Abstract
Background
In order to use aseptically prepared elastomeric infusers, outpatient parenteral antimicrobial therapy (OPAT) services require extended stability data for antimicrobial agents to assign a product shelf-life. In the UK, the relevant standards for stability testing and shelf-life assignment are published in ‘A Standard Protocol for Deriving and Assessment of Stability—Part 1 (Aseptic Preparations—Small Molecules), commonly called the Yellow Covered Document (YCD). A previous systematic review published in 2017 failed to identify data on the stability of antimicrobials in elastomeric devices for OPAT services that met YCD requirements in force at the time. The aim of this review was to update that search, following a subsequent change to YCD requirements in 2017 and 2019 and expand that dataset to identify progress made in providing assurance about the stability of antimicrobial agents for OPAT services.
Methods
Searches were undertaken for papers relating to extended stability of antimicrobials. Citations were included when antimicrobial shelf-life was assessed using a stability-indicating method and considered a period of storage, either refrigerated or at room temperature, followed by in-use testing at a temperature at or above 32°C.
Results
Of 267 initial citations, six met the inclusion criteria and underwent full text review for data extraction. Included antimicrobials were cefazolin, ceftazidime, piperacillin/tazobactam, flucloxacillin and ceftolozane/tazobactam. Of these, only flucloxacillin and piperacillin demonstrated YCD compliant stability over the 24-hour infusion period while cefazolin, ceftazidime and ceftolozane/tazobactam could be infused over 12-hour period.
Conclusions
Contrary to the position found in 2017 review, high-quality data are now available to support the use of a number of antimicrobial agents in extended infusion in elastomeric devices for OPAT services. There is a need to expand the dataset, as well as developing international consensus on the ideal parameters for stability assessment of such infusions in elastomeric devices.
Keywords: drug administration routes, microbiology, drug compounding, education, pharmacy, continuing, administration, intravenous
Introduction
Outpatient parenteral antimicrobial therapy (OPAT) services offer a means of treating patients who require intravenous therapy, usually provided as an inpatient, care delivery options which include home or clinic-based treatment.1–3
In order to balance the therapeutic benefits of OPAT with the convenience of outpatient administration, these services need to be able to access and use a range of agents. This includes both broad and narrow spectrum antimicrobials that can be given by convenient bolus injection or short infusion following immediate reconstitution, agents that are amenable to self-administration by parents and carers and agents that can be given by extended infusion in delivery systems such as elastomeric devices that infuse antimicrobials in solution over 12–24-hour periods, allowing one or two times a day administration of agents that are normally given three to four times a day.4–6
In order for UK National Health Service (NHS) hospital aseptic units to prepare elastomeric infusers for patient administration, stability data compliant with the NHS standards must be sourced. These standards are published in the National Health Service Pharmaceutical Quality Assurance Committee document ‘A Standard Protocol for Deriving and Assessment of Stability—Part 1 (Aseptic Preparations—Small Molecules)’ commonly referred to as the Yellow Covered Document (YCD).7
In 2017, a systematic review of antimicrobial stability identified 121 papers for full text screening to determine if they contained data that met the YCD standards, but none met the inclusion criteria.8 The 2017 review was conducted using the YCD edition 3 (December 2015) standards which required in-use testing at 37°C. Following an update in 2017, the temperature of in-use testing was amended and this review investigates papers that adhere to the new standard of 32°C.
The YCD acceptance criteria that confer stability for an antimicrobial intended for extended infusion is a key requisite in the clinical governance and quality assurance of OPAT services.8 9 The aims of this review were to update the 2017 systematic stability review by searching for data on the extended infusions of antimicrobial agents appropriate to the OPAT setting and which fulfil the requirements of the YCD. For most OPAT services, the stability of reconstituted antimicrobial agents for a period of time prior to administration—to allow for stock to be ordered and stored in advance of patient need—is a pragmatic requirement and was therefore an important addition to the previous protocol (see online supplemental information for full protocol).
ejhpharm-2021-002729supp001.pdf (195.6KB, pdf)
A previous review of publicly accessible data that met the YCD standards for the administration of antimicrobials via elastomeric devices did not find any studies which met the criteria.8 It is hoped that collating the recent published literature will support hospital aseptic units in expanding the provision of local preparative services in the OPAT setting.
Method
This paper updates the review of extended stability data for antimicrobial agents, published in 2017, which included papers published up until November 2015.8 This updated review searched the published literature initially from October 2015 to September 2020 with the search rerun in December 2020.
A systematic review was conducted using MEDLINE, EMBASE, CINAHL, Google Scholar and Google for published literature relating to extended antimicrobial stability (for full search strategy, see online supplemental information). The outputs of the searches were combined and any duplicates removed.
Each citation was reviewed by title and abstract for inclusion by two of the four authors. Any discrepancies were resolved by consideration by a third author. Citations were considered appropriate for inclusion if an antimicrobial shelf-life was assessed using a stability-indicating method and included a period of storage, either refrigerated or at room temperature, followed by in-use testing at a temperature at or above 32°C.
Remaining references were then similarly reviewed for inclusion in full-text. Final list of included papers was data extracted to a spread sheet including fields to assess compliance with the YCD.7 Data extraction fields included:
Author and date of publication.
Drug investigated.
Storage conditions (period of in-use temperature testing for example, 32°C following a period of appropriate storage).
Range of active pharmaceutical ingredient (API) to remain to confer stability.
Use of a stability indicating assay, for example, high performance liquid chromatography.
Physical stability testing, for example, pH, colorimetry, non-visible particulate assessment.
Number of time-points studied (four in addition to time zero).
Number of samples tested at each time-point (three).
Testing of low and high ‘clinically relevant’ concentrations.
Replicate samples tested (duplicate).
Text in parentheses indicates standards as outlined in the YCD.
The references of included articles were further reviewed for any potential additional citations of interest which were then reviewed for inclusion using the process above.
Results
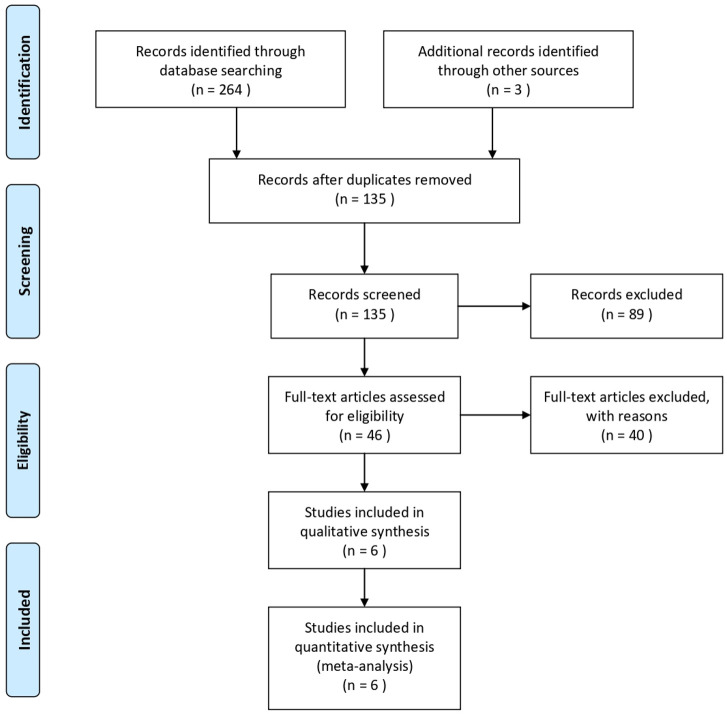
Searches in information retrieval sources yielded the following outputs: MEDLINE (119), EMBASE (126), CINAHL (19) and Google Scholar (3). Following deduplication, 135 citations remained (figure 1). Eighty-nine references were excluded on review of title and abstract alone, while a further 40 were eliminated on full-text consideration and reasons for exclusion documented in table 1.
Figure 1.
PRISMA 2009 flow diagram.24
Table 1.
Exclusion criteria for citations reviewed in full text
| Reason for exclusion | No. papers |
| Stability study conducted below 32°C | 27 |
| Stability study conducted at or above 32°C but does not include a period of prior storage under ambient or refrigerated condition | 11 |
| Not a stability study | 2 |
| Total | 40 |
Six citations went forward for data extraction, comprising three conference abstracts and three full-text articles. The lead author for each of the posters was contacted and each supplied additional and comprehensive supporting information.
Four out of six papers were conducted in the UK with one completed in each of Australia and Switzerland. The stability under storage and in-use conditions is presented for five medicines: two cephalosporins (cefazolin and ceftazidime), one penicillin (flucloxacillin) and two beta-lactam/beta-lactamase inhibitor combinations (ceftolozane-tazobactam and piperacillin-tazobactam).
Elastomeric infusors were the storage devices investigated in all papers, with the Baxter LV10 (Baxter Healthcare) used in five papers, the Easypump II (BBraun) in four citations and the Accufuser (WooYoung Medical) in one.
All papers used sodium chloride 0.9% as a diluent while three considered the addition of citrate buffer to sodium chloride 0.9% to enhance the stability of the beta-lactam studied.
The YCD states that the tolerance limit for the API of a manufactured product should be between 95% and 105% of the initial concentration at the end of the administration period, and the British Pharmacopoeia (BP) usually has a similar range for the API concentration which applies across the product shelf-life. This is not always the case, for example, ceftazidime has an BP API limit of 90%–110%. If there is no pharmacopoeial monograph for the drug, then the API range is limited to 95%–105% as set out in the YCD. Three of the included papers used the API limit 95%–105% (flucloxacillin, ceftolozane/tazobactam and piperacillin/tazobactam) and three used a range of 90%–110% (piperacillin/tazobactam, ceftazidime and cefazolin). Contrary to the 2017 review, papers reporting stability using an API range of 90%–110% were included to ensure reports for all drugs including ceftazidime were captured and also to determine if 95%–105% stability data could be interpolated from studies where the API limit was 90%–110%. Of the papers reporting stability using an API range of 90%–110%, the cefazolin paper also reported data for 95% API and the results from El Saghir’s study of piperacillin/tazobactam using a 90%–110% API range were similar to those of Jamieson et al using 95%–105% API range.
Cefazolin
Patel et al studied cefazolin 3 and 6 g in 240 mL, diluted in sodium chloride 0.9% or glucose 5% in elastomeric devices (Baxter LV10) stored at 2–8°C for 72 hours, followed by 12 hours at 35°C and 12 hours at 25°C. The authors report that all infusers retained at least 94% of the initial cefazolin concentration with both infusers diluted with sodium chloride 0.9% retaining in excess of 95% at the end of the 96-hour study period.10
Ceftazidime
While the YCD sets out the testing requirements in order the ascertain the shelf life of an aseptically prepared product, it also states that there may be additional parameters that need to be considered for individual drugs, for example, limits on levels of potentially toxic degradants. This is the case with ceftazidime where the BP states that pyridine levels must not exceed 0.5% of the starting ceftazidime concentration.
Jamieson et al studied the stability of ceftazidime 12.5 and 25 mg/mL diluted with sodium chloride 0.9% when stored in elastomeric devices (Baxter LV10 and Easypump II, BBraun Ltd.) filled to 240 mL. Formation of pyridine was also monitored. Infusors were stored at 2–8°C for 48 hours, allowed 3 hours to come to room temperature and then stored at 32°C. Although pyridine levels remained within monograph limits for at least 18 hours, 5% loss of ceftazidime was observed within 6–8 hours and more than 10% loss was observed after 18 hours. Prescoping work did not identify any benefit from buffering the solution to improve stability. The authors proposed that a 12-hour infusion of ceftazidime was acceptable to limit loss of the active ingredient and maintain pyridine limits within allowable tolerances.11 12
Ceftolozane-tazobactam
Jamieson et al report the YCD compliant stability assessment of ceftolozane-tazobactam 5 and 20 mg/mL in sodium chloride 0.9%, stored in elastomeric devices (Baxter LV10 and Easypump II). Less than 5% loss of either ceftolozane or tazobactam was observed following storage of the devices at 2–8°C for 8 days, followed by 3 hours warmup to room temperature and finally 12 hours storage at 32°C.13 14
Flucloxacillin
Allwood et al studied flucloxacillin 10 and 50 mg/mL, diluted with 0.3% w/v citrate buffered sodium chloride 0.9% in Baxter LV10 and Accufuser (WooYoung Medical) infusors. In YCD compliant studies, stability was demonstrated for 13 days stored at 2–8°C, followed by 24 hours at 32°C.15
Piperacillin-tazobactam
El Saghir studied the stability of branded and generic formulations of piperacillin-tazobactam in the absence or presence of citrate buffer in Easypump infusors. Optimal stability was achieved with piperacillin-tazobactam 13.5 g with 17 mL citrate buffer 4% made up to 240 mL with sodium chloride 0.9%. Less than 5% loss of the active ingredient was reported when elastomerics filled with this formulation were stored at 2–8°C for 7 days followed by 24 hours at 37°C. Jamieson et al also conducted a YCD compliant study using piperacillin-tazobactam 25 and 90 mg/mL diluted in 0.3% w/v citrate-buffered saline which demonstrated stability for 13 days stored 2–8°C, followed by 24 hours at 32°C.16–18
Discussion
This paper updates the 2017 review and summarises available data that meets the inclusion criteria and provides a useful yardstick to measure the progress of drug stability testing for OPAT services in the last few years.
The focus of this systematic review was on antimicrobial stability in elastomeric devices, as these are widely used in UK and worldwide OPAT services due to convenience and high patient acceptability.1 19 Our systematic review identified six good quality stability studies looking at five antimicrobial agents of use to the OPAT community. This is an improvement on the previous stability review published in 2015 and demonstrates that the research activity in this important therapeutic area is growing.
Three out of the six included papers used an API range of 90%–110% in order to determine product shelf-life. Compared with the previous systematic review by Jenkins et al, we note that experimental methodology and detail within the published reports have improved. For example, we note that in citations where an API range of 90%–110% was used, 95%–105% is frequently also reported or there are sufficient data to calculate this value. Additionally, testing at or above 32°C now seems commonplace for stability testing in elastomerics.
Of 40 excluded full-text articles, the in-use study temperature in 27 was less than 32°C. Temperature is a key influencer of stability particularly of beta-lactam antimicrobials.20 As a result, stability testing conducted at lower temperatures will show lower degradation rates and any data taken from studies should be used with caution.
We identified 11 excluded papers where the studied temperature was above 32°C; however, no preadministration storage was included in the study. Although these papers did not meet the inclusion criteria for this review, we suggest that they contain data valuable in the OPAT setting which warrant further investigation (manuscript in preparation). Furthermore, we speculate that there is increasing recognition that testing temperatures need to be more ‘real world’, and that the YCD 32°C is a sensible pragmatic temperature to test at, and based on evidence.7 21 Room temperature (usually defined as 15–25°C)22 testing does not seem to be a valid ‘in use’ testing temperature for OPAT, and recently published data suggest that the temperature of solutions inside elastomeric devices can approach at least 32°C, and if exposed to sunlight, can exceed 45°C.20 For some territories with higher ambient temperatures than the UK, a testing temperature of greater than 32°C has been proposed.9
Antimicrobial drug stability testing using nationally or internationally agreed standards is becoming increasingly important to support the further growth of OPAT services; the importance of OPAT services has recently been recognised in a review of NHS aseptic services conducted by NHS England.23 Perks et al recently proposed three criteria for the assessment of chemical stability of antimicrobial agents for extended infusion in OPAT—demonstration of stability at 20–25°C for standard room temperature, or 34°C or above for warmer climates, for the 24 hour ‘in use’ period; the nominal volume of 240 mL be used to reflect the dose of the antimicrobial used in clinical practice and assessment of stability following a period of refrigerated storage and the subsequent in use period.9
A compound that is acceptably stable at 34°C or higher during in-use period would satisfy the criteria for the YCD – however, that in itself is a challenging target for stability for many antimicrobial agents. Based on the data identified in the Perks review, and that which we identified from our research, only cefazolin and piperacillin/tazobactam have data that might meet this criterion.
We would agree with Perks et al with regard to the importance of assessment of stability following a period of refrigerated storage; this is a pragmatic requirement for many OPAT services where the near patient preparation of elastomeric infuser devices is neither practical nor desirable, given the potential risks associated with this practice.
Our review extends on the work done by Perks et al and we identified new data to support the use of flucloxacillin and cefazolin in elastomeric devices which meets YCD criteria. While the cefazolin study followed YCD for 72 hours storage between 2°C and 8°C and the initial 12 hours of in-use testing at 35°C, the researchers reduced the study temperature for the later 12 hours of the in-use period to 25°C. Although this study does include 24 hours at clinically relevant temperature, the temperature profile is not in line with that suggested in the YCD. Nevertheless, the study robustly supports a 12-hour infusion period and if the mean kinetic temperature is calculated for the infusion period, this is 31.2°C, within the stated range of the YCD for the 24-hour infusion period. In sodium chloride,0.9% over 95% of initial concentration is maintained for this period. Nevertheless, it would be prudent to confirm this with a study performed to YCD criteria.
Conclusion
This updated review of literature collates stability pertaining to extended stability of antimicrobials in elastomeric devices. Six reports were identified that demonstrate stability data compliant with the YCD to support OPAT services and hospital aseptic units in the preparation, storage and administration of these antibiotics via elastomeric infusion devices. Our review identified the acceptable stability of flucloxacillin and piperacillin/tazobactam for continuous infusion over 24 hours, ceftolozane-tazobactam for infusion over 12 hours and the potential for acceptable stability of cefazolin, subject to adequately performed stability testing.
Footnotes
Contributors: AJ conducted the literature search. AJ, CJ, MS and SS reviewed titles and abstracts of citations followed by full text review for included articles. AJ completed the data extraction. All authors contributed to the writing of the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Durojaiye OC, Bell H, Andrews D, et al. Clinical efficacy, cost analysis and patient acceptability of outpatient parenteral antibiotic therapy (OPAT): a decade of Sheffield (UK) OPAT service. Int J Antimicrob Agents 2018;51:26–32. 10.1016/j.ijantimicag.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 2. Fisher D, Michaels J, Hase R, et al. Outpatient parenteral antibiotic therapy (OPAT) in Asia: missing an opportunity. J Antimicrob Chemother 2017;72:1221–1226. 10.1093/jac/dkw551 [DOI] [PubMed] [Google Scholar]
- 3. Carter B, Carrol ED, Porter D, et al. Delivery, setting and outcomes of paediatric outpatient parenteral antimicrobial therapy (OPAT): a scoping review. BMJ Open 2018;8:e021603. 10.1136/bmjopen-2018-021603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slavik RS, Jewesson PJ. Selecting antibacterials for outpatient parenteral antimicrobial therapy : pharmacokinetic-pharmacodynamic considerations. Clin Pharmacokinet 2003;42:793–817. 10.2165/00003088-200342090-00002 [DOI] [PubMed] [Google Scholar]
- 5. Leggett JE. Ambulatory use of parenteral antibacterials: contemporary perspectives. Drugs 2000;59 Suppl 3:1–8. 10.2165/00003495-200059003-00001 [DOI] [PubMed] [Google Scholar]
- 6. Candel FJ, Julián-Jiménez A, González-Del Castillo J. Current status in outpatient parenteral antimicrobial therapy: a practical view. Rev Esp Quimioter 2016;29:55–68. [PubMed] [Google Scholar]
- 7. NHS Pharmaceutical Quality Assurance Committee . A standard protocol for deriving and assessment of Stability- Part 1 (aseptic Preparations- small molecules), 2020. Available: https://www.sps.nhs.uk/wp-content/uploads/2013/12/Stability-part-1-small-molecules-5th-Ed-Sept-19.pdf
- 8. Jenkins A, Hills T, Santillo M, et al. Extended stability of antimicrobial agents in administration devices. J Antimicrob Chemother 10.1093/jac/dkw556 [DOI] [PubMed] [Google Scholar]
- 9. Perks SJ, Lanskey C, Robinson N, et al. Systematic review of stability data pertaining to selected antibiotics used for extended infusions in outpatient parenteral antimicrobial therapy (OPAT) at standard room temperature and in warmer climates. Eur J Hosp Pharm 2020;27:65–72. 10.1136/ejhpharm-2019-001875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel RP, Jacob J, Sedeeq M, et al. Stability of cefazolin in polyisoprene elastomeric infusion devices. Clin Ther 2018;40:664–7. 10.1016/j.clinthera.2018.02.009 [DOI] [PubMed] [Google Scholar]
- 11. Jamieson Drummond C C, Ozolina L, Wilkinson A-S. Stability testing of ceftazidime solutions for injection in elastomeric devices at 12 mg/mL and 25 mg/mL in 0.9% w/v saline for safe use in Outpatient Parenteral Antimicrobial Therapy (OPAT). Available: https://e-opat.com/wp-content/uploads/2020/01/OPAT2019-CAZPoster-28Nov.pdf
- 12. Jamieson C. Personal communication regarding ceftazidime, 2020. [Google Scholar]
- 13. Jamieson Drummond C C, Ozolina L, Wilkinson A-S. Assessing the stability of ceftolozane/tazobactam (ZERBAXA®) at 5 mg/mL and 20 mg/mL following reconstitution and dilution in 0.9% saline in two commercially available elastomeric devices. Available: https://e-opat.com/wp-content/uploads/2020/01/OPAT2019-ZerbaxaPoster-28Nov.pdf
- 14. Jamieson C. Personal communication regarding ceftolozane/tazobactam, 2020. [Google Scholar]
- 15. Allwood MC, Stonkute D, Wallace A, et al. Assessment of the stability of citrate-buffered flucloxacillin for injection when stored in two commercially available ambulatory elastomeric devices: INfusor LV (Baxter) and Accufuser (Woo young medical): a study compliant with the NHS yellow cover document (yCD) requirements. Eur J Hosp Pharm 2020;27:90–4. 10.1136/ejhpharm-2018-001515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El Saghir F, Meier J, Bornand D, et al. Stability testing of piperacilline/tazobactam in elastomeric infusion pumps. Le Pharmacien Hospitalier et Clinicien 2017;52:e32. 10.1016/j.phclin.2017.01.081 [DOI] [Google Scholar]
- 17. El Saghir F. Implementation of prolonged infusion times for meropenem and Piperacillin/Tazobactam: stability testing and organizational aspects. Thesis from Basel University, 2018. [Google Scholar]
- 18. Jamieson C, Ozolina L, Seaton RA, et al. Assessment of the stability of citrate-buffered piperacillin/tazobactam for continuous infusion when stored in two commercially available elastomeric devices for outpatient parenteral antimicrobial chemotherapy: a study compliant with the NHS yellow cover document requirements. Eur J Hosp Pharm 2022;29:212–6. 10.1136/ejhpharm-2020-002340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saillen L, Arensdorff L, Moulin E, et al. Patient satisfaction in an outpatient parenteral antimicrobial therapy (OPAT) unit practising predominantly self-administration of antibiotics with elastomeric pumps. Eur J Clin Microbiol Infect Dis 2017;36:1387–92. 10.1007/s10096-017-2944-5 [DOI] [PubMed] [Google Scholar]
- 20. Voumard R, Van Neyghem N, Cochet C, et al. Antibiotic stability related to temperature variations in elastomeric pumps used for outpatient parenteral antimicrobial therapy (OPAT). J Antimicrob Chemother 2017;72:1462–5. 10.1093/jac/dkw582 [DOI] [PubMed] [Google Scholar]
- 21. Merwe SvanD, Green H. What is the maximum temperature reached in elastomeric devices under simulated OPAT conditions? Arch Dis Child 2016;101:e2. 10.1136/archdischild-2016-311535.32 [DOI] [PubMed] [Google Scholar]
- 22. ECA Academy . What are the regulatory Definitions for "Ambient", "Room Temperature" and "Cold Chain"? Available: https://www.gmp-compliance.org/gmp-news/what-are-the-regulatory-definitions-for-ambient-room-temperature-and-cold-chain
- 23. Carter PR. Transforming NHS aseptic services in England. A national report for the Department of health and social care. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/931195/aseptic-pharmacy.pdf
- 24. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2021-002729supp001.pdf (195.6KB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study.