Abstract
Eleven isolates of spotted fever group rickettsiae from the blood of patients or ixodid ticks from North and South America were characterized. All isolates were identified as Rickettsia rickettsii using restriction fragment length polymorphism analysis of a 532-bp rOmpA gene fragment obtained by PCR. The ability of the R. rickettsii isolates to elicit cytopathic effects and parameters of oxidative injury were examined in cultured human EA.hy 926 endothelial cells. Cytopathic effects were determined by direct observation of infected cultures, by measuring the release of cytoplasmic lactate dehydrogenase (LDH), and by determination of intracellular pools of peroxide and reduced glutathione. Four biotypes of R. rickettsii were defined. Group I included two highly cytopathic isolates from Montana, Bitterroot and Sheila Smith, and three isolates from Maryland, North Carolina, and Brazil. These isolates rapidly damaged cells, released large amounts of cytoplasmic LDH, caused accumulation of intracellular peroxide, and depleted intracellular pools of reduced glutathione. Group II contained three isolates, two from Montana, Hlp#2 and Lost Horse Canyon, and an isolate from Colombia, which were similar to group I but caused either lower responses in LDH release or smaller changes in intracellular peroxide levels. The group III isolates, Sawtooth from Montana and 84JG from North Carolina, caused lower cellular injury by all measures. Group IV isolate Price T from Montana was the least cytopathic and caused minimal alterations of all parameters measured. Understanding the molecular basis for the varied cellular injury caused by different isolates of R. rickettsii may contribute to improved treatment of Rocky Mountain spotted fever and to the rapid identification of those isolates which are more likely to cause fulminant disease.
Rocky Mountain spotted fever (RMSF) is a seasonal tick-borne rickettsial disease which is widely distributed in the Americas. While it was originally discovered in the Rocky Mountains and surrounding western states at the beginning of the 20th century, later more foci were detected in the eastern United States and in Central and South America (15, 19, 28, 48, 49, 51, 62). RMSF is caused by an obligate intracellular bacterium, Rickettsia rickettsii, which is transmitted to humans by the bite of ixodid ticks, particularly Dermacentor andersoni in the western United States, Dermacentor variabilis in the eastern United States, and Amblyomma cajennense in Central and South America (11, 20, 55). The rabbit tick, Haemaphysalis leporispalustris, may be also involved in the circulation of R. rickettsii in nature, but its role in the epidemiology of RMSF has not been established completely (38). RMSF is an acute disease which starts after a 3- to 10-day incubation period following a tick bite or an accidental laboratory exposure to an aerosol of the rickettsiae. Primary symptoms of RMSF are very nonspecific; they often include high fever, severe headache, and muscle pain and may be accompanied by nausea, vomiting, abdominal pain, and cough (58). A maculopapular rash appears on the lower extremities after 3 to 5 days of illness. Later during the course of infection, the rash develops into a petechial and even hemorrhagic presentation in severe cases, and it spreads from the palms and soles to the body trunk; however, approximately 10% of RMSF cases are spotless (lack a rash), particularly in older people (34, 36, 58). Neurologic and gastroenteric dysfunctions, retinal injury, cardiopulmonary pathology, and renal deficiency are among the syndromes which are detected during RMSF, further complicating its clinical recognition and, therefore, its diagnosis and treatment (17, 34, 35, 58). RMSF responds well to doxycycline treatment; however, delayed diagnosis and therapy continue to cause unnecessary fatalities. High proportions of fatal RMSF cases occur particularly among patients suffering from spotless forms of the disease and young children (5, 27, 32, 52, 58).
R. rickettsii strains vary in virulence for experimental animals, serological properties, cultural characteristics, and ability to cross-protect guinea pigs against challenge with rickettsiae or to cross-protect mice against large doses of rickettsiae (2–4, 9, 10, 43). Based on differences in virulence for guinea pigs, tick isolates of R. rickettsii from Montana were divided into four groups: R, S, T, and U (46). R-type isolates represented the most pathogenic strains, which caused severe infection, accompanied by long-lasting fever and scrotal reactions, and caused mortality in 30% of infected animals. Infection with strains of type S or type T caused significantly milder symptoms and shorter duration of fever; inoculation of guinea pigs with U-type strains did not induce any detectable symptoms of rickettsiosis. In a search for the molecular basis underlying these differences, Anacker and his colleagues detected only minor differences in protein, lipopolysaccharide, and antigenic profiles of selected isolates of R. rickettsii (1, 3); however, there are no significant correlations between these findings and the biological characteristics of the strains they examined.
Here we examined the ability of a variety of isolates of R. rickettsii to elicit different degrees of cellular injury as an in vitro method that could potentially be used to predict their in vivo pathogenicities. In particular, we developed standardized quantitative methods for comparing the damage caused by isolates of R. rickettsii by measuring cellular parameters of oxidative injury.
(This work was presented at the 15th Sesquiannual Meeting of the American Society for Rickettsiology, Captiva Island, Fla., May 2000.)
MATERIALS AND METHODS
Cell culture.
Vero cells (strain C1008, green monkey kidney cells) were obtained from the American Type Culture Collection (Manassas, Va.) and propagated in high-glucose Dulbecco's modified Eagle medium (GibcoBRL Life Technologies, Grand Island, N.Y.) supplemented with 4% heat-inactivated fetal bovine serum (GibcoBRL) and 1 mM l-glutamine (GibcoBRL) in a 5% CO2 atmosphere. The cells used were at 13 to 20 passages from the American Type Culture Collection stock.
EA.hy 926 human endothelial cells (kindly provided by Cora-Jean S. Edgell, University of North Carolina, Chapel Hill) were at the 13th passage in Dulbecco's modified Eagle medium supplemented with 4.5 g of glucose per liter, 10% fetal bovine serum (GibcoBRL), 1 mM l-glutamine (GibcoBRL), and hypoxanthine-aminopterin-thymidine (Sigma, St. Louis, Mo.). The origin of the EA.hy 926 cell line and its characteristics have been described previously (21, 25).
Rickettsiae.
The isolates studied, their origins, and their passage histories are listed in Table 1. Rickettsiae were propagated in Vero cell monolayers as described elsewhere (24, 25). Five to 6 days after inoculation, the intensity of infection was examined in smears stained by the method of Gimenez (31). Heavily infected cells were harvested by using 3-mm-diameter glass beads. Suspensions of Vero cells infected with isolates Brazil and Colombia were concentrated by centrifugation at 10,000 × g for 30 min, the pellet was resuspended in SRM buffer (0.218 M sucrose, 5 mM potassium glutamate buffer [pH 7.0] supplemented with 1% Renografin-76 [E.R. Squibb & Sons, Inc., Princeton, N.J.] and 5 mM MgCl2), and the suspensions were frozen at −83°C before purification. Other isolates were purified immediately after harvesting using a modification of a previously described procedure (24) as follows: the purification procedure included disruption of infected cells by sonication, differential centrifugation, centrifugation through a sucrose cushion, and filtration through an AP-20 glass fiber filter (Millipore). The final suspension of purified rickettsiae was prepared in SRM buffer, aliquoted, and frozen at −83°C. The viable titer of purified rickettsiae was determined by plaque titration on Vero cells as described previously (60).
TABLE 1.
R. rickettsii isolates studied
| Isolate | Geographical location and yr of isolation | Source of isolation | Passage historya | Association with human disease | Classification | Origin (collection)b | Reference |
|---|---|---|---|---|---|---|---|
| Sheila Smith (VR149) | Missoula, Mont., 1946 | Patient suffering from RMSF | 2GP + 8YS + 5V + 1GP + 3YS + 4V | Fulminant RMSF | R | ATCC (M. Bozeman, WRAIR) | 8 |
| Bitterroot (VR891) | Bitterroot Valley, Mont., 1945 | D. andersoni tick | GP? + 55YS + 3V | Likely | R | ATCC (M. Bozeman, WRAIR) | 8 |
| Sutkiewicz | Frederick, Md., 1973 | Patient blood | 3YS + 3V | RMSF | R | M. Bozeman, (WRAIR) | This study |
| Sawtooth | Sawtooth Canyon, Bitterroot Valley, Mont., 1961 | D. andersoni tick | >8T + 7V | Likely | R | C. Pretzman (from W. Burgdorfer) | 12 |
| Lost Horse Canyon | Montana, 1958 | D. andersoni tick | 6YS + 2GP + 5YS + 3V | Likely | R | M. Peacock (RML) | 12 |
| 84JG | North Carolina, 1984 | Human | GP? + 3V + 1YS + 5V | Fulminant RMSF | ? | C. Pretzman (ODPH) | Walker, personal communication |
| Morgan | North Carolina, 1975 | Human | 1M + 3YS + 5TC + 2–3YS + 8V | RMSF | S-R | G. McDonald (RML) | 3 |
| Hlp#2 | Western Montana, 1948 | H. leporispalustris tick | 50YS + 9TC + 3–4YS + 8V | ? | ? | G. McDonald (RML) | 38 |
| Colombia (Tobia) | Colombia, about 1935? | Human? | ? + 10YS + 1L + +2V | Likely | ? | C. Pretzman (ODPH) | 40 |
| Brazil (San Paulo) | Brazil, before 1943 | ? tick | ? + 10YS + 2L + 2V | Likely | ? | D. Kelly, WRAIR | 8 |
| Price T | Montana, before 1953 | D. andersoni tick | ? + 3YS + 2L + 2V | ? | T | J. Spielman, HSPH | 45 |
?, passage history not known; YS, passages in yolk sacs of embryonated eggs; GP, passages in guinea pigs; M, Microtus passage; T, tick passage; TC, tissue culture passages; V, passages in VERO cells; L, passages in L929 cells.
ATCC, American Type Culture Collection, Manassas, Va.; HSPH, Harvard School of Public Health, Cambridge, Mass.; RML, Rocky Mountain Laboratory, Hamilton, Mont.; ODPH, Ohio State Department of Public Health, Columbus; WRAIR, Walter Reed Army Institute of Research, Washington, D.C.
Genetic identification of R. rickettsii isolates.
Genetic identification of the R. rickettsii isolates was done by restriction fragment length polymorphism analysis of a PCR-amplified DNA as previously described (26, 47). Purified microorganisms were washed in distilled water, boiled, and used as a template for PCR amplification. PCR primers Rr190.70p and Rr190.602n were used to amplify a 532-bp fragment of the R. rickettsii rOmpA sequence. PCR amplification was performed using Perkin-Elmer PCR reagents (Roche Molecular Systems, Inc., Branchburg, N.J.) (26, 47). Twenty-five microliters of amplified product was digested with RsaI and PstI restriction nucleases (New England Biolabs, Beverly, Mass.) and analyzed on 10% polyacrylamide gels as described previously (26).
Infection of EA.hy 926 cells with R. rickettsii isolates.
The suspension of purified rickettsiae was diluted in cell culture medium, and EA.hy 926 cell monolayers were inoculated at a ratio of 0.25 to 1.0 PFU per cell in 0.3 ml per 35-mm-diameter dish. After rickettsiae were allowed to adhere for 2 h at room temperature with rocking, the volume of the cell culture medium was adjusted to 2 ml, and dishes were transferred to an incubator and maintained at 35°C in 5% CO2. The infection was monitored at 24-h intervals by examination of smears stained with acridine orange (37) and by visual observation of cytopathic effects by inverted phase microscopy of the dishes. At selected times the cell culture medium was aspirated, the monolayers were rinsed with phosphate-buffered saline, and the dishes were processed for further analysis as described below.
Determination of released LDH activity.
The activity of lactate dehydrogenase (LDH) was measured using an LDH-10 kit (Sigma). Supernatants from uninfected control and infected cells were collected in Eppendorf tubes held on ice and spun for 5 min at maximum speed (12,000 rpm) in a tabletop centrifuge (Sorvall Fresco Biofuge; Kendro Laboratory Products, Newton, Conn.). Fifty-microliter portions of cleared supernatants were used for the assay, which was performed at room temperature according to the manufacturer's instructions. The results of the assay were expressed as units of LDH per dish.
Determination of intracellular pools of reduced glutathione.
Glutathione concentrations were determined by the method of Saville (50) in 96-well plates (Fisher Scientific) as previously described (24). Cell proteins were precipitated with 6.5% (wt/vol) trichloroacetic acid, followed by colorimetric measurement of reduced glutathione in the supernatants. The color reaction was developed after 5 min of incubation with a substrate solution containing 0.2% N-(1-naphthyl)ethylenediamine dihydrochloride (ICN Biomedical Inc., Aurora, Ohio) in distilled water. The absorbance at 540 nm was read in a Multiscan Ascent microplate reader (LabSystems, Franklin, Mass.); the blank solution consisted of 6.5% trichloroacetic acid processed in the same manner as for the supernatant fractions. The standard curve was generated from triplicate samples of serial dilutions of reduced glutathione (Sigma), and the results were expressed as nanomoles of glutathione per dish.
Determination of pools of intracellular peroxide.
Endothelial cells were assayed for intracellular peroxide by a modification of the method of Cathcart et al. (16). For this, 5(and 6)-carboxy-2′,7′-dichlorofluorescein diacetate (Molecular Probes, Eugene, Oreg.) was used as a fluorescent probe, and total fluorescence was measured in a Shimadzu model RF-5301PC fluorescence spectrophotometer with an emission wavelength of 535 nm and an excitation wavelength of 505 nm. The background fluorescence, determined in the absence of added cell samples, was subtracted. Peroxide levels were expressed as fluorescence units per milligram of protein. The protein concentration was measured in 96-well plates by the method of Smith et al. (54) using the bicinchoninic acid protein assay reagent (Pierce, Rockford, Ill.).
Statistical analysis.
For each experiment, three replicate dishes were used for each time point and experimental variable. Measurements of glutathione were also done in triplicate on each sample. The mean, standard deviation, and standard error of the mean were calculated. Statistical significance was assessed by Student's t test (α = 0.05).
RESULTS
Genetic characterization of rickettsial isolates.
Genetic identification of all spotted fever group (SFG) isolates as R. rickettsii was confirmed before biochemical experiments were started. This step was necessary to avoid potential misidentification and/or mislabeling of the isolates included in this study at the times of their original isolation and of subsequent passages by different laboratories (Table 1). It was particularly important since R. montana, R. rhipicephali, R. parkeri, R. amblyommi, R. bellii, and several unnamed SFG rickettsiae also circulate in areas where R. rickettsii is endemic and share common tick vectors (7, 13, 14, 39, 41, 42, 59).
Restriction fragment length polymorphism-PCR analysis with the species-specific Rr190.70-602pn primer pair was used for the identification. This primer pair amplifies a 532-bp fragment of the rOmpA gene of R. rickettsii strain Sheila Smith and homologous sequences from other species of SFG rickettsiae. The amplicon has RsaI and PstI restriction sites which are unique to R. rickettsii and which distinguish it from other SFG species found in the United States, including R. montana, R. parkeri, and R. rhipicephali, and other known SFG species (26, 29, 47). A 532-bp fragment was obtained with DNAs from all isolates of SFG rickettsiae included in this study. Restriction profiles of the amplicons, obtained by digestion with RsaI or PstI, were identical to each other and to that of the type strain of R. rickettsii, Sheila Smith. For each isolate, as expected, the patterns contained three fragments with RsaI restriction endonuclease (a double band of 220 bp and a 106-bp fragment) and three fragments with PstI (226, 205, and 80 bp) (data not shown).
Cell culture characteristics of R. rickettsii isolates.
Inoculation of the EA.hy 926 endothelial cell line with R. rickettsii strain Sheila Smith results in productive infection (25). Progressive multiplication of rickettsiae results in accumulation of damaged cells in the monolayer. This is typically observed as cytopathic foci of rounded cells which then detach into the cell culture medium. Increased detachment resulted in the appearance of holes in the infected monolayers. Typically, infection with 0.25 PFU per cell with strain Sheila Smith causes significant damage to infected monolayers by 72 to 96 h after infection.
Isolates Bitterroot, Sutkiewicz, and Long Horse Canyon caused cytopathic effects similar to those observed for strain Sheila Smith. Isolates 84JG, Morgan, Hlp#2, Colombia, and Brazil usually caused cytopathic effects no more than 1 to 2 days later than those seen in the Sheila Smith-like group of strains. Isolates Price T and Sawtooth readily infected EA.hy 926 cells and multiplied; however, cytopathic effects were not observed in these cultures at all, since the visible damage caused by these isolates was very minimal. The infected viable cells in these cultures had the same appearance as uninfected cultures of the same age.
Dosage-dependent effects of R. rickettsii infection on biochemical parameters measured in EA.hy 926 endothelial cells.
Three isolates, Bitterroot, 84JG, and Price T, were selected for study as representative isolates which differed markedly in their morphological cytopathic effects. In preliminary experiments we selected appropriate experimental conditions for determining the biochemical measures of cellular injury. We then evaluated the rickettsial dosage dependence, reproducibility, and variability of these assays.
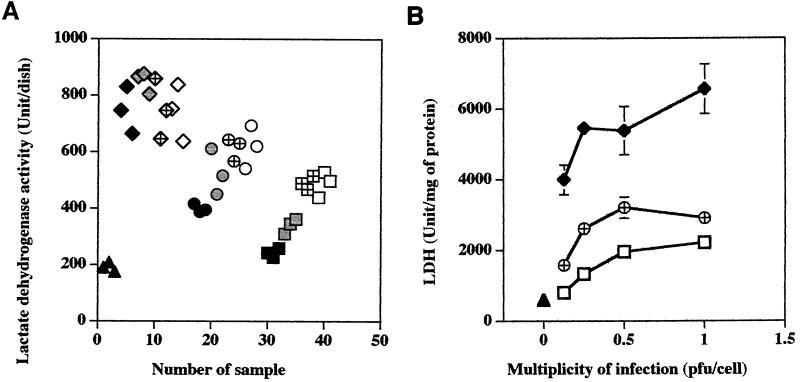
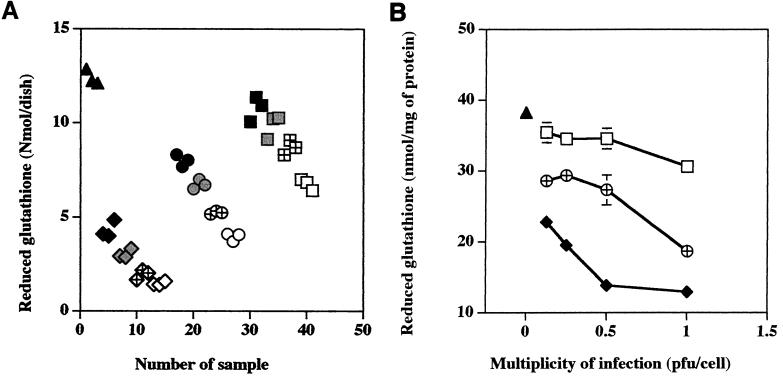
The EA.hy 926 endothelial cells were infected at ratios of 1:1, 1:2, 1:4, and 1:8 PFU per cell, and the levels of extracellular LDH and intracellular glutathione were measured at 96 h postinfection. The measurements were done at 96 h after infection because our previous experiments had demonstrated that at this time cultures infected with the highly cytotoxic isolates developed maximal changes (23, 25), and the earliest signs of infection are only then detectable in cultures infected with an isolate causing low cytotoxicity (this study). The results of these experiments are shown in Fig. 1 and 2. Figures 1A and 2A illustrate the scatterplot distribution of individual measurements obtained for a given condition for a single dish, and Fig. 1B and 2B show the dosage-dependent changes of the means of these replicate dish results normalized to the protein content as determined for each experimental variable. Very little deviation in either LDH or glutathione measurements was apparent from the scatterplots or the standard deviations of the measurements obtained for a single experimental condition.
FIG. 1.
Dosage-dependent release of LDH by EA.hy 926 endothelial cells infected with different isolates of R. rickettsii. Triangles, uninfected cells; diamonds, cells infected with isolate Bitterroot; circles, cells infected with isolate 84JG; squares, cells infected with isolate Price T. (A) Solid symbols, uninfected cells and cells infected at 0.125 PFU/cell; shaded symbols, cells inoculated at 0.25 PFU/cell; hatched symbols, cells inoculated at 0.5 PFU/cell; open symbols, cells inoculated at 1.0 PFU/cell. The data are given as individual measurements obtained for a single dish (A) or means ± standard errors of LDH units divided by the protein content for each dish (B). Many error bars are not seen due to the low standard error of the mean obtained. The statistical significance of the variables compared is given in the text.
FIG. 2.
Dosage-dependent changes of intracellular glutathione levels in EA.hy 926 endothelial cells infected with different isolates of R. rickettsii. Triangles, uninfected cells; diamonds, cells infected with isolate Bitterroot; circles, cells infected with isolate 84JG; squares, cells infected with isolate Price T. (A) Solid symbols, uninfected cells and cells infected at 0.125 PFU/cell; shaded symbols, cells inoculated at 0.25 PFU/cell; hatched symbols, cells inoculated at 0.5 PFU/cell; open symbols, cells inoculated at 1.0 PFU/cell. The data are given as means of triplicate measurements obtained for a single dish (A) or means ± standard errors normalized to the protein content for three dishes per experimental variable (B). Many error bars are not seen due to the low standard error of the mean obtained. The statistical significance of the variables compared is given in the text.
At 96 h after inoculation, infection of EA.hy 926 cells with isolate Price T induced a strongly dosage-dependent release of LDH into the cell culture medium, ranging from 1.3 to 2.6 times the amount of LDH released from control cells (Fig. 1A). The amounts of LDH released by all doses of Price T were significantly greater than the amount released by control cells (P < 0.01) (Fig. 1B). Even the lowest inoculation dose, 0.125 PFU per cell, caused significant damage to the EA.hy 926 cells (797 U/mg of protein) compared to control cells (586 U/mg of protein). Cell cultures infected with isolate 84JG also released a highly dosage-dependent quantity of cytoplasmic LDH into the cell culture medium. Its increase was greater than that observed for the Price T isolate at all equivalent doses of rickettsiae, and it ranged from 2.1- to 3.3-fold higher than the LDH quantity released from uninfected control cells during the same time frame (Fig. 1A). Cultures infected with different doses of isolate Bitterroot were damaged the most, except that the LDH release (3.9 to 4.5 times that of control cells [Fig. 1A]) was not dose dependent, as the amount released by the three highest doses was not significantly different (P > 0.21) (Fig. 1B).
Similar experiments were done to measure the levels of intracellular glutathione at 96 h postinfection (Fig. 2). In the scattergram plots of individual average measurements with dishes of cells infected with isolates Bitterroot, 84JG, and Price T, all exhibited dosage-dependent reductions in intracellular pools of reduced glutathione (Fig. 2A). Only the highest dose of the Price T isolate, 1 PFU per cell, caused changes in glutathione levels significantly different from those of uninfected cells (P < 0.001) (Fig. 2B). In contrast, all doses of the 84JG and Bitterroot isolates caused a significant reduction of glutathione pools (P < 0.01). At each dose of rickettsiae, Bitterroot caused the greatest reduction, 84JG caused an intermediate reduction, and Price T caused the least decrease in glutathione levels, and the differences between isolates at each dose were significant (P < 0.05) (Fig. 2B). No significant differences in glutathione levels were found with 0.5 and 1 PFU per cell following infection with isolate Bitterroot (P > 0.05), but this is not surprising, since both doses of rickettsiae nearly completely depleted the EA.hy 926 cells of glutathione (Fig. 2B).
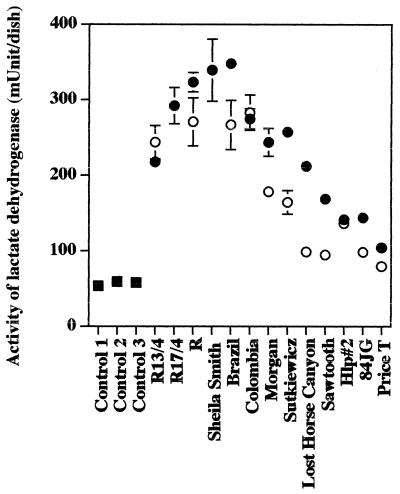
Release of LDH from EA.hy 926 endothelial cells by infection with 11 isolates of R. rickettsii.
Of the 11 isolates of R. rickettsii compared in this study at 1 PFU per cell, infection of EA.hy 926 cells with 7 isolates, i.e., Sheila Smith, Bitterroot (R), Brazil, Morgan, Sutkiewicz, Colombia, and Lost Horse Canyon, caused marked cellular injury which resulted in at least a fourfold increase in the release of cytoplasmic LDH into the cell culture medium, and 4 of these (Sheila Smith, Bitterroot, Brazil, and Colombia) also released similar amounts of LDH even after infection with only 0.25 PFU per cell (Fig. 1A and 3; Table 2). At 1 PFU/cell, three isolates, i.e., Hlp #2, Sawtooth, and 84JG, released at least 2.6 times the amount for control cells (P < 0.001) (Fig. 3), and at 0.25 PFU per cell they released lower (at least 1.66 times control levels) but still significant levels (P < 0.01) (Fig. 3). In this experiment isolate Price T released significant levels of LDH (1.84 times that for control cells) only following infection with 1 PFU/cell (P < 0.01) (Fig. 3).
FIG. 3.
Release of LDH from EA.hy 926 human endothelial cells following infection with different isolates of R. rickettsii. Squares, uninfected cells; solid circles, cells infected with 1 PFU/cell; open circles, cells inoculated with 0.25 PFU/cell. The data are presented as means ± standard errors (for three dishes per experimental variable). Some error bars are not visible due to the low standard error of the mean obtained. The statistical significance of the variables compared is given in the text.
TABLE 2.
Summary of changes in biochemical measures of cellular injury caused by infection with different isolates of R. rickettsii
| Isolate | Degree of change in each biochemical measurea at the indicated multiplicity of rickettsial infection (PFU/cell)
|
|||||
|---|---|---|---|---|---|---|
| LDH release
|
Intracellular peroxide
|
Intracellular glutathione
|
||||
| 1 | 0.25 | 1 | 0.25 | 1 | 0.25 | |
| Group I | ||||||
| Sheila Smith | 4+ | NDb | 3+ | ND | 4+ | ND |
| Bitterroot | 3–4+ | 3+ | 4+ | 1–2+ | 4+ | 3+ |
| Brazil | 4+ | 3+ | 3+ | 1+ | 4+ | 2+ |
| Morgan | 3+ | 2+ | 3+ | 3+ | 4+ | 2+ |
| Sutkiewicz | 3+ | 2+ | 3+ | 2+ | 4+ | 3+ |
| Group II | ||||||
| Colombia | 4+ | 4+ | 2+ | 1+ | 4+ | 2+ |
| Lost Horse Canyon | 3+ | 1+ | 2+ | 1+ | 4+ | 2+ |
| Hlp #2 | 2+ | 2+ | 3+ | 3+ | 4+ | 3+ |
| Group III | ||||||
| Sawtooth | 2+ | 1+ | 2+ | 2+ | 2+ | 1+ |
| 84JG | 2+ | 1+ | 2+ | 1+ | 2+ | 1+ |
| Group IV | ||||||
| Price T | 1+ | 1+ | 1+ | 1+ | 1+ | 1+ |
For each measure the quartile of the range between the largest change observed following infection with any isolate and the mean of the control cell culture values was determined from data in Fig. 3 to 5: 4+, 76 to 100% of range; 3+, 51 to 75% of range; 2+, 26 to 50% of range; 1+, 0 to 25% of range. Values in boldface indicate changes of lower magnitude than found with the other measures determined for that isolate.
ND, not determined.
Influence of different R. rickettsii isolates on the concentration of intracellular peroxide.
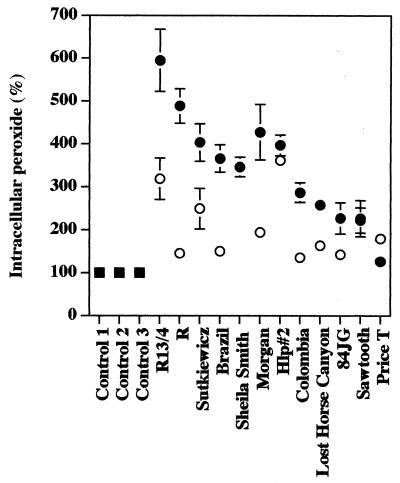
Multiplication of R. rickettsii Sheila Smith in cultured human endothelial cells causes significant accumulation of intracellular peroxide (53). In contrast to the release of LDH, this accumulation (at least 3.5 times the content of control cells) was strongly dosage dependent for all of the highly cytotoxic isolates (the Sheila Smith isolate was tested at 1 PFU/cell only) except Hlp#2, which caused this level of intracellular peroxide accumulation even at 0.25 PFU per cell (Fig. 4; Table 2). However, even infection with 0.25 PFU per cell caused accumulation of peroxide at levels significantly greater than that for controls; high levels of peroxide accumulated in cells infected with the isolates Sheila Smith, Bitterroot, Brazil, Morgan, Sutkiewicz, and Hlp#2. Infection at 1 PFU per cell with isolates Colombia, Lost Horse Canyon, Sawtooth, and 84JG caused intracellular accumulation of peroxide at levels 2.2 to 2.8 times those of control cells and, except for Sawtooth, much lower but still significant levels following infection at 0.25 PFU per cell (P < 0.04) (Fig. 4; Table 2). Isolate Price T caused only very small changes in intracellular peroxide compared to control cells at either multiplicity of infection (Fig. 4; Table 2).
FIG. 4.
Accumulation of peroxide in EA.hy 926 human endothelial cells infected with different isolates of R. rickettsii. Squares, uninfected cells; solid circles, cells infected with 1 PFU/cell; open circles, cells inoculated with 0.25 PFU/cell. The data are presented as means ± standard errors (for three dishes per experimental variable). Some error bars are not visible due to the low standard error of the mean obtained. The statistical significance of the variables compared is given in the text.
Influence of R. rickettsii isolates on levels of reduced glutathione.
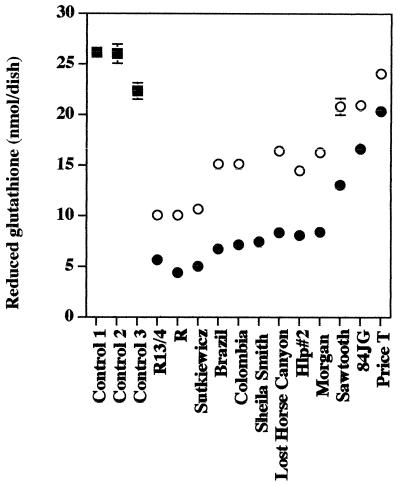
Eight of the R. rickettsii isolates, i.e., Sheila Smith, Bitterroot, Brazil, Morgan, Sutkiewicz, Colombia, Lost Horse Canyon, and Hlp#2, caused similar and large dosage-dependent reductions in the intracellular reduced thiol pools of EA.hy 926 cells following infection with either 1 or 0.25 PFU per cell (Fig. 5; Table 2). The quantity of reduced glutathione in cell cultures infected with 1 PFU per cell was only 18 to 34% of that in uninfected control cells (P < 0.001) (Fig. 5). Only three isolates, i.e., Sawtooth, 84JG, and Price T, were significantly different (P < 0.05) from this group, and the reduction in glutathione was again strongly dosage dependent. Infection at 1 PFU per cell with isolate Sawtooth and isolate 84JG resulted in 47 and 33% decreases of thiol levels, respectively, compared to that of uninfected control (P < 0.001) (Fig. 5; Table 2), while infection with isolate Price T caused very little change in the glutathione level, which was only 18% below that measured in uninfected control cells (P < 0.001). When the same experiments were performed using 0.25 PFU per cell, the reduction of thiol levels caused by isolates Sawtooth, 84JG, and Price T were not significant (P > 0.05) (Fig. 5; Table 2).
FIG. 5.
Effects of R. rickettsii isolates on glutathione levels in EA.hy 926 human endothelial cells. Squares, uninfected cells; solid circles, cells infected with 1 PFU/cell; open circles, cells inoculated with 0.25 PFU/cell. The data are presented as means ± standard errors (for three dishes per experimental variable). Some error bars are not visible due to the low standard error of the mean obtained. The statistical significance of the variables compared is given in the text.
DISCUSSION
The majority of the isolates of R. rickettsii included in this study have been characterized previously by several authors using a guinea pig model of infection which permits their categorization by the degree of their pathogenicity (3, 44, 46). However, because complex interactions undoubtedly underlie the pathogenesis of R. rickettsii infection in vivo, it is very difficult to define the causality of individual events which occur in animals. It is even more difficult to determine the nature of differences among rickettsial isolates in vivo and to quantify them objectively. In contrast, differences in the cellular damage elicited in cultured endothelial cells by R. rickettsii isolates were readily demonstrated by measuring any of three parameters: release of cytoplasmic LDH into the cell culture medium, which is correlated with cell viability and integrity of the host cell membrane (18), and the intracellular levels of reduced glutathione and peroxide, which correlate with the degree of oxidative injury of infected cells (53).
The 11 isolates of R. rickettsii could be separated into four groups based on the results obtained in this study (Table 2). Group I included the Sheila Smith human and Bitterroot tick isolates of R. rickettsii from Montana, isolate Sutkiewicz from Maryland, isolate Morgan from North Carolina, and isolate Brazil from South America. These isolates possessed very cytotoxic phenotypes regardless of the biochemical measurement used. Consequently, cytotoxic strains are not restricted to a single geographic region. The morphological cytopathic effects caused by these isolates developed very quickly in the EA.hy 926 human endothelial cells even at a relatively low multiplicity of infection (0.25 PFU per cell). They caused a dramatic destruction of cell structure and cell membrane integrity, which resulted in the release of cytoplasmic LDH into the extracellular milieu. These cells accumulated large quantities of intracytosolic hydrogen peroxide, and their intracellular pools of reduced glutathione were significantly depleted, possibly indicating the total inability of the cells to respond adequately to the oxidative injury caused by these isolates of rickettsiae. The Bitterroot isolate was classified in category R by Price (46) because it exhibited the most severe manifestations of virulence in guinea pigs.
Three additional isolates that were placed in group II possessed characteristics that were quite similar to those of the isolates placed in group I. Isolate Colombia from South America and two tick isolates from Montana, Hlp#2 and Lost Horse Canyon, each exhibited lower biochemical injury than group I isolates in at least one biochemical parameter, either LDH release or accumulation of intracellular peroxide (Table 2). These isolates exhibited similar morphological cytopathic effects which did not appear to differ significantly from those caused by group I isolates. However, Hlp-type strains are often categorized as being of lower virulence (2, 3, 38).
The third group included isolates 84JG and Sawtooth, which caused markedly less cellular damage than groups I and II as measured with any of the three biochemical measures at both of the multiplicities of infection that were used (Table 2). The 84JG strain was isolated from a patient who experienced fulminant RMSF in North Carolina (D. H. Walker, personal communication). This individual had hemolysis during the infection. Consequently, both host and microbial factors are probably important contributors to virulence of R. rickettsii in humans. Strain Sawtooth was isolated from a tick from Montana and was originally described as having a cytotoxic phenotype (12). However, Sawtooth, but not 84JG, exhibited markedly lower cytopathology when observed by microscopy.
A fourth group was represented solely by isolate Price T (Table 2). Its cytotoxicity for EA.hy 926 human endothelial cells was very low by all biochemical measures and by microscopic examination, a finding that correlates very well with the observed low pathogenicity of this isolate in guinea pigs (45).
Whether R. rickettsii has the intrinsic ability to adapt to different conditions which it experiences during the natural cycle and, particularly, whether these are recurrent, programmed, or permanent changes in the microorganism are unknown. Circulation of R. rickettsii in nature includes vertical transtadial and transovarial transmission with its tick vectors and horizontal passages through vertebrate hosts. R. rickettsii strains that have undergone multiple laboratory passages in guinea pigs, meadow voles, or cottontail rabbits lose their original virulent phenotype (44, 45). On the other hand, passage of these attenuated strains through embryonated chicken eggs or ticks reverted them to their original virulence phenotype in guinea pigs. Reversion of the virulent phenotype of rickettsiae (reactivation) also occurs in overwintered ticks after a blood meal or incubation at 37°C for 24 to 48 h (44, 45, 56). It is possible that the isolates of R. rickettsii which exhibit different virulence for guinea pigs differ primarily because they were isolated during different phases of a normal cycle of adaptation to their vertebrate and invertebrate hosts that is characteristic of the species. R. sibirica also has a complex cycle of maintenance in nature and distinct biotypes that differ in virulence for guinea pigs (6). Characterization of the changes in rickettsial gene expression that may occur immediately after tick-to-human or tick-to-animal transitions may require development of more complex experimental systems.
Several microbial factors probably contribute to the varied ability of R. rickettsii to cause cellular injury. Some of these factors may also contribute importantly to its virulence for vertebrate hosts. R. rickettsii isolates may differ in their abilities to adhere to and enter into a host cell. They may also differ in the rates of their intracellular growth and replication, in their abilities to adapt to different intracellular environments and to interact with different signaling systems of the host, and finally in their abilities to escape from the cytoplasm and spread to adjacent cells.
The initial uptake kinetics of R. rickettsii by chicken embryo cells and L929 cells resembled those established for R. prowazekii (61). Similarly, R. prowazekii strains differing in virulence are not readily distinguished by their ability to enter mouse fibroblasts, human and mouse macrophages, and macrophage-like cells (30, 57). The importance of some variability in adherence of our isolates of R. rickettsii may be very minimal as well. Our studies were standardized by plaque assay in Vero cells to ensure comparable levels of infection of the EA.hy 926 cells by the different isolates. Furthermore, the use of two dilutions of each isolate ensured that even if fourfold differences in titer or uptake occurred, it is unlikely that low levels of cytotoxicity would be due to inadequate numbers of viable rickettsiae being used. Although they were not quantitated in these experiments, we do not believe that the numbers of rickettsiae initially taken up by the EA.hy 926 were significantly different. Consequently, isolate differences in cytotoxicity are not likely due to differences in the initial adhesion and uptake of the rickettsiae. However, possibly rOmpA also plays another role during rickettsial growth in the cells where differences in its structure may be important.
Shortly after R. rickettsii enters the host cell, it begins exponential intracytoplasmic growth without a measurable lag period (61). Differences in the rate of intracellular growth of R. rickettsii might potentially affect the cell-to-cell spread of the microorganisms and the subsequent development of cytotoxic effects which are associated with oxidative injury (53). We have not compared the growth rates of the 11 isolates during this study, so the contribution of this factor to the development of cellular injury remains to be determined.
The ability of rickettsiae to produce lytic plaques does not correlate with animal virulence characteristics, since even supposedly avirulent or low-virulence species like R. parkeri and R. slovaca produce plaques of similar size to those of R. rickettsii (reviewed in reference 22). Plaques of virulent R. prowazekii and Orientia tsutsugamushi are also much smaller than those produced by R. rickettsii (22). We did not notice any significant differences in the rates of plaque formation or plaque size among the 11 isolates studied here (data not shown). Polymerization of actin tails, which has been implicated in the rapid cell-to-cell spread of R. rickettsii, is also not solely associated with virulent phenotypes, since different SFG rickettsiae that vary in their pathogenicities are all able to polymerize actin (23, 33). The role of other components of the cellular cytoskeleton besides actin, especially actin-binding proteins, may be more important for rickettsial infection in endothelial cells (reviewed in reference 23). These cytoskeletal proteins may interact to different degrees with variable proteins that may occur in different isolates of R. rickettsii.
In conclusion, different isolates of R. rickettsii exhibit marked differences in the cellular injury they induce in endothelial cells, which may readily be quantified by biochemical assays. Understanding the molecular basis for the varied cellular injury caused by different isolates of R. rickettsii may contribute to improved treatment of RMSF and to the rapid identification of those isolates which are more likely to cause fulminant disease.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI 17416 from the National Institute of Allergy and Infectious Diseases (to D.J.S. and M.E.E.) and by the Naval Medical Research and Development Command, Research Task 61102A.001.01.BJX.1293 (to G.A.D.).
We are greatly thankful to Cora-Jean S. Edgell for the gift of the EA.hy 926 endothelial cell line.
REFERENCES
- 1.Anacker R L, Mann R E, Gonzales C. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J Clin Microbiol. 1987;25:167–171. doi: 10.1128/jcm.25.1.167-171.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anacker R L, List R H, Mann R E, Wiedbrauk D L. Antigenic heterogeneity in high- and low-virulence strains of Rickettsia rickettsii revealed by monoclonal antibodies. Infect Immun. 1986;51:653–660. doi: 10.1128/iai.51.2.653-660.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anacker R L, Philip R N, Williams J C, List R H, Mann R E. Biochemical and immunochemical analysis of Rickettsia rickettsii strains of various degrees of virulence. Infect Immun. 1984;44:559–564. doi: 10.1128/iai.44.3.559-564.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anacker R L, McCaul T F, Burgdorfer W, Gerloff R K. Properties of selected rickettsiae of the spotted fever group. Infect Immun. 1980;27:468–474. doi: 10.1128/iai.27.2.468-474.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anonymous. Consequences of delayed diagnosis of Rocky Mountain spotted fever in children—West Virginia, Michigan, Tennessee, and Oklahoma, May-July 2000. Morb Mortal Wkly Rep. 2000;49:887–891. [PubMed] [Google Scholar]
- 6.Balayeva N M, Eremeeva M E, Ignatovich V F, Rudakov N K, Reschetnikova T A, Samoilenko I E, Yastrebov V K, Raoult D. Biological and genetic characterization of Rickettsia sibirica strains isolated in the endemic area of the North Asian tick typhus. Am J Trop Med Hyg. 1996;35:685–692. doi: 10.4269/ajtmh.1996.55.685. [DOI] [PubMed] [Google Scholar]
- 7.Bell E J, Kohls G M, Stoenner H G, Lackman D B. Nonpathogenic rickettsiae related to the spotted fever group isolated from ticks, Dermacentor variabilis and Dermacentor andersoni from eastern Montana. J Immunol. 1963;90:770–781. [PubMed] [Google Scholar]
- 8.Bell E J, Pickens E G. A toxic substance associated with the rickettsias of the spotted fever group. J Immunol. 1953;70:461–472. [PubMed] [Google Scholar]
- 9.Bell E J, Stoenner H G. Spotted fever vaccine; potency assay by direct challenge of vaccinated mice with toxin of Rickettsia rickettsii. J Immunol. 1961;87:737–746. [PubMed] [Google Scholar]
- 10.Bell E J, Stoenner H G. Immunologic relationships among the spotted fever group of rickettsias determined by toxin neutralization tests in mice with convalescent animal serums. J Immunol. 1960;84:171–182. [PubMed] [Google Scholar]
- 11.Burgdorfer W. A review of Rocky Mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. J Med Entomol. 1975;12:269–278. doi: 10.1093/jmedent/12.3.269. [DOI] [PubMed] [Google Scholar]
- 12.Burgdorfer W, Brinton L P. Mechanisms of transovarial infection of spotted fever rickettsiae in ticks. Ann NY Acad Sci. 1975;266:61–72. doi: 10.1111/j.1749-6632.1975.tb35088.x. [DOI] [PubMed] [Google Scholar]
- 13.Burgdorfer W, Hayes S F, Thomas L A, Lancaster J L., Jr . A new spotted fever group rickettsia from the lone star tick, Amblyomma americanum. In: Burgdorfer W, Anacker R L, editors. Rickettsiae and rickettsial diseases. New York, N.Y: Academic Press, Inc.; 1981. pp. 595–602. [Google Scholar]
- 14.Burgdorfer W, Brinton L P, Krynski W L, Philip R N. Rickettsia rhipicephali, a new spotted fever group rickettsia from the brown dog tick, Rhipicephalus sanguineus. In: Kazar J, Ormsbee R A, Tarasevich I, editors. Rickettsiae and rickettsial diseases. Bratislava, Czechoslovakia: VEDA Publishing House of the Slovak Academy of Sciencesi; 1978. pp. 307–316. [Google Scholar]
- 15.Campbell C C, Hobbs J H, Marranghello L, Vargas M, Shepard C, Feldmann R A. An apparent outbreak of rickettsial illness in Costa Rica, 1974. Bull PAHO. 1978;12:104–111. [PubMed] [Google Scholar]
- 16.Cathcart R, Schwiers E, Ames B N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Biochim Biophys Acta. 1983;963:558–561. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- 17.Conlon P J, Procop G W, Fowler V, Ali Eloubeidi M, Smith S R, Sexton D J. Predictors of prognosis and risk of acute renal failure in patients with Rocky Mountain spotted fever. Am J Med. 1996;101:621–626. doi: 10.1016/s0002-9343(96)00332-4. [DOI] [PubMed] [Google Scholar]
- 18.Cook J A, Mitchell J B. Viability measurements in mammalian systems. Anal Biochem. 1989;179:1–7. doi: 10.1016/0003-2697(89)90191-7. [DOI] [PubMed] [Google Scholar]
- 19.Dalton M J, Clarke M J, Holman R C, Krebs J W, Fishbein D B, Olson J G, Childs J E. National surveillance for Rocky Mountain spotted fever, 1981–1992: epidemiologic summary and evaluation of risk factors for fatal outcome. Am J Trop Med Hyg. 1995;52:405–413. doi: 10.4269/ajtmh.1995.52.405. [DOI] [PubMed] [Google Scholar]
- 20.deLemos E R, Machado R D, Coura J R, Guimaraes M A, Freire N M S, Amorim M, Gazeta G S. Epidemiological aspects of the Brazilian spotted fever: seasonal activity of ticks collected in an endemic area in Sao Paulo, Brazil. Rev Soc Bras Med Trop. 1997;30:181–185. doi: 10.1590/s0037-86821997000300002. [DOI] [PubMed] [Google Scholar]
- 21.Edgell C-J S, McDonald C C, Graham J B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eremeeva M E, Dasch G A, Silverman D J. Interaction of rickettsiae with eukaryotic cells. Adhesion, entry, intracellular growth, and host cell responses. In: Oelschlaeger T, Hacker J, editors. Subcellular biochemistry. 33. Bacterial invasion into eukaryotic cells. New York, N.Y: Kluwer Academic/Plenum Publishers; 2000. pp. 479–516. [PubMed] [Google Scholar]
- 23.Eremeeva M E, Santucci L A, Popov V L, Walker D H, Silverman D J. Rickettsia rickettsii infection of human endothelial cells: oxidative injury and reorganization of cytoskeleton. In: Raoult D, Brouqui P, editors. Rickettsiae and rickettsial diseases at the turn of the third millennium. Paris, France: Elsevier; 1999. pp. 128–144. [Google Scholar]
- 24.Eremeeva M E, Silverman D J. Effects of the antioxidant alpha-lipoic acid on human umbilical vein endothelial cells infected with Rickettsia rickettsii. Infect Immun. 1998;66:2290–2299. doi: 10.1128/iai.66.5.2290-2299.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eremeeva M E, Silverman D J. Rickettsia rickettsii infection of the EA.hy 926 endothelial cell line: morphological response to infection and evidence for oxidative injury. Microbiology. 1998;144:2037–2048. doi: 10.1099/00221287-144-8-2037. [DOI] [PubMed] [Google Scholar]
- 26.Eremeeva M, Yu X-J, Raoult D. Differentiation among spotted fever group rickettsia species by analysis of restriction fragment length polymorphism of PCR-amplified DNA. J Clin Microbiol. 1994;32:803–810. doi: 10.1128/jcm.32.3.803-810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fishbein D B, Frontini M G, Giles R, Vernon L L. Fatal cases of Rocky Mountain spotted fever in the United States, 1981–1988. Ann NY Acad Sci. 1990;590:246–247. doi: 10.1111/j.1749-6632.1990.tb42227.x. [DOI] [PubMed] [Google Scholar]
- 28.Fuentes L. Ecological study of Rocky Mountain spotted fever in Costa Rica. Am J Trop Med Hyg. 1986;35:192–196. doi: 10.4269/ajtmh.1986.35.192. [DOI] [PubMed] [Google Scholar]
- 29.Gage K L, Schrumpf M E, Karstens R H, Burgdorfer W, Schwan T G. DNA typing of rickettsiae in naturally infected ticks using a polymerase chain reaction/restriction fragment length polymorphism system. Am J Trop Med Hyg. 1994;50:247–260. doi: 10.4269/ajtmh.1994.50.247. [DOI] [PubMed] [Google Scholar]
- 30.Gambrill M R, Wisseman C L., Jr Mechanisms of immunity in typhus infections. II. Multiplication of typhus rickettsiae in human macrophage cell cultures in the nonimmune system: influence of virulence of rickettsial strains and of chloramphenicol. Infect Immun. 1973;8:519–527. doi: 10.1128/iai.8.4.519-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gimenez D F. Staining rickettsiae in yolk sac culture. Stain Technol. 1964;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- 32.Hattwick M A W, Retailliau H, O'Brien R J, Slutzker M, Fontaine R E, Hanson B. Fatal Rocky Mountain spotted fever. JAMA. 1978;240:1499–1503. [PubMed] [Google Scholar]
- 33.Heinzen R A, Hayes S F, Peacock M G, Hackstadt T. Directional actin polymerization associated with spotted fever group rickettsia infection of Vero cells. Infect Immun. 1993;61:1926–1935. doi: 10.1128/iai.61.5.1926-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helmick C G, Bernard K W, D'Angelo L J. Rocky Mountain spotted fever: clinical, laboratory, and epidemiological features of 262 cases. J Infect Dis. 1984;150:480–488. doi: 10.1093/infdis/150.4.480. [DOI] [PubMed] [Google Scholar]
- 35.Kirkland K B, Wilkinson W E, Sexton D J. Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. Clin Infect Dis. 1995;20:1118–1121. doi: 10.1093/clinids/20.5.1118. [DOI] [PubMed] [Google Scholar]
- 36.Latham R H, Schaffner W. Rocky Mountain spotted (and spotless) fever. Compr Ther. 1992;18:18–21. [PubMed] [Google Scholar]
- 37.Lauer B A, Reller L B, Mirrett S. Comparison of acridine orange and Gram stains for detection of microorganisms in cerebrospinal fluid and other clinical specimens. J Clin Microbiol. 1981;14:201–205. doi: 10.1128/jcm.14.2.201-205.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker R R, Pickens E G, Lackman D B, Bell E J, Thraikill F B. Isolation and characterization of Rocky Mountain spotted fever rickettsiae from the rabbit tick Haemaphysalis leporis-palustris Packard. Public Health Rep. 1951;66:455–463. [PMC free article] [PubMed] [Google Scholar]
- 39.Parker R R, Kohls G M, Cox G W, Davis G E. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep. 1939;54:1482–1484. [Google Scholar]
- 40.Patino L, Afandar A, Paul J H. A spotted fever in Tobia, Colombia. Preliminary report. Am J Trop Med. 1937;17:639. [Google Scholar]
- 41.Philip R N, Casper E A, Anacker R L, Cory J, Hayes S F, Burgdorfer W, Yunker C E. Rickettsia bellii sp. nov.: a tick-borne rickettsia, widely distributed in the United States, that is distinct from the spotted fever and typhus biogroups. Int J Syst Bacteriol. 1983;33:94–106. [Google Scholar]
- 42.Philip R N, Casper E A. Serotypes of spotted fever group rickettsiae isolated from Dermacentor andersoni (Stiles) ticks in Western Montana. Am J Trop Med Hyg. 1981;30:230–238. doi: 10.4269/ajtmh.1981.30.230. [DOI] [PubMed] [Google Scholar]
- 43.Philip R N, Casper E A, Burgdorfer W, Gerloff R K, Hughes L E, Bell E J. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978;121:1961–1968. [PubMed] [Google Scholar]
- 44.Price W H. Variation in virulence of “Rickettsia rickettsii” under natural and experimental conditions. In: Hartman F W, Horsfall F L Jr, Kidd J G, editors. The dynamics of virus and rickettsial infections. New York, N.Y: Blakiston; 1954. pp. 164–183. [Google Scholar]
- 45.Price W H. The epidemiology of Rocky Mountain spotted fever. II. Studies on the biological survival mechanism of Rickettsia rickettsii. Am J Hyg. 1954;60:292–319. doi: 10.1093/oxfordjournals.aje.a119723. [DOI] [PubMed] [Google Scholar]
- 46.Price W H. The epidemiology of Rocky Mountain spotted fever. I. The characterization of strain virulence of Rickettsia rickettsii. Am J Hyg. 1953;58:248–268. doi: 10.1093/oxfordjournals.aje.a119604. [DOI] [PubMed] [Google Scholar]
- 47.Regnery R L, Spruill C L, Plikaytis B D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991;173:1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ripoll C M, Remondegui C E, Ordonez G, Arazamendi R, Fusaro H, Hyman M J, Paddock C D, Zaki S R, Olson J G, Santos-Buch C A. Evidence of rickettsial spotted fever and ehrlichial infections in a subtropical territory of Jujuy, Argentina. Am J Trop Med Hyg. 1999;61:350–354. doi: 10.4269/ajtmh.1999.61.350. [DOI] [PubMed] [Google Scholar]
- 49.Rucker W C. Rocky Mountain spotted fever. Public Health Rep. 1912;27:1465–1482. [Google Scholar]
- 50.Saville B. A scheme for the colorimetric determination of microgram amounts of thiols. Analyst. 1958;83:670–672. [Google Scholar]
- 51.Sexton D J, Muniz M, Corey G R, Breitschwerdt E B, Hegarty B C, Dumler S, Walker D H, Pecanha P M, Dietze R. Brazilian spotted fever in Espirito Santo, Brazil: description of a focus of infection in a new endemic region. Am J Trop Med Hyg. 1993;49:222–226. doi: 10.4269/ajtmh.1993.49.222. [DOI] [PubMed] [Google Scholar]
- 52.Sexton D J, Corey G R. Rocky Mountain “spotless” and “almost spotless” fever: a wolf in sheep's clothing. Clin Infect Dis. 1992;15:439–448. doi: 10.1093/clind/15.3.439. [DOI] [PubMed] [Google Scholar]
- 53.Silverman D J. Oxidative cell injury and spotted fever group rickettsiae. In: Anderson B, Bendenelli M, Friedman H, editors. Rickettsial infection and immunity. New York, N.Y: Plenum Publishing Corp.; 1997. pp. 79–98. [Google Scholar]
- 54.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 55.Sonenshine D E, Atwood E L, Lamb J T., Jr The ecology of ticks transmitting Rocky Mountain spotted fever in a study area in Virginia. Ann Entomol Soc Am. 1966;59:1234–1262. doi: 10.1093/aesa/59.6.1234. [DOI] [PubMed] [Google Scholar]
- 56.Spencer H R, Parker R R. Rocky Mountain spotted fever: infectivity of fasting and recently fed ticks. Public Health Rep. 1923;38:333–339. [Google Scholar]
- 57.Turco J, Winkler H H. Differentiation between virulent and avirulent strains of Rickettsia prowazekii by macrophage-like cell lines. Infect Immun. 1982;35:783–791. doi: 10.1128/iai.35.3.783-791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker D H, Lane T W. Rocky Mountain spotted fever: clinical signs, symptoms, and pathophysiology. In: Walker D H, editor. Biology of rickettsial diseases. Boca Raton, Fla: CRC Press, Inc.; 1988. pp. 63–78. [Google Scholar]
- 59.Weller S J, Baldridge G D, Munderloh U G, Noda H, Simser J, Kurtti T J. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J Clin Microbiol. 1998;36:1305–1317. doi: 10.1128/jcm.36.5.1305-1317.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wike D A, Burgdorfer W. Plaque formation in tissue cultures by Rickettsia rickettsii isolated directly from whole blood and tick hemolymph. Infect Immun. 1972;6:736–738. doi: 10.1128/iai.6.5.736-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wisseman C L, Jr, Edlinger E A, Waddell A D, Jones M R. Infection cycle of Rickettsia rickettsii in chicken embryo and L-929 cells in culture. Infect Immun. 1976;14:1052–1064. doi: 10.1128/iai.14.4.1052-1064.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zavala-Velazquez J E, Ruiz-Sosa J, Vado-Solis I, Billings A N, Walker D H. Serologic study of the prevalence of rickettsiosis in Yucatan: evidence for a prevalent spotted fever group rickettsiosis. Am J Trop Med. 1999;61:405–408. doi: 10.4269/ajtmh.1999.61.405. [DOI] [PubMed] [Google Scholar]