Abstract
Public health vaccination recommendations for COVID-19 primary series and boosters in previously infected individuals differ worldwide. As infection with SARS-CoV-2 is often asymptomatic, it remains to be determined if vaccine immunogenicity is comparable in all previously infected subjects. This study presents detailed immunological evidence to clarify the requirements for one- or two-dose primary vaccination series for naturally primed individuals. The main objective was to evaluate the immune response to COVID-19 mRNA vaccination to establish the most appropriate vaccination regimen to induce robust immune responses in individuals with prior SARS-CoV-2 infection. The main outcome measure was a functional immunity score (zero to three) before and after vaccination, based on anti-RBD IgG levels, serum capacity to neutralize live virus and IFN-γ secretion capacity in response to SARS-CoV-2 peptide pools. One point was attributed for each of these three functional assays with response above the positivity threshold. The immunity score was compared based on subjects’ symptoms at diagnosis and/or serostatus prior to vaccination. None of the naïve participants (n=14) showed a maximal immunity score of three following one dose of vaccine compared to 84% of the previously infected participants (n=55). All recovered individuals who did not have an immunity score of three were seronegative prior to vaccination, and 67% had not reported symptoms resulting from their initial infection. Following one dose of vaccine, their immune responses were comparable to naïve individuals, with significantly weaker responses than individuals who were symptomatic during infection. These results indicate that the absence of symptoms during initial infection and negative serostatus prior to vaccination predict the strength of immune responses to COVID-19 mRNA vaccine. Altogether, these findings highlight the importance of administering the complete two-dose primary regimen and following boosters of mRNA vaccines to individuals who experienced asymptomatic SARS-CoV-2 infection.
Keywords: SARS-CoV-2, COVID-19, mRNA vaccination, hybrid immunity, adaptive immune response
Introduction
As of March 2022, more than 450 million people have been infected by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), leading to over six million deaths from Coronavirus Infectious Disease 2019 (COVID-19) (1–3). The range of symptoms associated with SARS-CoV-2 infection is highly diverse, and the scale of symptoms spans from asymptomatic to severe (4). Public health vaccination guidelines of adults who have recovered from SARS-CoV-2 infection differ depending on the country. In Canada and the United States, the National Advisory Committee on Immunization (NACI) and the Centers for Disease Control and Prevention (CDC) recommend a two-dose primary regimen for every adult (5–8), regardless of previous infection status, while many countries such as France, Germany, Italy, and ten other countries from the European Union consider that infection with SARS-CoV-2 is equivalent to one vaccine dose (9). It remains to be established if vaccine requirements for previously infected subjects differ from those for naïve individuals.
Up to one year after their infection, recovered individuals present strong and slowly decaying humoral and cellular responses (10–13). Importantly, it was suggested that the strength of the immune response following natural infection is proportional to the disease severity (14–16). In previously infected individuals, one vaccine dose a few months after infection acts as a booster of immune responses (17, 18). It elicits much stronger B- and T-cell-specific responses in previously infected individuals, compared to naïve individuals, with minimal benefits observed when a second dose is given 21 days after the first one (10, 11, 18–22). However, it is unknown if individuals who were previously infected, but did not experience symptoms, show similar heightened immune responses after a single mRNA vaccine dose. We hypothesized that the symptom severity during natural infection impacts the response to vaccination and that individuals who did not experience symptoms during infection may present suboptimal protection after one dose of vaccine.
In this report, we used three biomarkers of immunity to characterize an effective immune response to SARS-CoV-2. We assessed (1) circulating anti-RBD IgG levels, (2) functional serum capacity to neutralize live SARS-CoV-2 virus and (3) IFN-γ secretion by memory T cells in response to mega pools of SARS-CoV-2 peptides in a cohort of naïve and previously infected individuals before and after COVID-19 mRNA vaccination. We compared the impact of symptoms at the time of primary infection and serology at enrollment (prior to vaccination) on the immune responses to one and two vaccine doses.
Methods
Study participants
Our cohort consisted of 55 previously infected health care workers (HCWs) who were recruited following a PCR-confirmed SARS-CoV-2 infection as part of the RECOVER study (n=569) (23), and 14 naïve HCWs with neither history of SARS-CoV-2 infection nor the presence of anti-N or anti-RBD antibodies against SARS-CoV-2 prior to vaccination with BNT162b2 mRNA vaccine (Pfizer-BioNTech, 30 μg per dose). PCR screening was not part of the study itself, and infections were therefore detected through provincial and institutional PCR screening policies, including following exposure to a confirmed SARS-CoV-2 infection case. Participants included in this study were selected based on their reported symptomatology at the time of infection and their serostatus at enrollment in the study. All RECOVER subjects who were asymptomatic (n=28/569) and for whom samples were available before and after vaccination were included (n=19). Participants who were symptomatic during infection (n=26) and for whom samples were available before and after each dose of vaccine were selected from the rest of the RECOVER cohort based on their serostatus at enrollment after being matched with participants from the asymptomatic group for sex, age, ethnicity, time since infection, time between infection and vaccination as well as time between vaccination and each sample available. Characteristics of the study population are shown in ( Table 1 in Supplemental 1 ). The severity of the initial SARS-CoV-2 infection was determined using the WHO clinical progression scale (4). Blood samples for humoral and cellular immunity were collected at enrollment and at different time points after vaccination as described in Figure S1 in Supplemental 1 . Participants were recruited from August 17, 2020, to April 8, 2021, and followed for one year after enrollment.
Sample collection and processing
Blood samples were collected into serum separation tubes (SST™, BD) and acid–citrate–dextrose tubes (ACD, BD). They were shipped to the Rare Pediatric Disease (RaPID) biobank at the Sainte-Justine University Hospital Research Center, where serum and peripheral blood mononuclear cells (PBMCs) were isolated according to standard operation procedures (SOPs) using SepMate™ tubes (Stemcell Technologies, Canada) for PBMCs isolation. Serum was cryopreserved at -80°C, and PBMCs were cryopreserved in complete RPMI (Gibco) with 10% DMSO and stored in liquid nitrogen until used.
Exposures and outcome
Exposure was defined as vaccination with Pfizer BioNTech BNT162b2 mRNA vaccine. Vaccine administration was not part of the study itself, as participants received their vaccines through routine public health programs. The primary outcome was the multi-functionality of the immune response developed after each vaccine dose, represented as an immunity score from zero to three. One point was attributed for each of the following immune assays above positive threshold: anti-RBD IgG levels by ELISA, plasma capacity to neutralize live SARS-CoV-2 (ancestral strain) by micro-neutralization assay, and IFN-γ secretion by PBMCs in response to SARS-CoV-2 mega pool of Spike peptides, determined by ELISpot.
Enzyme-linked immunosorbent assay
An ELISA was performed on serum samples to detect specific IgG antibodies for the Receptor Binding Domain (anti-RBD) of SARS-CoV-2 Spike Glycoprotein as previously described (24, 25). Seropositivity was defined as an OD490 ratio greater than one with the experimentally determined cut-off, which corresponds to the mean of 10 negative controls plus three times the standard deviation of the negative controls for each experiment. This anti-RBD IgG ELISA was validated on the Canadian National Microbiology Laboratory SARS-CoV-2 National Serology Panel (23, 26). Absence of previous infection to SARS-CoV-2 was confirmed in the naïve cohort by performing an ELISA detecting specific IgG antibodies for the Nucleoprotein (anti-N) of SARS-CoV-2 and the anti-RBD ELISA before vaccination.
Micro-neutralization assay
Neutralizing antibody titers were assessed using SARS-CoV-2/Québec City/21697/2020 strain (ancestral Wuhan-1 like SARS-CoV-2), isolated from a clinical sample in March 2020 in Québec City, Canada. Two-fold dilutions of heat-inactivated serum were prepared, starting with 1:20 dilution. Equal volumes of serum and virus were mixed and incubated for one hour at room temperature. The residual infectivity of those mixtures was assessed in quadruplicate wells of African green monkey kidney E6 cell line (Vero ATCC® CRL-1586™). Neutralizing antibody titers were defined as the reciprocal of the serum dilution that completely neutralized the infectivity of 100 TCID50 of SARS-CoV-2, determined by the absence of cytopathic effect on cells after four days (27). The neutralizing antibody titer is calculated using the Reed/Muench method (28). These studies were performed in the Containment Level 3 (CL3) laboratory at the Centre Hospitalier Universitaire de Québec - Université Laval Research Center.
Enzyme-linked immunospot assay
Cell-mediated immune (CMI) response was estimated by ELISpot. Frozen PBMCs were rapidly thawed and rested overnight. They were then stimulated with 1 µg/mL of spike glycoprotein (S), nucleocapsid protein (NCAP) or membrane protein (VME1) mega pools of SARS-CoV-2 peptides from the ancestral Wuhan-1 like strain (JPT Peptide Technologies, JPT) as described elsewhere (29). Spots were revealed using BIO-RAD Alkaline Phosphatase Conjugate Substrate Kit. The resulting ELISpots were analyzed using CTL ImmunoSpot® S5 UV Analyzer (Cellular Technology Ltd, OH). Culture media and phytohemagglutinin (PHA, Sigma) were used as negative and positive controls, respectively. The positive threshold response was defined as more than 25 spot-forming units (SFU) per million cells.
Activation induced markers assay
Thawed and rested PBMCs were stimulated for 20 hours with 1 µg/mL of the CD4-Spike peptides mega pool developed and synthesized by the Sette laboratory (30, 31). Constituted of 246 HLA-class II restricted 15-mers spanning the whole Spike Glycoprotein, the CD4-Spike mega pool stimulates both CD4 and CD8 T cells. Cells were then stained with panels of fluorescent monoclonal antibodies ( Table 3 in Supplemental 1 ). Data were acquired with an LSR FORTESSA II with High Throughput Sampler (HTS) from BD Biosciences ( Figure S2 in Supplemental 1 for gating strategy). FlowJo software, version 10.7.1, was used to perform all data analysis. Fluorescence Minus One (FMO), unstimulated (negative control) and PMA-Ionomycine (positive control) conditions were used to set the gates for each participant. Results are represented by the frequency of activated cells following peptide stimulation minus the frequency of activated cells in the unstimulated condition.
Statistics
All statistical tests were performed using Prism 9, version 9.2.0 (2021 GraphPad Software, LLC) and data were displayed as median [IQR, 25th-75th percentile]. Mann-Whitney or Kruskal-Wallis unpaired nonparametric tests were used to evaluate statistical significance between groups for variables that did not follow a normal distribution. Simple linear regression is presented with Pearson correlation (R2) and P-value (p). Significance was set as *P < .05; **P < .01; ***P < .001; ****P < .0001.
Ethics
RECOVER protocols were approved by the Research Ethics Board (REB) at the Sainte-Justine University Hospital and Research Center under study MP-21-2021-3035 and in each of the five participating centers in the Province of Québec. Written informed consent was obtained from all participants during the recruitment period, and ongoing consent was reviewed at each subsequent visit.
Results
Participants characteristics and natural immunity to SARS-CoV-2 infection
The RECOVER cohort consists of 569 HCWs infected with SARS-CoV-2 during the first and second pandemic waves in the province of Québec, Canada (23). Based on surveillance data from public health authorities, most participants enrolled in the study were infected when the original Wuhan-1-like strain was in circulation (23). Some HCWs enrolled were infected at the beginning of 2021, when both the original strain and the Alpha variant were in circulation in our province. Overall, 95% of participants had symptoms at the time of diagnosis (n=541), and very few were asymptomatic (n=28). Among asymptomatic subjects, only 43% were seropositive at enrollment, in contrast to 77% of symptomatic individuals ( Figure S3A in Supplemental 1 ). The presence of symptoms at the time of initial infection correlated with more robust anti-RBD IgG levels (median [IQR, 25th-75th percentile], 1.5 [1.0-2.2] vs. 0.7 [0.5-1.5], P<.0001) in contrast to subjects who did not experience symptoms. Further, antecedent infection led to long-lasting humoral immune responses in most HCWs (up to 12 months post-infection; Figure S3B in Supplemental 1 ). Disease severity, assessed from 1 to 5 using the WHO clinical progression score (4), increased the strength of this humoral response in HCWs ( Figure S3C in Supplemental 1 ). Cell-mediated immune responses to the major components of SARS-CoV-2 were also assessed in almost half of the RECOVER cohort (n=200) by ELISpot, prior to any vaccination. Responses to Spike, NCAP and VME1 were present and maintained up to one year after natural infection in the majority of unvaccinated participants (84%, 80% and 72% respectively), with the most potent response being specific for Spike antigens (85.0 [45.0-177.5] SFU/106 PBMCs, compared to 65.0 [30.0-110.0] and 55.0 [20.0-115.0] SFU/106 PBMCs for NCAP and VME1) ( Figure S4 in Supplemental 1 ). From this cohort, we selected a subset of symptomatic and asymptomatic individuals (n=55) to study the impact of previous infection on the immune response to vaccination. Of that subset, 93% (51/55) were infected when only the Wuhan-1-like strain was circulating. All subjects received the BNT162b2 Pfizer BioNTech COVID-19 vaccine, with a first dose administered (mean time ± SD) 8.4 ± 2.3 months (range: 0.8-13.1 months) after infection ( Table 2 in Supplemental 1 ). The second dose was offered 16 ± 6 weeks after the first dose. Samples were collected before vaccination, around 45 ± 25 days and 57 ± 30 days after the first and second dose, respectively. They were compared to 14 previously naïve individuals before vaccination, 29 ± 2 days after the first vaccine dose and 35 ± 5 days after the second dose.
One vaccine dose strongly boosts humoral and cellular responses in recovered individuals
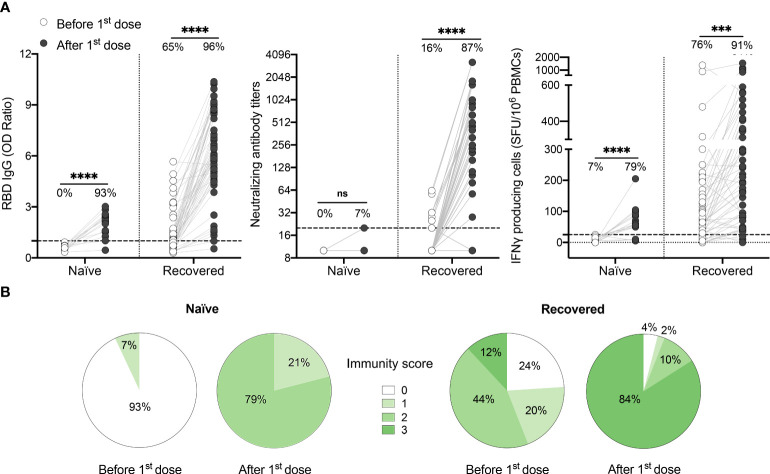
Prior to vaccination, most recovered HCWs presented detectable anti-RBD IgG levels (1.1 [0.6-1.7]) and IFN-γ responses to Spike antigens (70.0 [25.0-155.0] SFU/106 PBMCs), while serum neutralization capability was mainly absent or low. Following one vaccine dose, recovered individuals demonstrated a striking increase in their anti-RBD IgG levels, neutralization capacity and cell-mediated immune response (1.1 [0.6-1.7] to 6.1 [4.8-8.7], P<.0001, 10.0 [10.0-10.0] to 254.0 [113.0-640.0] P<.0001, 70.0 [25.0-155.0] to 195.0 [70.0-360.0] SFU/106 PBMCs, P<.001) ( Figure 1A ). In contrast, only one naïve individual showed detectable neutralization titers following the first vaccine dose. In naïve subjects, the breadth of humoral and cellular immune response was also significantly reduced compared to previously infected subjects, with reduced IgG levels (2.2 [1.5-2.5]) and a lower number of IFN-γ secreting cells (60.0 [40.0-92.5] SFU/106 PBMCs) compared to recovered HCWs (6.1 [4.8-8.7] IgG OD ratio and 195.0 [70.0-360.0] SFU/106 PBMCs respectively; P<.0001 and P=.003) ( Figure S5 in Supplemental 1 ).
Figure 1.
One dose of COVID-19 mRNA-vaccine strongly boosts SARS-CoV-2-specific humoral and cellular responses in most HCWs who recovered from SARS-CoV-2 infection. (A) SARS-CoV-2 Spike RBD–specific binding IgG levels assessed by ELISA (left panel), SARS-CoV-2 neutralizing antibody titers (middle panel), IFN-γ secreting cells per million in response to SARS-CoV-2 Spike glycoprotein peptides (right panel) assessed by ELISpot for naïve (n=14) and recovered HCWs (n=55) before (white circles) and after one dose of vaccine (black circles). Dashed black lines indicate the positive threshold value, and the percentage of individuals with responses above positive threshold value is indicated for each time point. Statistical significance was assessed by Mann-Whitney tests. Not significant (ns) P >.05; ***P <.001; ****P <.0001. (B) Pie charts of calculated immunity score for naïve and recovered HCWs before and after one dose of vaccine.
It has been demonstrated that a well-coordinated humoral and cellular immune response, with the development of both antibody neutralizing capacity and cell-mediated immune responses, protects against severe disease (14, 15). In previously infected HCWs vaccinated with one dose, we observed that the strength of the cellular response, as assessed by ELISpot assay, correlates positively with both RBD-binding IgG and SARS-CoV-2 neutralizing antibody titers ( Figure S6 in Supplemental 1 ), with the strongest correlation being between IFN-γ response and neutralization capacity. This suggests that vaccine-induced immune protection relies on a synchronized adaptive immune response (32, 33). To better depict the global functionality of the immune response of each subject, we calculated an immunity score based on the presence or absence of RBD-binding antibodies, SARS-CoV-2 neutralizing antibodies and CMI response for each participant. We showed that none of the naïve individuals had a global immunity score of three after one dose of vaccine ( Figure 1B ). In contrast, 84% of the previously infected individuals reached the maximal immunity score, the remainder showing an incomplete response and 6% demonstrating nearly no response ( Figure 1B ).
Symptomatology at the time of infection impacts the immune response to one vaccine dose
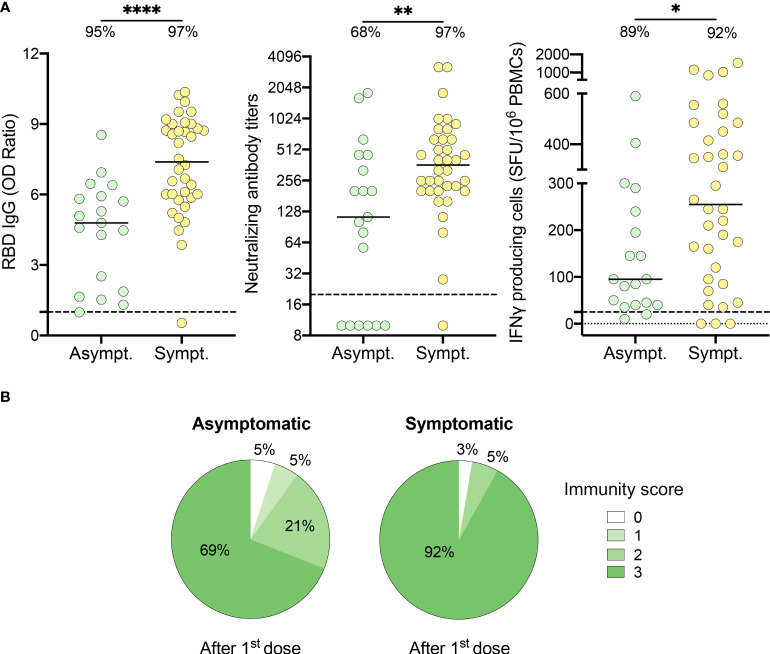
As 16% of recovered participants had an incomplete immune response after one dose of vaccine, we questioned if the presence of symptoms at the time of infection influenced the immune response to vaccination. Compared to symptomatic subjects, individuals who remained asymptomatic during their initial infection exhibited reduced anti-RBD IgG levels (7.4 [5.9-9.0] vs. 4.8 [1.9-5.9], P<.0001), neutralizing antibody titers (361.5 [202.0-640.0] vs. 113.0 [10.0-453.0], P=.007) and IFN-γ secreting cells (255.0 [101.3-476.3] vs. 95.0 [40.0-240.0] SFU/106 PBMCs, P=.02) ( Figure 2A ). Importantly, asymptomatic individuals were less likely to neutralize SARS-CoV-2 after one dose of vaccine compared to symptomatic subjects (68% vs. 97%, P=.007). Furthermore, 92% of symptomatic individuals showed a multi-functional immune response with an immunity score of three after their first dose of vaccine compared to 69% of asymptomatic individuals ( Figure 2B ).
Figure 2.
HCWs who recovered from an asymptomatic SARS-CoV-2 infection demonstrate partial immune response after one dose of vaccine. (A) SARS-CoV-2 Spike RBD–specific binding IgG levels assessed by ELISA (left panel), SARS-CoV-2 neutralizing antibody titers (middle panel), IFN-γ secreting cells per million in response to SARS-CoV-2 Spike glycoprotein peptides (right panel) assessed by ELISpot for asymptomatic (Asympt.) (n=19) and symptomatic (Sympt.) (n=36) recovered HCWs after one dose of vaccine. Dashed black lines indicate the positive threshold value, and the percentage of HCWs with responses above positive threshold value is indicated for each assay. Horizontal bars indicate the median of each group. Statistical significance was assessed by Mann-Whitney tests. *P <.05; **P <.01; ****P <.0001. (B) Pie charts of calculated immunity score for asymptomatic (left panel) and symptomatic (right panel) recovered HCWs after one dose of vaccine.
Serology status six months after infection influences the strength of immune responses following one vaccine dose
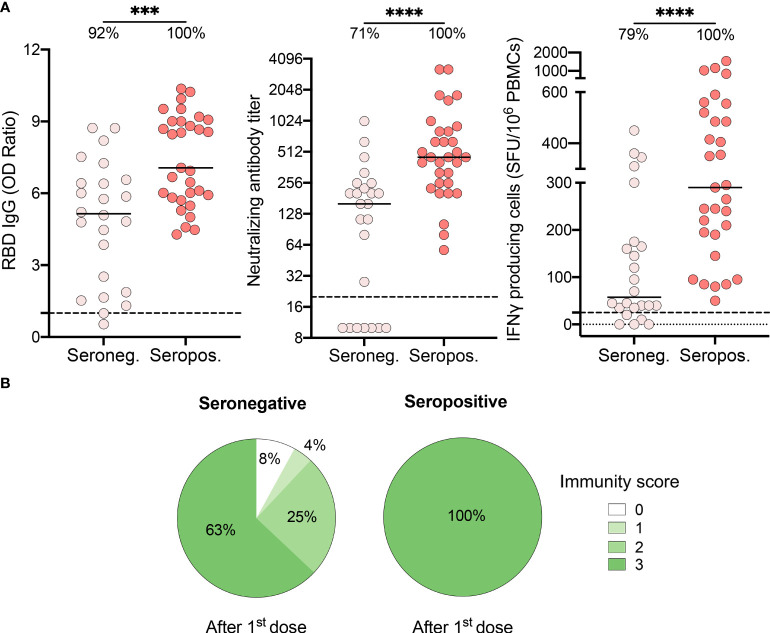
In our RECOVER cohort, 24.6% (n = 140) of recovered HCWs were seronegative at enrollment in the study (23). We thus questioned if serostatus at enrollment, 3.0 ± 1.5 months prior to vaccination, could predict the immune response to the vaccine. Compared to seropositive individuals, seronegative subjects presented reduced levels of IgG (7.1 [5.8-9.0] vs. 5.2 [2.0-6.5], P=.0003), neutralizing antibodies (453.0 [254.0-806.0] vs. 160.0 [10.0-247.0], P<.0001), and IFN-γ producing cells (290.0 [190.0-520.0] vs. 57.5 [35.0-172.5], P<.0001) after one dose of vaccine ( Figure 3A ). Importantly, subjects who did not mount a positive humoral and/or cellular response after one dose of vaccine were all seronegative before vaccination. One dose of vaccine was only sufficient to boost immunity to SARS-CoV-2 in 63% of seronegative participants ( Figure 3B ). The others showed reduced capability to mount adequate neutralization of the virus and/or cell-mediated immune response, and two subjects remained nonresponsive.
Figure 3.
Recovered HCWs who were seronegative prior to vaccination showed suboptimal immune responses after one vaccine dose. (A) SARS-CoV-2 Spike RBD–specific binding IgG levels assessed by ELISA (left panel), SARS-CoV-2 neutralizing antibody titers (middle panel), IFN-γ secreting cells per million in response to SARS-CoV-2 Spike glycoprotein peptides (right panel) assessed by ELISpot for seronegative (Seroneg.) (n=24) and seropositive (Seropos.) (n=31) recovered HCWs after one dose of vaccine. Dashed black lines indicate the positive threshold value, and the percentage of HCWs with responses above positive threshold value is indicated for each condition. Horizontal bars indicate the median of each group. Statistical significance was assessed by Mann-Whitney tests. ***P <.001; ****P <.0001. (B) Pie charts of calculated immunity score for seronegative (left panel) and seropositive (right panel) recovered HCWs after one dose of vaccine.
Combined effect of symptomatology and serostatus on the immunogenicity of the vaccine
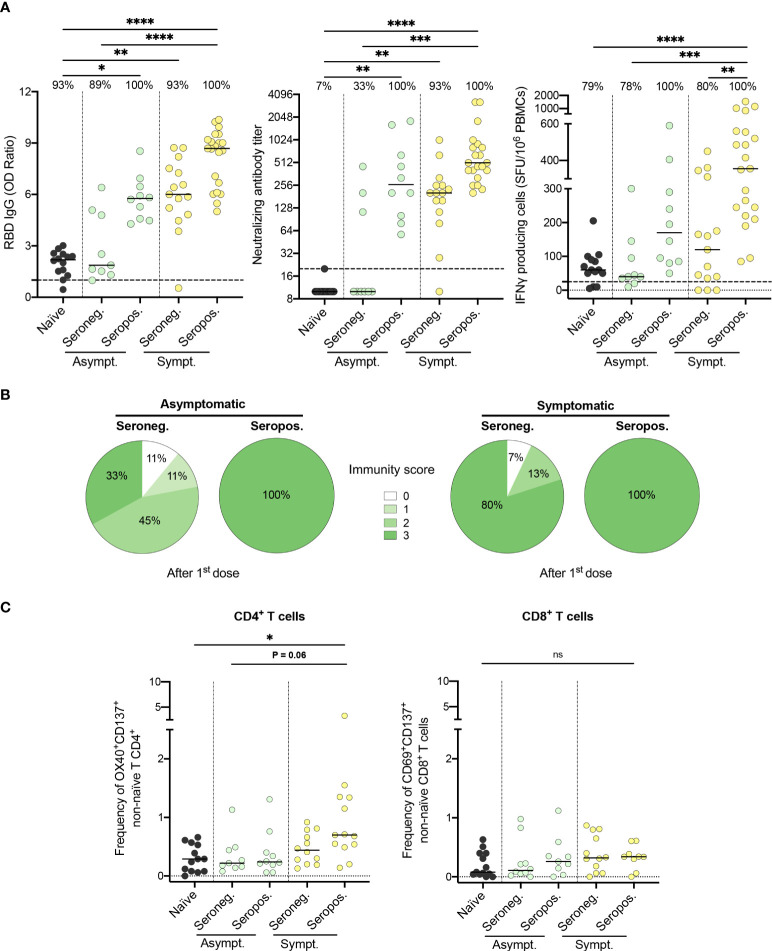
Most previously infected HCWs who did not develop an immunity score of three after the first dose of vaccine had an asymptomatic SARS-CoV-2 infection (6 out of 9; Figure 2B ). To better depict the impact of seropositivity and symptomatology on the immune response to one dose of vaccine, we compared levels of induced immunity within each subgroup. Symptomatic individuals who remained seropositive following natural infection developed the most vigorous humoral and cellular immune response to one dose of vaccine ( Figure 4A, B ). Furthermore, their frequency of SARS-CoV-2 specific CD4+ T cells, as assessed by the expression of activation markers, was the highest (0.7 [0.5-1.3] vs. 0.3 [0.1-0.6], P=.02) for naïve subjects ( Figure 4C ). In contrast, asymptomatic individuals who were seronegative at enrollment had significantly lower anti-RBD IgG levels (1.9 [1.4-4.9] vs. 8.7 [6.4-9.4], P<.0001), neutralizing antibody titers (10.0 [10.0-157.5] vs. 508.0 [361.5-855.5], P<.001) as well as IFN-γ secreting cells (40.0 [27.5-120.0] vs. 355.0 [232.5-557.5] SFU/106 PBMCs, P<.001) following one dose of the vaccine, compared to seropositive symptomatic subjects. Notably, this subgroup of asymptomatic seronegative individuals was the only group to demonstrate an immune response following one dose of vaccine that was similar to naïve individuals. In fact, prior to vaccination, these individuals lacked immunological markers of previous antigen encounter, as did SARS-CoV-2 naïve subjects ( Figure S7 in Supplemental 1 ).
Figure 4.
Both symptomatology during infection and serostatus prior to vaccination influence the immunogenicity of the first vaccine dose. (A) SARS-CoV-2 Spike RBD–specific binding IgG levels assessed by ELISA (left panel), SARS-CoV-2 neutralizing antibody titers (middle panel), and IFN-γ secreting cells per million in response to SARS-CoV-2 Spike glycoprotein peptides assessed by ELISpot (right panel) for naïve (black circles), seronegative (Seroneg.) (n=24) and seropositive (Seropos.) (n=31) recovered HCWs after one dose of vaccine. Asymptomatic recovered HCWs (Asympt.) are represented with green circles (n=19) and symptomatic HCWs (Sympt.) with yellow circles (n=36). Dashed black lines indicate the positive threshold value, and the percentage of HCWs with responses above positive threshold value is indicated for each assay. (B) Pie charts of calculated immunity score for asymptomatic (left panel) and symptomatic (right panel) recovered HCWs after one dose of vaccine. (C) Frequency of SARS-CoV-2−Spike-specific T cells measured as percentage of OX40+CD137+ non-naïve CD4+ (left panel) and CD69+CD137+ non-naïve CD8+ T cells (right panel) after stimulation of PBMCs with CD4-S mega pool of peptides from the Spike glycoprotein for naïve (n=13), asymptomatic seronegative (n=9) or seropositive (n=10) and symptomatic seronegative (n=12) or seropositive (n=13) recovered HCWs after one dose of vaccine. Horizontal bars indicate the median of each group. Kruskal-Wallis tests assessed statistical significance (A-C). Not significant (ns) P >.05; *P < .05; **P <.01; ***P < .001; ****P < .0001.
A two-dose primary series of mRNA vaccine is required in previously infected individuals who did not experience symptoms
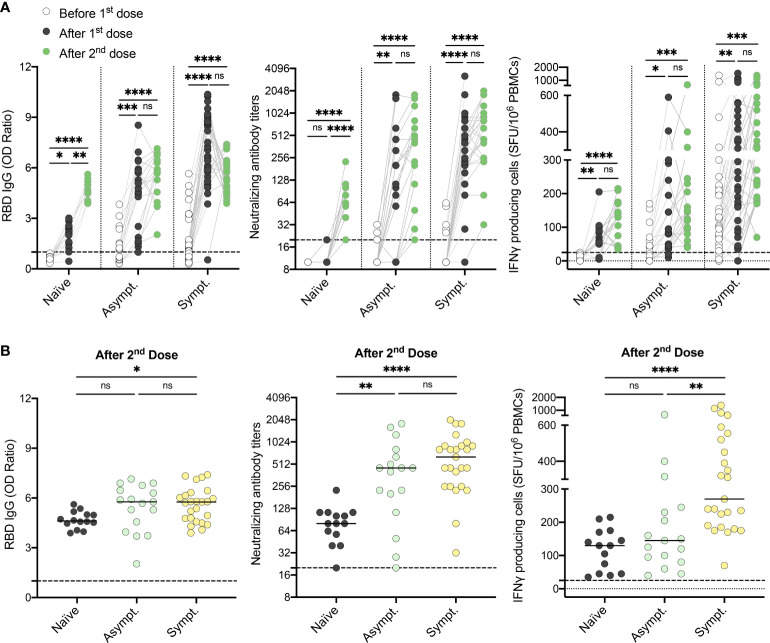
Among HCWs who recovered from SARS-CoV-2 infection, 16% did not demonstrate a well-coordinated humoral and cellular immune response after one vaccine dose ( Figure 1B ), with asymptomatic, seronegative subjects being the least responsive. Therefore, we assessed humoral and cellular responses after the second dose of vaccine. Strikingly, all subjects displayed a strong humoral and cellular immune response after two doses of vaccine, including naïve and asymptomatic participants, as demonstrated by elevated anti-RBD IgG levels, neutralizing capability, as well as IFN-γ secreting cells ( Figure 5A and Figure S8 in Supplemental 1 ). After two doses of vaccine, naïve individuals still showed slightly lower anti-RBD IgG levels compared to both asymptomatic and symptomatic recovered individuals ( Figure 5B ). Cellular responses remained lower in asymptomatic HCWs compared to their symptomatic counterparts ( Figure 5B ). Our results collectively demonstrate that the severity of initial infection leads to the development of a more robust and persistent immune memory response after a vaccination challenge. In contrast, individuals who did not experience symptoms during infection with SARS-CoV-2 are less likely to develop long-term humoral and cellular immunity.
Figure 5.
Two doses of vaccine are required for individuals who recovered from an asymptomatic SARS-CoV-2 infection to generate a global and coordinated adaptive immune response. (A) SARS-CoV-2 Spike RBD–specific binding IgG levels assessed by ELISA (left panel), SARS-CoV-2 neutralizing antibody titers (middle panel), IFN-γ secreting cells per million in response to SARS-CoV-2 Spike glycoprotein peptides (right panel) assessed by ELISpot for naïve (n=14), asymptomatic (n=17) and symptomatic recovered HCWs (n=25) before (white circles), after one dose (black circles) and after two doses of vaccine (green circles). (B) SARS-CoV-2 Spike RBD–specific binding IgG levels assessed by ELISA (left panel), SARS-CoV-2 neutralizing antibody titers (middle panel), and IFN-γ secreting cells per million in response to SARS-CoV-2 Spike glycoprotein peptides assessed by ELISpot (right panel) for naïve (black circles), asymptomatic (n=17) and symptomatic (n=25) recovered HCWs after two doses of vaccine. Dashed black lines indicate the positive threshold value. Horizontal bars indicate the median of each group. Kruskal-Wallis tests assessed statistical significance. Not significant (ns) P >.05; *P <.05; **P <.01; ***P <.001; ****P <.0001.
Discussion
The ultimate goal of vaccination is to generate broad and long-lasting immune responses that will protect the host from severe clinical outcomes if infected. The most appropriate vaccination regimen to be used in previously infected individuals remains undefined, leading to discrepancies in public health recommendations worldwide. Based on prior studies suggesting that previously infected individuals did not benefit from their second dose of mRNA vaccine (18, 22), many countries have moved forward with the recommendation that a single dose of mRNA vaccine was sufficient for individuals who were previously infected (9). In this report, we demonstrate that one dose of vaccine is sufficient to induce sustained humoral and cellular immune responses in 92% of previously infected individuals who experienced symptoms during their infection. In contrast, only 69% in individuals who remained asymptomatic during their primary infection mounted robust immune responses to one dose of vaccine. In fact, infected individuals who remained asymptomatic and were seronegative at enrollment in our study harbored an immune response comparable to naïve subjects before and after vaccination.
Our results differ from one American HCWs cohort that concluded that humoral immune responses shortly after one mRNA vaccine dose were comparable in individuals who recovered from symptomatic and asymptomatic infections (34). However, HCWs from that cohort were all seropositive at the time of vaccination. Here, we show that previously infected but asymptomatic individuals who harbor a negative serostatus at vaccination mount reduced serum neutralization capabilities and cell-mediated memory responses following one vaccine dose. Furthermore, our study is the first one to describe both cellular and humoral immunity developed after vaccination in recovered subjects from an asymptomatic infection.
Knowledge gained from this study is particularly important in the current context of booster vaccination and protection against recent variants of concerns (VOCs). Waning immunity has been reported six months after a two-dose primary series in naïve individuals (35, 36). In these naïve individuals, three doses of vaccine increase protection against symptomatic and severe B.1.617.2 (Delta) and B.1.1.529 (Omicron) infections (37–42). Notably, despite strengthened immune responses following the booster dose, the capacity to neutralize the B.1.1.529 variant appears weaker (43–46). A German study reported that neutralizing antibodies against the Omicron variant were highest in individuals who had encountered the antigen three times, whether it was from receiving three doses of mRNA vaccine for uninfected individuals or two doses for individuals who had been infected with SARS-CoV-2 either prior to or subsequent to vaccination (45). Based on our current report, we want to highlight that this affirmation is true only in subjects who experienced symptoms during infection. Indeed, our results suggest that infected individuals who did not develop symptoms should receive additional booster doses, as should previously uninfected individuals, since we demonstrated that a fraction of these individuals harbors immune responses to vaccination comparable to naïve individuals. Further studies will be required to evaluate the vaccine requirements for boosters in recently infected Omicron cases, as people infected with this variant are more likely to be asymptomatic (47).
Limitations of this study are mainly related to the fact that results were obtained from a cohort of primarily middle-aged Caucasian females infected with SARS-CoV-2 eight months before vaccination. The immunization strategy in our province led to an important delay of sixteen weeks between the first and the second doses in these previously infected individuals, compared to ten weeks in uninfected individuals, which should be taken into consideration when interpreting the results. Indeed, an extended vaccination regimen was shown to favor better immune memory (48). It therefore remains to be evaluated if a shorter interval between infection and vaccination, or between the two vaccine doses, lead to similar results. In addition, samples available before and after each dose of vaccine being an important constraint, the time window between vaccination and analysis had to be extended for previously infected HCWs, while uninfected individuals were closer to one-month post-vaccination. Further, the protection induced by booster doses against infection by VOCs in previously infected, but asymptomatic individuals remain to be evaluated. The low frequency of asymptomatic individuals in our HCWs cohort and attrition with time prevented us to evaluate persistence and expansion of immune memory before and after booster vaccination. This study did not assess the impact of other approved vaccines against SARS-CoV-2 (49, 50), as primary series or boosters, as well as natural infection with emerging VOCs, as most of the cohort was infected at the beginning of 2020, with the original strain (51). While the immunity score we developed allows to easily report on the multi-functionality of the immune response induced by hybrid immunity, it does not report on the strength of the response. Evidence is currently lacking to establish the threshold at which humoral and cellular responses are optimal for heightened protection. This limitation could eventually be overcome by refining our metric to be more informative on protective immunity based on new evidence and evaluation of larger cohorts. Detailed functional, transcriptomic and repertoire analyses will be essential to fully grasp the immune memory differences between individuals who experienced symptoms or not during infection.
Conclusions
This report reveals the critical importance of the impact of an individual’s symptomatology at the time of infection and serostatus at the time of vaccination on the capacity to mount adequate immune protection, including cell-mediated responses. In contrast to individuals who were symptomatic during infection, we demonstrated that a third of the individuals who recovered from an asymptomatic infection responded like naïve individuals. We recommend that the two-dose primary series, and subsequent boosters, be offered to all previously infected individuals who were asymptomatic, in order to elicit a global and sustained adaptive immune response following mRNA vaccination.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Research Ethics Board (REB) at the Sainte-Justine University Hospital and Research Center. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HD, CQ: Conceptualized the project, designed the study and experiments, and obtained funding. SN, BB, JuC, HR, M-EH: Performed the experiments. SN, BB, HD, MB: Analyzed the data. SN, BB, HD: Interpreted the results and prepared the figures. KA, DM: Performed the clinical follow-up of the study. SN, BB, HD: Wrote the manuscript. CQ, VG, MC, PS, GS, JaC, YL, MB, GB: Edited the manuscript and provided critical input. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Canadian Institutes of Health Research (CIHR) (VR2172712) and the Public Health Agency of Canada, through the Vaccine Surveillance Reference Group and the COVID-19 Immunity Task Force. This work was also supported by NIH contract 75N93019C00065 (A.S, D.W).
Acknowledgments
We want to thank Valérie Villeneuve, Jocelyne Ayotte, and the Rare Pediatric Disease (RaPID) Biobank personnel at the Sainte-Justine University Hospital and Research Center for their technical help and expertise. Special thanks to Fazia Tadount and the team at the Vaccine Study Center for coordinating the RECOVER studies, and to Zineb Laghdir for administrative support for the RECOVER studies. We are grateful to the nurses and research coordinators at each University Hospital for their involvement in this study and the healthcare workers who agreed to participate in this research. We also thank Alessandro Sette and Daniela Weiskopf for providing the mega pool of peptides utilized in the AIM assays.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.930252/full#supplementary-material
References
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet (London England) (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. New Engl J Med (2020) 382(13):1199–207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection . A minimal common outcome measure set for COVID-19 clinical research. The Lancet Infectious diseases. (2020) 20(8):e192–e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis (2020) 20(8):e192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. (CDC) CfDCaP . Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the united states. (2022). [Google Scholar]
- 6. (NACI) NACoI . NACI rapid response: Updated guidance on COVID-19 vaccination timing for individuals previously infected with SARS-CoV-2. (2022). [Google Scholar]
- 7. (NIH) NIoH . COVID-19 treatment guidelines – prevention of SARS-CoV-2 infection, prevention of SARS-CoV-2 infection. (2022). [Google Scholar]
- 8. (PHAC) PHAoC . Canadian Immunization guide – COVID-19 vaccin. (2022). [Google Scholar]
- 9. (ECDC) ECfDPaC . Overview of the implementation of COVID-19 vaccination strategies and deployment plans in the EU/EEA. (2022). [Google Scholar]
- 10. Breton G, Mendoza P, Hagglof T, Oliveira TY, Schaefer-Babajew D, Gaebler C, et al. Persistent cellular immunity to SARS-CoV-2 infection. bioRxiv preprint Server Biol (2020) 2020.12.08.416636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science (2021) 371(6529):eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai L, Mantus G, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory b and T cells. medRxiv (2021) 2021.04.19.21255739. doi: 10.1101/2021.04.19.21255739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y, Yang M, Peng Y, Liang Y, Wei J, Xing L, et al. Longitudinal analysis of antibody dynamics in COVID-19 convalescents reveals neutralizing responses up to 16 months after infection. Nat Microbiol (2022) 7(3):423–43. doi: 10.1038/s41564-021-01051-2 [DOI] [PubMed] [Google Scholar]
- 14. McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature (2021) 590(7847):630–4. doi: 10.1038/s41586-020-03041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell (2020) 183(4):996–1012.e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol (2020) 5(12):1598–607. doi: 10.1038/s41564-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levi R, Azzolini E, Pozzi C, Ubaldi L, Lagioia M, Mantovani A, et al. One dose of SARS-CoV-2 vaccine exponentially increases antibodies in individuals who have recovered from symptomatic COVID-19. J Clin Invest (2021) 131(12): e149154. doi: 10.1172/JCI149154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mazzoni A, Di Lauria N, Maggi L, Salvati L, Vanni A, Capone M, et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J Clin Invest (2021) 131(12):e149150. doi: 10.1172/JCI149150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stamatatos L, Czartoski J, Wan YH, Homad LJ, Rubin V, Glantz H, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Sci (New York NY) (2021) 372(6549): 1413–8. doi: 10.1101/2021.02.05.21251182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med (2021) 27(6):981–4. doi: 10.1038/s41591-021-01325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goel RR, Apostolidis SA, Painter MM, Mathew D, Pattekar A, Kuthuru O, et al. Distinct antibody and memory b cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol (2021) 6(58):eabi6950. doi: 10.1126/sciimmunol.abi6950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lozano-Ojalvo D, Camara C, Lopez-Granados E, Nozal P, Del Pino-Molina L, Bravo-Gallego LY, et al. Differential effects of the second SARS-CoV-2 mRNA vaccine dose on T cell immunity in naive and COVID-19 recovered individuals. Cell Rep (2021) 36(8):109570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Racine É, Boivin G, Longtin Y, McCormack D, Decaluwe H, Savard P, et al. The REinfection in COVID-19 estimation of risk (RECOVER) study: Reinfection and serology dynamics in a cohort of Canadian healthcare workers. Influenza Other Respir Viruses 16(5):916–25. doi: 10.1111/irv.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amanat F, Stadlbauer D, Strohmeier S, Nguyen THO, Chromikova V, McMahon M, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med (2020) 26(7):1033–6. doi: 10.1038/s41591-020-0913-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stadlbauer D, Amanat F, Chromikova V, Jiang K, Strohmeier S, Arunkumar GA, et al. SARS-CoV-2 seroconversion in humans: A detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol (2020) 57(1):e100. doi: 10.1002/cpmc.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dibernardo A, Toledo NPL, Robinson A, Osiowy C, Giles E, Day J, et al. Evaluation of the performance of multiple immunoassay diagnostic platforms on the national microbiology laboratory SARS-CoV-2 national serology panel. Off J Assoc Med Microbiol Infect Dis Canada Preprint (2022). doi: 10.3138/jammi-2021-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baz M, Paskel M, Matsuoka Y, Zengel JR, Cheng X, Treanor JJ, et al. A single dose of an avian H3N8 influenza virus vaccine is highly immunogenic and efficacious against a recently emerged seal influenza virus in mice and ferrets. J Virol (2015) 89(13):6907–17. doi: 10.1128/JVI.00280-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg 1938(27):493–7. doi: 10.1093/oxfordjournals.aje.a118408 [DOI] [Google Scholar]
- 29. Salem Fourati I, Grenier AJ, Jolette É, Merindol N, Ovetchkine P, Soudeyns H. Development of an IFN-γ ELISpot assay to assess varicella-zoster virus-specific cell-mediated immunity following umbilical cord blood transplantation. J Visualized Experiments JoVE (2014) (89):51643. doi: 10.3791/51643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grifoni A, Sidney J, Zhang Y, Scheuermann RH, Peters B, Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe (2020) 27(4):671–680.e672. doi: 10.1016/j.chom.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell (2020) 181(7):1489–1501.e1415. doi: 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, et al. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity (2021) 54(9):2133–2142.e2133. doi: 10.1016/j.immuni.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tauzin A, Nayrac M, Benlarbi M, Gong SY, Gasser R, Beaudoin-Bussières G, et al. A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits fc-mediated antibody effector functions and T cell responses. Cell Host Microbe (2021) 29(7):1137–1150.e1136. doi: 10.1016/j.chom.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saadat S, Rikhtegaran Tehrani Z, Logue J, Newman M, Frieman MB, Harris AD, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. Jama (2021) 325(14):1467–9. doi: 10.1001/jama.2021.3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. New Engl J Med (2021) 385(24):e84. doi: 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shrotri M, Navaratnam AMD, Nguyen V, Byrne T, Geismar C, Fragaszy E, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet (London England) (2021) 398(10298):385–7. doi: 10.1016/S0140-6736(21)01642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. Jama (2022) 327(7):639–51. doi: 10.1001/jama.2022.0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson AG, Amin AB, Ali AR, Hoots B, Cadwell BL, Arora S, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of delta and omicron variant emergence - 25 U.S. jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep (2022) 71(4):132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, et al. Clinical severity and mRNA vaccine effectiveness for omicron, delta, and alpha SARS-CoV-2 variants in the united states: A prospective observational study. medRxiv (2022) 7:2022.02.06.22270558. doi: 10.1101/2022.02.06.22270558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-Associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION network, 10 states, august 2021-January 2022. MMWR Morb Mortal Wkly Rep (2022) 71(4):139–45. doi: 10.15585/mmwr.mm7104e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Veneti L, Bøås H, Bråthen Kristoffersen A, Stålcrantz J, Bragstad K, Hungnes O, et al. Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 omicron BA.1 variant compared with the delta variant, Norway, December 2021 to January 2022. Euro Surveill (2022) 27(4): 2200077 doi: 10.2807/1560-7917.ES.2022.27.4.2200077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buchan SA, Chung H, Brown KA, Austin PC, Fell DB, Gubbay JB, et al. Effectiveness of COVID-19 vaccines against omicron or delta symptomatic infection and severe outcomes. medRxiv (2022) 12.30.21268565. doi: 10.1101/2021.12.30.21268565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheng SMS, Mok CKP, Leung YWY, Ng SS, Chan KCK, Ko FW, et al. Neutralizing antibodies against the SARS-CoV-2 omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med (2022) 28: 486–9. doi: 10.1038/s41591-022-01704-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, et al. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. medRxiv (2021) 12.07.21267432. doi: 10.1101/2021.12.07.21267432 [DOI] [Google Scholar]
- 45. Wratil PR, Stern M, Priller A, Willmann A, Almanzar G, Vogel E, et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med (2022) 28:496–503. doi: 10.1038/s41591-022-01715-4 [DOI] [PubMed] [Google Scholar]
- 46. GeurtsvanKessel CH, Geers D, Schmitz KS, Mykytyn AZ, Lamers MM, Bogers S, et al. Divergent SARS CoV-2 omicron-reactive T- and b cell responses in COVID-19 vaccine recipients. Sci Immunol 2022:eabo2202. doi: 10.1126/sciimmunol.abo2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garrett N, Tapley A, Andriesen J, Seocharan I, Fisher LH, Bunts L, et al. High rate of asymptomatic carriage associated with variant strain omicron. medRxiv (2022) 2021.12.20.21268130. doi: 10.1101/2021.12.20.21268130 [DOI] [Google Scholar]
- 48. Payne RP, Longet S, Austin JA, Skelly DT, Dejnirattisai W, Adele S, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell (2021) 184(23):5699–5714.e5611. doi: 10.1016/j.cell.2021.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. Jama (2021) 325(13):1318–20. doi: 10.1001/jama.2021.3199 [DOI] [PubMed] [Google Scholar]
- 50. Forni G, Mantovani A. COVID-19 Commission of Accademia Nazionale dei Lincei, Rome. COVID-19 vaccines: where we stand and challenges ahead. Cell Death & Differentiation. (2021) 28(2):626–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Organization WH. Tracking SARS-CoV-2 variants (2021). Available at: https://wwwwhoint/en/activities/tracking-SARS-CoV-2-variants/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Inquiries can be directed to the corresponding author.