Works by blocking the SARS-CoV-2 3a-channel. |
Covid-19 patients | It is used to derail the entry of the COVID-19 virus into a host | It acts by blocking target cells of viral binding | 50 μM. | (Tomar et al., 2021, 2021, 2021) |
| Darapladib non-enzymatic targets |
Fusion agent Works by blocking the SARS-CoV-2 3a-channel. |
Leukemia patient | It is an inhibitor of the fusion process | It functions by inhibiting protein fusion of Bcr-Abl | 50 μM. | (Tomar et al., 2021, 2021, 2021) |
| Floxuridine non-enzymatic targets |
Therapeutic agent Works by blocking the SARS-CoV-2 3a-channel. |
Influenza patients | It is used to cure influenza. | It works as a chain terminator during the incorporation of viral RNA and hence reducing the viral load. | 50 μM. | (Tomar et al., 2021, 2021, 2021) |
| Fludarabine non-enzymatic targets |
Antidepressant agent Works by blocking the SARS-CoV-2 3a-channel. |
Mentally disorder patients | It reduces panic disorder and attention deficit hyperactivity | It acts by reducing norepinephrine reuptake inhibitor | 50 μM. | (Tomar et al., 2021, 2021, 2021) |
| Ciclesonide RNA-dependent RNA polymerase |
Antitussive agent Works by suppressing the replication of SARS-CoV-2 in cultured cells. |
Bronchus infections | It cures coughs associated with a bronchial infection. | It performs through inhibiting cell migration and angiogenesis reactions that cause tumor invasiveness | 5.1 μM. | Matsuyama et al. (2020) |
| Exebryl-1 RNA-dependent RNA polymerase |
Mebutate agent Work by promoting SARS-CoV-2 antiviral activity in Vero 76, Caco-2, and Calu-3 cells. |
Keratosis patients | It cures skin conditions | It is applied to the skin to kill cells causing scaly skin patches. | 10–66 μM. | Choi et al. (2021) |
| Sofosbuvir RNA-dependent RNA polymerase |
Selective inhibitor Works by inhibiting SARS-CoV-2 replication in brain and lung cells. |
It is administered to patients | It inhibits tumor invasiveness in cancer patients | It performs through inhibiting cell migration and angiogenesis reactions that cause tumor invasiveness | 6.2–9.5 μM (EC50) | Shabani et al. (2021) |
| Alovudine RNA-dependent RNA polymerase |
Anticancer agent Works by terminating RNA synthesis of SARS-CoV-2 virus. |
Cancer patients | It inhibits the conversions of 2-anilino-2, 4-pyrimidine from palbociclib | It performs by inhibiting the activities of proteases such as plasmin, kallikrein, and trypsin. | 100 μM | Kumar et al. (2021) |
| Tenofovir alafenamide RNA-dependent RNA polymerase |
FXa agent Works by blocking the SARS-CoV-2 polymerase extension. |
Patients with acute coronary diseases | This is an activated factor X (FXa) inhibitor involved applied in acute coronary syndrome patients | It acts through a high selection of FXa compounds to inhibit the generation of thrombin. | (Kocabaş and Uslu, 2021; Zanella et al., 2021) | |
| Zidovudine RNA-dependent RNA polymerase |
Prodrug ethyl ester Can work by inhibiting SARS-CoV-2 replication and transcription. |
Patients with inflammatory reactions. | It is only active in its acidic form (E64c). | It hydrolyzed from E64d to E64c in the gut to inhibit cysteine proteases | Matsuyama et al. (2020) | |
| Suramin RNA-dependent RNA polymerase |
Malononitilamide agent Works by inhibiting SARS-CoV-2 replication. |
Rheumatoid arthritis. | It decreases inflammation and slows the rate of arthritis inflammation. | It acts by blocking target cells of viral binding. | 20 μM (EC50) | Mostafa (2020) |
| Atorvastatin RNA-dependent RNA polymerase |
Anti-infective agent Works by inhibiting SARS-CoV-2 replication. |
Pneumonia patients | It treats pneumonia caused by organisms. | It performs through inhibiting cell migration and angiogenesis reactions that cause tumor invasiveness | 3.9–15.7 μM | Zapata-Cardona et al. (2021) |
| Flupenthixol RNA-dependent RNA polymerase |
Novel furin inhibitor agent Works by preventing SARS-CoV-2 spike protein pseudovirus cell entry in the host cell. |
It is administered to patients | It inhibits the synthesis of bacterial peptidoglycan | It acts by inhibiting the conversion of furin. | 0.56 μM | Devi et al. (2022) |
| Raloxifene RNA-dependent RNA polymerase |
Mebutate agent Works by modulating SARS-CoV-2 replication. |
Keratosis patients | It cures skin conditions | It is applied to the skin to kill cells causing scaly skin patches. | 40 μM–0.31 μM | Nicastri et al. (2022) |
| Disulfiram Papain-like proteinases |
Selective inhibitor Works by inhibiting SARS-CoV-2 papain-like proteases |
It is administered to patients | It inhibits tumor invasiveness in cancer patients | It works by reducing dopamine release and blocking dopamine reuptake. | 9.35 μM | Fillmore et al. (2021) |
| GRL0617 Papain-like proteinases |
Serotonin agent Works by inhibiting SARS-CoV-2 PLpro. |
Mentally disorder patients | It is used to treat depression | It acts by preventing serotonin reuptake. | 2.1 μM | (Fu et al., 2021, p. 202) |
| Maprotiline Papain-like proteinases |
Antitussive agent Works by preventing SARS-CoV-2 infection on Vero cells. |
Bronchus infections | It cures coughs associated with a bronchial infection. | It acts by reducing norepinephrine reuptake inhibitor | 5 μM–35 μM | Carpinteiro et al. (2020) |
| Reserpine Papain-like proteinases |
Anti-infective agent Works by inhibiting SARS-CoV-2 activities. |
Pneumonia patients | It is used to cure influenza. | It works as a chain terminator during the incorporation of viral RNA and hence reducing the viral load. | 3.4–6.0 μM. | Xian et al. (2020) |
| Levothyroxine Papain-like proteinases |
Therapeutic agent Works by inhibiting SARS-CoV-2 PLpro |
Influenza patients | It is used to cure influenza. | It works by reducing dopamine release and blocking dopamine reuptake. | 5.0 ± 1.9 to 11±3 μM | Brewitz et al. (2022) |
| Proanthocyanidin Papain-like proteinases |
Antiviral agent Works by inhibiting SARS-CoV-2. |
Covid-19 patients | It is used to derail the entry of COVID-19 virus into a host. | It acts by blocking target cells of viral binding. | Sugamoto et al. (2022) | |
| Sepantronium bromide Papain-like proteinases |
Novel furin inhibitor agent | It is administered to patients | It inhibits the synthesis of bacterial peptidoglycan | It acts by inhibiting the conversion of furin. | Devi et al. (2022) | |
| Cryptotanshinone Papain-like proteinases |
Bacteriostatic agent Works by inhibiting SARS-CoV-2 protease |
Bacterial infection | It inhibits the synthesis of bacterial peptidoglycan | It acts by blocking target cells of viral binding. | 13.6 μM | Zhao et al. (2021) |
| Tanshinone I Papain-like proteinases |
Anti-infective agent Works by inhibiting viral protease, SARS-CoV-2 3CLpro, and PLpro |
Bronchus infections | It cures coughs associated with a bronchial infection. | It is applied to the skin to kill cells causing scaly skin patches. | 0.7 μM | Elebeedy et al. (2021) |
| Ranitidine Bismuth citrate Helicase |
Oral diphenylbutylpiperidine antipsychotic agent Works by suppressing SARS-CoV-2 replication. |
Butyrophenones patients | It induces stress in the endoplasmic | It acts through HIV protease inhibition | 0.69 μM | Shu et al. (2020) |
10.5. Host receptors
ACE2 is the most abundant and highest affinity receptor of SARS-CoV-2 spike protein and is the first step in viral entry into the host cell. There are multiple reports that ACE2 polymorphisms and Spike protein modulate viral infectivity (Suryamohan et al., 2021). Various known ACE2 inhibitors, as well as expression modulators, have been proposed to be viable anti-COVID-19 therapeutics. There is another novel approach of molecular mimicry where B38-CAP an ACE2 homolog carboxypeptidase of bacterial origin protected patients from lung injury without apparent viral neutralization, but through a mechanism of RAS inactivation and decreased Acute Respiratory Distress Syndrome (ARDS) (Yamaguchi et al., 2021). This is coherent with the previous reports of lung damage protection with recombinant soluble ACE2 in animal models (Imai et al., 2005, p. 20). Also, soluble recombinant human ACE2 has a high SARS-CoV-2 neutralizing potential as shown in vitro (Monteil et al., 2020). Giapreza, the angiotensin II substrate of ACE2, had variable outcomes from different studies. The conclusive multicentric trial concluded a decrease in blood pressure and improved fraction of inspired oxygen (FiO2) levels but there was no apparent benefit in terms of mortality among severe ARDS patients. ACE2 agonists have also shown a decrease in Spike-ACE2 interaction as their binding site is closer to the interface compare to antagonists e.g. Losartan/Valsartan that bind in the catalytic core and have no positive effect as reported in multiple trials (Geriak et al., 2021; Puskarich et al., 2021, 2022). A small randomized trial with 51 patients receiving C21 and an ACE2 agonist showed a significant reduction in the requirement of mechanical ventilation (Tornling et al., 2021). Methylene Blue is a nonspecific ACE2-Spike interaction inhibitor and has been used to inactivate residual viruses in convalescent plasma (Table 4 ) (Alemany et al., 2022). Ceftazidime is an injectable broad-spectrum beta-lactam antibiotic that is a third-generation cephalosporin. Ceftazidime was found to effectively block ACE-2 spike interactions in vitro (C. Lin et al., 2021). It was trialed on 136 patients in a study and showed a significant reduction in recovery (PCR negativity) (Eid et al., 2021). On the contrary, Ramipril is highly contraindicated in COVID-19 patients as it is known to highly up-regulate ACE2 and increase SARS-CoV-2 virion loads (Theodorakopoulou et al., 2022).
Neuropilin-1 (NRP1) is another host surface receptor mediating SARS-CoV-2 entry (Cantuti-Castelvetri et al., 2020; Kyrou et al., 2021) and has been associated with neurological morbidities seen in COVID-19 (Davies et al., 2020). Apart from protein receptor binding spike protein also interacts with cell surface heparan sulfate and is the basis for antiviral activity of heparin (Gupta et al., 2021c) and sulfated polysaccharides (Kwon et al., 2020) abundant in many natural products. There is a high interest in using sulfated polysaccharides as anti-COVID-19 also due to the reduction in coagulopathy seen in COVID-19 patients (B. Tu et al., 2022). There is still a possibility of SARS-CoV-2 variants evolving or already evolved to use different receptors like other coronaviruses (Nassar et al., 2021).
11. Spike processing enzymatic targets
11.1. Cathepsin L
Cathepsin L (CTSL) is a transmembrane peptidase/serine subfamily member 2/4 and plays an important role in spike activation in endosomes. The widespread now-dominant mutation in the SARS-CoV-2 Spike glycoprotein D614G is predicted to confer a site loss for CTSL (Gobeil et al., 2020; Y. Gupta et al., 2022). Amantadine acts as a lysosomotropic agent by disturbing Cathepsin L's functional environment(Smieszek et al., 2020). A few reports are showing decreased leukopathy (Lam et al., 2022) and the slowdown of neurodegeneration presentations of COVID-19 by amantadine (Rejdak and Grieb, 2020).
11.2. Furin
Furin is a Ca2+−dependent endopeptidase that processes many secretory proteins as well as protein digestion (Than et al., 2005). During hypoxia, furin can translocate to the cell surface and is thought to be responsible for the rapid worsening of hypoxia patients in COVID-19 by increased spike processing at the cell surface resulting in direct fusion (Arsenault et al., 2012; Y. Gupta et al., 2022). Both Furin is essential for SARS-CoV-2 invasion (Bestle et al., 2020) and known furin inhibitors MI-1851 and E-64d have both shown in vitro efficacy against SARS-CoV-2 (Table 3).
11.3. TMPRSS2
Transmembrane serine protease 2 (TMPRSS2) is a cell surface activator of spike protein essential to exposing and activating the viral fusion domain (Bestle et al., 2020; Hoffmann et al., 2020). Nafamostat (CKD-314/Nafabelltan) a TMPRSS2 inhibitor was found to instigate a significantly higher recovery rate among treated patients and was well tolerated (Table 4) (Zhuravel et al., 2021). Another TMPRSS2 inhibitor Camostat mesylate (FOY-305) in contrast didn't show any positive effect in a phase III trial (Kinoshita et al., 2022). One speculation for inconclusive outcome with Camostat is the drug might need a better dosage formulation for effective treatment (Kosinsky et al., 2022). There are additional inhibitors of TMPRSS2 with promising results in vitro e.g. Aprotinin, MI-1900, MI-432, E-64d, PCI-27483, and Otamixaban.
11.4. Targets associated with host immune response
The TLR 2/6/9 agonist PUL-042 is a phase III investigational compound that can induce epithelial resistance to SARS-CoV-2 in animal models (Evans et al., 2020). Famotidine is a selective histamine H2-receptor (H2R) antagonist (Malone et al., 2021) that also inhibits 3CLpro of SARS-CoV-2 (Loffredo et al., 2021). Famotidine had a positive effect with a reduced risk of clinical deterioration leading to intubation or death when tested in a small retrospective cohort (Freedberg et al., 2020). Currently, famotidine is part of multiple combinations in various trials. There are hypothetical reports of targeting different immune components such as Basigin CD_antigen: CD147, 5F7, Collagenase stimulatory factor, Leukocyte activation antigen M6, Extracellular matrix metalloproteinase inducer, Tumor cell-derived collagenase stimulatory factor, GCSF-Receptor Signaling Complex CSF3, IL-1β, leukocytic pyrogen, leukocytic endogenous mediator, and mononuclear cell factor, yet discussing all of these is beyond the scope of the current review. Major confounding comorbidity arising in a portion of the SARS-CoV-2 infected populations is the activation of a cytokine storm leading to the development of ARDS. To block the cytokine storm from activating in COVID-19 patients, various antibody cocktails blocking these factors have been used in ongoing trials (Elahi et al., 2022; Harrison, 2020; Harrison et al., 2021). Many recombinant proteins e.g. Recombinant TNF (INB03) and Recombinant human interferon α1β (Novaferon) have also been tried (Drożdżal et al., 2021). Other targets include Peginterferon Lambda-1a, and Chemokine Receptor Type 2 (CCR2) (Hu et al., 2021). The Interleukin-1 receptor-associated Kinase 4 (IRAK4) Inhibitor PF-06650833 is predicted to restore immunological balance (Gupta and Chun, 2021) and is under trial (Franchin, 2021). Sigma-1 receptor (sigma non-opioid intracellular receptor 1) is an important factor associated with the mortality of COVID-19 patients (Lehrer and Rheinstein, 2021) several inhibitors have been predicted to be anti-COVID-19 e.g. Haloperidol, PD-144418, clemastine, Cloperastine, and progesterone. Naringenin, targeting the endo-lysosomal Two-Pore Channels (TPCs) has been shown as having anti-SARS-CoV-2 activity (Clementi et al., 2021).
12. Mechanistic targets
12.1. Dihydroorotate dehydrogenase
Dihydroorotate dehydrogenase (mitochondrial DHODH), is a Dihydroorotate oxidase involved in pyrimidine synthesis within cells. DHODH inhibition has been shown to decrease viral replication/turnover rates (Kaur et al., 2021) as well as increase the incorporation of nucleoside analog antivirals such as N4-hydroxycytidine (NHC) which is an activated metabolite of Molnupiravir (Stegmann et al., 2021). Brequinar (DUP 785, NSC 368390) in combination with nucleoside analog Dipyridamole has shown high in vitro efficacy (Demarest et al., 2022; Xiong et al., 2020) and is in Phase II trials. There are many more DHODH inhibitors showing high anti-SARS-CoV-2 activities e.g. PTC299 (Luban et al., 2021), Teriflunomide (Maghzi et al., 2020), and Leflunomide (Hu et al., 2020). Leflunomide also showed faster PCR negativity in COVID-19 patients in a small trial (Hu et al., 2020).
12.2. Cathepsin B
Cathepsin B (APP secretase/Cathepsin B1) is an important enzyme overexpressed in hyperimmune inflammatory disorders and hence can be a target for ARDS mitigation (Ding et al., 2022).
12.3. Caspase
COVID-19 inflammasome causes cell death through caspase pathways, specifically caspase 8 (Li et al., 2020). Belnacasan and Emricasan are Caspase inhibitors that showed inhibition of inflammasome in vitro (Jeong et al., 2022).
12.4. Calpain
Calpain inhibitor BLD-2660 is an anti-fibrotic and part of many ongoing trials shown to mitigate lung fibrosis in combinations with antivirals (Djordje et al., 2021).
12.5. Ferroportin
Multiple reports point to SARS-CoV-2 mediated lung injury being mediated by ferroptosis with a portion of spike protein mimicking hepcidin hormone (Y. Gupta et al., 2022). Vitamin D is known to induce ferroportin overexpression which effluxes out the excess iron thereby preventing ferroptosis to reduce lung injury (Moran-Lev et al., 2018). Low levels of vitamin D were associated with higher COVID-19 mortality and it has been part of various combinations as an inexpensive therapeutic supplement for COVID-19 patients (Z. Wang et al., 2022).
12.6. Eukaryotic elongation factor 1A2 (eEF1A2)
Nitazoxanide is a thiazolide chemical compound that induces eIF2α (eukaryotic translation initiation factor-2) overexpression and PKR (double-stranded-RNA-activated protein kinase) phosphorylation, which has been used clinically to control Japanese encephalitis virus replication (Elazor et al., 2008; Shi et al., 2014). Nitazoxanide has been part of various combinations for SARS-CoV-2 infections and has shown depression in disease trajectory if started early on (Mendieta Zerón et al., 2021; Miorin et al., n.d.; Rocco et al., 2021). Paradoxically, Plitidepsin (dehydrodidemnin B/Aplidin) is a marine-derived cyclic depsipeptide inhibiting eEF1A2 that is authorized in a few countries for treating refractory multiple myeloma. Preclinical and randomized phase-I trials showed Plitidepsin to be well tolerated and block the SARS-CoV-2 virus at the nanomolar range (Varona et al., 2022). Both eEF1A2 inhibition and overexpression seem to be detrimental to SARS-CoV-2 pathogenesis.
12.7. Inosine-5′-monophosphate dehydrogenase (IMPDH)
Merimepodib (MMPD) is a IMPDH inhibitor that showed 2.5-log decrease in viral titers (p-value = 0.0004) with 4hr pretreatment (Bukreyeva et al., 2020). When used in combination with Remdesivir, there was a rapid undetected level of achievement of viral load in vitro; a trial with the same combination is ongoing (Wimmer and Keestra, 2022).
13. Target independent drugs
13.1. NSAIDs
Indomethacin is an NSAID that inhibits prostaglandin E synthase 2 (PGES-2) (Lucas, 2016). Its mechanism of action is still an enigma, while its primary target is IL6 suppression through PGES-2 inhibition, it is also proposed to block multiple factors for severe COVID-19 e.g. suppressing ACE2, TMPRSS2, cytokines, and inflammation in general (Alkotaji and Al-Zidan, 2021). Indomethacin has shown 100% protection from the development of hypoxia/desaturation with SpO2 ≤ 93 compared to 16–22% in the untreated pool of patients (Ravichandran et al., 2022).
14. Newer approaches to drugging targets
A variety of novel targets are being investigated with non-standard drug targeting. Ensovibep (MP0420) is a DARPins derivative that is an emerging class of novel therapeutics. This molecule's three distinct DARPin domains are designed to simultaneously target the receptor binding ridge on each RBD of the spike trimer (Chonira et al., 2022). MP0420 had an IC50 of an average of 2.3 ng/ml except for the mutation F486V, it was twice as effective as neutralizing antibodies; REGN10933 and REGN10987, and had a better efficacy against variants of concern (Reichen et al., n.d.).
A novel therapeutic paradigm is a proteolysis-targeting chimera (PROTAC), an application of targeted protein degradation, which has successfully been applied toward COVID-19 targets (Shaheer et al., 2021). Essentially, PROTACs have a region that binds the viral target and the same region that binds a ubiquitin ligase, thereby positioning it to traffic the target for degradation. Since the virus must enter the cell, it is thereafter susceptible to PROTACs. Viral proteins are also exogenous, making them good targets from a standpoint of specificity. Furthermore, fragments generated from degradation can result in novel antigens that stimulate the host immune response. MPRO in particular has been selected as a viable candidate for PROTACs (Shaheer et al., 2021). Other potential targets include viral envelope proteins, PLpro, and RNA-dependent RNA polymerase (RdRp). PROTACs use a ligand as the basis for targeted protein degradation, novel therapeutics can be based on existing drugs or those in development, for the appropriate intracellular targets. For example, indomethacin has gained attention after drug repurposing studies identified its antiviral capabilities (Shekhar et al., 2022; Zeng et al., 2020). A recent study investigated the effectiveness of indomethacin-based PROTACs in pan anti-coronavirus therapy (Desantis et al., 2021). Their findings indicated the indomethacin-PROTAC was more potent at inhibiting coronavirus, as well as was able to be effective against multiple strains of coronavirus (Table 4).
A major limitation of PROTACs is that they are only useable for intracellular targets, or at least ones with an intracellular component; this limitation precludes a vast range of potential targets of high importance. A very recent technique called molecular degraders of extracellular proteins through the asialoglycoprotein receptor (MoDE-As) addresses the glaring weakness of targeted protein degradation. MoDE-As can target extracellular proteins for degradation (Caianiello et al., 2021). This is accomplished via the formation of a ternary complex between a target protein, the ligand, and hepatocyte ASGPRs; this complex is then endocytosed, trafficked to the lysosome and the target protein is degraded by the host machinery. While MoDE-As has not yet been applied to COVID-19 therapy, it is a viable technique to intervene with viral protein targets before they enter the cells. Furthermore, there is evidence that the SARS-CoV-2 spike protein interacts with the ASGPR in hepatocytes through a lesser-known mechanism of entry (Collins and Steer, 2021; Gu et al., 2022).
15. Conclusion
COVID-19 disease can be safely called a virus-induced hyper-immune disorder. There are thus numerous factors still being discovered from the host point of view which can be mitigated by various therapeutics to reduce the severe clinical presentations (W. Zhang et al., 2022). Also with new roles assigned to various viral components essential in pathogenesis and severe disease progression, numerous virus-coded proteins have been proposed as drug targets albeit only a few have bioactive inhibitors (Martin et al., 2020). Although there are numerous agents with known in vitro activity, there is an urgent need to form suitable combinations based on the synergy of the agents, a stratified patient population taking into consideration important pathways leading to either ARDS or Long-haul disorders. Also, various trialed agents with borderline protection or a population-specific activity can be used to fortify newly discovered strong antivirals like Nirmatrelvir or Molnopiravir. As there is no single pathway in this COVID-19 sequela, there is an urgent need for utilizing personalized medicine combinations composed of the most tolerated and active agent combinations.
Intriguingly, when viewing from a drug discovery perspective, there is a learning phase we must endeavor to better understand the druggability of identified viral targets with known and potential inhibitors to continue developing new antivirals to be better prepared for the emergence of drug resistance to current candidates and therapeutics (Gandhi et al., 2022), especially when it's now known as immunocompromised patients are the source of new resistant variant emergence (Chen et al., 2021; Gandhi et al., 2022; Leung et al., 2022).
Within this realm of rapidly advancing, technology is a convergent race between computational and experimental methods, which furthers the acceleration of drug discovery(Dara et al., 2022; Hinton, 2007; Jiménez-Luna et al., 2021; Lima et al., 2016; Patel et al., 2020; Sherrington and Kirkpatrick, 1975; Talevi et al., 2020). We are using ML increasingly in multiple areas of science and even in other areas (e.g. social science), whilst we are making stronger strides in computational design techniques. ML is now commonplace in digital pathology, search engines, recognition (voice, facial, pattern), market and financial predictions, astronomy, cryptography, agriculture, and more. The use of AL, ML, and deep learning techniques is to better find and rapidly identify data from multiple sources, extract valuable insights, visualize the data meaningfully, and give context. Within drug discovery, there is an ongoing explosion of the use of ML with molecular modeling for protein structure prediction and drug-protein interaction analyses. For example, the pioneering of Boltzmann machines using decision trees and then adaptive rules for protein structures was a crucial development that allowed the generation of predetermined global variables on molecular structures to dictate conformational searches in directions under the reinforced learning pattern dictated (Caulfield and Devkota, 2012; Caulfield, 2011; Caulfield et al., 2011; Coban et al., 2021b; Kayode et al., 2016; von Roemeling et al., 2018). The particularly useful application of this allowed such things as cryo-EM fitting and rapid space searches (Caulfield and Devkota, 2012; Caulfield et al., 2011) using entropy as the controller.
Particularly of note is the emergence of AI and ML to the forefront of protein structural modeling, conformational dynamics exploratory mission of many labs to find key druggable states, and the determination of the human genomic variance as a contributing factor to the way viruses capitalize on variation. Virus exploitation of human genetic variance is also being tackled by computationalists to better understand how genetics plays a role in virus proliferation, which will allow better tools to predict potential virus offshoots in the future. One can imagine a day when there will be a virtual medicine cabinet pre-stocked with the needed antivirals specific to the patient's genetic predispositions and particular cell pathways. In such a scenario, we will have AI-based medicine that has the genetic profile, molecular structures for the targets needed, rapidly available custom chemistry, and rapid safety-profiling needed for the new chemical entities to be used in humans on-demand with acceptable safety tolerances. While this particular view of AI and ML is not anytime soon, the palatability of this particular star trek viewpoint is very realizable and within our horizon.
References
- Abidi S.H., Almansour N.M., Amerzhanov D., Allemailem K.S., Rafaqat W., Ibrahim M.A., la Fleur P., Lukac M., Ali S. Repurposing potential of posaconazole and grazoprevir as inhibitors of SARS-CoV-2 helicase. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-021-89724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abolhassani H., Bashiri G., Montazeri M., Kouchakzadeh H., Shojaosadati S.A., Siadat S.E.R. COVID-19. Springer; 2021. Ongoing clinical trials and the potential therapeutics for COVID-19 treatment; pp. 27–89. [Google Scholar]
- Ahmed M., Farag A., Boys I.N., Wang P., L. Eitson J., Ohlson M.B., Fan W., McDougal M.B., Menendez-Montez I., Uyen Nhi Nguyen N.U.N.N., Mar K., Ortiz F., Young Kim S., Williams N., lemoff A.L., DeBerardinis R., Schoggins, J W., Sadek H. Identification of atovaquone and mebendazole as repurposed drugs with antiviral activity against SARS-CoV-2. Version 6Chemistry. 2021 doi: 10.26434/chemrxiv-2021-b3fv1-v7. ) (preprint) [DOI] [Google Scholar]
- Alemany A., Millat-Martinez P., Corbacho-Monné M., Malchair P., Ouchi D., Ruiz-Comellas A., Ramírez-Morros A., Codina J.R., Simon R.A., Videla S., others High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial. Lancet Respir. Med. 2022;10:278–288. doi: 10.1016/S2213-2600(21)00545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkotaji M., Al-Zidan R.N. Indomethacin: can it counteract bradykinin effects in COVID-19 patients? Curr. Pharm. Rep. 2021;7:102–106. doi: 10.1007/s40495-021-00257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amani Bahman, Khanijahani A., Amani Behnam, Hashemi P. Lopinavir/ritonavir for COVID-19: a systematic review and meta-analysis. J. Pharm. Pharmaceut. Sci. 2021;24:246–257. doi: 10.18433/jpps31668. [DOI] [PubMed] [Google Scholar]
- Amani Behnam, Amani Bahman, Zareei S., Zareei M. Efficacy and safety of arbidol (umifenovir) in patients with COVID‐19: a systematic review and meta‐analysis. Immun. Inflamm. Dis. 2021;9:1197–1208. doi: 10.1002/iid3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreana I., Bincoletto V., Milla P., Dosio F., Stella B., Arpicco S. Nanotechnological approaches for pentamidine delivery. Drug Deliv. and Transl. Res. 2022;12:1911–1927. doi: 10.1007/s13346-022-01127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angamo M.T., Mohammed M.A., Peterson G.M. Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis. Infection. 2022;50:27–41. doi: 10.1007/s15010-021-01671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum F., Mohammad T., Asrani P., Shafie A., Singh S., Yadav D.K., Uversky V.N., Hassan M.I. Identification of intrinsically disorder regions in non-structural proteins of SARS-CoV-2: new insights into drug and vaccine resistance. Mol. Cell. Biochem. 2022;477:1607–1619. doi: 10.1007/s11010-022-04393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault D., Lucien F., Dubois C.M. Hypoxia enhances cancer cell invasion through relocalization of the proprotein convertase furin from the trans-golgi network to the cell surface. J. Cell. Physiol. 2012;227:789–800. doi: 10.1002/jcp.22792. [DOI] [PubMed] [Google Scholar]
- Auwul M.R., Rahman M.R., Gov E., Shahjaman M., Moni M.A. Bioinformatics and machine learning approach identifies potential drug targets and pathways in COVID-19. Briefings Bioinf. 2021;22 doi: 10.1093/bib/bbab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Ye F., Feng Y., Liao H., Song H., Qi J., Gao G.F., Tan W., Fu L., Shi Y. Structural basis for the inhibition of the SARS-CoV-2 main protease by the anti-HCV drug narlaprevir. Signal Transduct. Targeted Ther. 2021;6:1–3. doi: 10.1038/s41392-021-00468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J., McLauchlan H., Elliott M., Cohen P. The specificities of protein kinase inhibitors: an update. Biochem. J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J.D., Uhrich R.L., Kraemer G.C., Love J.E., Kraemer B.C. A drug repurposing screen identifies hepatitis C antivirals as inhibitors of the SARS-CoV2 main protease. PLoS One. 2021;16 doi: 10.1371/journal.pone.0245962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balli F., Kara E., Demirkan S.K. The another side of COVID-19 in Alzheimer’s disease patients: drug-drug interactions. Int. J. Clin. Pract. 2020;74(10) doi: 10.1111/ijcp.13596. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt-Due A., Olsen I.C., Henriksen K.N., K\aasine T., Lund-Johansen F., Hoel H., Holten A.R., Tveita A., Mathiessen A., Haugli M., others . 2021. Evaluation of Remdesivir and Hydroxychloroquine on Viral Clearance in COVID-19 Patients: Results from the NOR-Solidarity Randomised Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerman R., Gori A., Jeyakumar S., Malin J.J., Paredes R., Póvoa P., Smith N.J., Teixeira-Pinto A. Remdesivir for the treatment of patients hospitalized with COVID-19 receiving supplemental oxygen: a targeted literature review and meta-analysis. Sci. Rep. 2022;12:9622. doi: 10.1038/s41598-022-13680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., others Remdesivir for the treatment of covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- Bestle D., Heindl M.R., Limburg H., Pilgram O., Moulton H., Stein D.A., Hardes K., Eickmann M., Dolnik O., Rohde C., others TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life science alliance. 2020;3 doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch A. Penguin; 2003. Murphy's Law. [Google Scholar]
- Blum V.F., Cimerman S., Hunter J.R., Tierno P., Lacerda A., Soeiro A., Cardoso F., Bellei N.C., Maricato J., Mantovani N., Vassao M., Dias D., Galinskas J., Janini L.M.R., Santos-Oliveira J.R., Da-Cruz A.M., Diaz R.S. Nitazoxanide superiority to placebo to treat moderate COVID-19 – a Pilot prove of concept randomized double-blind clinical trial. eClinicalMedicine. 2021;37 doi: 10.1016/j.eclinm.2021.100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojkova D., Bechtel M., McLaughlin K.-M., McGreig J.E., Klann K., Bellinghausen C., Rohde G., Jonigk D., Braubach P., Ciesek S., Münch C., Wass M.N., Michaelis M., Cinatl J. Aprotinin inhibits SARS-CoV-2 replication. Cells. 2020;9:2377. doi: 10.3390/cells9112377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boodhoo N., Matsuyama-Kato A., Shojadoost B., Behboudi S., Sharif S. The severe acute respiratory syndrome coronavirus 2 non-structural proteins 1 and 15 proteins mediate antiviral immune evasion. Current Research in Virological Science. 2022;3 doi: 10.1016/j.crviro.2022.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewitz L., Kamps J.J.A.G., Lukacik P., Strain‐Damerell C., Zhao Y., Tumber A., Malla T.R., Orville A.M., Walsh M.A., Schofield C.J. Mass spectrometric assays reveal discrepancies in inhibition profiles for the SARS‐CoV‐2 papain‐like protease. ChemMedChem. 2022;17 doi: 10.1002/cmdc.202200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukreyeva N., Mantlo E.K., Sattler R.A., Huang C., Paessler S., Zeldis J. The IMPDH inhibitor merimepodib suppresses SARS-CoV-2 replication in vitro. bioRxiv. 2020 [Google Scholar]
- Caianiello D.F., Zhang M., Ray J.D., Howell R.A., Swartzel J.C., Branham E.M.J., Chirkin E., Sabbasani V.R., Gong A.Z., McDonald D.M., Muthusamy V., Spiegel D.A. Bifunctional small molecules that mediate the degradation of extracellular proteins. Nat. Chem. Biol. 2021;17:947–953. doi: 10.1038/s41589-021-00851-1. [DOI] [PubMed] [Google Scholar]
- Cairns D.M., Dulko D., Griffiths J.K., Golan Y., Cohen T., Trinquart L., Price L.L., Beaulac K.R., Selker H.P. Efficacy of niclosamide vs placebo in SARS-CoV-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19: a phase 2 randomized clinical trial. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2021.44942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. ‘The entire protein universe’: AI predicts shape of nearly every known protein. nature news article. 2022 doi: 10.1038/d41586-022-02083-2. [DOI] [PubMed] [Google Scholar]
- Calonico S., Di Tella R., Del Valle J.C.L. National Bureau of Economic Research; 2022. Causal Inference during a Pandemic: Evidence on the Effectiveness of Nebulized Ibuprofen as an Unproven Treatment for COVID-19 in Argentina. [Google Scholar]
- Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., others Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., others A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraco Y., Crofoot G.E., Moncada P.A., Galustyan A.N., Musungaie D.B., Payne B., Kovalchuk E., Gonzalez A., Brown M.L., Williams-Diaz A., others Phase 2/3 trial of molnupiravir for treatment of Covid-19 in nonhospitalized adults. NEJM Evidence. 2022;1 doi: 10.1056/EVIDoa2100043. [DOI] [PubMed] [Google Scholar]
- Carpinteiro A., Edwards M.J., Hoffmann M., Kochs G., Gripp B., Weigang S., Adams C., Carpinteiro E., Gulbins A., Keitsch S., Sehl C., Soddemann M., Wilker B., Kamler M., Bertsch T., Lang K.S., Patel S., Wilson G.C., Walter S., Hengel H., Pöhlmann S., Lang P.A., Kornhuber J., Becker K.A., Ahmad S.A., Fassbender K., Gulbins E. Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells. Cell Rep. Med. 2020;1 doi: 10.1016/j.xcrm.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Garit J.A., Cañizares-Carmenate Y., Pérez-Giménez F. Biosynthetic enzymes of the SARS-CoV-2 as potential targets for the discovery of new antiviral drugs. Nereis. Interdisciplinary Ibero-American Journal of Methods, Modelling and Simulation. 2021:17–23. doi: 10.46583/nereis_2021.13.844. [DOI] [Google Scholar]
- Caulfield T., Devkota B. Motion of transfer RNA from the A/T state into the A-site using docking and simulations. Proteins. 2012;80:2489–2500. doi: 10.1002/prot.24131. [DOI] [PubMed] [Google Scholar]
- Caulfield T., Medina-Franco J.L. Molecular dynamics simulations of human DNA methyltransferase 3B with selective inhibitor nanaomycin A. J. Struct. Biol. 2011;176:185–191. doi: 10.1016/j.jsb.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Caulfield T.R. Inter-ring rotation of apolipoprotein A-I protein monomers for the double-belt model using biased molecular dynamics. J. Mol. Graph. Model. 2011;29:1006–1014. doi: 10.1016/j.jmgm.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Caulfield T.R., Devkota B., Rollins G.C. Examinations of tRNA range of motion using simulations of cryo-EM microscopy and X-ray data. J. Biophys. 2011;2011 doi: 10.1155/2011/219515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty C., Bhattacharya M., Mallick B., Sharma A.R., Lee S.-S., Agoramoorthy G. SARS-CoV-2 protein drug targets landscape: a potential pharmacological insight view for the new drug development. Expet Rev. Clin. Pharmacol. 2021;14:225–238. doi: 10.1080/17512433.2021.1874348. [DOI] [PubMed] [Google Scholar]
- Chan H.-T., Chao C.-M., Lai C.-C. Sofosbuvir/daclatasvir in the treatment of COVID-19 infection: a meta-analysis. J. Infect. 2021;82:e34–e35. doi: 10.1016/j.jinf.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. 4′-Modified nucleosides for antiviral drug discovery: achievements and perspectives. Accounts Chem. Res. 2022;55:565–578. doi: 10.1021/acs.accounts.1c00697. [DOI] [PubMed] [Google Scholar]
- Chejfec-Ciociano J.M., Martínez-Herrera J.P., Parra-Guerra A.D., Chejfec R., Barbosa-Camacho F.J., Ibarrola-Peña J.C., Cervantes-Guevara G., Cervantes-Cardona G.A., Fuentes-Orozco C., Cervantes-Pérez E., others Misinformation about and interest in chlorine Dioxide during the COVID-19 pandemic in Mexico identified using google trends data: infodemiology study. JMIR Infodemiology. 2022;2 doi: 10.2196/29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Zhang Z., Wang L., Huang Z., Gong F., Li X., Chen Y., Wu J.J. First clinical study using HCV protease inhibitor danoprevir to treat COVID-19 patients. Medicine. 2020;99 doi: 10.1097/MD.0000000000023357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Malone B., Llewellyn E., Grasso M., Shelton P.M., Olinares P.D.B., Maruthi K., Eng E.T., Vatandaslar H., Chait B.T., others Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex. Cell. 2020;182:1560–1573. doi: 10.1016/j.cell.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Zody M.C., Di Germanio C., Martinelli R., Mediavilla J.R., Cunningham M.H., Composto K., Chow K.F., Kordalewska M., Corvelo A., others Emergence of multiple SARS-CoV-2 antibody escape variants in an immunocompromised host undergoing convalescent plasma treatment. mSphere. 2021;6 doi: 10.1128/mSphere.00480-21. e00480-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi R., Zhou M., Shek R., Wilson J.W., Tillery L., Craig J.K., Salukhe I.A., Hickson S.E., Kumar N., James R.M., others High-throughput screening of the ReFRAME, Pandemic Box, and COVID Box drug repurposing libraries against SARS-CoV-2 nsp15 endoribonuclease to identify small-molecule inhibitors of viral activity. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonira V.K., Kwon Y.-D., Gorman J., Case J.B., Ku Z., Simeon R., Casner R.G., Harris D.R., Olia A.S., Stevens T., others . bioRxiv; 2022. Potent and Pan-Neutralization of SARS-CoV-2 Variants of Concern by DARPins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimolai N. Potentially repurposing adamantanes for COVID-19. J. Med. Virol. 2020;92:531–532. doi: 10.1002/jmv.25752. [DOI] [PubMed] [Google Scholar]
- Clemency B.M., Varughese R., Gonzalez-Rojas Y., Morse C.G., Phipatanakul W., Koster D.J., Blaiss M.S. Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial. JAMA Intern. Med. 2022;182:42–49. doi: 10.1001/jamainternmed.2021.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi N., Scagnolari C., D'Amore A., Palombi F., Criscuolo E., Frasca F., Pierangeli A., Mancini N., Antonelli G., Clementi M., others Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacol. Res. 2021;163 doi: 10.1016/j.phrs.2020.105255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban M.A., Blackburn P.R., Whitelaw M.L., Haelst M.M. van, Atwal P.S., Caulfield T.R. Structural models for the dynamic effects of loss-of-function variants in the human SIM1 protein transcriptional activation domain. Biomolecules. 2020;10:E1314. doi: 10.3390/biom10091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban M.A., Fraga S., Caulfield T.R. Structural and computational perspectives of selectively targeting mutant proteins. Curr. Drug Discov. Technol. 2021;18:365–378. doi: 10.2174/1570163817666200311114819. [DOI] [PubMed] [Google Scholar]
- Coban M.A., Morrison J., Maharjan S., Hernandez Medina D.H., Li W., Zhang Y.S., Freeman W.D., Radisky E.S., Le Roch K.G., Weisend C.M., Ebihara H., Caulfield T.R. Attacking COVID-19 progression using multi-drug therapy for synergetic target engagement. Biomolecules. 2021;11:787. doi: 10.3390/biom11060787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D.P., Steer C.J. Binding of the SARS-CoV-2 spike protein to the asialoglycoprotein receptor on human primary hepatocytes and immortalized hepatocyte-like cells by confocal analysis. Hepat. Med. 2021;13:37–44. doi: 10.2147/HMER.S301979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway S.R., Keller M.D., Bollard C.M. Cellular therapies for the treatment and prevention of SARS-CoV-2 infection. Blood. 2022 doi: 10.1182/blood.2021012249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M., De Giglio M.A.R., Roviello G.N. SARS-CoV-2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr. Med. Chem. 2020 doi: 10.2174/0929867327666200416131117. [DOI] [PubMed] [Google Scholar]
- Cox R.M., Wolf J.D., Plemper R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat. Microbiol. 2021;6:11–18. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva M.F., de Araújo-Júnior J.X., da Silva-Júnior E.F., Heimfarth L., Martins-Filho P.R., Quintans J. de S.S., Quintans-Júnior L.J. Bradykinin-target therapies in SARS-CoV-2 infection: current evidence and perspectives. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022:1–9. doi: 10.1007/s00210-022-02206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W., Zhang B., Jiang X.-M., Su H., Li J., Zhao Y., Xie X., Jin Z., Peng J., Liu F., Li C., Li Y., Bai F., Wang H., Cheng X., Cen X., Hu S., Yang X., Wang J., Liu X., Xiao G., Jiang H., Rao Z., Zhang L.-K., Xu Y., Yang H., Liu H. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020;368:1331–1335. doi: 10.1126/science.abb4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang M., Song J. A review of the effects of ATP and hydroxychloroquine on the phase separation of the SARS-CoV-2 nucleocapsid protein. Biophys. Rev. 2022:1–7. doi: 10.1007/s12551-022-00957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J.A., Chau N., Abdel-Hamid M.K., Hu L., von Kleist L., Whiting A., Krishnan S., Maamary P., Joseph S.R., Simpson F., Haucke V., McCluskey A., Robinson P.J. Phenothiazine-derived antipsychotic drugs inhibit dynamin and clathrin-mediated endocytosis: antipsychotic drugs inhibit dynamin. Traffic. 2015;16:635–654. doi: 10.1111/tra.12272. [DOI] [PubMed] [Google Scholar]
- Dara S., Dhamercherla S., Jadav S.S., Babu C.M., Ahsan M.J. Machine learning in drug discovery: a review. Artif. Intell. Rev. 2022;55:1947–1999. doi: 10.1007/s10462-021-10058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Randeva H.S., Chatha K., Hall M., Spandidos D.A., Karteris E., Kyrou I. Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Mol. Med. Rep. 2020;22:4221–4226. doi: 10.3892/mmr.2020.11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest J.F., Kienle M., Boytz R., Ayres M., Kim E., Chung D., Gandhi V., Davey R.A., Sykes D.B., Shohdy N., others Brequinar and Dipyridamole in combination exhibits synergistic antiviral activity against SARS-CoV-2 in vitro: rationale for a host-acting antiviral treatment strategy for COVID-19. bioRxiv. 2022 doi: 10.1016/j.antiviral.2022.105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Zhou F., Heybati K., Ali S., Zuo Q.K., Hou W., Dhivagaran T., Ramaraju H.B., Chang O., Wong C.Y., others Efficacy of chloroquine and hydroxychloroquine for the treatment of hospitalized COVID-19 patients: a meta-analysis. Future Virol. 2022;17:95–118. doi: 10.2217/fvl-2021-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desantis J., Mercorelli B., Celegato M., Croci F., Bazzacco A., Baroni M., Siragusa L., Cruciani G., Loregian A., Goracci L. Indomethacin-based PROTACs as pan-coronavirus antiviral agents. Eur. J. Med. Chem. 2021;226 doi: 10.1016/j.ejmech.2021.113814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi K.P., Pourkarim M.R., Thijssen M., Sureda A., Khayatkashani M., Cismaru C.A., Neagoe I.B., Habtemariam S., Razmjouei S., Khayat Kashani H.R. A perspective on the applications of furin inhibitors for the treatment of SARS-CoV-2. Pharmacol. Rep. 2022;74:425–430. doi: 10.1007/s43440-021-00344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka P., Singh A., Choudhary S., Kumar P., Sharma G.K., Tomar S. bioRxiv; 2022. Discovery of Anti-SARS-CoV-2 Molecules Using Structure-Assisted Repurposing Approach Targeting N-Protein. [Google Scholar]
- Ding X., Ye N., Qiu M., Guo H., Li J., Zhou X., Yang M., Xi J., Liang Y., Gong Y., others Cathepsin B is a potential therapeutic target for coronavirus disease 2019 patients with lung adenocarcinoma. Chem. Biol. Interact. 2022;353 doi: 10.1016/j.cbi.2022.109796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar M., Lee J.S., Whig K., Segrist E., Li M., Kamalia B., Castellana L., Ayyanathan K., Cardenas-Diaz F.L., Morrisey E.E., others Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-Cov-2. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.108959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordje A., Avila S.V., Forat L., Xiaoxuan F., Sanchez-Petitto G., Siglin J., Baddley J., Mannuel H.D., Hanan A., Hankey K.G., others Deep dissection of the antiviral immune profile of patients with COVID-19. Commun. Biol. 2021;4 doi: 10.1038/s42003-021-02852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayman N., DeMarco J.K., Jones K.A., Azizi S.-A., Froggatt H.M., Tan K., Maltseva N.I., Chen S., Nicolaescu V., Dvorkin S., others Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science. 2021;373:931–936. doi: 10.1126/science.abg5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drożdżal S., Rosik J., Lechowicz K., Machaj F., Szostak B., Przybyciński J., Lorzadeh S., Kotfis K., Ghavami S., Los M.J. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist. Updates. 2021;59 doi: 10.1016/j.drup.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R.J., Olivier D.S., Amaral M.S., Gering I., Willbold D., Arni R.K., Coronado M.A. The repurposed drugs suramin and quinacrine cooperatively inhibit SARS-CoV-2 3CLpro in vitro. Viruses. 2021;13:873. doi: 10.3390/v13050873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi Chaharom F., Pourafkari L., Ebrahimi Chaharom A.A., Nader N.D. Effects of corticosteroids on Covid-19 patients: a systematic review and meta-analysis on clinical outcomes. Pulm. Pharmacol. Therapeut. 2022;72 doi: 10.1016/j.pupt.2021.102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid R.A., Elgendy M.O., El-Gendy A.O., Elgendy S.O., Belbahri L., Sayed A.M., Rateb M.E. Efficacy of ceftazidime and cefepime in the management of COVID-19 patients: single center report from Egypt. Antibiotics. 2021;10:1278. doi: 10.3390/antibiotics10111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi R., Karami P., Heidary A.H., Esmaeilzadeh A. An updated overview of recent advances, challenges, and clinical considerations of IL-6 signaling blockade in severe coronavirus disease 2019 (COVID-19) Int. Immunopharm. 2022 doi: 10.1016/j.intimp.2022.108536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazor M., Liu M., McKenna S., Liu P., Gehrig E.A., Elfert A., Puglisi J., Rossignol J.-F., Glenn J.S. Hepatology. JOHN WILEY & SONS INC 111 RIVER ST; HOBOKEN, NJ 07030 USA: 2008. Nitazoxanide (ntz) is an inducer Eif2A and Pkr phosphorylation. 1151A-1151A. [Google Scholar]
- El-Behery H., Attia A.-F., El-Feshawy N., Torkey H. Efficient machine learning model for predicting drug-target interactions with case study for Covid-19. Comput. Biol. Chem. 2021;93 doi: 10.1016/j.compbiolchem.2021.107536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elebeedy D., Elkhatib W.F., Kandeil A., Ghanem A., Kutkat O., Alnajjar R., Saleh M.A., Abd El Maksoud A.I., Badawy I., Al-Karmalawy A.A. Anti-SARS-CoV-2 activities of tanshinone IIA, carnosic acid, rosmarinic acid, salvianolic acid, baicalein, and glycyrrhetinic acid between computational and in vitro insights. RSC Adv. 2021;11:29267–29286. doi: 10.1039/D1RA05268C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger B., Bojkova D., Zaliani A., Cinatl J., Claussen C., Westhaus S., Keminer O., Reinshagen J., Kuzikov M., Wolf M., others A SARS-CoV-2 cytopathicity dataset generated by high-content screening of a large drug repurposing collection. Sci. Data. 2021;8:1–10. doi: 10.1038/s41597-021-00848-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S.E., Tseng C.-T.K., Scott B.L., Höök A.M., Dickey B.F. Inducible epithelial resistance against coronavirus pneumonia in mice. Am. J. Respir. Cell Mol. Biol. 2020;63:540–541. doi: 10.1165/rcmb.2020-0247LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezer N., Belga S., Daneman N., Chan A., Smith B.M., Daniels S.-A., Moran K., Besson C., Smyth L.Y., Bartlett S.J., others Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial. Br. Med. J. 2021;375 doi: 10.1136/bmj-2021-068060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag A., Wang P., Boys I.N., L. Eitson J., Ohlson M.B., Fan W., McDougal M.B., Ahmed M., Schoggins, J W., Sadek H. Identification of atovaquone, Ouabain and mebendazole as FDA approved drugs tar-geting SARS-CoV-2 (version 4) (preprint) Chemistry. 2020 doi: 10.26434/chemrxiv.12003930.v4. [DOI] [Google Scholar]
- Farhangnia P., Dehrouyeh S., Safdarian A.R., Farahani S.V., Gorgani M., Rezaei N., Akbarpour M., Delbandi A.-A. Recent advances in passive immunotherapies for COVID-19: the Evidence-Based approaches and clinical trials. Int. Immunopharm. 2022 doi: 10.1016/j.intimp.2022.108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima S.W., Alam S., Khare S.K. Molecular and structural insights of β-boswellic acid and glycyrrhizic acid as potent SARS-CoV-2 Envelope protein inhibitors. Phytomedicine. 2022;2 doi: 10.1016/j.phyplu.2022.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenizia C., Galbiati S., Vanetti C., Vago R., Clerici M., Tacchetti C., Daniele T. Cyclosporine A inhibits viral infection and release as well as cytokine production in lung cells by three SARS-CoV-2 variants. Microbiol. Spectr. 2022;10:e01504–e01521. doi: 10.1128/spectrum.01504-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore N., Bell S., Shen C., Nguyen V., La J., Dubreuil M., Strymish J., Brophy M., Mehta G., Wu H., Lieberman J., Do N., Sander C. Disulfiram associated with lower risk of Covid-19: a retrospective cohort study (preprint) Epidemiology. 2021 doi: 10.1101/2021.03.10.21253331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink K., Nitsche A., Neumann M., Grossegesse M., Eisele K.-H., Danysz W. Amantadine inhibits SARS-CoV-2 in vitro. Viruses. 2021;13:539. doi: 10.3390/v13040539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fintelman-Rodrigues N., Sacramento C.Q., Ribeiro Lima C., Souza da Silva F., Ferreira A.C., Mattos M., de Freitas C.S., Cardoso Soares V., da Silva Gomes Dias S., Temerozo J.R., others Atazanavir, alone or in combination with ritonavir, inhibits SARS-CoV-2 replication and proinflammatory cytokine production. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00825-20. e00825-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C. Eigenenergy; Adelaide, Australia: 2020. Covid-19: Predicting Inhibition of SARS-CoV-2 in Caco-2 Cells: Viral Cellular Entry (PhD Thesis) [Google Scholar]
- Foo C.S., Abdelnabi R., Kaptein S.J., Zhang X., ter Horst S., Mols R., Delang L., Rocha-Pereira J., Coelmont L., Leyssen P., others . bioRxiv; 2021. Nelfinavir Markedly Improves Lung Pathology in SARS-CoV-2-Infected Syrian Hamsters Despite a Lack of an Antiviral Effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasier E., Macchia M.L., Giachin G., Sosic A., Pavan M., Sturlese M., Salata C., Moro S., Gatto B., Bellanda M., others A new inactive conformation of SARS-CoV-2 main protease. Acta Crystallogr. D: Struct. Biol. 2022;78 doi: 10.1107/S2059798322000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchin G. 2021. IRAK 4 Inhibitor (PF-06650833) in Hospitalized Patients with COVID-19 Pneumonia and Exuberant Inflammation. [Google Scholar]
- Fred S.M., Kuivanen S., Ugurlu H., Casarotto P.C., Levanov L., Saksela K., Vapalahti O., Castrén E. Antidepressant and antipsychotic drugs reduce viral infection by SARS-CoV-2 and fluoxetine shows antiviral activity against the novel variants in vitro. Front. Pharmacol. 2022;12 doi: 10.3389/fphar.2021.755600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg D.E., Conigliaro J., Wang T.C., Tracey K.J., Callahan M.V., Abrams J.A., Sobieszczyk M.E., Markowitz D.D., Gupta A., O'Donnell M.R., others Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020;159:1129–1131. doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., Huang B., Tang J., Liu S., Liu M., Ye Y., Liu Z., Xiong Y., Zhu W., Cao D., others The complex structure of GRL0617 and SARS-CoV-2 PLpro reveals a hot spot for antiviral drug discovery. Nat. Commun. 2021;12:1–12. doi: 10.1038/s41467-020-20718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung S.-Y., Siu K.-L., Lin H., Chan C.-P., Yeung M.L., Jin D.-Y. SARS-CoV-2 NSP13 helicase suppresses interferon signaling by perturbing JAK1 phosphorylation of STAT1. Cell Biosci. 2022;12:1–12. doi: 10.1186/s13578-022-00770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez-Romero J.L., Palmeros-Rojas O., Real-Ramírez F.A., Sánchez-Romero S., Tome-Maxil R., Ramírez-Sandoval M.P., Olivos-Rodríguez R., Flores-Encarnación S.E., Cabrera-Estrada A.A., Ávila-Morales J., others Cyclosporine A plus low-dose steroid treatment in COVID-19 improves clinical outcomes in patients with moderate to severe disease: a pilot study. J. Intern. Med. 2021;289:906–920. doi: 10.1111/joim.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S., Klein J., Robertson A.J., Peña-Hernández M.A., Lin M.J., Roychoudhury P., Lu P., Fournier J., Ferguson D., Mohamed Bakhash S.A., others De novo emergence of a remdesivir resistance mutation during treatment of persistent SARS-CoV-2 infection in an immunocompromised patient: a case report. Nat. Commun. 2022;13:1–8. doi: 10.1038/s41467-022-29104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Zhang L., Liu X., Li F., Ma R., Zhu Z., Zhang J., Wu J., Shi Y., Pan Y., Ge Y., Ruan K. Repurposing low-molecular-weight drugs against the main protease of severe acute respiratory syndrome coronavirus 2. J. Phys. Chem. Lett. 2020;11:7267–7272. doi: 10.1021/acs.jpclett.0c01894. [DOI] [PubMed] [Google Scholar]
- Garcia G., Sharma A., Ramaiah A., Sen C., Purkayastha A., Kohn D.B., Parcells M.S., Beck S., Kim H., Bakowski M.A., Kirkpatrick M.G., Riva L., Wolff K.C., Han B., Yuen C., Ulmert D., Purbey P.K., Scumpia P., Beutler N., Rogers T.F., Chatterjee A.K., Gabriel G., Bartenschlager R., Gomperts B., Svendsen C.N., Betz U.A.K., Damoiseaux R.D., Arumugaswami V. Antiviral drug screen identifies DNA-damage response inhibitor as potent blocker of SARS-CoV-2 replication. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.108940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile I., Schiano Moriello N. COVID-19 prophylaxis in immunosuppressed patients: beyond vaccination. PLoS Med. 2022;19 doi: 10.1371/journal.pmed.1003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geriak M., Haddad F., Kullar R., Greenwood K.L., Habib M., Habib C., Willms D., Sakoulas G. Randomized prospective open label study shows no impact on clinical outcome of adding losartan to hospitalized COVID-19 patients with mild hypoxemia. Infect Dis. Therp. 2021;10:1323–1330. doi: 10.1007/s40121-021-00453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemiyeh P., Mortazavi N., Karimzadeh I., Vazin A., Mahmoudi L., Moghimi Sarani E., MohammadSadeghi A., Shahisavandi M., Kheradmand A., Mohammadi-Samani S. Psychiatric adverse drug reactions and potential anti-COVID-19 drug interactions with psychotropic medications. IJPR. 2021;20 doi: 10.22037/ijpr.2021.114717.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno A., Mestres-Truyol J., Ojeda-Montes M.J., Macip G., Saldivar-Espinoza B., Cereto-Massagué A., Pujadas G., Garcia-Vallvé S. Prediction of novel inhibitors of the main protease (M-pro) of SARS-CoV-2 through consensus docking and drug reposition. IJMS. 2020;21:3793. doi: 10.3390/ijms21113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giossi R., Menichelli D., Pani A., Tratta E., Romandini A., Roncato R., Nani A., Schenardi P., Diani E., Fittipaldo V.A., Farcomeni A., Scaglione F., Pastori D. A systematic review and a meta-analysis comparing prophylactic and therapeutic low molecular weight heparins for mortality reduction in 32,688 COVID-19 patients. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.698008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil S., Janowska K., McDowell S., Mansouri K., Parks R., Manne K., Stalls V., Kopp M., Henderson R., Edwards R.J., others . bioRxiv; 2020. D614G Mutation Alters SARS-CoV-2 Spike Conformational Dynamics and Protease Cleavage Susceptibility at the S1/S2 Junction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa A.A., Mohamed H.S., Abd-Ellatief R.B., Gomaa M.A., Hammam D.S. Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: a randomized clinical trial. Inflammopharmacology. 2022;30:477–486. doi: 10.1007/s10787-022-00939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good S.S., Westover J., Jung K.H., Zhou X.-J., Moussa A., La Colla P., Collu G., Canard B., Sommadossi J.-P. AT-527, a double prodrug of a guanosine nucleotide analog, is a potent inhibitor of SARS-CoV-2 in vitro and a promising oral antiviral for treatment of COVID-19. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.02479-20. e02479-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grellet E., Goulet A., Imbert I. Replication of the coronavirus genome: a paradox among positive-strand RNA viruses. J. Biol. Chem. 2022 doi: 10.1016/j.jbc.2022.101923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Cao J., Zhang X., Gao H., Wang Y., Wang J., He J., Jiang X., Zhang J., Shen G., Yang J., Zheng X., Hu G., Zhu Yuanfei, Du S., Zhu Yunkai, Zhang R., Xu J., Lan F., Qu D., Xu G., Zhao Y., Gao D., Xie Y., Luo M., Lu Z. Receptome profiling identifies KREMEN1 and ASGR1 as alternative functional receptors of SARS-CoV-2. Cell Res. 2022;32:24–37. doi: 10.1038/s41422-021-00595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Chun H.J. Interleukin-1-Receptor kinase 4 inhibition: achieving immunomodulatory synergy to mitigate the impact of COVID-19. Front. Immunol. 2021;2483 doi: 10.3389/fimmu.2021.693085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T., Thakkar P., Kalra B., Kannan S. Hydroxychloroquine in the treatment of coronavirus disease 2019: rapid updated systematic review and meta-analysis. Rev. Med. Virol. 2022;32 doi: 10.1002/rmv.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Y., Kumar S., Zak S.E., Jones K.A., Upadhyay C., Sharma N., Azizi S.-A., Kathayat R.S., Herbert A.S., Durvasula R., others Antiviral evaluation of hydroxyethylamine analogs: inhibitors of SARS-CoV-2 main protease (3CLpro), a virtual screening and simulation approach. Bioorg. Med. Chem. 2021;116393 doi: 10.1016/j.bmc.2021.116393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Y., Maciorowski D., Medernach B., Becker D.P., Durvasula R., Libertin C.R., Kempaiah P. Iron dysregulation in COVID-19 and reciprocal evolution of SARS-CoV-2: natura nihil frustra facit. J. Cell. Biochem. 2022 doi: 10.1002/jcb.30207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Y., Maciorowski D., Zak S.E., Jones K.A., Kathayat R.S., Azizi S.-A., Mathur R., Pearce C.M., Ilc D.J., Husein H., others Bisindolylmaleimide IX: a novel anti-SARS-CoV2 agent targeting viral main protease 3CLpro demonstrated by virtual screening pipeline and in-vitro validation assays. Methods. 2021 doi: 10.1016/j.ymeth.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta Y., Maciorowski D., Zak S.E., Kulkarni C.V., Herbert A.S., Durvasula R., Fareed J., Dye J.M., Kempaiah P. Heparin: a simplistic repurposing to prevent SARS-CoV-2 transmission in light of its in-vitro nanomolar efficacy. Int. J. Biol. Macromol. 2021;183:203–212. doi: 10.1016/j.ijbiomac.2021.04.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy R., Mostafa A., Abo Shama N.M., Soliman S.S., Fayed B. Phytotherapy Research; 2022. Comparative Evaluation of Flavonoids Reveals the Superiority and Promising Inhibition Activity of Silibinin against SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., Baniecki M., Hendrick V.M., Damle B., Simón-Campos A., Pypstra R., Rusnak J.M. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N. Engl. J. Med. 2022;386:1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Duan X., Yang L., Nilsson-Payant B.E., Wang P., Duan F., Tang X., Yaron T.M., Zhang T., Uhl S., Bram Y., Richardson C., Zhu J., Zhao Z., Redmond D., Houghton S., Nguyen D.-H.T., Xu D., Wang X., Jessurun J., Borczuk A., Huang Y., Johnson J.L., Liu Y., Xiang J., Wang H., Cantley L.C., tenOever B.R., Ho D.D., Pan F.C., Evans T., Chen H.J., Schwartz R.E., Chen S. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature. 2021;589:270–275. doi: 10.1038/s41586-020-2901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Halim D.A., Rosalind J., Gunawan C., Kurniawan A. Ivermectin and outcomes from Covid-19 pneumonia: a systematic review and meta-analysis of randomized clinical trial studies. Rev. Med. Virol. 2022;32:e2265. [Google Scholar]
- Harrison C. Focus shifts to antibody cocktails for COVID-19 cytokine storm. Nat. Biotechnol. 2020;38:905–909. doi: 10.1038/s41587-020-0634-9. [DOI] [PubMed] [Google Scholar]
- Harrison S., Thumm L., Nash T.E., Nutman T.B., O'Connell E.M. The local inflammatory profile and predictors of treatment success in subarachnoid neurocysticercosis. Clin. Infect. Dis. 2021;72:e326–e333. doi: 10.1093/cid/ciaa1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel T., Elez K., Krüger N., Raich L., Shrimp J.H., Danov O., Jonigk D., Braun A., Shen M., Hall M.D., Pöhlmann S., Hoffmann M., Noé F. Synergistic inhibition of SARS-CoV-2 cell entry by otamixaban and covalent protease inhibitors: pre-clinical assessment of pharmacological and molecular properties. Chem. Sci. 2021;12:12600–12609. doi: 10.1039/D1SC01494C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata A., Shionyu-Mitsuyama C., Nakae S., Shionyu M., Ota M., Kanaya S., Hirokawa T., Nakajima S., Watashi K., Shirai T. Evaluating cepharanthine analogues as natural drugs against SARS-CoV-2. FEBS Open bio. 2022;12:285–294. doi: 10.1002/2211-5463.13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines S.L., Mohammad A.N., Jackson J., Macklin S., Caulfield T.R. Integrative data fusion for comprehensive assessment of a novel CHEK2 variant using combined genomics, imaging, and functional-structural assessments via protein informatics. Mol Omics. 2019;15:59–66. doi: 10.1039/c8mo00137e. [DOI] [PubMed] [Google Scholar]
- Hines S.L., Richter J.E., Mohammad A.N., Mahim J., Atwal P.S., Caulfield T.R. Protein informatics combined with multiple data sources enriches the clinical characterization of novel TRPV4 variant causing an intermediate skeletal dysplasia. Mol Genet Genomic Med. 2019;7:e566. doi: 10.1002/mgg3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton G. Boltzmann machine. Scholarpedia. 2007;2:1668. doi: 10.4249/scholarpedia.1668. [DOI] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., others SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M., Mandala V., McKay M., Shcherbakov A., Dregni A., Kolocouris A. 2020. Structure and Drug Binding of the SARS-CoV-2 Envelope Protein in Phospholipid Bilayers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S., Wang H., Zhang Z., Qiao L. The roles of methylprednisolone treatment in patients with COVID-19: a systematic review and meta-analysis. Steroids. 2022;183 doi: 10.1016/j.steroids.2022.109022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.-K., Chen C.-Y., Chen W.-C., Lai C.-C., Hung S.-H., Lin W.-T. The effect of sofosbuvir-based treatment on the clinical outcomes of patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Int. J. Antimicrob. Agents. 2022;106545 doi: 10.1016/j.ijantimicag.2022.106545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Wang M., Zhao Y., Zhang Y., Wang T., Zheng Z., Li X., Zeng S., Zhao D., Li H., others A small-scale medication of leflunomide as a treatment of COVID-19 in an open-label blank-controlled clinical trial. Virol. Sin. 2020;35:725–733. doi: 10.1007/s12250-020-00258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., van der Ploeg K., Chakraborty S., Arunachalam P., Mori D., Jacobson K., Bonilla H., Parsonnet J., Andrews J., Hedlin H., others Early immune responses have long-term associations with clinical, virologic, and immunologic outcomes in patients with COVID-19. Research Square. 2021 [Google Scholar]
- Hung D.T., Ghula S., Aziz J.M.A., Makram A.M., Tawfik G.M., Abozaid A.A.-F., Pancharatnam R.A., Ibrahim A.M., Shabouk M.B., Turnage M., others The efficacy and adverse effects of favipiravir on COVID-19 patients: a systematic review and meta-analysis of published clinical trials and observational studies. Int. J. Infect. Dis. 2022 doi: 10.1016/j.ijid.2022.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., others Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K., Sakurai Y., Enami T., Shibukawa R., Nishi Y., Ohta A., Shu T., Kawaguchi J., Okada S., Hoenen T., Yasuda J., Inoue H. iPSC screening for drug repurposing identifies anti‐RNA virus agents modulating host cell susceptibility. FEBS Open Bio. 2021;11:1452–1464. doi: 10.1002/2211-5463.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova N., Sotirova Y., Gavrailov G., Nikolova K., Andonova V. Advances in the prophylaxis of respiratory infections by the nasal and the Oromucosal route: relevance to the fight with the SARS-CoV-2 pandemic. Pharmaceutics. 2022;14:530. doi: 10.3390/pharmaceutics14030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashchenko A.A., Dmitriev K.A., Vostokova N.V., Azarova V.N., Blinow A.A., Egorova A.N., Gordeev I.G., Ilin A.P., Karapetian R.N., Kravchenko D.V., Lomakin N.V., Merkulova E.A., Papazova N.A., Pavlikova E.P., Savchuk N.P., Simakina E.N., Sitdekov T.A., Smolyarchuk E.A., Tikhomolova E.G., Yakubova E.V., Ivachtchenko A.V. AVIFAVIR for treatment of patients with moderate coronavirus disease 2019 (COVID-19): interim results of a phase II/III multicenter randomized clinical trial. Clin. Infect. Dis. 2021;73:531–534. doi: 10.1093/cid/ciaa1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang K.-J., Jeong S., Kang D.Y., Sp N., Yang Y.M., Kim D.-E. A high ATP concentration enhances the cooperative translocation of the SARS coronavirus helicase nsP13 in the unwinding of duplex RNA. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-61432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroenram W., Chatnuntawech I., Kampeera J., Pengpanich S., Leaungwutiwong P., Tondee B., Sirithammajak S., Suvannakad R., Khumwan P., Dangtip S., Arunrut N., Bantuchai S., Nguitragool W., Wongwaroran S., Khanchaitit P., Sattabongkot J., Teerapittayanon S., Kiatpathomchai W. One-step colorimetric isothermal detection of COVID-19 with AI-assisted automated result analysis: a platform model for future emerging point-of-care RNA/DNA disease diagnosis. Talanta. 2022;249 doi: 10.1016/j.talanta.2022.123375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martín-Quirós A., Caraco Y., Williams-Diaz A., Brown M.L., others Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N. Engl. J. Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong G.U., Lyu J., Kim K.-D., Chung Y.C., Yoon G.Y., Lee S., Hwang I., Shin W.-H., Ko J., Lee J.-Y., others SARS-CoV-2 infection of microglia elicits proinflammatory activation and apoptotic cell death. Microbiol. Spectr. 2022 doi: 10.1128/spectrum.01091-22. e01091-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Luna J., Grisoni F., Weskamp N., Schneider G. Artificial intelligence in drug discovery: recent advances and future perspectives. Expet Opin. Drug Discov. 2021;16:949–959. doi: 10.1080/17460441.2021.1909567. [DOI] [PubMed] [Google Scholar]
- Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang Xinglou, You T., Liu Xiaoce, Yang Xiuna, Bai F., Liu H., Liu Xiang, Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- Kalantari S., Fard S.R., Maleki D., Taher M.T., Yassin Z., Alimohamadi Y., Minaeian S. Comparing the effectiveness of Atazanavir/Ritonavir/Dolutegravir/Hydroxychloroquine and Lopinavir/Ritonavir/Hydroxychloroquine treatment regimens in COVID-19 patients. J. Med. Virol. 2021;93:6557–6565. doi: 10.1002/jmv.27195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel W.A., Kamel M.I., Alhasawi A., Elmasry S., AlHamdan F., Al-Hashel J.Y. Effect of pre-exposure use of amantadine on COVID-19 infection: a hospital-based cohort study in patients with Parkinson's disease or multiple sclerosis. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.704186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeel M., Yamamoto M., Tani H., Kobayashi A., Gohda J., Kawaguchi Y., Park B.K., Kwon H.-J., Inoue J., Alkattan A. Discovery of new fusion inhibitor peptides against SARS-CoV-2 by targeting the spike S2 subunit. Biomolecules & therapeutics. 2021;29:282. doi: 10.4062/biomolther.2020.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., Sarma P., Bhattacharyya A., Sharma S., Chhimpa N., Prajapat M., Prakash A., Kumar S., Singh A., Singh R., others Efficacy and safety of dihydroorotate dehydrogenase (DHODH) inhibitors “leflunomide” and “teriflunomide” in Covid-19: a narrative review. Eur. J. Pharmacol. 2021;906 doi: 10.1016/j.ejphar.2021.174233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayode O., Wang R., Pendlebury D.F., Cohen I., Henin R.D., Hockla A., Soares A.S., Papo N., Caulfield T.R., Radisky E.S. An acrobatic substrate metamorphosis reveals a requirement for substrate conformational dynamics in trypsin proteolysis. J. Biol. Chem. 2016;291:26304–26319. doi: 10.1074/jbc.M116.758417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern, D.M., Sorum, B., Hoel, C.M., Sridharan, S., Remis, J.P., Toso, D.B., Brohawn, S.G., n.d. Cryo-EM structure of the SARS-CoV-2 3a ion channel in lipid nanodiscs. 10.1101/2020.06.17.156554. [DOI]
- Kinoshita T., Shinoda M., Nishizaki Y., Shiraki K., Hirai Y., Kichikawa Y., Tsushima K., Sinkai M., Komura N., Yoshida K., others . medRxiv; 2022. Phase 3, Multicentre, Double-Blind, Randomised, Parallel-Group, Placebo-Controlled Study of Camostat Mesilate (FOY-305) for the Treatment of COVID-19 (CANDLE Study) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M., Jeon S., Ryu W.-S., Kim S. Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells. J. Med. Virol. 2021;93:1403–1408. doi: 10.1002/jmv.26397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocabaş F., Uslu M. The current state of validated small molecules inhibiting SARS-CoV-2 non-structural proteins. Turkish J. Biol. 2021;45:469–483. doi: 10.3906/biy-2106-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinsky Y., Peskov K., Stanski D.R., Wetmore D., Vinetz J. Semi-Mechanistic pharmacokinetic-pharmacodynamic model of Camostat mesylate-predicted efficacy against SARS-CoV-2 in COVID-19. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.02167-21. e02167-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow C.S., Javed A., Ramachandram D., Hasan S.S. Clinical outcomes of sofosbuvir-based antivirals in patients with COVID-19: a systematic review and meta-analysis of randomized trials. Expert Rev. Anti-infect. Ther. 2022;20:567–575. doi: 10.1080/14787210.2022.2000861. [DOI] [PubMed] [Google Scholar]
- Kumar Prateek, Bhardwaj T., Garg N., Giri R. Microsecond simulations and CD spectroscopy reveals the intrinsically disordered nature of SARS-CoV-2 spike-C-terminal cytoplasmic tail (residues 1242–1273) in isolation. Virology. 2022;566:42–55. doi: 10.1016/j.virol.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Prateek, Bhardwaj T., Giri R. Mitoxantrone dihydrochloride, an FDA approved drug, binds with SARS-CoV-2 NSP1 C-terminal. RSC Adv. 2022;12:5648–5655. doi: 10.1039/D1RA07434B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Prabhakaran, Mathayan M., Smieszek S.P., Przychodzen B.P., Koprivica V., Birznieks G., Polymeropoulos M.H., Prabhakar B.S. Identification of potential COVID-19 treatment compounds which inhibit SARS Cov2 prototypic, Delta and Omicron variant infection. Virology. 2022 doi: 10.1016/j.virol.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Singh B., Kumari P., Kumar P.V., Agnihotri G., Khan S., Kant Beuria T., Syed G.H., Dixit A. Identification of multipotent drugs for COVID-19 therapeutics with the evaluation of their SARS-CoV2 inhibitory activity. Comput. Struct. Biotechnol. J. 2021;19:1998–2017. doi: 10.1016/j.csbj.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon P.S., Oh H., Kwon S.-J., Jin W., Zhang F., Fraser K., Hong J.J., Linhardt R.J., Dordick J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell. Discov. 2020;6:1–4. doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou I., Randeva H.S., Spandidos D.A., Karteris E. Not only ACE2—the quest for additional host cell mediators of SARS-CoV-2 infection: neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal Transduct. Targeted Ther. 2021;6:1–3. doi: 10.1038/s41392-020-00460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.Y., Bukhari F.D.M., Zulkefli N.Z., Ismail I., Mustapa N.I., Soh T.S.T., Hassan A.H., Peariasamy K.M., Lee Y.L., Suppiah J., Thayan R., Lau Y.L. Colorimetric detection of SARS-CoV-2 by uracil-DNA glycosylase (UDG) reverse transcription loop-mediated isothermal amplification (RT-LAMP) Int. J. Infect. Dis. 2022;120:132–134. doi: 10.1016/j.ijid.2022.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E., Sayedy N., Dasgupta N., Akella J., Iqbal J. B24. REPORTING ON COVID-19 AND ITS COMPLICATIONS. American Thoracic Society; 2022. COVID-19-Related Diffuse Leukoencephalopathy clinical improvement with amantadine therapy. A2478–A2478. [Google Scholar]
- Lamb Y.N. Nirmatrelvir plus Ritonavir: first approval. Drugs. 2022:1–7. doi: 10.1007/s40265-022-01692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Q., Wang C., Zhou J., Wang L., Jiao F., Zhang Y., Cai Y., Lu L., Xia S., Jiang S. 25-Hydroxycholesterol-Conjugated EK1 peptide with potent and broad-spectrum inhibitory activity against SARS-CoV-2, its variants of concern, and other human coronaviruses. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222111869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laponogov I., Gonzalez G., Shepherd M., Qureshi A., Veselkov D., Charkoftaki G., Vasiliou V., Youssef J., Mirnezami R., Bronstein M., Veselkov K. Network machine learning maps phytochemically rich “Hyperfoods” to fight COVID-19. Hum. Genom. 2021;15:1. doi: 10.1186/s40246-020-00297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau E.Y., Negrete O.A., Bennett W.F.D., Bennion B.J., Borucki M., Bourguet F., Epstein A., Franco M., Harmon B., He S., Jones D., Kim H., Kirshner D., Lao V., Lo J., McLoughlin K., Mosesso R., Murugesh D.K., Saada E.A., Segelke B., Stefan M.A., Stevenson G.A., Torres M.W., Weilhammer D.R., Wong S., Yang Y., Zemla A., Zhang X., Zhu F., Allen J.E., Lightstone F.C. Discovery of small-molecule inhibitors of SARS-CoV-2 proteins using a computational and experimental pipeline. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.678701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S., Rheinstein P.H. Homozygosity for rs17775810 minor allele associated with reduced mortality of COVID-19 in the UK Biobank Cohort. In Vivo. 2021;35:965–968. doi: 10.21873/invivo.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.F., Chorlton S., Tyson J., Al-Rawahi G.N., Jassem A.N., Prystajecky N., Masud S., Deans G.D., Chapman M.G., Mirzanejad Y., others COVID-19 in an immunocompromised host: persistent shedding of viable SARS-CoV-2 and emergence of multiple mutations: a case report. Int. J. Infect. Dis. 2022;114:178–182. doi: 10.1016/j.ijid.2021.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.S., Ho J., Wills S., Kawall A., Sharma A., Chavada K., Ebert M.C., Evoli S., Singh A., Rayalam S., others Aloin isoforms (A and B) selectively inhibits proteolytic and deubiquitinating activity of papain like protease (PLpro) of SARS-CoV-2 in vitro. Sci. Rep. 2022;12:1–11. doi: 10.1038/s41598-022-06104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chen G., Meng Z., Wu Z., Gan H., Zhu X., Han P., Liu T., Wang F., Gu R., others Bioavailability enhancement of cepharanthine via pulmonary administration in rats and its therapeutic potential for pulmonary fibrosis associated with COVID-19 infection. Molecules. 2022;27:2745. doi: 10.3390/molecules27092745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhang Y., Guan Z., Li H., Ye M., Chen X., Shen J., Zhou Y., Shi Z.-L., Zhou P., others SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Targeted Ther. 2020;5:1–10. doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima A.N., Philot E.A., Trossini G.H.G., Scott L.P.B., Maltarollo V.G., Honorio K.M. Use of machine learning approaches for novel drug discovery. Expet Opin. Drug Discov. 2016;11:225–239. doi: 10.1517/17460441.2016.1146250. [DOI] [PubMed] [Google Scholar]
- Lin C., Li Yue, Zhang Y., Liu Z., Mu X., Gu C., Liu J., Li Yutang, Li G., Chen J. Ceftazidime is a potential drug to inhibit SARS-CoV-2 infection in vitro by blocking spike protein–ACE2 interaction. Signal Transduct. Targeted Ther. 2021;6:1–4. doi: 10.1038/s41392-021-00619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Cherukupalli S., Feng D., Gao S., Kang D., Zhan P., Liu X. SARS-CoV-2 Entry inhibitors targeting virus-ACE2 or virus-TMPRSS2 interactions. Curr. Med. Chem. 2022;29:682–699. doi: 10.2174/0929867328666210420103021. [DOI] [PubMed] [Google Scholar]
- Lin Y., Zhang Z., Mahjour B., Wang D., Zhang R., Shim E., McGrath A., Shen Y., Brugger N., Turnbull R., others Reinforcing the supply chain of umifenovir and other antiviral drugs with retrosynthetic software. Nat. Commun. 2021;12:1–8. doi: 10.1038/s41467-021-27547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Wang X.-J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. Journal of Genetics and Genomics. 2020;47:119. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo H.S., Hui K.P.Y., Lai H.-M., He X., Khan K.S., Kaur S., Huang J., Li Z., Chan A.K., Cheung H.H.-Y., others Simeprevir potently suppresses SARS-CoV-2 replication and synergizes with remdesivir. ACS Cent. Sci. 2021;7:792–802. doi: 10.1021/acscentsci.0c01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo M., Lucero H., Chen D.-Y., O'Connell A., Bergqvist S., Munawar A., Bandara A., De Graef S., Weeks S.D., Douam F., others The in-vitro effect of famotidine on sars-cov-2 proteases and virus replication. Sci. Rep. 2021;11:1–9. doi: 10.1038/s41598-021-84782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luban J., Sattler R.A., Mühlberger E., Graci J.D., Cao L., Weetall M., Trotta C., Colacino J.M., Bavari S., Strambio-De-Castillia C., others The DHODH inhibitor PTC299 arrests SARS-CoV-2 replication and suppresses induction of inflammatory cytokines. Virus Res. 2021;292 doi: 10.1016/j.virusres.2020.198246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas S. The pharmacology of indomethacin. Headache. The Journal of Head and Face Pain. 2016;56:436–446. doi: 10.1111/head.12769. [DOI] [PubMed] [Google Scholar]
- Ma C., Hu Y., Townsend J.A., Lagarias P.I., Marty M.T., Kolocouris A., Wang J. Ebselen, disulfiram, carmofur, PX-12, tideglusib, and shikonin are nonspecific promiscuous SARS-CoV-2 main protease inhibitors. ACS Pharmacol. Transl. Sci. 2020;3:1265–1277. doi: 10.1021/acsptsci.0c00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Sacco M.D., Hurst B., Townsend J.A., Hu Y., Szeto T., Zhang X., Tarbet B., Marty M.T., Chen Y., Wang J. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–692. doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghzi A.H., Houtchens M.K., Preziosa P., Ionete C., Beretich B.D., Stankiewicz J.M., Tauhid S., Cabot A., Berriosmorales I., Schwartz T.H., others COVID-19 in teriflunomide-treated patients with multiple sclerosis. J. Neurol. 2020;267:2790–2796. doi: 10.1007/s00415-020-09944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi M., Mótyán J.A., Szojka Z.I., Golda M., Miczi M., Tőzsér J. Analysis of the efficacy of HIV protease inhibitors against SARS-CoV-2’s main protease. Virol. J. 2020;17:190. doi: 10.1186/s12985-020-01457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A., Mostafa A., Al-Karmalawy A.A., Zidan A., Abulkhair H.S., Mahmoud S.H., Shehata M., Elhefnawi M.M., Ali M.A. Telaprevir is a potential drug for repurposing against SARS-CoV-2: computational and in vitro studies. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e07962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchair P., Otero A., Giol J., Solanich X., Carnaval T., Fernández-Nistal A., Sánchez-Gabriel A., Montoto C., Lleonart R., Videla S. A multicenter, open-label, randomized, proof-of-concept phase II clinical trial to assess the efficacy and safety of icatibant in patients infected with SARS-CoV-2 (COVID-19) and admitted to hospital units without invasive mechanical ventilation: study protocol (ICAT-COVID) Trials. 2022;23:1–15. doi: 10.1186/s13063-022-06219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R.W., Tisdall P., Fremont-Smith P., Liu Y., Huang X.-P., White K.M., Miorin L., Moreno E., Alon A., Delaforge E., others COVID-19: famotidine, histamine, mast cells, and mechanisms. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.633680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala V.S., McKay M.J., Shcherbakov A.A., Dregni A.J., Kolocouris A., Hong M. Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers. Nat. Struct. Mol. Biol. 2020;27:1202–1208. doi: 10.1038/s41594-020-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna S., Chowdhury T., Baindara P., Mandal S.M. Fusion protein targeted antiviral peptides: fragment-based drug design (FBDD) guided rational design of dipeptides against SARS-CoV-2. Curr. Protein Pept. Sci. 2020;21:938–947. doi: 10.2174/1389203721666200908164641. [DOI] [PubMed] [Google Scholar]
- Martin R., Löchel H.F., Welzel M., Hattab G., Hauschild A.-C., Heider D. CORDITE: the curated CORona drug InTERactions database for SARS-CoV-2. iScience. 2020;23 doi: 10.1016/j.isci.2020.101297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashayekhi-Sardoo H., Hosseinjani H. A new application of mTOR inhibitor drugs as potential therapeutic agents for COVID-19. J. Basic Clin. Physiol. Pharmacol. 2022;33:17–25. doi: 10.1515/jbcpp-2020-0495. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., Shimojima M., Fukushi S. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J. Virol. 2020;95 doi: 10.1128/JVI.01648-20. e01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaherpour H., Sofian M., Farahani E., Abdi A., Mazaherpour S., Larijani M.S., abd Ramezani A. Higher rate of hyperbilirubinemia and arrythmia in COVID-19 cases receiving combination therapy atazanavir/ritonavir vs. Lopinavir/ritonavir. 2021 [Google Scholar]
- Mellott D.M., Tseng C.-T., Drelich A., Fajtová P., Chenna B.C., Kostomiris D.H., Hsu J., Zhu J., Taylor Z.W., Kocurek K.I., Tat V., Katzfuss A., Li L., Giardini M.A., Skinner D., Hirata K., Yoon M.C., Beck S., Carlin A.F., Clark A.E., Beretta L., Maneval D., Hook V., Frueh F., Hurst B.L., Wang H., Raushel F.M., O'Donoghue A.J., de Siqueira-Neto J.L., Meek T.D., McKerrow J.H. A clinical-stage cysteine protease inhibitor blocks SARS-CoV-2 infection of human and monkey cells. ACS Chem. Biol. 2021;16:642–650. doi: 10.1021/acschembio.0c00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendieta Zerón H., Meneses Calderón J., Paniagua Coria L., Meneses Figueroa J., Vargas Contreras M.J., Vives Aceves H.L., Carranza Salazar F.M., Californias Hernández D., Miraflores Vidaurri E., Carrillo González A., others Nitazoxanide as an early treatment to reduce the intensity of COVID-19 outbreaks among health personnel. World Academy of Sciences Journal. 2021;3:1–6. doi: 10.3892/wasj.2021.94. [DOI] [Google Scholar]
- Merat S. SD1000: high sustained viral response rate in 1361 patients with hepatitis C genotypes 1, 2, 3, and 4 using a low-cost, fixed-dose combination tablet of generic sofosbuvir and daclatasvir: a multicenter, phase III clinical trial. Clin. Infect. Dis. 2020;70:2206–2212. doi: 10.1093/cid/ciz628. [DOI] [PubMed] [Google Scholar]
- Messina V., Nevola R., Izzi A., De Lucia Sposito P., Marrone A., Rega R., Fusco R., Lumino P., Rinaldi L., Gaglione P., others Efficacy and safety of the sofosbuvir/velpatasvir combination for the treatment of patients with early mild to moderate COVID-19. Sci. Rep. 2022;12:1–6. doi: 10.1038/s41598-022-09741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Chiaravalli J., Gellenoncourt S., Brownridge P., Bryne D.P., Daly L.A., Grauslys A., Walter M., Agou F., Chakrabarti L.A., Craik C.S., Eyers C.E., Eyers P.A., Gambin Y., Jones A.R., Sierecki E., Verdin E., Vignuzzi M., Emmott E. Characterising proteolysis during SARS-CoV-2 infection identifies viral cleavage sites and cellular targets with therapeutic potential. Nat. Commun. 2021;12:5553. doi: 10.1038/s41467-021-25796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minasov G., Rosas-Lemus M., Shuvalova L., Inniss N.L., Brunzelle J.S., Daczkowski C.M., Hoover P., Mesecar A.D., Satchell K.J. Mn2+ coordinates Cap-0-RNA to align substrates for efficient 2′-O-methyl transfer by SARS-CoV-2 nsp16. Sci. Signal. 2021;14 doi: 10.1126/scisignal.abh2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miorin, L., Mire, C., Ranjbar, S., Hume, A., Huang, J., Crossland, N., White, K., Laporte, M., Kehrer, T., Haridas, V., others, n.d. The Oral Drug Nitazoxanide Restricts SARS-CoV-2 Infection and Attenuates Disease Pathogenesis in Syrian Hamsters (preprint).
- Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Del Pozo C.H., Prosper F., others Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Lev H., Weisman Y., Cohen S., Deutsch V., Cipok M., Bondar E., Lubetzky R., Mandel D. The interrelationship between hepcidin, vitamin D, and anemia in children with acute infectious disease. Pediatr. Res. 2018;84:62–65. doi: 10.1038/s41390-018-0005-0. [DOI] [PubMed] [Google Scholar]
- Mostafa A., Kandeil A., A. M. M. Elshaier Y., Kutkat O., Moatasim Y., Rashad A.A., Shehata M., Gomaa M.R., Mahrous N., Mahmoud S.H., GabAllah M., Abbas H., Taweel A.E., Kayed A.E., Kamel M.N., Sayes M.E., Mahmoud D.B., El-Shesheny R., Kayali G., Ali M.A. FDA-approved drugs with potent in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2. Pharmaceuticals. 2020;13:443. doi: 10.3390/ph13120443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa M.A. 2020. Role of Zidovudine and Candesartan in the Novel SARS-CoV-2 Treatment Trials: Theoretical Study (Preprint) [DOI] [Google Scholar]
- Narayanan A., Narwal M., Majowicz S.A., Varricchio C., Toner S.A., Ballatore C., Brancale A., Murakami K.S., Jose J. Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay. Commun Biol. 2022;5:169. doi: 10.1038/s42003-022-03090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar A., Ibrahim I.M., Amin F.G., Magdy M., Elgharib A.M., Azzam E.B., Nasser F., Yousry K., Shamkh I.M., Mahdy S.M., others A review of human coronaviruses' receptors: the host-cell targets for the crown bearing viruses. Molecules. 2021;26:6455. doi: 10.3390/molecules26216455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastri E., Marinangeli F., Pivetta E., Torri E., Reggiani F., Fiorentino G., Scorzolini L., Vettori S., Marsiglia C., Gavioli E.M., Beccari A.R., Terpolilli G., De Pizzol M., Goisis G., Mantelli F., Vaia F., Allegretti M. A phase 2 randomized, double-blinded, placebo-controlled, multicenter trial evaluating the efficacy and safety of raloxifene for patients with mild to moderate COVID-19. eClinicalMedicine. 2022;48 doi: 10.1016/j.eclinm.2022.101450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi H., Watashi K., Saso W., Shionoya K., Iwanami S., Hirokawa T., Shirai T., Kanaya S., Ito Y., Kim K.S., Nomura T., Suzuki Tateki, Nishioka K., Ando S., Ejima K., Koizumi Y., Tanaka T., Aoki S., Kuramochi K., Suzuki Tadaki, Hashiguchi T., Maenaka K., Matano T., Muramatsu M., Saijo M., Aihara K., Iwami S., Takeda M., McKeating J.A., Wakita T. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience. 2021;24 doi: 10.1016/j.isci.2021.102367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono J., Koshimizu U., Fukunishi Y., Nakai H. Multiple protonation states in ligand-free SARS-CoV-2 main protease revealed by large-scale quantum molecular dynamics simulations. Chem. Phys. Lett. 2022;794 doi: 10.1016/j.cplett.2022.139489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P., Prasad K., Prakash A., Kumar V. Insights into the biased activity of dextromethorphan and haloperidol towards SARS-CoV-2 NSP6: in silico binding mechanistic analysis. J. Mol. Med. 2020;98:1659–1673. doi: 10.1007/s00109-020-01980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel L., Shukla T., Huang X., Ussery D.W., Wang S. Machine learning methods in drug discovery. Molecules. 2020;25:E5277. doi: 10.3390/molecules25225277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patidar V., Sharma A., Garg V., Tripathi A.P., Dhaneriya S. Methylene blue in management of COVID19. J. Assoc. Phys. India. 2022;70:11–12. [PubMed] [Google Scholar]
- Pearlman B.L. Protease inhibitors for the treatment of chronic hepatitis C genotype-1 infection: the new standard of care. Lancet Infect. Dis. 2012;12:717–728. doi: 10.1016/S1473-3099(12)70060-9. [DOI] [PubMed] [Google Scholar]
- Pfefferle S., Schöpf J., Kögl M., Friedel C.C., Müller M.A., Carbajo-Lozoya J., Stellberger T., von Dall'Armi E., Herzog P., Kallies S., others The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts J., Li J., Perry J.K., Du Pont V., Riola N., Rodriguez L., Lu X., Kurhade C., Xie X., Camus G., others Remdesivir and GS-441524 retain antiviral activity against Delta, Omicron, and other emergent SARS-CoV-2 variants. Antimicrob. Agents Chemother. 2022 doi: 10.1128/aac.00222-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G., Roli L., Oliva G., Manfredini M., Trenti T., Kaleci S., Iannella R., Balzano B., Coppola A., Fiorentino G., others Homocysteine (Hcy) assessment to predict outcomes of hospitalized Covid-19 patients: a multicenter study on 313 Covid-19 patients. Clin. Chem. Lab. Med. 2021;59:e354–e357. doi: 10.1515/cclm-2021-0168. [DOI] [PubMed] [Google Scholar]
- Prajapat M., Sarma P., Shekhar N., Avti P., Sinha S., Kaur H., Kumar S., Bhattacharyya A., Kumar H., Bansal S., Medhi B. Drug targets for corona virus: a systematic review. Indian J. Pharmacol. 2020;52:56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. Protein kinase inhibitors. [Google Scholar]
- Puschmann A., Fiesel F.C., Caulfield T.R., Hudec R., Ando M., Truban D., Hou X., Ogaki K., Heckman M.G., James E.D., Swanberg M., Jimenez-Ferrer I., Hansson O., Opala G., Siuda J., Boczarska-Jedynak M., Friedman A., Koziorowski D., Rudzińska-Bar M., Aasly J.O., Lynch T., Mellick G.D., Mohan M., Silburn P.A., Sanotsky Y., Vilariño-Güell C., Farrer M.J., Chen L., Dawson V.L., Dawson T.M., Wszolek Z.K., Ross O.A., Springer W. Heterozygous PINK1 p.G411S increases risk of Parkinson's disease via a dominant-negative mechanism. Brain. 2017;140:98–117. doi: 10.1093/brain/aww261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskarich M.A., Cummins N.W., Ingraham N.E., Wacker D.A., Reilkoff R.A., Driver B.E., Biros M.H., Bellolio F., Chipman J.G., Nelson A.C., others A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine. 2021;37 doi: 10.1016/j.eclinm.2021.100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskarich M.A., Ingraham N.E., Merck L.H., Driver B.E., Wacker D.A., Black L.P., Jones A.E., Fletcher C.V., South A.M., Murray T.A., others Efficacy of losartan in hospitalized patients with COVID-19–induced lung injury: a randomized clinical trial. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.2735. e222735–e222735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie A.M. Teriflunomide: a possible effective drug for the comprehensive treatment of COVID-19. Current Research in Pharmacology and Drug Discovery. 2021;2 doi: 10.1016/j.crphar.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R., Bhosale V., Reddy H., Atam V., Faridi M., Fatima J., Shukla V., Khan Z.A., Khan H., Singh V., Negi M.P.S., Srivastava M., Srivastava A.K., Tripathi C.B., Ghosh N., Majumdar N., Tripathi R.K., Rath S.K., Mishra P.R., Sharma S., Kundu T.K. Phase III, randomized, double-blind, placebo controlled trial of efficacy, safety and tolerability of antiviral drug umifenovir vs standard care of therapy in non-severe COVID-19 patients. Int. J. Infect. Dis. 2022;115:62–69. doi: 10.1016/j.ijid.2021.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F., Jr., Zeze V., Velut S., Jan M. Cystic meningiomas. Practical value of a radio-surgical classification. J. Neuroradiol. 1987;14(3):271–286. [PubMed] [Google Scholar]
- Rapicavoli R.V., Alaimo S., Ferro A., Pulvirenti A. Computational methods for drug repurposing. Adv. Exp. Med. Biol. 2022;1361:119–141. doi: 10.1007/978-3-030-91836-1_7. [DOI] [PubMed] [Google Scholar]
- Ravichandran R., Mohan S.K., Sukumaran S.K., Kamaraj D., Daivasuga S.S., Ravi S.O.A.S., Vijayaraghavalu S., Kumar R.K. An open label randomized clinical trial of Indomethacin for mild and moderate hospitalised Covid-19 patients. Sci. Rep. 2022;12:1–10. doi: 10.1038/s41598-022-10370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichen, F.R., Dawson, K.M., Lewis, S., Steiner, D., Amstutz, P., Engler, O., Stumpp, M.T., Stumpp, M.T., n.d. Multi-specific DARPin® Therapeutics Demonstrate Very High Potency against Mutated SARS-CoV-2 Variants in Vitro.
- Reina J., Iglesias C. Revista Espanola de Quimioterapia: Publicacion Oficial de la Sociedad Espanola de Quimioterapia; 2022. Nirmatrelvir Plus Ritonavir (Paxlovid) a Potent SARS-CoV-2 3CLpro Protease Inhibitor Combination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis G., Silva E.A.S.M., Silva D.C.M., Thabane L., Milagres A.C., Ferreira T.S., dos Santos C.V.Q., Campos V.H.S., Nogueira A.M.R., de Almeida A.P.F.G., Callegari E.D., Neto A.D.F., Savassi L.C.M., Simplicio M.I.C., Ribeiro L.B., Oliveira R., Harari O., Forrest J.I., Ruton H., Sprague S., McKay P., Guo C.M., Rowland-Yeo K., Guyatt G.H., Boulware D.R., Rayner C.R., Mills E.J. Effect of early treatment with ivermectin among patients with covid-19. N. Engl. J. Med. 2022;386:1721–1731. doi: 10.1056/NEJMoa2115869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejdak K., Grieb P. Adamantanes might be protective from COVID-19 in patients with neurological diseases: multiple sclerosis, parkinsonism and cognitive impairment. Multiple sclerosis and related disorders. 2020;42 doi: 10.1016/j.msard.2020.102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z., Luo H., Yu Z., Song J., Liang L., Wang L., Wang H., Cui G., Liu Y., Wang J., others A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv. Sci. 2020;7 doi: 10.1002/advs.202001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikov L.R., Norris M.H., Vashisht R., Bluhm A.P., Li D., Liao Y.-S.J., Brown A., Butte A.J., Ostrov D.A. Identification of antiviral antihistamines for COVID-19 repurposing. Biochem. Biophys. Res. Commun. 2021;538:173–179. doi: 10.1016/j.bbrc.2020.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A.A., Sullivan E.D., Copeland W.C. Activation of the SARS-CoV-2 NSP14 3′–5′ exoribonuclease by NSP10 and response to antiviral inhibitors. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2021.101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P.C., Liew D.F., Tanner H.L., Grainger J.R., Dwek R.A., Reisler R.B., Steinman L., Feldmann M., Ho L.-P., Hussell T., others COVID-19 therapeutics: challenges and directions for the future. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2119893119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco P.R., Silva P.L., Cruz F.F., Melo-Junior M.A.C., Tierno P.F., Moura M.A., De Oliveira L.F.G., Lima C.C., Dos Santos E.A., Junior W.F., others Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial. Eur. Respir. J. 2021;58 doi: 10.1183/13993003.03725-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J.-F., Bardin M.C., Fulgencio J., Mogelnicki D., Bréchot C. A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19. eClinicalMedicine. 2022;45 doi: 10.1016/j.eclinm.2022.101310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero V., Aquino R.P., Del Gaudio P., Campiglia P., Russo P. Post-COVID syndrome: the research progress in the treatment of pulmonary sequelae after COVID-19 infection. Pharmaceutics. 2022;14:1135. doi: 10.3390/pharmaceutics14061135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhm C.J. Excess deaths in the United States during the first year of COVID-19. Prev. Med. 2022;107174 doi: 10.1016/j.ypmed.2022.107174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo L.C., Tomasin R., Matos I.A., Manucci A.C., Sowa S.T., Dale K., Caldecott K.W., Lehtiö L., Schechtman D., Meotti F.C., others The SARS-CoV-2 Nsp3 macrodomain reverses PARP9/DTX3L-dependent ADP-ribosylation induced by interferon signaling. J. Biol. Chem. 2021;297 doi: 10.1016/j.jbc.2021.101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvarani C., Massari M., Costantini M., Franco Merlo D., Lucia Mariani G., Viale P., Nava S., Guaraldi G., Dolci G., Boni L., Savoldi L., Bruzzi P., Turrà C., Catanoso M., Maria Marata A., Barbieri C., Valcavi A., Franzoni F., Cavuto S., Mazzi G., Corsini R., Trapani F., Bartoloni A., Barisione E., Barbieri C., Jole Burastero G., Pan A., Inojosa W., Scala R., Burattini C., Luppi F., Codeluppi M., Eldin Tarek K., Cenderello G., Salio M., Foti G., Dongilli R., Bajocchi G., Alberto Negri E., Ciusa G., Fornaro G., Bassi I., Zammarchi L., Aloè T., Facciolongo N. Intravenous methylprednisolone pulses in hospitalised patients with severe COVID-19 pneumonia, A double-blind, randomised, placebo-controlled trial. Eur. Respir. J. 2022 doi: 10.1183/13993003.00025-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savytskyi O.V., Yesylevskyy S.O., Kornelyuk A.I. Asymmetric structure and domain binding interfaces of human tyrosyl-tRNA synthetase studied by molecular dynamics simulations. J. Mol. Recogn. 2013;26(2):113–120. doi: 10.1002/jmr.2259. https://doi:10.1002/jmr.2259 [DOI] [PubMed] [Google Scholar]
- Savytskyi O., Kornelyuk A. Computational modeling of the complex between glycyrrhizin and SARS-CoV-2 protease 3CLpro as a target for the development of antiviral drugs. Reports of the National Academy of Sciences of Ukraine. 2022 doi: 10.15407/dopovidi2022.01.115. [DOI] [Google Scholar]
- Schloer S., Brunotte L., Mecate-Zambrano A., Zheng S., Tang J., Ludwig S., Rescher U. Drug synergy of combinatory treatment with remdesivir and the repurposed drugs fluoxetine and itraconazole effectively impairs SARS-CoV-2 infection in vitro. Br. J. Pharmacol. 2021;178:2339–2350. doi: 10.1111/bph.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans M.M., Hage R. Cyclosporine A and COVID-19–The COQUIMA cohort. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpa Neto A., Landoni G., Ostermann M., Lumlertgul N., Forni L., Alvarez-Belon L., Trapani T., Alliegro P.V., Zacharowski K., Wiedenbeck C., others Angiotensin II infusion in COVID-19: an international, multicenter, registry-based study. J. Med. Virol. 2022;94:2079–2088. doi: 10.1002/jmv.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani M., Sadegh Ehdaei B., Fathi F., Dowran R. A mini-review on sofosbuvir and daclatasvir treatment in coronavirus disease 2019. New Microbes and New Infections. 2021;42 doi: 10.1016/j.nmni.2021.100895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee A., Teymouri Athar M.M., Kohandel Gargari O., Jafarabady K., Siahvoshi S., Mozhgani S.-H. Ivermectin under scrutiny: a systematic review and meta-analysis of efficacy and possible sources of controversies in COVID-19 patients. Virol. J. 2022;19:102. doi: 10.1186/s12985-022-01829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S.B. COVID-19 and Progesterone: Part 1. SARS-CoV-2, Progesterone and its potential clinical use. Endocrine and Metabolic Science. 2021;5 doi: 10.1016/j.endmts.2021.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheer M., Singh R., Sobhia M.E. Protein degradation: a novel computational approach to design protein degrader probes for main protease of SARS-CoV-2. J. Biomol. Struct. Dyn. 2021:1–13. doi: 10.1080/07391102.2021.1953601. [DOI] [PubMed] [Google Scholar]
- Shannon A., Fattorini V., Sama B., Selisko B., Feracci M., Falcou C., Gauffre P., El Kazzi P., Delpal A., Decroly E., others A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase. Nat. Commun. 2022;13:1–9. doi: 10.1038/s41467-022-28113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., others Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar N., Kaur H., Sarma P., Prakash A., Medhi B. Indomethacin: an exploratory study of antiviral mechanism and host-pathogen interaction in COVID-19. Expert Rev. Anti Infect. Ther. 2022;20:383–390. doi: 10.1080/14787210.2022.1990756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z., Halberg A., Fong J.Y., Guo J., Song G., Louie B., Luedtke G.R., Visuthikraisee V., Protter A., Koh X., Baik T., Lum P.Y. Elucidating host cell response pathways and repurposing therapeutics for SARS-CoV-2 and other coronaviruses using gene expression profiles of chemical and genetic perturbations (preprint) Genomics. 2022 doi: 10.1101/2022.04.18.488682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington D., Kirkpatrick S. Solvable model of a spin-glass. Phys. Rev. Lett. 1975;35:1792–1796. doi: 10.1103/PhysRevLett.35.1792. [DOI] [Google Scholar]
- Shi Z., Wei J., Deng X., Li S., Qiu Y., Shao D., Li B., Zhang K., Xue F., Wang X., others Nitazoxanide inhibits the replication of Japanese encephalitis virus in cultured cells and in a mouse model. Virol. J. 2014;11:1–10. doi: 10.1186/1743-422X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T., Huang M., Wu D., Ren Y., Zhang X., Han Y., Mu J., Wang R., Qiu Y., Zhang D.-Y., others SARS-coronavirus-2 Nsp13 possesses NTPase and RNA helicase activities that can be inhibited by bismuth salts. Virol. Sin. 2020;35:321–329. doi: 10.1007/s12250-020-00242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeoni M., Cavinato T., Rodriguez D., Gatfield D. I (nsp1) ecting SARS-CoV-2–ribosome interactions. Communications biology. 2021;4:1–5. doi: 10.1038/s42003-021-02265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragusa L., Menna G., Buratta F., Baroni M., Desantis J., Cruciani G., Goracci L. CROMATIC: cro ss-Relationship Ma p of Cavi ti es from C oronaviruses. J. Chem. Inf. Model. 2022 doi: 10.1021/acs.jcim.2c00169. [DOI] [PubMed] [Google Scholar]
- Smieszek S.P., Przychodzen B.P., Polymeropoulos M.H. Amantadine disrupts lysosomal gene expression: a hypothesis for COVID19 treatment. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.106004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.-Y., Kim Y.-S., Eom J.-S., Kim J.-Y., Lee J.-S., Lee J., Choi W.-S., Heo J.-Y., Sohn J.-W., Lee K.-D., Cho D., Cho I., Kim W.-J. Oral antiviral clevudine compared with placebo in Korean COVID-19 patients with moderate severity (preprint) Infectious Diseases (except HIV/AIDS) 2021 doi: 10.1101/2021.12.09.21267566. [DOI] [Google Scholar]
- Stegmann K.M., Dickmanns A., Gerber S., Nikolova V., Klemke L., Manzini V., Schlösser D., Bierwirth C., Freund J., Sitte M., others The folate antagonist methotrexate diminishes replication of the coronavirus SARS-CoV-2 and enhances the antiviral efficacy of remdesivir in cell culture models. Virus Res. 2021;302 doi: 10.1016/j.virusres.2021.198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L.J., Pruijssers A.J., Lee H.W., Gordon C.J., Tchesnokov E.P., Gribble J., George A.S., Hughes T.M., Lu X., Li J., others Mutations in the SARS-CoV-2 RNA dependent RNA polymerase confer resistance to remdesivir by distinct mechanisms. Science translational medicine eabo0718. 2022 doi: 10.1126/scitranslmed.abo0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamoto K., Tanaka Y.L., Saito A., Goto Y., Nakayama T., Okabayashi T., Kunitake H., Morishita K. Highly polymerized proanthocyanidins (PAC) components from blueberry leaf and stem significantly inhibit SARS-CoV-2 infection via inhibition of ACE2 and viral 3CLpro enzymes. Biochem. Biophys. Res. Commun. 2022;615:56–62. doi: 10.1016/j.bbrc.2022.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.J., Velez G., Parsons D.E., Li K., Ortiz M.E., Sharma S., McCray P.B., Bassuk A.G., Mahajan V.B., others Structure-based phylogeny identifies avoralstat as a TMPRSS2 inhibitor that prevents SARS-CoV-2 infection in mice. J. Clin. Invest. 2021;131 doi: 10.1172/JCI147973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryamohan K., Diwanji D., Stawiski E.W., Gupta R., Miersch S., Liu J., Chen C., Jiang Y.-P., Fellouse F.A., Sathirapongsasuti J.F., others Human ACE2 receptor polymorphisms and altered susceptibility to SARS-CoV-2. Communications biology. 2021;4:1–11. doi: 10.1038/s42003-021-02030-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccone F.S., Gorham J., Vincent J.-L. Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talevi A., Morales J.F., Hather G., Podichetty J.T., Kim Sarah, Bloomingdale P.C., Kim Samuel, Burton J., Brown J.D., Winterstein A.G., Schmidt S., White J.K., Conrado D.J. Machine learning in drug discovery and development Part 1: a primer. CPT Pharmacometrics Syst. Pharmacol. 2020;9:129–142. doi: 10.1002/psp4.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X., Zhang L., Du L., Liao R., Cai H., Lu K., Zhao Z., Xie Y., Wang P.-H., Pan J.-A., others Allosteric inhibition of SARS-CoV-2 3CL protease by colloidal bismuth subcitrate. Chem. Sci. 2021;12:14098–14102. doi: 10.1039/D1SC03526F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thachil J. The versatile heparin in COVID-19. J. Thromb. Haemostasis. 2020;18:1020–1022. doi: 10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than M.E., Henrich S., Bourenkov G.P., Bartunik H.D., Huber R., Bode W. The endoproteinase furin contains two essential Ca2+ ions stabilizing its N-terminus and the unique S1 specificity pocket. Acta Crystallogr. Sect. D Biol. Crystallogr. 2005;61:505–512. doi: 10.1107/S0907444905002556. [DOI] [PubMed] [Google Scholar]
- Theodorakopoulou M.P., Alexandrou M.-E., Boutou A.K., Ferro C.J., Ortiz A., Sarafidis P. Renin–angiotensin system blockers during the COVID-19 pandemic: an update for patients with hypertension and chronic kidney disease. Clinical kidney journal. 2022;15:397–406. doi: 10.1093/ckj/sfab272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft-Bertelsen T.L., Jeppesen M.G., Tzortzini E., Xue K., Giller K., Becker S., Mujezinovic A., Bentzen B.H., B Andreas L., Kolocouris A., others Amantadine inhibits known and novel ion channels encoded by SARS-CoV-2 in vitro. Commun. Biol. 2021;4:1–10. doi: 10.1038/s42003-021-02940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar P.P.S., Krugliak M., Arkin I.T. Identification of sars-cov-2 e channel blockers from a repurposed drug library. Pharmaceuticals. 2021;14:604. doi: 10.3390/ph14070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornling G., Batta R., Porter J.C., Williams B., Bengtsson T., Parmar K., Kashiva R., Hallberg A., Cohrt A.K., Westergaard K., others Seven days treatment with the angiotensin II type 2 receptor agonist C21 in hospitalized COVID-19 patients; a placebo-controlled randomised multi-centre double-blind phase 2 trial. EClinicalMedicine. 2021;41 doi: 10.1016/j.eclinm.2021.101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J.A., Sanders H.M., Rolland A.D., Park C.K., Horton N.C., Prell J.S., Wang J., Marty M.T. Influenza AM2 channel Oligomerization is sensitive to its chemical environment. Anal. Chem. 2021;93:16273–16281. doi: 10.1021/acs.analchem.1c04660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D.P., Taira Y., Ogawa T., Misu R., Miyazawa Y., Kitao A. Inhibition of the hexamerization of SARS-CoV-2 endoribonuclease and modeling of RNA structures bound to the hexamer. Sci. Rep. 2022;12:1–15. doi: 10.1038/s41598-022-07792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu B., Wang H., An X., Qu J., Li Q., Gao Y., Shi M., Qiu H., Huang Y. Inhaled heparin polysaccharide nanodecoy against SARS-CoV-2 and variants. Acta Pharm. Sin. B. 2022 doi: 10.1016/j.apsb.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J., Mo X., Zhang X., Xun J., Chen X., Liu Y., Jing W., Xie T. Effects of different corticosteroid therapy on severe COVID-19 patients: a meta-analysis of randomized controlled trials. Expet Rev. Respir. Med. 2022;16:79–89. doi: 10.1080/17476348.2021.1983429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich S., Ekanayake K.B., Otting G., Nitsche C. Main protease mutants of SARS-CoV-2 variants remain susceptible to nirmatrelvir. Bioorg. Med. Chem. Lett. 2022;62 doi: 10.1016/j.bmcl.2022.128629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandyck K., Deval J. Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Current Opinion in Virology. 2021 doi: 10.1016/j.coviro.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varona J.F., Landete P., Lopez-Martin J.A., Estrada V., Paredes R., Guisado-Vasco P., de Orueta L.F., Torralba M., Fortun J., Vates R., others . vol. 5. 2022. (Preclinical and Randomized Phase I Studies of Plitidepsin in Adults Hospitalized with COVID-19. Life Science Alliance). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela J.M. Repurposing sigma-1 receptor ligands for COVID-19 therapy? Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.582310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma J.S., Libertin C.R., Gupta Y., Khanna G., Kumar R., Arora B.S., Krishna L., Fasina F.O., Hittner J.B., Antoniades A., others Multi-cellular immunological interactions associated with COVID-19 infections. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.794006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Roemeling C.A., Caulfield T.R., Marlow L., Bok I., Wen J., Miller J.L., Hughes R., Hazlehurst L., Pinkerton A.B., Radisky D.C., Tun H.W., Kim Y.S.B., Lane A.L., Copland J.A. Accelerated bottom-up drug design platform enables the discovery of novel stearoyl-CoA desaturase 1 inhibitors for cancer therapy. Oncotarget. 2018;9:3–20. doi: 10.18632/oncotarget.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong W., Khan M.B., Fischer C., Arutyunova E., Lamer T., Shields J., Saffran H.A., McKay R.T., van Belkum M.J., Joyce M.A., Young H.S., Tyrrell D.L., Vederas J.C., Lemieux M.J. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 2020;11:4282. doi: 10.1038/s41467-020-18096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A., Gralinski L.E., Johnson C.E., Yao W., Kovarova M., Dinnon K.H., Liu H., Madden V.J., Krzystek H.M., De C., others SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–457. doi: 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Li H., Xiao S., Li Z., Zhao X., Xie J., Ye C., Xia L., Lou X., Zhou X. Abnormal dynamic ventilation function of COVID-19 survivors detected by pulmonary free-breathing proton MRI. Eur. Radiol. 2022:1–11. doi: 10.1007/s00330-022-08605-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fang S., Wu Y., Cheng X., Zhang L., Shen X., Li S., Xu J., Shang W., Gao Z., others Discovery of SARS-CoV-2-E channel inhibitors as antiviral candidates. Acta Pharmacol. Sin. 2022;43:781–787. doi: 10.1038/s41401-021-00732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Joshi A., Leopold K., Jackson S., Christensen S., Nayfeh T., Mohammed K., Creo A., Tebben P., Kumar S. Association of vitamin D deficiency with COVID-19 infection severity: systematic review and meta-analysis. Clin. Endocrinol. 2022;96:281–287. doi: 10.1111/cen.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C. Rise of the preprint: how rapid data sharing during COVID-19 has changed science forever. Nat. Med. 2022;28:2–5. doi: 10.1038/s41591-021-01654-6. [DOI] [PubMed] [Google Scholar]
- Whitley R. Molnupiravir—a step toward orally bioavailable therapies for Covid-19. N. Engl. J. Med. 2022 doi: 10.1056/NEJMe2117814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO coronavirus disease (COVID-19) https://covid19.who.int Dashboard [WWW Document], n.d. URL. accessed 1.20.21.
- Wimmer S., Keestra S.M. Public risk-taking and rewards during the COVID-19 pandemic-a case study of remdesivir in the context of global health equity. Int. J. Health Pol. Manag. 2022;11:567–578. doi: 10.34172/ijhpm.2020.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C.K., Au I.C., Lau K.T., Lau E., Cowling B.J., Leung G.M. medRxiv; 2022. Real-world Effectiveness of Molnupiravir and Nirmatrelvir/ritonavir Among COVID-19 Inpatients during Hong Kong's Omicron BA. 2 Wave: an Observational Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian Y., Zhang J., Bian Z., Zhou H., Zhang Z., Lin Z., Xu H. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharm. Sin. B. 2020;10:1163–1174. doi: 10.1016/j.apsb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang R., Yu Z., Wang Y., Wang L., Huo S., Li Y., Liang R., Hao Q., Ying T., Gao Y., Yu F., Jiang S. Recent advances in developing small-molecule inhibitors against SARS-CoV-2. Acta Pharm. Sin. B. 2022;12:1591–1623. doi: 10.1016/j.apsb.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R., Zhang L., Li S., Sun Y., Ding M., Wang Y., Zhao Y., Wu Y., Shang W., Jiang X., others Novel and potent inhibitors targeting DHODH are broad-spectrum antivirals against RNA viruses including newly-emerged coronavirus SARS-CoV-2. Protein & cell. 2020;11:723–739. doi: 10.1007/s13238-020-00768-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcinkaya M., Liu W., Islam M.N., Kotini A.G., Gusarova G.A., Fidler T.P., Papapetrou E.P., Bhattacharya J., Wang N., Tall A.R. Modulation of the NLRP3 inflammasome by sars-CoV-2 envelope protein. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-04133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T., Hoshizaki M., Minato T., Nirasawa S., Asaka M.N., Niiyama M., Imai M., Uda A., Chan J.F.-W., Takahashi S. ACE2-like carboxypeptidase B38-CAP protects from SARS-CoV-2-induced lung injury. Nat. Commun. 2021;12:1–13. doi: 10.1038/s41467-021-27097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M., Kinoshita N., Ohmagari N., Gohda J., Semba K., Matsuda Z., Kawaguchi Y., Kawaoka Y., Inoue J. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12:629. doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Zheng Y., Zeng X., He B., Cheng W. Structural biology of SARS-CoV-2: open the door for novel therapies. Signal Transduct. Targeted Ther. 2022;7:1–28. doi: 10.1038/s41392-022-00884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Pan X., Huang Y., Cheng C., Xu X., Wu Y., Xu Y., Shang W., Niu X., Wan Y., others Drug repurposing of itraconazole and Estradiol benzoate against COVID-19 by blocking SARS-CoV-2 spike protein-mediated membrane fusion. Advanced therapeutics. 2021;4 doi: 10.1002/adtp.202000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.-W., Peng T.-T., Hsu H.-Y., Lee Y.-Z., Wu S.-H., Lin W.-H., Ke Y.-Y., Hsu T.-A., Yeh T.-K., Huang W.-Z., Lin J.-H., Sytwu H.-K., Chen C.-T., Lee S.-J. Repurposing old drugs as antiviral agents for coronaviruses. Biomed. J. 2020;43:368–374. doi: 10.1016/j.bj.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., West A.M., Silletti S., Corbett K.D. Architecture and self-assembly of the SARS-CoV-2 nucleocapsid protein. Protein Sci. 2020;29:1890–1901. doi: 10.1002/pro.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim S.-K., Kim K., Kim I., Chun S., Oh T., Kim J.-U., Kim J., Jung W., Moon H., Ku B., Jung K. Inhibition of SARS-CoV-2 virus entry by the crude polysaccharides of seaweeds and abalone viscera in vitro. Mar. Drugs. 2021;19:219. doi: 10.3390/md19040219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Pan T., Huang F., Ying R., Liu J., Fan H., Zhang J., Liu W., Lin Y., Yuan Y., others Glycopeptide antibiotic teicoplanin inhibits cell entry of SARS-CoV-2 by suppressing the proteolytic activity of cathepsin L. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.884034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Granberg T., Shams R., Petersson S., Skold M., Nyren S., Lundberg J. medRxiv; 2022. Lung Perfusion Disturbances Detected with MRI in Non-hospitalized Post-COVID-19 Individuals with Dyspnea 3-13 Months after the Acute Disease. [Google Scholar]
- Yuan S., Wang R., Chan J.F.-W., Zhang A.J., Cheng T., Chik K.K.-H., Ye Z.-W., Wang S., Lee A.C.-Y., Jin L., others Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters. Nat. Microbiol. 2020;5:1439–1448. doi: 10.1038/s41564-020-00802-x. [DOI] [PubMed] [Google Scholar]
- Zamai L. Upregulation of the renin–angiotensin system pathways and SARS-CoV-2 infection: the rationale for the administration of zinc-chelating agents in COVID-19 patients. Cells. 2021;10:506. doi: 10.3390/cells10030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella I., Zizioli D., Castelli F., Quiros-Roldan E. Tenofovir, another inexpensive, well-known and widely available old drug repurposed for SARS-COV-2 infection. Pharmaceuticals. 2021;14:454. doi: 10.3390/ph14050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata-Cardona M.I., Flórez-Álvarez L., Zapata-Builes W., Guerra-Sandoval A.L., Guerra-Almonacid C.M., Hincapié-García J., Rugeles M.T., Hernandez J.C. Atorvastatin effectively inhibits late replicative cycle steps of SARS-CoV-2 in vitro (preprint) Microbiology. 2021 doi: 10.1101/2021.03.01.433498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zein A.F.M.Z., Sulistiyana C.S., Raffaello W.M., Wibowo A., Pranata R. Sofosbuvir with daclatasvir and the outcomes of patients with COVID-19: a systematic review and meta-analysis with GRADE assessment. Postgrad. Med. 2022;98:509–514. doi: 10.1136/postgradmedj-2021-140287. [DOI] [PubMed] [Google Scholar]
- Zein J.G., Strauss R., Attaway A.H., Hu B., Milinovich A., Jawhari N., Chamat S.S., Ortega V.E. Eosinophilia is associated with improved COVID-19 outcomes in inhaled corticosteroid-treated patients. J. Allergy Clin. Immunol. Pract. 2022;10:742–750. doi: 10.1016/j.jaip.2021.12.034. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendehdel A., Bidkhori M., Ansari M., Jamalimoghaddamsiyahkali S., Asoodeh A. Efficacy of oseltamivir in the treatment of patients infected with Covid-19. Annals of Medicine and Surgery. 2022;77 doi: 10.1016/j.amsu.2022.103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Song X., Ma T., Pan X., Zhou Y., Hou Y., Zhang Z., Li K., Karypis G., Cheng F. Repurpose open data to discover therapeutics for COVID-19 using deep learning. J. Proteome Res. 2020;19:4624–4636. doi: 10.1021/acs.jproteome.0c00316. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yang Y., Li J., Wang M., Saravanan K.M., Wei J., et al. A novel virtual screening procedure identifies Pralatrexate as inhibitor of SARS-CoV-2 RdRp and it reduces viral replication in vitro. PLoS Comput. Biol. 2020;16(12) doi: 10.1371/journal.pcbi.1008489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang T., Saravanan K.M., Liao L., Wu H., Zhang H., et al. DeepBindBC: a practical deep learning method for identifying native-like protein-ligand complexes in virtual screening. Methods. 2022;205:247–262. doi: 10.1016/j.ymeth.2022.07.009. [DOI] [PubMed] [Google Scholar]
- Zhang J., Ejikemeuwa A., Gerzanich V., Nasr M., Tang Q., Simard J.M., Zhao R.Y. Understanding the role of SARS-CoV-2 ORF3a in viral pathogenesis and COVID-19. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.854567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Radvak P., Lee J., Xu Y., Cao-Dao V., Xu M., Zheng W., Chen C.Z., Xie H., Ye Y. Mitoxantrone modulates a heparan sulfate-spike complex to inhibit SARS-CoV-2 infection. Sci. Rep. 2022;12:1–12. doi: 10.1038/s41598-022-10293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhang Y., Min Z., Mo J., Ju Z., Guan W., Zeng B., Liu Y., Chen J., Zhang Q., others COVID19db: a comprehensive database platform to discover potential drugs and targets of COVID-19 at whole transcriptomic scale. Nucleic Acids Res. 2022;50:D747–D757. doi: 10.1093/nar/gkab850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Du X., Duan Y., Pan X., Sun Y., You T., Han L., Jin Z., Shang W., Yu J., others High-throughput screening identifies established drugs as SARS-CoV-2 PLpro inhibitors. Protein & cell. 2021;12:877–888. doi: 10.1007/s13238-021-00836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Karki R., Williams E.P., Yang D., Fitzpatrick E., Vogel P., Jonsson C.B., Kanneganti T.-D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021;22:829–838. doi: 10.1038/s41590-021-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.-X., Wang L., Kong W.-S., Chen H., Wang X.-N., Meng Q., Zhang H.-N., Guo S.-J., Jiang H.-W., Tao S.-C. Nsp2 has the potential to be a drug target revealed by global identification of SARS-CoV-2 Nsp2-interacting proteins. Acta Biochim. Biophys. Sin. 2021;53:1134–1141. doi: 10.1093/abbs/gmab088. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Vedantham P., Lu K., Agudelo J., Carrion R., Jr., Nunneley J.W., Barnard D., Pöhlmann S., McKerrow J.H., Renslo A.R., others Protease inhibitors targeting coronavirus and filovirus entry. Antivir. Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuravel S.V., Khmelnitskiy O.K., Burlaka O.O., Gritsan A.I., Goloshchekin B.M., Kim S., Hong K.Y. Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: a randomised Phase II clinical trial. EClinicalMedicine. 2021;41 doi: 10.1016/j.eclinm.2021.101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak M., Kirschner L., Hilpert H., Geiger N., Danov O., Oberwinkler H., Steinke M., Sewald K., Seibel J., Bodem J. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci. Rep. 2021;11:5890. doi: 10.1038/s41598-021-85049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]