Abstract
Background
Reperfusion therapy is the most effective treatment for acute ischaemic stroke (AIS) but remains underutilised in China. There is an urgent need to develop tailored strategies to increase adherence to intravenous thrombolysis (IVT) and endovascular thrombectomy (EVT) within the guideline-recommended time window for eligible patients.
Aims
This study aims to investigate the efficacy of a comprehensive quality improvement intervention on adherence to guideline-recommended reperfusion therapy for patients with AIS in China.
Design
The Improve Acute Reperfusion Treatment Quality for Stroke in China (IMPROVE Stroke Care in China) trial is designed as a stepped wedge cluster randomised trial within 51 hospitals. We developed the comprehensive intervention ‘STEP’ (Strategies, Toolkit, Exploration, Paradigm) to promote the reconstruction of workflow in stroke centres and shorten in-hospital delay of reperfusion treatment for patients with AIS. The participating hospitals (clusters) were randomised to three groups (cohorts) for different predefined steps to intervention implementation. The primary outcome was the adherent rate of IVT or EVT for eligible patients within the time window. The sample size was estimated to be 7644, and was determined by the number of cases to be enrolled in five study periods to detect a relative increase of 30% (from 19% to 25%) with 90% power and intraclass correlation coefficient of 0.03. All efficacy analyses will be conducted based on the intention-to-treat principle. The primary outcome will be analysed using a mixed-effects logistic regression with a random effect for the cluster (hospital), and a fixed effect for the strategy and period.
Conclusions
If the efficacy is well established, this targeted comprehensive intervention STEP will inform national strategies to increase adherence to guideline-recommended performance on reperfusion therapy.
Trial registration number
clinicaltrials.gov Identifier: NCT003578107
Keywords: stroke, clinical trial
Summary box.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Reperfusion therapy is the most effective treatment for acute ischaemic stroke (AIS). The best practice strategies of ‘Target: Stroke’ have achieved tremendous success in improving in-hospital healthcare quality of AIS, however, these strategies have not been proved to be effective in China.
WHAT THIS STUDY ADDS
In the ‘IMPROVE Stroke Care in China’, we developed the comprehensive intervention ‘STEP’ (Strategies, Toolkit, Exploration, Paradigm) based on the strategies of ‘Target: Stroke’ to promote the reconstruction of workflow in stroke centres and to shorten in-hospital delay of reperfusion treatment for patients with AIS.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
We hypothesise that the STEP intervention will increase adherence to reperfusion therapy and improve 3 month clinical outcomes in patients with AIS in China.
Introduction
Stroke is a leading cause of death and disability worldwide.1 As the most populous country in the world with a rapidly ageing population, China faces an immense healthcare challenge due to stroke. The burden of stroke in China has risen steeply in the past three decades, with disability-adjusted life-years increasing by 24.4% between 1990 and 2017. One such way to mitigate this burden is through reperfusion therapy.
Reperfusion therapy is the most effective treatment for acute ischaemic stroke (AIS).2 Several large randomised controlled trials have shown that intravenous recombinant tissue plasminogen activator (IV rt-PA) and endovascular thrombectomy (EVT) significantly improve functional outcomes.3–9 Intravenous thrombolysis (IVT) for eligible patients within 4.5 hours after onset and EVT for eligible patients within 6 hours after onset were recommended highest by the American Heart Association/American Stroke Association (AHA/ASA) and Chinese Stroke Association (CSA), respectively.10 11 However, rate of IVT therapy in China was only 18.3% in 2012, which was still far from the adherence reported by the Get With The Guidelines-Stroke (GWTG-Stroke) Study in the USA (72.8%).12 13 Additionally, the proportion of eligible patients undergoing EVT within 6 hours was only 2.17% in data from the Chinese Stroke Center Alliance (CSCA) in March 2018.14 Given the low adherence to both IVT and EVT, there is an urgent need to develop tailored strategies to increase adherence for eligible patients within the guideline-recommended time window.
As an effective quality improvement programme for reperfusion therapy, ‘Target: Stroke’ has achieved tremendous success in improving in-hospital healthcare quality of AIS, especially in shortening door-to-needle time and increasing the thrombolysis rate.15 Accordingly, multidimensional quality improvement initiatives targeting reperfusion therapy, including clinical decision support tools, facilitating hospital participation and sharing best practices, which may be effective in improving adherence to evidence-based performance measures. However, there is a lack of evidence for effective interventions in low-income and middle-income countries.
We developed the comprehensive intervention ‘STEP’ (Strategies, Toolkit, Exploration, Paradigm) to promote the reconstruction of workflow in stroke centres and to shorten in-hospital delay of reperfusion treatment for patients with AIS. We hypothesise that the STEP intervention will increase adherence to IVT or EVT and improve 3-month clinical outcomes in patients with AIS in China. In addition, we seek to evaluate the influence of different intervention durations on adherence to reperfusion therapy guidelines.
Methods
Study design
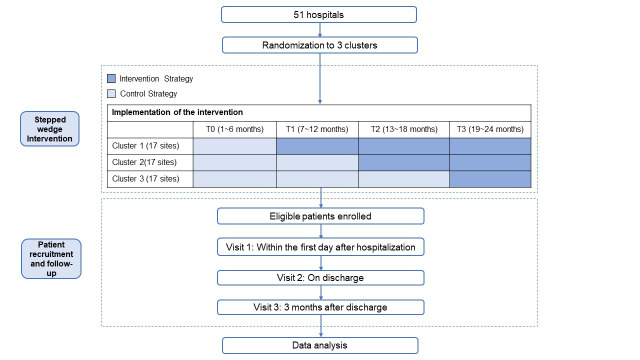
The Improve Acute Reperfusion Treatment Quality for Stroke in China (IMPROVE: Stroke Care in China) trial is a stepped wedge cluster randomised trial to assess the effectiveness of the STEP intervention on adherence to guideline-recommended reperfusion therapy for patients with AIS in China. Using the stepped wedge cluster randomised trial design,16 participating hospitals will be randomised to three cohorts for different predefined steps to receiving the intervention. After a 6-month initial period, the STEP intervention will be implemented in cohort 1 through the end of the trial. The intervention will be implemented in cohort 2 at 13 months through the end of the trial, and in cohort 3 at 19 months through the end of the trial. There will not be a transition period for any of the cohorts (figure 1). All patients in each cohort will be included consecutively and undergo a 3-month follow-up period.
Figure 1.
The flow chart of the IMPROVE trial. IMPROVE, Improve Acute Reperfusion Treatment Quality for Stroke in China
Study participants
Participating hospitals will be enrolled from Mainland China according to the economic-geographic regions: Eastern, Central and Western. To ensure the diversity and representativeness of the included clusters, the number of hospitals enrolled from each stratum will be proportional to the number of secondary and tertiary hospitals in each region. Each participating hospital will designate a principal investigator and a physician or nurse as the quality improvement research coordinator. The inclusion and exclusion criteria of participating hospitals are shown in box 1. Hospitals (clusters) participating in the CSCA and that meet the inclusion and exclusion criteria are eligible for inclusion in the IMPROVE Stroke Care in China. The CSCA, launched by the CSA and National Center of Quality Management in Stroke Care, includes 1576 hospitals from 27 provinces and 4 municipalities in Mainland China.16 A total of 51 secondary or tertiary hospitals (clusters) were selected voluntarily from the CSCA hospitals and located in 29 provinces, autonomous regions or municipalities (Anhui, Beijing, Fujian, Gansu, Guangdong, Guangxi, Guizhou, Hainan, Hebei, Henan, Heilongjiang, Hubei, Hunan, Inner Mongolia, Jilin, Jiangsu, Jiangxi, Liaoning, Ningxia, Qinghai, Shandong, Shanxi, Shannxi, Sichuan, Tianjin, Xinjiang, Yunnan, Zhejiang and Chongqing).
Box 1. The specific hospital inclusion and exclusion criteria.
Inclusion criteria
Voluntary.
Secondary or tertiary public hospitals with an emergency department and neurologic wards that admit patients with AIS.
Has a 24×7 on-call stroke team.
Has the capacity for IVT and/or EVT.
Implemented at least 10 patients’ IVT and/or EVT during the last year.
Admitted at least five patients within 6 hours after onset each month.
Desire to improve treatment workflow of AIS.
Good cooperation among Neurology Department, Emergency Department, Interventional Department, Neurosurgery Department, Laboratory and Radiology Department.
Exclusion criteria
Refusal to participate.
Primary or private hospitals.
Hospitals participated in other stroke care quality improvement projects or related clinical trials.
Patients eligible for inclusion are those over 18 years of age, present with symptoms of AIS and confirmed by CT and/or MRI and arrive at the hospital within 6 hours after stroke onset. Patients diagnosed with transient ischaemic attack, haemorrhagic stroke and non-cerebrovascular diseases will not be included in this trial. Informed consent will be obtained from patients or their proxies prior to enrolment.
Cluster randomizsation
Cluster randomisation at the hospital level will be performed centrally using a computer-generated random number sequence that will only be known by two independent biostatisticians (YP and HG). Hospital location and grade will be matched during the randomisation to minimise imbalances throughout the trial. A confirmation letter will be sent to all the hospitals in a particular cohort 1 month before the implementation of the intervention to ensure preparedness. If the selected hospital cannot complete the research study, it will be replaced by an eligible hospital of the same capacity in the same economic-geographic region stratum. The status of hospitals in each cluster will not be shared with hospitals outside the cohort.
Quality improvement interventional strategies
Strategies for the STEP intervention will be performed to promote the reconstruction of workflow in stroke centres and to shorten in-hospital delay of reperfusion treatment for patients with AIS. The main intervention objects contain managers, physicians (including emergency doctors, radiologists, neurologists or vascular neurologists, interventionists or neurosurgeons, anaesthesiologists) and nurses in the participating hospitals.
One group of best practice Strategies is based on ‘Target: Stroke’ and aims to optimise the Green Channel.15 It includes hospital prenotification by the emergency medical system (120/999 in China), rapid triage, a single call activation of the stroke team, a designated staff supporting the whole process (including taking patients to CT scan, helping to pay for therapy, and so on), a patient-specific chart with time record, a principle of CT scan priority for patients with stroke, a stroke team that directly goes to CT room, rapid acquisition and interpretation of brain imaging, stroke first for laboratory testing (including point of care testing if indicated), rt-PA regularly stored in the thrombolysis room of the emergency department, receiving IVT as soon as possible, and some other written documents from CSA or National Center for Healthcare Quality Management in Neurological Diseases to receive support from hospital management.
One integrated Toolkit for AIS treatment is presented as a handbook and designed according to the AHA/ASA and CSA guidelines for AIS management.10 11 It is integrated with the key tools that have been used and updated in the Green Channel of Beijing Tiantan Hospital, Capital Medical University. It includes the rapid triage protocol, clinical pathways, recommended time intervals, brain CT/MRI interpretation protocol, indications and contraindications of IVT/EVT, stroke-specific order sets, rt-PA application process, calculated dosage by different weights, peri-IVT/EVT management, stroke scales (National Institute of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS)), updated guidelines, informed consent for IVT/EVT and relevant contact information.
The Exploration component provides guidance by experts on improving the intervention. Given the experience of improving clinical practice in Beijing Tiantan Hospital, Capital Medical University, a regular point-to-point guidance to hospitals will be provided by experienced experts through teleconferences when each cohort crosses from the control to the interventional steps. Some remote technical trainings and workshops will be held as well.
The Paradigm of the feedback system serves to make a Plan–Do–Study–Act circle. Weekly data feedback will be provided to sites through the online data feedback platform, WeChat App, telephone or email by the assigned quality coordinator of this trial. A weekly or biweekly meeting will be encouraged in hospitals receiving the intervention to analyse the reasons for in-hospital delays in receiving IVT/EVT or not receiving IVT/EVT at all. A biweekly or monthly communication with the site contact person will be performed and some improvement strategies will be recommended by the quality coordinator. On-site monitoring will be conducted on the establishment and improvement of IVT/EVT procedures in each hospital.
Outcomes
The primary outcome is the rate of reperfusion treatment, including IVT for eligible patients who arrived within 3.5 hours and are treated within 4.5 hours after onset or that of EVT for eligible patients who arrived within 4.5 hours and are treated within 6 hours after onset. The secondary outcomes include the proportion of patients receiving IVT in those who arrived in the hospital within 4.5 hours after onset; the proportion of patients receiving EVT in those who arrived at the hospital within 6 hours after onset; door-to-needle time; door-to-puncture time; in-hospital mortality; and 3-month disability as measured by mRS 3–5.
Organizational structure
This study is administered by an international academic steering committee composed of coprinciple investigators and members. This committee is responsible for the study design, research protocol, academic support and phased progress by Senior Management Group meetings. The quality improvement committee is composed of clinical and management experts and responsible for supervising the current adherence of IVT/EVT, determining measures to improve the adherent rate and providing technical support for workshops, remote training and point-to-point guidance to the hospitals receiving the intervention. The executive committee is composed of senior experts from the international academic steering committee. The purpose of this committee is to assess the progress and safety of the study and make decisions regarding early termination, modification or continuation of the trial.
Data collection and quality control
The variables in the case report form of this trial are mainly sourced from the China National Stroke Registries and the GWTG-Stroke, and are confirmed by the executive committee.13 17 All variables of baseline data on demographics, medical history, vascular risk factors, prestroke mRS, key time points (time of onset, to door, to image, to needle, to puncture and to recanalisation), NIHSS score on admission, imaging data and information of IVT/EVT are obtained at admission by trained research coordinators in each site. The second follow-up will be performed at discharge to collect information about the final diagnosis, NIHSS score and mRS at discharge, aetiological classification, in-hospital complications, discharge secondary prevention medications, hospitalisation expenses and other in-hospital medical treatments. The aetiological classification will use the Trial of Org 10 172 in Acute Stroke Treatment criteria.18 A centralised follow-up interview by telephone will be conducted at 3 months after onset to collect data on current mRS, medication use and compliance, stroke recurrence and all-cause mortality. All the interviewers will be blinded to which cluster patients are in.
All information of included patients will be collected and uploaded by a web-based Patient Management Tool (GaiDe, Inc, Beijing, China) at each site. To be compliant with the national privacy standards, all data will be encrypted and transmitted to the China National Clinical Research Center for Neurological Diseases, which will serve as the data analysis and feedback centre. If crossing from control to the interventional step, all feedback data of current adherent rate can be checked by each participating hospital online; all data will be sent to the principal investigator of each hospital via WeChat App and email.
Sample estimation
Based on the CSCA data up to March 2018, we assume that the baseline adherent rate of IVT and EVT for eligible patients within 6 hours is approximately 19%. As such, 51 participating hospitals (17 for each cluster) enrolling 31 patients per time period (6 months) per site will be required to detect a relatively 30% improvement (from 19% to 25%) with 90% power, alpha=0.05, and an intracluster correlation coefficient of 0.03. That would be 6324 patients totally. Considering the increasing enrolment volumes of participating sites in clinical practice, the final sample size was increased to 7644. It is a comprehensive consideration of statistical and operational aspects.
Statistical analyses
All efficacy analyses will use the intention-to-treat principle.19 Continuous variables are presented as the means and SD or medians with IQRs, and categorical variables are presented as counts and percentages. The baseline characteristics will be compared by analysis of variance or Kruskal-Wallis test for continuous variables and χ2 test or Fisher exact test for categorical variables. The primary outcome will be analysed using a mixed-effects logistic regression with a random effect for the cluster (hospital) and a fixed time effect for every step. For categorical secondary outcomes, data will be analysed using the same strategy for the primary outcome. For continuous secondary outcomes, mixed-effects linear regression with a random effect for the cluster (hospital) and a fixed time effect for the step will be used. All tests will be performed in SAS software V.9.4 (SAS Institute, Cary, North Carolina, USA).
Baseline information
This trial was launched in July 2018. During the inclusion process, the number of patients enrolled in each month was more than expected. Two hospitals were ruled out from this trial because they were unable to follow the research protocol; one was from cluster 2 at 3 months and the other was from cluster 3 at 5 months. Among the 49 hospitals, 32 (65.3%) were tertiary hospital and 22 (44.9%) from Eastern region (table 1).
Table 1.
Baseline characteristics of the enrolled hospitals
| Variables (n, %) | Statistics (n=49) |
| Hospital grade | |
| Secondary | 17 (34.7) |
| Tertiary | 32 (65.3) |
| Region | |
| Eastern | 22 (44.9) |
| Central | 12 (24.5) |
| Western | 15 (30.6) |
Discussion
In recent years, with the clinical application of perfusion imaging, the time window for IVT/EVT has continued to expand and benefit more patients with AIS.20–23 In addition, among patients with AIS from a proximal large-vessel occlusion within 4.5 hours after symptom onset, some newly updated trials have shown that endovascular treatment alone was non-inferior to IVT bridging with EVT for the functional outcome at 3 months, which could reduce time to save more ischaemic lesions based on the time-related benefits of reperfusion therapy.24–26
Recently, the Randomization of Endovascular Treatment with Stent-retriever and/or Thrombo-aspiration versus Best Medical Therapy in Acute Ischemic Stroke due to Large Vessel Occlusion Trial (RESILIENT) Study provided evidence of mechanical thrombectomy in low-income and middle-income countries. Although the between-group differences in functional outcomes in the RESILIENT trial were similar to those in previous trials from high-income countries, the RESILIENT trial still had substantially higher mortality and worse prognosis. In view of the sharp contrast between suboptimal guideline adherence and continuously evolving reperfusion therapy research, improving acute stroke care quality is a national priority for developing countries with heavy stroke burdens.
The low adherence to IVT or EVT may be attributed to many reasons, including the unintegrated hospital system, insufficient health resources and high treatment costs.12 A previous study showed that quality improvement initiatives were highly cost-effective in China.27 We hope to improve clinical performance in AIS through comprehensive quality improvement inventions on reperfusion therapy, with the aim to reduce stroke disability and mortality. Additionally, analysing data before and after the quality improvement intervention will provide insights into ischaemic stroke care in different regions and hospital grades, drive improvements to bridge any gaps that are identified and facilitate the integration of scientific evidence into clinical practice. If efficacy is established, this targeted comprehensive intervention will help develop national strategies to increase the adherence to guideline-recommended treatment, with important implications in addressing the disproportionate and growing burden of stroke in China.
Acknowledgments
We thank all investigators and participating hospitals of the IMPROVE trial.
Footnotes
Twitter: @braindoc_mgh
ZL, CW and XZ contributed equally.
Contributors: ZL, CW and XZ analysed and interpreted the data and drafted the manuscript. LZ, HongyuZ, HaifenZ, XY, MW and YX assisted to promote the project progress. HG, YJ, QZ and YP completed the statistical work. XM, XZ, YW, LL, XM, LM, YX, LHS and YW conceived and designed the research.
Funding: This trial was funded by Boeheringer Ingelheim (2017–2019) and Medrtonic Inc (2017–2018), the Ministry of Science and Technology of the People’s Republic of China (2017YFC1310901), the National Natural Science Foundation of China (92046016), Beijing Talents Project (2018000021223ZK03), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-029) and Beijing Municipal Committee of Science and Technology (Z201100005620010).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request. http://paper.ncrcnd.ttctrc.com/default/projects.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
Ethics approval
This study involves human participants and was approved by the Ethics Committee of Beijing Tiantan Hospital (KY2018-044-01). Participants gave informed consent to participate in the study before taking part. The protocol of this study was approved by the central institutional review board at Beijing Tiantan Hospital, Capital Medical University and participating hospitals, and written informed consent will be obtained prior to enrolment. This trial has been registered online (www.clinicaltrials.gov identifier NCT003578107).
References
- 1. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campbell BCV, Khatri P. Stroke. Lancet 2020;396:129–42. 10.1016/S0140-6736(20)31179-X [DOI] [PubMed] [Google Scholar]
- 3. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–8. 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 4. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–29. 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 5. Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 6. Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 7. Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30. 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 8. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306. 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 9. Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 10. Powers WJ, Rabinstein AA, Ackerson T. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/american stroke association. Stroke 2018;2018:e46–110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 11. Association CS . 2018 guideline for endovascular treatment in acute ischemic stroke patients. Chin J Stroke 2018;2018:706–29. [Google Scholar]
- 12. Li Z, Wang C, Zhao X, et al. Substantial progress yet significant opportunity for improvement in stroke care in China. Stroke 2016;47:2843–9. 10.1161/STROKEAHA.116.014143 [DOI] [PubMed] [Google Scholar]
- 13. Schwamm LH, Fonarow GC, Reeves MJ, et al. Get with the Guidelines–Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation 2009;119:107–15. 10.1161/CIRCULATIONAHA.108.783688 [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Li Z, Wang Y, et al. Chinese stroke center alliance: a national effort to improve healthcare quality for acute stroke and transient ischaemic attack: rationale, design and preliminary findings. Stroke Vasc Neurol 2018;3:256–62. 10.1136/svn-2018-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 2014;311:1632–40. 10.1001/jama.2014.3203 [DOI] [PubMed] [Google Scholar]
- 16. Hemming K, Haines TP, Chilton PJ, et al. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ 2015;350:h391. 10.1136/bmj.h391 [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Jing J, Meng X, et al. The third China national stroke registry (CNSR-III) for patients with acute ischaemic stroke or transient ischaemic attack: design, rationale and baseline patient characteristics. Stroke and Vascular Neurology 2019;4:158–64. 10.1136/svn-2019-000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST. trial of ORG 10172 in acute stroke treatment. Stroke 1993;24:35–41. 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 19. Jo B, Asparouhov T, Muthén BO. Intention-To-Treat analysis in cluster randomized trials with noncompliance. Stat Med 2008;27:5565–77. 10.1002/sim.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma H, Campbell BCV, Parsons MW, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med 2019;380:1795–803. 10.1056/NEJMoa1813046 [DOI] [PubMed] [Google Scholar]
- 21. Campbell BCV, Ma H, Ringleb PA, et al. Extending thrombolysis to 4·5–9 H and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. The Lancet 2019;394:139–47. 10.1016/S0140-6736(19)31053-0 [DOI] [PubMed] [Google Scholar]
- 22. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 23. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med 2020;382:1981–93. 10.1056/NEJMoa2001123 [DOI] [PubMed] [Google Scholar]
- 25. Zi W, Qiu Z, Li F, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke. JAMA 2021;325:234–43. 10.1001/jama.2020.23523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, atlantis, NINDS, and epithet trials. The Lancet 2010;375:1695–703. 10.1016/S0140-6736(10)60491-6 [DOI] [PubMed] [Google Scholar]
- 27. Pan Y, Zhang L, Li Z, et al. Cost-Effectiveness of a multifaceted quality improvement intervention for acute ischemic stroke in China. Stroke 2020;51:1265–71. 10.1161/STROKEAHA.119.027980 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request. http://paper.ncrcnd.ttctrc.com/default/projects.