Abstract
Objectives
Topical resorcinol 15% is a self-treatment for painful hidradenitis suppurativa nodules and abscesses with good results in reducing pain and lesion duration. The aim of this study is to establish a 15% topical resorcinol formula, to develop a physicochemical and microbiological stability study and to further determine the compounding shelf-life in different package conditions following the European Pharmacopoeia (Ph. Eur.) specifications.
Methods
Physicochemical and microbiological stability studies of the formulation were conducted for 12 months at room temperature (25°C±2°C) in different package conditions: aluminium tubes (aluminium A7-99.7% varnish DF-6172), plastic tubes (low density polyethylene) and amber plastic containers (polyethylene terephthalate). High performance liquid chromatography (HPLC) was developed as a method of indicating the stability of the resorcinol formulation. A microbiological growth assay was also validated according to the Ph. Eur. Physical properties were inspected to determine parameters such as odour, colour, pH, emulsion phase and extensibility index and its evolution.
Results
The HPLC method was validated according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines. At day 365, visual inspection remained unchanged only for preparations packaged in aluminium tubes. The pH did not vary by more than 0.3 units in all conditions. The extensibility index decreased in the preparations packaged in plastic and amber plastic containers. HPLC analysis conducted over 1 year did not show a degradation greater than 7% of resorcinol in the preparation in plastic and aluminium packages. The ability of ATCC strains to grow in resorcinol formulation was confirmed under the suitability test. Resorcinol packed in aluminium tubes achieved microbiological stability at day 365.
Conclusions
Only the formulation package in aluminium tubes showed physicochemical and microbiological stability of resorcinol for 12 months at room temperature (25°C±2°C).
Keywords: dermatology, pharmaceutical preparations, pharmacy service, hospital, microbiology, drug compounding
Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory dermatological disease of the pilosebaceous follicle.1 Manifestations of the disease are heterogeneous and generally appear after puberty, although most include painful and itchy nodules or abscesses with malodorous suppuration.2–4 The disease occurs in the form of recurrent outbreaks of variable frequency.2 5 6 Symptoms during each episode include increased pain and suppuration which generates a notable decrease in the quality of life.7 8 Finally, the condition evolves into a chronic inflammatory state leading to fistula formation.9 Although HS is not considered an infection, bacteria are thought to play a role in the pathogenesis. The most commonly encountered pathogens found in HS lesions are Gram-positive bacteria including Staphylococus aureus, Coagulase-negative staphylococci and Corynebacterium spp. The inflammation process results in the formation of a biofilm which severely stimulates the skin immune system response and aggravates the course of the disease.10 Treatment options for acute lesions include topical or systemic antibiotics, intralesional corticosteroids and surgical intervention, with limited evidence in favour of their efficiency.11 Due to the recurrence of the outbreaks of HS, the use of antibiotherapy becomes chronic. As a result, it enhances the microbiological virulence of Gram-positive bacteria, causing resistance to commonly used antibiotics, increased biofilm production, and resistance to biofilm eradication in patients with HS.12
Topical resorcinol 15% has been used as a self-treatment for painful HS nodules and abscesses with good results in reducing pain and lesion duration.13 14 Resorcinol is a phenolic compound that acts as a peeling agent at a concentration rate of 15%, in addition to its antiseptic, antifungal and antipruritic properties.
In hospital this formula is generally prepared and dispensed to outpatients who do not have a reference pharmacy for pharmaceutical compounding. Specifically, in Spain several pharmacies do not have space in which to formulate compounding. Since it is not yet commercially available, topical resorcinol 15% needs to be compounded in pharmaceutical laboratories with no standard formulation available. Moreover, no stability studies have been found to support its shelf-life period for safe and effective administration over time. Determining the shelf-life is also essential to produce this formulation in batches to be stored under optimal conditions. This will reduce the delay between prescription and dispensing, which can be especially important when the patient has an active outbreak. Furthermore, the eighth edition of the European Pharmacopoeia (Ph. Eur.) has no high performance liquid chromatography (HPLC) method for resorcinol analysis.
The aim of this study is to establish a 15% topical resorcinol formula, to develop a physicochemical and microbiological stability study and to further determine the shelf-life of the compound in different package conditions following Ph. Eur. specifications.15
Materials and methods
Reagents
Resorcinol and metabisulfite were provided by Acofarma (Barcelona, Spain) and Lannette II cream was provided by Fagron (Barcelona, Spain). All formulation components were Ph. Eur. grade. The analytical grade chemical reagents resorcinol and methanol were purchased from Sigma-Aldrich (Darmstadt, Germany). Milli-Q grade deionised water was used as the source of water in the study.
Formulation
To prepare the proposed formulation of 15% topical resorcinol, 15 g of resorcinol were dissolved in 15 mL of water. After resorcinol was dissolved, 0.1 g of sodium metabisulfite was added. The next step was to incorporate the prepared solution in a sufficient quantity of Lannette II cream to obtain 100 g of the desired final weight. Finally, different package conditions were filled with the compound.
Storage conditions
Preparations were produced and packaged in three different containers: varnished aluminium tubes (aluminium A7-99.7% varnish DF-6172), plastic tubes (low density polyethylene) and amber plastic containers (polyethylene terephthalate). Batch production was made in real work environments according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q2(R1) guidelines16 and stored at room temperature (25°C±2°C) for 1 year.
Physicochemical stability and chromatographic method
The chemical stability of prepared topical 15% resorcinol was tested for 1 year by HPLC. The 8th edition of Ph. Eur. has no HPLC method for resorcinol analysis, so the reported method was adapted from a previously published one.17 18 The HPLC system used was an Agilent Infinity 1260 (Waldbronn, Germany) equipped with an autosampler, quaternary pump, thermostat column compartment and a diode array as ultraviolet detector. The column used was a BDS Hypersil C18 250×4.6 mm, 5 µm (Thermo Scientific, USA). Chromatographic conditions of the HPLC system were set as follows: a mixture of methanol and water (40:60 v/v) as mobile phase, 10 µL injection volume, flow rate of 1 mL/min, column temperature of 25°C and detection wavelength of 280 nm. Sample preparation consists of a dilution of 0.1 g of the resorcinol cream in 10 mL methanol before 1:10 dilution with mobile phase. Recovery between 90% and 110% resorcinol was set as the acceptance criterion for chemical stability.15
The physical properties were inspected to determine parameters such as odour, colour, pH, emulsion phase and extensibility index. For emulsion phase determination, methylene blue was used as dye indicator. The pH was measured using a WTW-534 pH-metre (InoLab, Germany). The extensibility index was evaluated according to the Spanish National Formulary19 as follows: the surface increase was measured as 1 g of sample progressively exposed between two glass plates to increasing levels of pressure. Pressure was introduced by using different weights on the glass top plate at equal time intervals: 1 min of 0.24 Pa, 0.73 Pa and 1.22 Pa of pressure per m2. The values of the two larger diameters of the circle formed were noted and the average diameter calculated. For the average diameter, the mean extension surface is determined using the equation: surface=πr². The testing assay was carried out at 25°C±2°C. The extensibility index was then calculated based on the slope of the curve obtained. Sample measurements were carried out in three replicates on days 0, 7, 14, 21, 28, 60, 90, 120, 180, 240, 300 and 365.
Physical stability was defined as the non-variation of the pH of more than 1 unit, non-variation of the visual inspection and the non-inversion of the emulsion sign. Since there are no reference values with which to compare the extensibility index, we propose a non-variation of more than 10% as the stability acceptance criterion.
Validation of HPLC method
The chromatographic method described was validated for resorcinol quantification according to the ICH Q2(R1) guidelines16 as follows. The specificity was tested studying the peak purity obtained, the two-dimensional and three-dimensional spectral analysis between 190 and 400 nm. The following three factors were determined to define the analytical characteristics of the chromatographic method: relative standard deviation (%RSD=SD×100/mean measured concentration), column efficiency measured as theoretical plates and USP tailing factor. They were calculated by analysing 10 identical injections of the 150 µg/mL resorcinol standard solution. The calibration line was set by using six standard resorcinol solutions of 75, 100, 125, 150, 175 and 200 µg/mL, each used in three replicates. Linearity was studied by the correlation coefficient (R2) and the variance was analysed using an ANOVA. All statistical tests were performed with 95% confidence intervals. The limit of detection (LOD) was calculated using the formula (3.3 × (SD75/S)) and the limit of quantification (LOQ) was calculated as (10 × (SD75/S)). SD75 represents the SD of the mean recovery percentage corresponding to 75 µg/mL of the calibration point. S corresponds to the slope obtained. The precision was tested by using intra-day repeatability and inter-day intermediate precision. It was expressed as relative standard deviation (%RSD). In order to obtain inter-day accuracy, the minimum, medium and maximum standard concentrations were analysed in quintuplicate on the same working day. To study inter-day intermediate precision, three replicates of the same standard concentrations were measured on five consecutive days. The accuracy was studied at three different standard concentrations (low, medium and high) and calculated based on the recovery percentage.
Accelerated degradation study
Our resorcinol formulation was exposed to several forced degradation conditions to show that the results obtained by the method described were stability-indicating. The experiments were performed for a total duration of 72 hours. 1N HCl and 1N NaOH were used to simulate acid–base catalysed degradation. H2O2 15% v/v and heat (90°C) were used to force oxidative and thermal degradation conditions.
Microbiological stability
Acceptance criteria for compounding microbiological quality control were taken from Ph. Eur. For a non-sterile cutaneous use preparation, the total aerobic microbial count must be less than 102 colony forming units (cfu)/mL and the total combined yeast counts less than 101 cfu/mL. In addition, the preparation must demonstrate absence of Staphylococcus aureus and Pseudomonas aeruginosa.
Before performing the microbiological quality assay, the Ph. Eur. also requires testing the suitability of the method to determine the ability of microorganisms to grow in the studied formulation. Several reference strains were selected according to the Ph. Eur: Pseudomonas aeruginosa (ATCC9027), Candida albicans (ATCC10231), Aspergillus brasiliensis (ATCC16404) and Staphylococcus aureus (ATCC6538). To perform the growth assay, Tryptic Soy Agar (TSA) was used for P. aeruginosa and S. aureus and Saboureaud Agar (SAB) for C. albicans and A. brasilensis. The test was performed using a 1:1000 dilution of 1 g topical resorcinol in a 0.1% Tween 80® and phosphate-buffered saline (PBS) solution, to which 100 µL of a suspension equivalent was added at 1×103 cfu/mL of each ATCC strain. These strains were previously inoculated in SAB and TSA. All tests were performed in duplicate and intermediate readings were taken every 48 hours.
According to the Ph. Eur., the microbial count in the agar plate was considered to be the method of choice to test the microbiological stability. The microbial count was assessed by calculating the average number of cfu found in the agar. Two conditions were tested: open and closed tubes. They were both tested in three replicates. The microbiological formulation test was then performed at 0, 30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330 and 365 days for both closed and open aluminium tubes. Open tubes were opened twice a day to simulate regular use for the treatment of suppurative hidradenitis. The test was performed using a 1:1000 dilution of 1 g topical resorcinol in a 0.1% Tween 80® and PBS solution. One mL was spread across SAB and TSA using the surface-spread method. All tests were performed in duplicate. The SAB and TSA were incubated at 35–37°C for 2 days for TSA plates and 5 days for SAB plates. Plastic tubes and plastic amber containers were not tested since they are known to have worse physicochemical properties than aluminium.
Results
Validation of the HPLC method
The specificity of the HPLC method was validated by studying the resorcinol peak obtained. This peak is characterised by a mean retention time of 3.774±0.004 min and a mean asymmetry indicator of 0.850±0.005. The analytical characteristics of the chromatograph were set with a %RSD peak area of 0.042%, a theoretical column plate value of 11 942 and a USP tailing factor of 1.01. The calibration line was set in the range of 75–200 µg/mL. The equation calculated by linear regression was y=8.6585 x − 11.857 (n=18; R²=0.9999). The linearity was tested by ANOVA and was confirmed with a 95% confidence interval. The LOD calculated was 0.41 µg/mL and the LOQ was 1.25 µg/mL. Accuracy, measured as recovery percentage, was between 97.5% and 102.5% at all points tested, confirming the overall accuracy of the method (online supplemental table 2). By analysing the precision of the method, we found that the %RSD obtained was less than 1% for all points tested.
ejhpharm-2020-002534supp001.pdf (35.8KB, pdf)
Accelerated degradation study
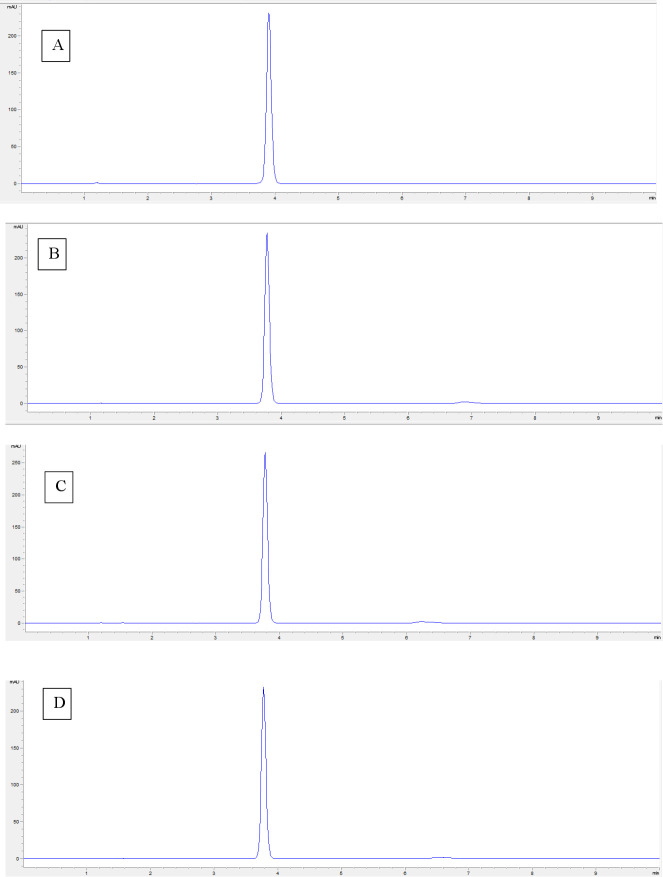
Chromatograms obtained after 72 hours of stress conditions are shown in figure 1. These results prove the method as stability-indicating, since complete separation of the degradation products was observed from the resorcinol peak. The most destabilising condition observed was oxidative condition.
Figure 1.
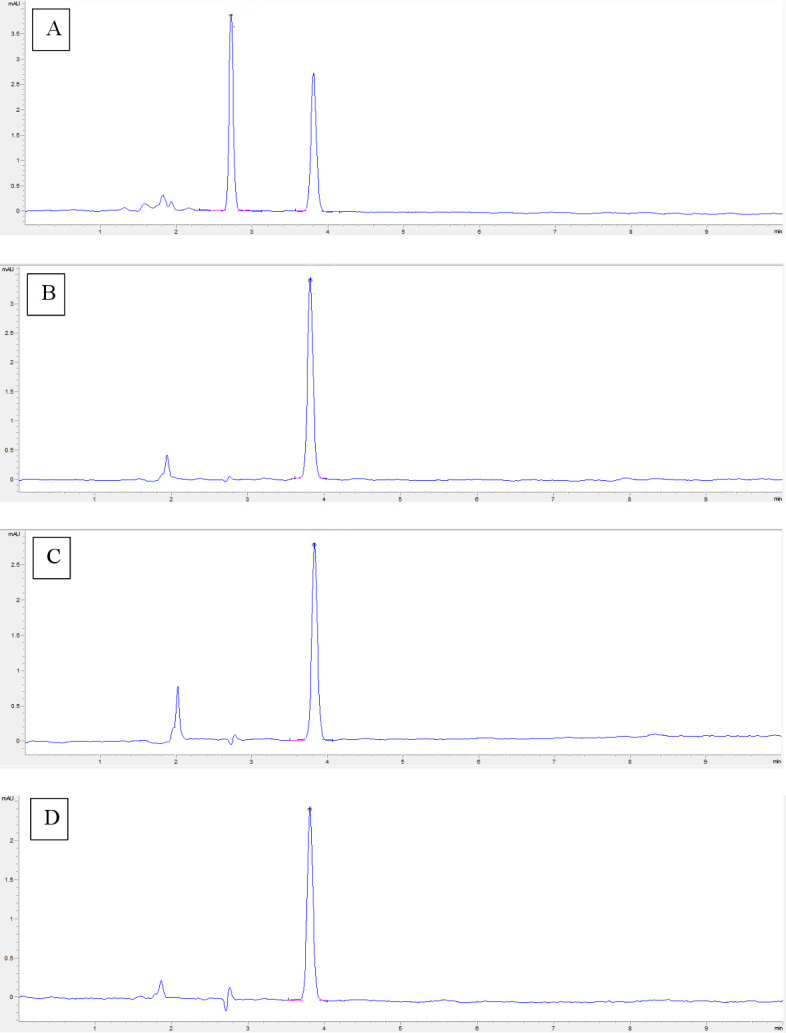
Chromatogram of 15% resorcinol formulation exposed for 72 hours to different stress conditions: (A) HCl 1N; (B) NaOH 1N; (C) H 2 O2 15%; and (D) 90°C.
Physicochemical stability
The results of visual inspections, pH and emulsion phase changes during 12 months of storage are summarised in table 1. On day 0 the preparation had a white/slightly orange colouration with no odour. This property remained unchanged to day 365 in the preparations packaged in aluminium tubes.
Table 1.
Visual inspection, emulsion phase and pH evolution of 15% resorcinol formulation packaged in aluminium tubes (AT), plastic tubes (PT) and plastic amber containers (PAC)
| Day 0 | Day 120 | Day 240 | Day 365 | |||||||
| AT | PT | PAC | AT | PT | PAC | AT | PT | PAC | ||
| Colour | White/ slightly orange | White/ slightly orange | White/ slightly orange | White/ slightly orange | White/ slightly orange | Orange | Orange | White/ slightly orange | Orange/brown | Brown |
| Odour | None | None | None | None | None | None | None | None | None | None |
| Emulsion phase | O/W | O/W | O/W | O/W | O/W | O/W | O/W | O/W | O/W | O/W |
| pH | 4.13±0.07 | 4.07±0.03 | 4.15±0.07 | 4.12±0.03 | 3.96±0.08 | 4.03±0.10 | 3.94±0.01 | 3.94±0.07 | 3.86±0.01 | 3.92±0.02 |
O/W, oil/water.
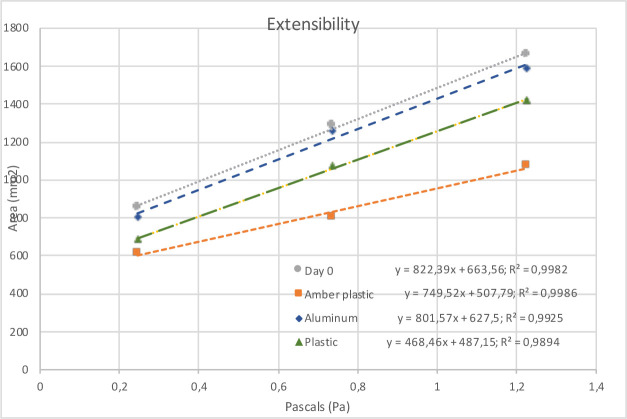
No gas formation or emulsion phase inversion was observed in any of the preparations. The formulation maintained an oil/water (O/W) sign on day 365. The pH did not vary by more than 0.3 units, which complies with the desired maximum variation of 1 unit. A decrease in extensibility was observed over time (figure 2).
Figure 2.
Extensibility curves for preparation at day 0, day 365 packaged in aluminium tubes, day 365 packaged in plastic tubes and day 365 packaged in plastic amber containers.
The HPLC analysis conducted over 1 year did not show a degradation of more than 7% of resorcinol in the preparation in plastic and aluminium packages (figure 3). Rather, an increase of more than 10% in the resorcinol concentration was observed in amber container packages starting from month 8 of the study. Chromatograms on days 0 and 365 (figure 4) did not show new peaks or degradation products. This can be confirmed by the findings of the stressed conditions discussed in the HPLC analysis.
Figure 3.
Changes in resorcinol concentration determined over 12 months for plastic tubes, aluminium tubes and amber plastic containers.
Figure 4.
Chromatogram of 15% resorcinol formulation diluted in mobile phase to 150 µg/mL at (A) day 0, (B) day 365 in aluminium packaging, (C) day 365 in plastic packaging and (D) day 365 in plastic amber packaging.
After analysing the three-dimensional spectra, no degradation products were observed in the entire ultraviolet spectrum at day 365 (online supplemental figure 1). There were no compounds that absorb in the same retention time as resorcinol.
ejhpharm-2020-002534supp002.pdf (294.5KB, pdf)
Microbiological stability
The ability of ATCC strains to grow in the resorcinol formulation was confirmed under the suitability test conditions defined. There was a mean growth of 17×104 cfu/mL for S. aureus and 11×104 cfu/mL for P. aeruginosa in TSA. Mean growth for A. brasilensis and C. albicans was less significant with 1×104 cfu/mL and 2×104 cfu/mL detected, respectively. The need to increase the dilution of resorcinol from 1:100 to 1:1000 in the suitability test shows the antiseptic effect of resorcinol. This effect favours microbiological stability by inhibiting bacterial growth.
Topical resorcinol packed in aluminium tubes achieved microbiological stability at day 365 under the storage conditions for aluminium tubes according to the Ph. Eur. In opened and closed tubes, the absence of the specified microorganisms S. aureus and P. aeruginosa was certified under the acceptance criteria for colony counts for aerobic and anaerobic microorganisms.
Discussion
Given the lack of commercially available topical 15% resorcinol, compounding is the only way to ensure availability of this treatment for patients with HS. Shelf-life evaluation allows storage of the formulation in optimal conditions in order to provide it quickly to ambulatory patients. According to the formulation (online supplemental table 1), we include sodium metabisulfite to reduce oxidation. Despite the high solubility of resorcinol in water (1.23 g/mL), an O/W cream was selected instead of a W/O. Indeed, a W/O emulsion could protect resorcinol against oxidation and facilitate microbiological stability. However, O/W cream was used because the high affinity of resorcinol for fatty bases (log POW 0.79–0.93) can interact with the external oil phase, interfering with or delaying absorption.
ejhpharm-2020-002534supp003.pdf (296.5KB, pdf)
The accelerated degradation study was based on the paper by Ngwa.20 Stronger conditions were used instead of NaOH 0.1N, HCl 0.1N, H2O2 3% and 60°C in order to reduce the end time to 72 hours. We did not set a target resolution to define complete separation. It shows that the oxidative condition is the main degradation pathway of resorcinol, in accordance with the resorcinol data sheet.21 The only package condition that remained physiochemically stable throughout the 365 days of the study at room temperature (25°C±2°C) was the aluminium packaging. A change in colour was noticed in the plastic and amber plastic container packages during storage for 6 months, and the preparations in these containers continued to become increasingly darker in colour over time up to 1 year. In amber plastic containers the colour became brown. The change in colour described can be explained by the oxidation effect in the fatty acids of Lanette cream. This change in colour does not seem to be related to a decrease in resorcinol concentration or any microbiological growth.
The rheology of the compound could not be determined by a viscometer. The extensibility index was used to approximate changes in viscosity during storage. The decrease in the extensibility index was found to be greater for the preparations packaged in plastic amber containers. Compared with the extensibility recorded by the cream on day 0, a 43% decrease was observed in the slope of the extensibility curve at day 365 (figure 2). On the other hand, the decrease in the extensibility of the formulation in the aluminium tubes and plastic tubes was much lower, with a variation of less than 2.5% and 8.8%, respectively.
In fact, the greater reduction in the extensibility found in amber plastic packaging was related to an increase in viscosity. This decrease in extensibility can be explained by evaporation. As a consequence of this reduction, absorption can be affected. Furthermore, application can be difficult and the preparation can feel more unpleasant to touch, which might negatively interfere with the patient’s adherence to the treatment. The increase in the resorcinol concentration observed and the lack of degradation products support the evaporation explanation. Also, due to their shape and wider base, amber plastic containers generate a wider cream-to-air contact surface. Meanwhile, as cream comes out of the aluminium tube, the packaging gets deformed and takes up the space left by the cream, allowing for a smaller cream-to-air contact surface compared with the amber container.
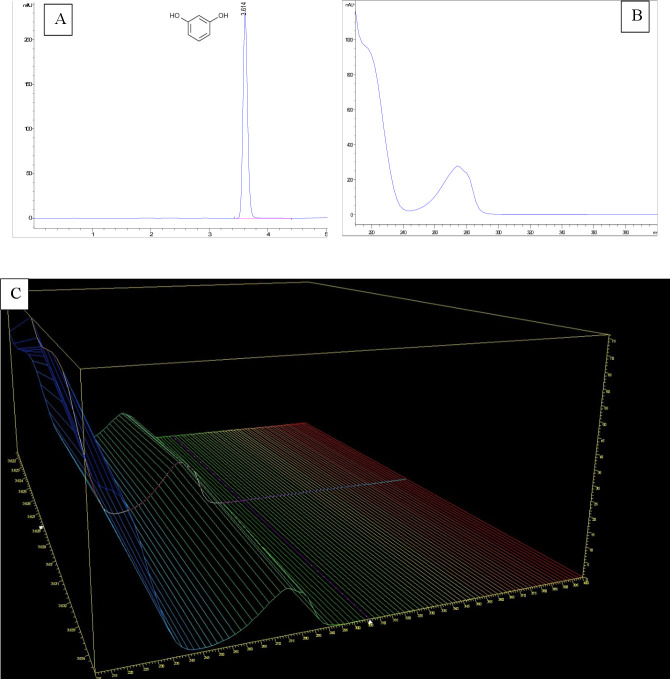
The specificity of the HPLC method proposed is demonstrated by the peak purity and the two-dimensional and three-dimensional spectral analysis (figure 5) in which a maximum absorption band at 280 nm matched with the wavelength set for analysis. The analytical characteristics obtained were compared with other chromatographic methods published such as that of De et al.17 It seems that the use of a combination of methanol:water instead of acetonitrile:water offers better results with a 71% increase in theoretical plates, a 89% decrease in the tailing factor and a similar %RSD. In addition, methanol:water reduces the cost of the analysis and the potential risk of toxicity by eliminating exposure to acetonitrile.
Figure 5.
(A) Typical chromatogram of 150 µg/mL resorcinol. (B) Two-dimensional and (C) three-dimensional ultraviolet spectral analysis of resorcinol in the 200–400 nm range.
Conclusion
In this study we developed a formula and performed a standard operation procedure for topical 15% resorcinol compounding. The physicochemical and microbiological analysis showed stability of the proposed topical 15% resorcinol formula for a period of 12 months, packaged in aluminium A7-99.7% varnish DF-6172 and stored at 25°C±2°C. Furthermore, an isocratic method of HPLC analysis for quality control of resorcinol topical formulation has been successfully developed. This method is fast, precise, specific and was developed and validated according to the ICH Q2(R1) guidelines.
What this paper adds.
What is already known on this subject
Topical resorcinol 15% is a self-treatment for mild to moderate hidradenitis suppurativa nodules and abscesses with good results in reducing pain and lesion duration.
Topical resorcinol for suppurative hidradenitis is not yet available commercially. It can be compounded at 15% concentration in an oil/water emulsion.
There is no assay for the routine control of topical resorcinol formulations suitable for hospital pharmacies.
What this study adds
The proposed formula of 15% topical resorcinol adding metabisulfite as an antioxidant is physicochemically and microbiologically stable when packed in aluminum tubes for 12 months.
An isocratic HPLC method was developed and validated for the routine control of topical resorcinol formulations and HPLC stability-indicating method.
Acknowledgments
We thank Antonio Gajardo González and Cynthia Adaimé for assistance and for making possible the publication of this work.
Footnotes
Contributors: Design of the work: JC-R, VM-B, MD-V, MAC. Data collection: RB-T, JC-R, VM-B, MD-V. Data analysis and interpretation: JC-R, VM-B, MD-V, MC-F. Drafting the article: JC-R, RB-T. Critical revision of the article: VM-B, JC-R, RB-T, MC-F, MD-V, MAC. Final approval of the version to be published: MAC.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Fimmel S, Zouboulis CC. Comorbidities of hidradenitis suppurativa (acne inversa). Dermatoendocrinol 2010;2:9–16. 10.4161/derm.2.1.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Revuz JE, Jemec GBE. Diagnosing hidradenitis suppurativa. Dermatol Clin 2016;34:1–5. 10.1016/j.det.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 3. Martorell A, García-Martínez FJ, Jiménez-Gallo D, et al. An update on hidradenitis suppurativa (Part I): epidemiology, clinical aspects, and definition of disease severity. Actas Dermosifiliogr 2015;106:703–15. 10.1016/j.ad.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 4. Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015;29:619–44. 10.1111/jdv.12966 [DOI] [PubMed] [Google Scholar]
- 5. Napolitano M, Megna M, Timoshchuk E, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment 2017;10:105–15. 10.2147/CCID.S111019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol 2013;68:412–9. 10.1016/j.jaad.2012.07.027 [DOI] [PubMed] [Google Scholar]
- 7. Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life and psychosocial implications in patients with hidradenitis suppurativa. Dermatology 2016;232:687–91. 10.1159/000453355 [DOI] [PubMed] [Google Scholar]
- 8. Alavi A, Farzanfar D, Rogalska T, et al. Quality of life and sexual health in patients with hidradenitis suppurativa. Int J Womens Dermatol 2018;4:74–9. 10.1016/j.ijwd.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. García-Martínez FJ, Pascual JC, López-Martín I, et al. Actualización en hidrosadenitis supurativa en Atención Primaria. SEMERGEN - Medicina de Familia 2017;43:34–42. 10.1016/j.semerg.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 10. Ring HC, Emtestam L. The microbiology of hidradenitis suppurativa. Dermatol Clin 2016;34:29–35. 10.1016/j.det.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 11. Martorell A, García FJ, Jiménez-Gallo D, et al. Actualización en hidradenitis supurativa (II): aspectos terapéuticos. Actas Dermo-Sifiliográficas 2015;106:716–24. 10.1016/j.ad.2015.06.005 [DOI] [PubMed] [Google Scholar]
- 12. Ardon CB, Prens EP, Fuursted K, et al. Biofilm production and antibiotic susceptibility of Staphylococcus epidermidis strains from hidradenitis suppurativa lesions. J Eur Acad Dermatol Venereol 2019;33:170–7. 10.1111/jdv.15183 [DOI] [PubMed] [Google Scholar]
- 13. Boer J, Jemec GBE. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol 2010;35:36–40. 10.1111/j.1365-2230.2009.03377.x [DOI] [PubMed] [Google Scholar]
- 14. Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: an uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol 2017;77:1175–8. 10.1016/j.jaad.2017.07.008 [DOI] [PubMed] [Google Scholar]
- 15. Pharmacopoeia E . European Pharmacopoeia. 8th Edition, 2013. www.edqm.eu [Google Scholar]
- 16. European Medicines Agency . ICH topic Q2 (R1) validation of analytical procedures: text and methodology. Prescrire Int 1995;20:278. [Google Scholar]
- 17. De AK, Chowdhury PP, Chattapadhyay S. Quantitative analysis of resorcinol from marketed hair tonic using liquid chromatographic technique. Int Sch Res Notices 2014;2014:1–5. 10.1155/2014/632591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y-H, Avonto C, Avula B, et al. Quantitative determination of α-arbutin, β-arbutin, kojic acid, nicotinamide, hydroquinone, resorcinol, 4-methoxyphenol, 4-ethoxyphenol, and ascorbic acid from skin whitening products by HPLC-UV. J AOAC Int 2015;98:5–12. 10.5740/jaoacint.14-123 [DOI] [PubMed] [Google Scholar]
- 19. Escribano Romero B, Tarno Fernández L, Alcaraz Tomás J, et al. Determinación de extensibilidad. In: Formulario Nacional. Madrid: Ministerio de Sanidad, Consumo y Bienestar Social, 2019: 202–5. [Google Scholar]
- 20. Ngwa G. Forced degradation as an integral part of HPLC stability-indicating method development. Drug Deliv Technol 2010;10:56–9. [Google Scholar]
- 21. National Center for Biotechnology Information . PubChem database. Resorcinol, 2020. Available: https://pubchem.ncbi.nlm.nih.gov/compound/5054 [Accessed 5 Jun 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2020-002534supp001.pdf (35.8KB, pdf)
ejhpharm-2020-002534supp002.pdf (294.5KB, pdf)
ejhpharm-2020-002534supp003.pdf (296.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request.