Abstract
Previously a Brucella protein named CP28, BP26, or Omp28 has been identified as an immunodominant antigen in infected cattle, sheep, goats, and humans. In the present study we evaluated antibody responses of infected and B. melitensis Rev.1-vaccinated sheep to the BP26 protein using purified recombinant BP26 protein produced in Escherichia coli in an indirect enzyme-linked immunosorbent assay (I-ELISA). The specificity of the I-ELISA determined with sera from healthy sheep (n = 106) was 93%. The sensitivity of the I-ELISA assessed with sera from naturally infected and suspected sheep found positive in the current conventional diagnostic tests was as follows: 100% for bacteriologically and serologically positive sheep (n = 50), 88% for bacteriologically negative but serologically and delayed-type hypersensitivity-positive sheep (n = 50), and 84% for bacteriologically and serologically negative but delayed-type hypersensitivity-positive sheep (n = 19). However, the absorbance values observed did not reach those observed in an I-ELISA using purified O-polysaccharide (O-PS) as an antigen. In sheep experimentally infected with B. melitensis H38 the antibody response to BP26 was delayed and much weaker than that to O-PS. Nevertheless, the BP26 protein appears to be a good diagnostic antigen to be used in confirmatory tests and for serological differentiation between infected and B. melitensis Rev.1-vaccinated sheep. Weak antibody responses to BP26 in some of the latter sheep suggest that a B. melitensis Rev.1 bp26 gene deletion mutant should be constructed to ensure this differentiation.
Brucellae are gram-negative intracellular bacterial pathogens of both humans and animals. The main etiologic agent in ovine brucellosis is Brucella melitensis, which may cause abortion in sheep, resulting in huge economic losses, particularly in Mediterranean countries. The live attenuated strain B. melitensis Rev.1 is considered the best vaccine available for the prophylaxis of brucellosis in sheep (1, 6). However, its use is known to stimulate antibody responses in sheep indistinguishable by the current conventional serological tests from those observed in B. melitensis-infected sheep (7). These tests, of which the most commonly used are the Rose Bengal test, the seroagglutination test, and the complement fixation test, principally measure antibodies against the immunodominant smooth lipopolysaccharide (S-LPS) (7). Antibody responses to S-LPS in B. melitensis Rev.1-vaccinated sheep have been demonstrated either by indirect enzyme-linked immunosorbent assay (I-ELISA) (7, 11, 14) or by immunoblotting (14). Therefore, a major goal in immunological studies of brucellosis has been the identification of protein antigens useful for diagnosis and possibly useful for distinguishing the immunological responses of infected animals from those of animals vaccinated with live attenuated strains.
Previously a Brucella protein named CP28, BP26, or Omp28 has been identified independently by three research groups as an immunodominant antigen in infected cattle, sheep, goats, and humans (3, 4, 5, 9, 10, 11, 12). We decided to name the protein BP26 according to the nomenclature of Rossetti et al. (10), who were the first to publish the nucleotide sequence of the bp26 gene. They localized the protein in the periplasm. The outer membrane localization reported by Lindler et al. (9) seems unlikely, since we also, by use of monoclonal antibodies (MAbs), found this protein to be localized exclusively intracellularly as a soluble protein (3). The BP26 protein appeared particularly useful for the differentiation of serological responses of infected and Rev.1-vaccinated sheep, since in the latter no detectable antibody responses against BP26 were observed either by immunoblotting, I-ELISA using the partially purified native protein, or competitive ELISA (C-ELISA) using MAbs against BP26 (5, 11, 12).
In the present study we evaluated purified recombinant BP26 protein produced in Escherichia coli as a diagnostic antigen in an I-ELISA for ovine infections caused by B. melitensis.
MATERIALS AND METHODS
Production and purification of recombinant BP26 protein.
The cloning and expression of the bp26 gene of B. melitensis 16M in E. coli have been described previously (4). E. coli cells carrying plasmid pCP2800 containing the bp26 gene were grown overnight at 37°C in 100 ml of Luria-Bertani (LB) medium supplemented with ampicillin (100 μg/ml). E. coli cells were harvested by centrifugation (at 6,000 × g for 10 min at 4°C) and washed twice with phosphate-buffered saline (PBS). After washing, pelleted bacteria were immediately resuspended in 2 ml of distilled water and lysed by sonication. Following sonication, lysed bacteria were centrifuged at 12,000 × g for 10 min at 4°C, and the supernatant was recovered. Purification of recombinant BP26 protein was further achieved by anion-exchange chromatography (M. S. Zygmunt et al., submitted for publication). Briefly, 1 ml of the supernatant was loaded (at 1 ml/min) onto a Mono-Q (HR 10/10) (Pharmacia Biotech Inc., Uppsala, Sweden) anion-exchange column equilibrated with 20 mM phosphate buffer. Recombinant BP26 protein was eluted using a nonlinear salt gradient of 1.5 M NaCl. Several fractions were collected and analyzed for the presence of BP26 by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with an anti-BP26 MAb. Purity was further assessed by SDS-PAGE and Coomassie blue staining. The fraction with the highest and purest BP26 content was further used for I-ELISA.
Sera.
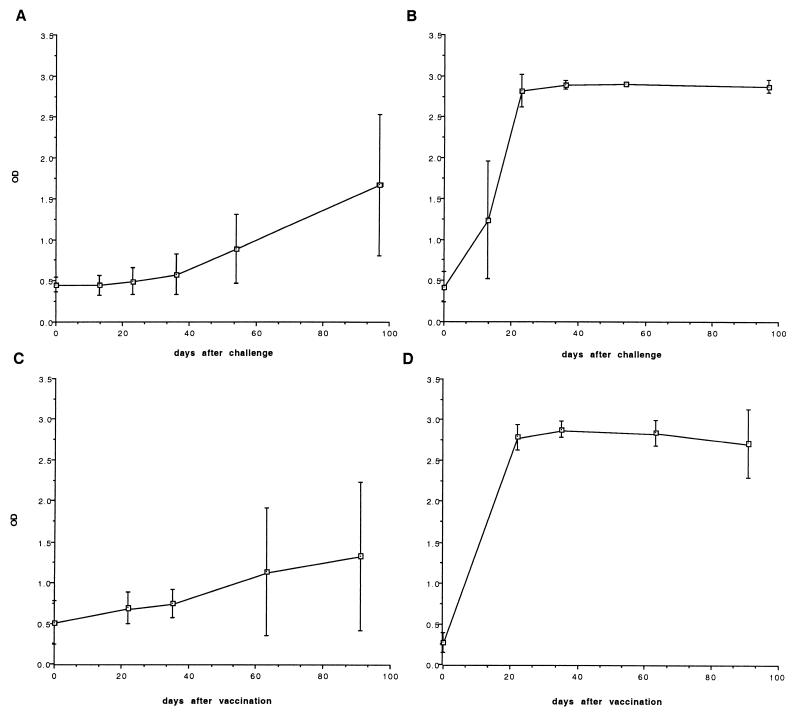
Sera used have been described in a previous study (12) and were from naturally infected, B. melitensis H38 experimentally infected (n = 8), and B. melitensis Rev.1-vaccinated (n = 8) sheep. Vaccination of sheep was performed at the age of 3 months by the conjunctival route with 109 CFU of B. melitensis Rev.1 vaccine. Animals were bled before vaccination and at several weeks postvaccination, and sera were collected (see Fig. 2). For experimental infection, 12-month-old sheep were conjunctivally infected, when 133 to 135 days pregnant, with 5.2 × 107 CFU of the virulent B. melitensis strain H38. Sera were collected before infection and at several weeks postinfection (see Fig. 2). All experimentally infected sheep yielded B. melitensis at slaughter (bacteriologically positive).
FIG. 2.
Kinetics of antibody responses to BP26 (A and C) and O-PS (B and D) in sheep experimentally infected with B. melitensis H38 (A and B) (n = 8) and B. melitensis Rev.1-vaccinated (C and D) (n = 8) sheep.
The sera from naturally infected sheep were from B. melitensis-infected flocks and could be subdivided into two groups. Group A comprised true infected animals, i.e., bacteriologically (isolation of a B. melitensis strain) and serologically (Rose Bengal and complement fixation tests) positive sheep (n = 50). Group B comprised suspected animals. B1 sheep were bacteriologically negative, serologically positive, and delayed-type hypersensitivity (DTH) test positive (n = 50). B2 sheep were bacteriologically and serologically negative but DTH test positive (n = 19).
Sera from 106 healthy sheep (group C) were also used to determine the cutoff and the specificity of the I-ELISA.
I-ELISA.
Antibody responses to recombinant BP26 protein and O-polysaccharide (O-PS) from B. melitensis 16M (15) were assessed by an I-ELISA performed as described previously (13, 14). Briefly, microtiter plates were coated with purified recombinant BP26 protein or O-PS by passive adsorption, at a concentration of 2 or 1 μg/ml, respectively, in PBS, overnight at room temperature. Ovine sera were tested on these plates at a dilution of 1/50 in PBS containing 0.05% Tween 20 (PBS-T). After incubation for 1 h at 37°C, binding of antibodies was detected by a further incubation for 1 h at room temperature with peroxidase-labeled rabbit anti-sheep immunoglobulin G (IgG) (heavy and light chain specific; Jackson Laboratories). The substrate used to reveal binding was 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS).
RESULTS AND DISCUSSION
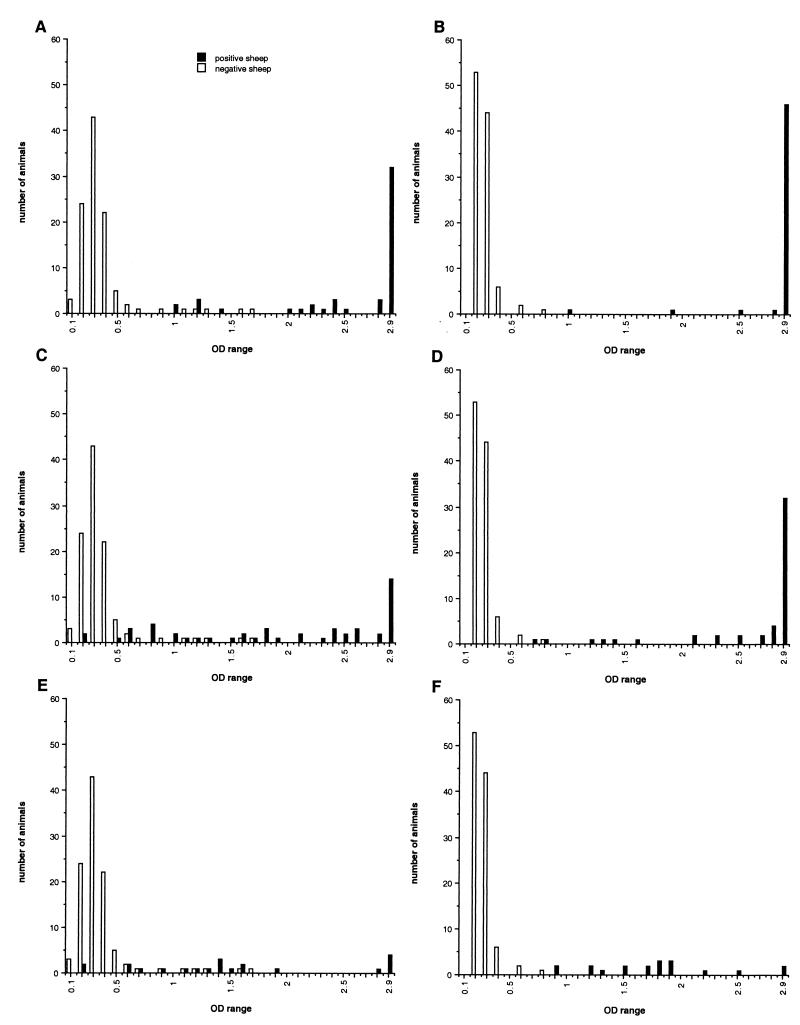
The cutoff of the recombinant BP26 I-ELISA was determined with sera from 106 healthy sheep (group C) at an absorbance value of 0.6. Under these conditions, the specificity of the recombinant BP26 I-ELISA was 93% (Fig. 1). The sensitivity of the I-ELISA assessed with sera from naturally infected (bacteriologically positive) and suspected (serologically and/or DTH positive) sheep found positive by conventional diagnostic tests was as follows: 100% for bacteriologically and serologically positive sheep (group A) (n = 50), 88% for bacteriologically negative but serologically and DTH-positive sheep (group B1) (n = 50), and 84% for bacteriologically and serologically negative but DTH-positive sheep (group B2) (n = 19) (Table 1). The sensitivity of the I-ELISA was higher than that of the C-ELISA using anti-BP26 MAbs reported in a previous study (12). The sensitivity of the recombinant BP26 ELISA was also higher than that from the study reported by Letesson et al. (8) using the same sheep sera but other recombinant proteins such as p15, p17, and p39 in I-ELISAs. Also higher absorbance values were observed in the recombinant BP26 I-ELISA than those reported in the study of Letesson et al. (8). For some sera, however, the absorbance values observed did not reach those observed in the I-ELISA using purified O-PS as the antigen (Fig. 1). All sera from the naturally infected and suspected sheep, including those that were serologically negative in the conventional tests, were found positive in the O-PS I-ELISA. This indicated, in addition, that the O-PS I-ELISA, by its high sensitivity, could possibly be a better primary screening method than the conventional serological tests.
FIG. 1.
Distribution of the recombinant BP26 (A, C, and E) and O-PS (B, D, and F) I-ELISA OD values in sera from 106 Brucella-free sheep (negative) and B. melitensis-infected and suspected sheep (positive) classified by conventional tests as bacteriologically and serologically positive (A and B) (n = 50), bacteriologically negative but serologically and DTH positive (C and D) (n = 50), or bacteriologically and serologically negative but DTH positive (E and F) (n = 19).
TABLE 1.
Detection levels of BP26 and O-PS I-ELISAs for different groups of animals
| Groupa (n) | Test resultb
|
% Positive in:
|
|||
|---|---|---|---|---|---|
| Bacteriological | Serological | DTH | BP26 I-ELISA | O-PS I-ELISA | |
| A (50) | + | + | ND | 100 | 100 |
| B1 (50) | − | + | + | 88 | 100 |
| B2 (19) | − | − | + | 84 | 100 |
| C (106) | ND | − | ND | 7 | 0 |
As described in Materials and Methods.
ND, not determined.
In sheep experimentally infected with B. melitensis H38, the antibody response to BP26 was delayed and much weaker than that to O-PS (Fig. 2). However, it is noteworthy that no antibody response at all against other interesting diagnostic protein antigens such as p15, p17, and p39 was detected in these experimentally infected sheep (8). In B. melitensis Rev.1-vaccinated sheep the antibody response to BP26 was also weak and highly heterogeneous, with some sheep showing antibody reactivities in the I-ELISA while others showed none at all (Fig. 2). The antibody response to O-PS in B. melitensis Rev.1-vaccinated sheep was as intense as that in sheep experimentally infected with B. melitensis H38 (Fig. 2).
Taken together, the results of the present study indicate that BP26 is a better diagnostic antigen for ovine brucellosis than other protein antigens reported in previous studies (8, 13, 14). Although the BP26 protein does not reach the diagnostic value of O-PS, this protein nevertheless appears promising for use in confirmatory tests and for serological differentiation between infected and B. melitensis Rev.1-vaccinated sheep. Weak antibody responses to BP26 in some of the latter sheep suggest that a B. melitensis Rev.1 bp26 gene deletion mutant should be constructed and used as a vaccine to ensure this differentiation. A bp26 gene mutant of Brucella abortus vaccine strain B19 has been successfully obtained and was shown in a mouse model to protect against a virulent B. abortus challenge as well as its parental strain (2).
ACKNOWLEDGMENTS
We thank J. M. Blasco, Zaragoza, Spain, for supplying sera from naturally infected sheep.
REFERENCES
- 1.Blasco J M. A review of the use of B. melitensis Rev.1 vaccine in adult sheep and goats. Prev Vet Med. 1997;31:275–283. doi: 10.1016/s0167-5877(96)01110-5. [DOI] [PubMed] [Google Scholar]
- 2.Boschiroli M L, Cravero S L, Arese A I, Campos E, Rossetti O L. Protection against infection in mice vaccinated with a Brucella abortus mutant. Infect Immun. 1997;65:798–800. doi: 10.1128/iai.65.2.798-800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cloeckaert A, Salih-Alj Debbarh H, Zygmunt M S, Dubray G. Production and characterisation of monoclonal antibodies to Brucella melitensis cytosoluble proteins that are able to differentiate antibody responses of infected sheep from Rev.1 vaccinated sheep. J Med Microbiol. 1996;45:206–213. doi: 10.1099/00222615-45-3-206. [DOI] [PubMed] [Google Scholar]
- 4.Cloeckaert A, Salih-Alj Debbarh H, Vizcaino N, Saman E, Dubray G, Zygmunt M S. Cloning, nucleotide sequence, and expression of the Brucella melitensis bp26 gene coding for a protein immunogenic in infected sheep. FEMS Microbiol Lett. 1996;140:139–144. doi: 10.1016/0378-1097(96)00169-3. [DOI] [PubMed] [Google Scholar]
- 5.Debbarh H S A, Cloeckaert A, Zygmunt M S, Dubray G. Identification of sero-reactive Brucella melitensis cytosoluble proteins which discriminate between antibodies elicited by infection and Rev.1 vaccination in sheep. Vet Microbiol. 1995;44:37–48. doi: 10.1016/0378-1135(94)00058-5. [DOI] [PubMed] [Google Scholar]
- 6.Garin-Bastuji B, Blasco J M, Grayon M, Verger J M. Brucella melitensis infection in sheep: present and future. Vet Res. 1998;29:255–274. [PubMed] [Google Scholar]
- 7.Jiménez de Bagüés M P, Marin C M, Blasco J M, Moriyon I, Gamazo C. An ELISA with Brucella lipopolysaccharide antigen for the diagnosis of B. melitensis infection in sheep and for the evaluation of serological responses following subcutaneous or conjunctival B. melitensis strain Rev.1 vaccination. Vet Microbiol. 1992;30:233–241. doi: 10.1016/0378-1135(92)90117-c. [DOI] [PubMed] [Google Scholar]
- 8.Letesson J J, Tibor A, van Eynde G, Wansard V, Weynants V, Denoel P, Saman E. Humoral immune responses of Brucella-infected cattle, sheep, and goats to eight purified recombinant Brucella proteins in an indirect enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 1997;4:556–564. doi: 10.1128/cdli.4.5.556-564.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindler L E, Hadfield T L, Tall B D, Snellings N J, Rubin F A, Van De Verg L L, Hoover D, Warren R L. Cloning of a Brucella melitensis group 3 antigen gene encoding Omp28, a protein recognized by the humoral immune response during human brucellosis. Infect Immun. 1996;64:2490–2499. doi: 10.1128/iai.64.7.2490-2499.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossetti O L, Arese A I, Boschiroli M L, Cravero S L. Cloning of Brucella abortus gene and characterization of expressed 26-kilodalton periplasmic protein: potential use for diagnosis. J Clin Microbiol. 1996;34:165–169. doi: 10.1128/jcm.34.1.165-169.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salih-Alj Debbarh H, Cloeckaert A, Bézard G, Dubray G, Zygmunt M S. Enzyme-linked immunosorbent assay with partially purified cytosoluble 28-kilodalton protein for serological differentiation between Brucella melitensis-infected and B. melitensis Rev.1-vaccinated sheep. Clin Diagn Lab Immunol. 1996;3:305–308. doi: 10.1128/cdli.3.3.305-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salih-Alj Debbarh H, Zygmunt M S, Dubray G, Cloeckaert A. Competitive enzyme-linked immunosorbent assay using monoclonal antibodies to the Brucella melitensis BP26 protein to evaluate antibody responses in infected and B. melitensis Rev.1 vaccinated sheep. Vet Microbiol. 1996;53:325–337. doi: 10.1016/s0378-1135(96)01265-5. [DOI] [PubMed] [Google Scholar]
- 13.Zygmunt M S, Cloeckaert A, Dubray G. Brucella melitensis cell envelope protein and lipopolysaccharide epitopes involved in humoral immune responses of naturally and experimentally infected sheep. J Clin Microbiol. 1994;32:2514–2522. doi: 10.1128/jcm.32.10.2514-2522.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zygmunt M S, Salih-Alj Debbarh H, Cloeckaert A, Dubray G. Antibody response to Brucella melitensis outer membrane antigens in naturally infected and Rev1 vaccinated sheep. Vet Microbiol. 1994;39:33–46. doi: 10.1016/0378-1135(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 15.Zygmunt M S, Dubray G, Bundle D R, Perry M B. Purified native hapten of Brucella abortus B19 and B. melitensis 16M reveal the lipopolysaccharide origin of the antigens. Ann Inst Pasteur/Microbiol. 1988;139:421–433. doi: 10.1016/0769-2609(88)90105-6. [DOI] [PubMed] [Google Scholar]