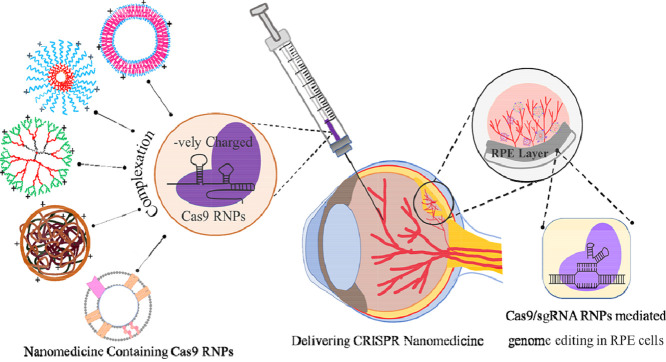
Abstract
CRISPR/Cas, an adaptive immune system in bacteria, has been adopted as an efficient and precise tool for site-specific gene editing with potential therapeutic opportunities. It has been explored for a variety of applications, including gene modulation, epigenome editing, diagnosis, mRNA editing, etc. It has found applications in retinal dystrophic conditions including progressive cone and cone-rod dystrophies, congenital stationary night blindness, X-linked juvenile retinoschisis, retinitis pigmentosa, age-related macular degeneration, leber's congenital amaurosis, etc. Most of the therapies for retinal dystrophic conditions work by regressing symptoms instead of reversing the gene mutations. CRISPR/Cas9 through indel could impart beneficial effects in the reversal of gene mutations in dystrophic conditions. Recent research has also consolidated on the approaches of using CRISPR systems for retinal dystrophies but their delivery to the posterior part of the eye is a major concern due to high molecular weight, negative charge, and in vivo stability of CRISPR components. Recently, non-viral vectors have gained interest due to their potential in tissue-specific nucleic acid (miRNA/siRNA/CRISPR) delivery. This review highlights the opportunities of retinal dystrophies management using CRISPR/Cas nanomedicine.
Keywords: CRISPR/Cas9, Gene editing, Retinal dystrophies, Non-viral nanocarriers
Graphical abstract
1. Introduction
Prokaryotes, especially bacteria, dwell in a variety of environments, including many unfavorable conditions. This means they have many methods by which they adapt to survive in the harsh habitat. The defense systems acting in an undefined, natural way include the restriction-modification, abortive infection, and surface exclusion systems [1]. Recent studies have also shown an acquired immune system, such as the clustered regularly interspaced short palindromic repeats (CRISPR), in prokaryotic organisms, both in bacteria and archaea. These are repeat sequence elements with, 21–37 bp in length, separated by spacers of similar size but varying composition. It forms a part of the adaptive immune system developed for protection against the attacking phage. The bacterium cleaves the genome of the invading virus and assimilates short viral genetic segments amongst its CRISPR sequences, which constitutes the pathogen-specific spacer elements. Thus, when the same virus attacks the bacterium subsequently, the CRISPR RNA (crRNA) and trans-activating CRISPR RNA (tracrRNA) guide the organism's CRISPR-associated (Cas) endonuclease to the foreign DNA complementary to its sequence, thereby degrading the invading viral genome [2]. A protospacer adjacent motif (PAM) that is present only in the viral genome and not in the bacterium helps it to differentiate self from non-self, thus cleaving and inactivating the virus [3].
CRISPR/Cas was discovered in 1987 and firstly demonstrated as therapeutic gene editing tool in mammalian cells in 2013 by Zhang and Church [4]. Since then, it has been identified as a potential therapeutic tool for genome editing and has been extensively studied for its application in many genetic and non-genetic diseases, including retinal dystrophies, cancer, hematological disorders, muscular dystrophies, neurodegenerative diseases, etc. The updated classification of CRISPR-Cas systems is based on the sequences of the Cas genes, the order of the repeats within the CRISPR arrays, and the organization of the Cas operons [5]. According to this system, there are three classes of CRISPR-Cas i.e. types I–III. Each type is further divided into subtypes, ranging from I-A to I-F, II-A to II-C, III-A and III-B. The Cas1 and Cas2 genes are present in all CRISPR-Cas types and the presence or absence of specific Cas proteins is the main basis of classification. For example, the Cas3, Cas9 and Cas10 proteins are hallmarks of CRISPR/Cas types I, II and III, respectively. There are systems that do not have the distinct Cas proteins of types I-III, are termed as unclassified (type U) [6].
The mechanism of action involves the formation of a ribonucleoprotein complex (RNP) that consists of the Cas9 protein and a guide RNA (gRNA) that can bind to the location directed by the gRNA on the genomic DNA. Upon binding, Cas9 cleaves the viral DNA, creating a double-stranded break that allows additional DNA modifications on the site [7]. The Cas9 nucleases are designed to lead to a DNA double‐strand break (DSB) at the target site. Repair of the strands takes place through error‐prone non-homologous end joining (NHEJ) or homology‐directed repair (HDR). When a template is absent, NHEJ is activated usually, resulting in insertions and/or deletions (indels) that damage the target genome loci. When a donor template is present with homology to the targeted locus, the HDR pathway follows, enabling precise edits [8].
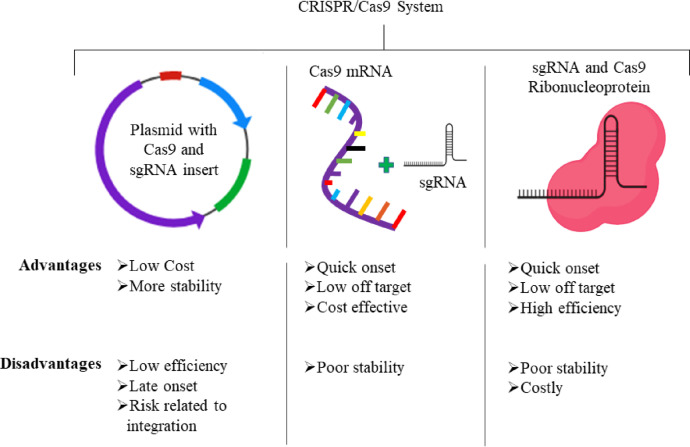
Recently, the CRISPR-Cas system has progressed as a remarkable engineering tool for carrying out precise and regulated genetic modifications in many microorganisms such as Escherichia coli, Staphylococcus aureus, Lactobacillus reuteri, Clostridium beijerinckii, Streptococcus pneumonia and Saccharomyces cerevisiae [9]. There are many steps involved in the application of CRISPR/Cas for bacterial genome editing. The first one being the selection of the target space in the genome which will also decide the guide RNA to be developed. Until now, various parameters such as sequence setting, gRNA binding stability, chromatin accessibility and PAM sequence have been discussed as important factors. Many software tools have been developed to forecast the on-site and off-site cleavage efficiency of sgRNAs, including CRISPOR, JATAYU and CHOPCHOP, amongst many others. The tool generates a series of sgRNA at different PAM sites within the targeted gene, which are then aligned based on their efficiency in terms of the expected on-target and off-target binding potential, as well as the other variables discussed above [10]. There are also many other parameters, such as specificity and mismatch concerns that must be investigated while creating it as a therapeutic tool. Moving forward, CRISPR/Cas9 system was adopted in three major forms i.e. plasmid, mRNA, and purified active RNP. All these forms have their inherent advantages and limitations (Fig. 1) and are therefore utilized accordingly for the therapeutic purposes.
Fig. 1.
Various forms of CRISPR/Cas9 including plasmid, mRNA and RNPs that could be delivered to achieve significant gene editing to treat retinal dystrophies.
Eye related diseases, especially retinal dystrophies are degenerative conditions marked with clinical and genetic heterogeneity and affect 1 out of every 2000 people all over the globe. More than 238 mutant genes that decide the phenotype are explored till now. The complexity of the neuronal pathways, physiological barrier due to anatomy of the eyes, the structure of each cell, and the diversity of functions of each retinal layer create many challenges in development of therapeutic strategies for these diseases. Most common site where the therapeutic agents need to work is the posterior part of the eyes which is quite inaccessible through conventional routes. Intravitreal route is beneficial in such cases with some risk of eye damage and requires expertise. There are available treatments for dystrophic conditions, such as wet age-related macular degeneration (wAMD), wherein anti-VEGF antibodies are injected through intravitreal route and were found to be beneficial. But the treatment needs multiple dosing over time and can cause eye damage due to multiple intravitreal injections. Therefore, a more relevant system needs to be developed to overcome such hurdles. Gene editing in recent times has grown to treat diseases characterized with gene mutation. CRISPR/Cas9 system could be directed towards a specific gene sequence to correct a mutation. This technique has been explored for retinal diseases, since the unique anatomical position, immune-privileged nature, blood-retinal barrier and identified underlying mutation makes eye, and specifically retina, amenable for therapeutic gene editing [11,12]. Further, it provides immense potential because of its one-time treatment possibilities via gene editing. CRISPR/Cas9 tool, however, is facing several delivery difficulties due to its large molecular weight. Although some viral vectors are available with limitations (Table 1), development of an efficient delivery vehicle for CRISPR/Cas is the need of the hour. Nanotechnology based non-viral carriers such as polymeric nanoparticles, liposomes, micelles, dendrimers etc. are currently being explored for delivery of CRISPR/Cas9. This review highlights the recent scenario of retinal dystrophic conditions and potential CRISPR/Cas based nanomedicines used in the treatment.
Table 1.
Pros and cons of the viral and non-viral delivery carrier used for CRISPR/Cas9 delivery to the eye.
| Delivery vehicle | Pros | Cons |
|---|---|---|
| Viral vectors (Lentivirus, Adenovirus, baculovirus) |
|
|
| Non-Viral vectors (Polymeric nanoparticles, dendrimers, exosomes, Liposomes, Lipid nanoparticle, polymeric micelles) |
|
|
2. Applications of CRISPR/Cas system: Beyond double-strand break
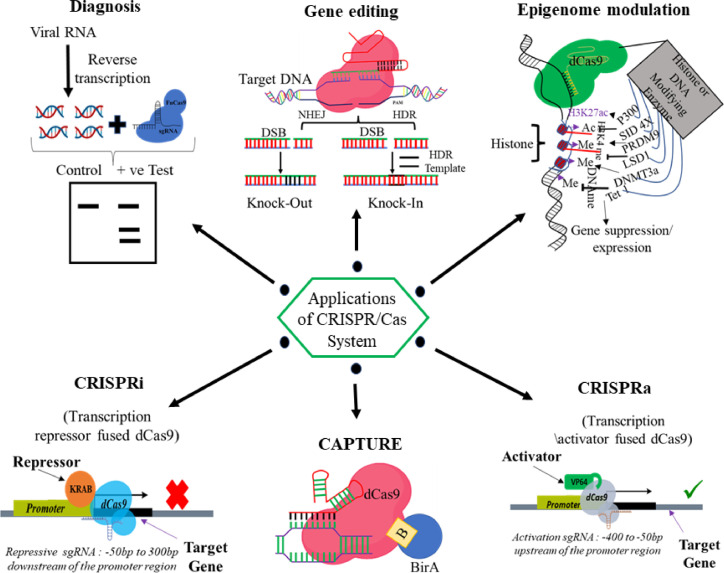
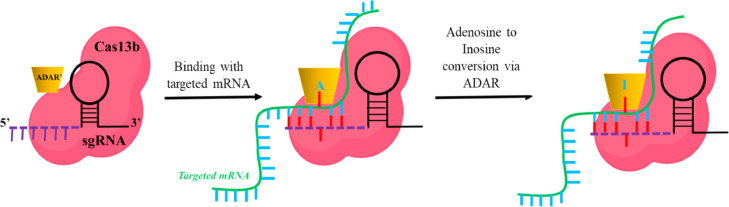
After the success of the spCas9 as a gene-editing tool, it has been explored more for several other applications as well. Despite the specificity, the large size of spCas9 makes it difficult to deliver using viral vectors. Therefore, ample variants or orthologs have been discovered till now. Campylobacter jejuni (CjCas9) (984 amino acid) is a smaller Cas9 nuclease discovered in 2017 [15]. Later in 2017, the Zhang group discovered Cas13 as a new orthologue having RNA targeting potential [16]. In 2013, Qi et al. used a dead version of Cas9 (i.e. dCas9) RNPs to suppress the gene expression by interfering with the RNA polymerase binding mechanism [17]. Additionally, the same group reported the application of dCas9 protein fused with transcriptional repressor KRAB (dCas9-KRAB) in gene silencing (CRISPRi) [18]. dCas9 protein fused with transcriptional activators VP64 was explored as gene activator i.e. CRISPRa, and therefore make CRISPR a suitable tool for transcriptional programming [19]. In 2017, the Liu group reported CRISPR as a base editor without introducing DSB [20]. dCas9 fused with epigenome modifier or the fluorescent tag was also used for the epigenome editing [21] and imaging [22] respectively. Recently, CRISPR/Cas system was also utilized as a diagnostic tool for the detection of Covid-19 infection [23]. Collectively, CRISPR/Cas has ample application beyond the DSB mediated gene editing. CRISPR has been potentially recognized as a tool for diagnostics, epigenome editing, gene regulation (CRISPRi/CRISPRa), imaging, etc. (Fig. 2).
Fig. 2.
Applications of CRISPR/Cas technology.
3. Retinal dystrophies
The mammalian retina is being widely studied for genetic disorders for several reasons. Multiple phenotypes of the retina can be directly observed, and photographs can be recorded. The effects of psychophysical parameters (acuity, field, color contrast) can be documented and retinal electrophysiology can be used to assess retinal functions [24]. Lastly, the ease of visualization of the retina has led to the development of many animal models that have led to a better understanding of pathways leading to photoreceptor death [25].
Worldwide, 1 in every 2000 people suffers from inherited retinal dystrophies (IRD). Individuals with IRD typically present with progressive vision loss that ultimately results in blindness. Since these diseases are genetic and clinically heterogeneous, hardly any effective treatments are available. Multiple cells, genes and drug-based therapies are in different phases of clinical trials for IRD [26]. IRD are distinguished by continuous degeneration of retinal pigment epithelium (RPE) and the neural retina. These dystrophies are of various types such as cone-dominant dystrophy (cone-rod/cone dystrophy), rod-dominant dystrophy (retinitis pigmentosa/rod-cone dystrophy), pattern dystrophy, macular dystrophy (Best macular dystrophy, Stargardt disease, Sors by fundus dystrophy), photoreceptors and bipolar cells abnormality (congenital stationary night blindness, X-linked retinoschisis), hereditary choroidal diseases, and vitreoretinopathies (Stickler syndrome, Wagner syndrome) [27,28].
3.1. CRISPR/Cas for correcting retinal dystrophies
Genome therapy using CRISPR-Cas in ophthalmic diseases may be promising, considering the scale of impact on society and various monogenic disorders of the eye [29]. Hopes are high to attenuate inherited retinal disorders due to the multiple clinical trials which have been initiated for specific retinal conditions with advancing gene therapy technology [30]. Table 2 showing various pre-clinical studies related to the use of CRISPR/Cas system for the treatment of retinal dystrophies. Over the last two decades, eye tissue has become a frontline organ for gene therapy. It is achieved either by using viral vectors to transfer correct cDNA copies or through the use of RNA intrusion to knockdown proteins with dominant-negative traits or toxic inclusion of functionalities via gene silencing [31].
Table 2.
Retinal dystrophies treated via genome engineering approach using different delivery strategies.
| RD | Mutant Gene | Therapeutic approach | Host | Mode of delivery | Result | Year | Ref |
|---|---|---|---|---|---|---|---|
| Leber's Congenital Amaurosis (LCA10) | RPE65 | Gene therapy | Homo sapiens | AAV Vector | Slight visual function improvement | 2008 | [77] |
| RPE65 | CRISPR/Cas9 | Rd12 mice | AAV Vector | RPE65 mutation correction | 2019 | [78] | |
| CEP290 | CRISPR/Cas9 | iPSCs | Plasmid vector | Successful repairment of mutations | 2017 | [79] | |
| Age-related macular degeneration (wAMD) | VEGFA gene | CRISPR/Cas9 | Mouse | Subretinal injection of Cas9 RNPs | Reduction in CNV area after Cas9 RNPs injection in mice bearing laser induced AMD | 2017 | [48] |
| VEGFA gene | CRISPR/Cas9 | Mice | Lentiviral vector | In vivo disruption of VEGFA gene in vivo with 84% indel efficiency | 2017 | [80] | |
| VEGFA/HIF1a gene | CRISPR- LbCpf1 | Mouse | AAV vector | A long term effect was seen when LbCpf1 targeted to Vegfa or Hif1a were introduced as a therapeutic in CNV to avoid the hurdles during multiple injection strategies. CNV, potentially avoiding repetitive injections. | 2018 | [81] | |
| RP1L1 | CRISPR/Cas9 | Zebrafish | Direction injection of gRNA and Cas9 into the embryo | Generated model of RP1L1-associated photoreceptor disease and the first zebrafish model of photoreceptor degeneration with reported subretinal drusenoid deposits, a feature of age-related macular degeneration. | 2020 | [82] | |
| Retinitis Pigmentosa (RP) | RPGR gene (exon 8) | CRISPR/Cas9 | Mice | AAV vector | Successful development of Rpgr knock out mouse model | 2020 | [83] |
| RHO gene (P23H mutation) | CRISPR/Cas9 | Mice | Plasmid vector | Successful editing in mutant P23H allele with a rate of ∼45%. | 2018 | [84] | |
| PDE6B gene | CRISPR/Cas9 | Mice | Plasmid Vector | Repaired mutation efficiently | 2016 | [85] | |
| RHO gene | CRISPR/Cas9 | Xenopus laevis | Co-injection of Cas9, eGFP mRNAs, and sgRNAs into fertilized eggs. | introduced and characterized in-frame and out-of-frame Indel in three genes encoding rhodopsin. | 2017 | [86] | |
| RHO gene | CRISPR/Cas9 | Rat | Plasmid vector | Improvement in visual function by preventing retinal degeneration | 2016 | [61] | |
| NRL gene | CRISPR/Cas9 | Mouse | AAV vector | Successfully preserve cone cell function and improved survival of rod cells | 2017 | [87] | |
| RPGR | CRISPR/Cas9 | Patient-derived iPSCs | Plasmid vector | Approx 13% of RPGR gene copies showed mutation correction and conversion to the wild-type allele. | 2016 | [62] | |
| Usher Syndrome | USH2A gene | CRISPR/Cas9 | HEK293 cells | pX330 vector | Repaired mutations efficiently | 2017 | [88] |
| USH2A gene | CRISPR/Cas9 | iPSCs,HEK293T Cells | Plasmid vector | Repaired mutations efficiently with minimal off target effects. | 2020 | [89] | |
| Best disease | BEST1 gene mutations | CRISPR/Cas9 | Ipsc | Lentiviral vector | Successfully reversal of the mutation with minimal off target effects | 2020 | [73] |
| X-linked Juvenile retinoschisis (XLRS) | RS1 gene mutations (C625T) | CRISPR/Cas9 | hiPSCs | Plasmid vector | Showed a high efficiency of mutation repair | 2019 | [74] |
| RS1gene mutation (p.Y65X) | TALEN | Mice | TALEN mRNA was co-injected into fertilized eggs | RS1-KI mice were viable, fertile and did not show notable physical abnormalities. | 2018 | [90] | |
| Achromatopsia | CNG B3 | Gene therapy | Mouse and Dog | AAV-hCNGB3 vectors via injection | Successfully rescued the function of cone photoreceptors. | 2016 | [91] |
| Progressive cone and cone-rod dystrophies | RPGIP1 | Gene therapy | Dog | rAAV mediated Rpgrip1 gene transfer via injection | Successfully rescued both cone and rod functions. | 2014 | [92] |
Before the arrival of gene therapy, retinal dystrophies were incurable [32]. Indeed, IRDs have been demonstrated as ideal candidates for gene therapy because: (i) they are inherited diseases linked to multiple genes and a subset of them show monogenic inheritance [33], (ii) the cells which are affected (PRs and RPE) can be accessed by various clinical and surgical procedures [34], (iii) the non-invasive diagnosis methods used in the clinics for IR patients could be translated to animal models and (iv) availability of animals models to study the eye conditions. The Phase III data for Spark Therapeutics’ gene therapy product (i.e. SPK-RPE65) for the treatment of patients with visual impairment caused by RPE65 gene defects provides hope for clinical translation opportunities. SPK-RPE65 is an AAV2 gene therapy that delivers the RPE65 gene via subretinal injection to patients with a defective RPE65 gene. Clinical trial outcomes were found beneficial and the therapy, SPK-RPE65, was approved by FDA in 2019 with trade name of LUXTURNA for the treatment of vision loss in the patient [35]. The therapy is based on recombinant adeno-associated virus (AAV) vectors expressing the human RPE65 cDNA using a viral promoter as a control [35]. Although, gene therapy provides immense potential for treatment of various genetic diseases, it poses some disadvantages like off-target effects and risk of mutation in the DNA. These disadvantages limit their application in several cases. Therefore, gene editing tools such as zinc finger nuclease (ZFN) and transcription activator-like effector nucleases (TALEN) have been developed for the treatment of genetic diseases. Moreover, in recent times CRISPR/Cas9 based gene editing tool is being explored for the treatment of genetic disease through its unique site-specific gene editing efficiency. Here, multiple guide RNAs are being used to simultaneously target various sites in the genome, which is a striking feature of the CRISPR/Cas system [36]. A major advantage of deploying CRISPR-Cas is that it is a RNA-based system; thus, custom guide RNAs can be easily designed to target within the genome. Whereas ZFN and TALEN systems, which are protein-DNA interfaces, are protein-dependent making it difficult to engineer for a given target [37]. The potential for multiplexed genome surgery is another interesting feature of the CRISPR-Cas system using several gRNAs for the concomitant editing of multiple sites within the genome [36,38].
Some of the major mutation based retinal dystrophic conditions are discussed below.
3.2. Leber's congenital amaurosis
Leber's Congenital Amaurosis (LCA) has been known to be the most severe retinal dystrophy as it potentially leads to congenital blindness in less than one year of age. Fourteen mutated genes have been identified by homozygosity mapping, linkage analysis and genome analysis in LCA patients and children with retinal degeneration constituting approximately 70% of the cases [39]. LCA is mostly associated with severe defects which includes roving movements of the eye called nystagmus. Also, slow reactions of the pupil and lack of electroretinographic reactions are some of the symptoms in children [40,41]. Genes involved in LCA encode proteins which are responsible for retinal functions includes photoreceptor morphogenesis (CRB1, CRX), vitamin A cycling (LRAT, RPE65, RDH12), phototransduction (AIPL1, GUCY2D), guanine synthesis (IMPDH1), and outer segment phagocytosis (MERTK) and also intra-photoreceptor ciliary transport processes (CEP290, RPGRIP1, LCA5, TULP1) [39]. The most prominently studied gene for LCA is mutations in the RPE (RPE65) gene, which is responsible for encoding retinoid isomerase [29] whereas, the most frequently occurring mutations are associated with the CEP290 (15%), GUCY2D (12%), and CRB1 (10%). Around 20% of patients in north-western Europe have an intronic CEP290 mutation (p.Cys998X). An AVV-CRISPR system has been developed for in vivo treatment of autosomal dominant retinitis pigmentosa (adRP) and LCA10 in mice. In this study, AAV-SpCas9 vector was delivered via subretinal-injections that targets the rhodopsin (RHO) or CEP290 and Nrl (neural retina leucine zipper transcription factor) gene in mouse models for adRP. The outcomes of the study showed the expression of spCas9 protein in retinal cells of the mice for 9.5 months. While the authors have deployed different AAV serotypes as well as different vector doses, the results proved effective restoration of RP or LCA10 phenotype without off-target effects and adverse toxic reactions [42,43]. Later, the strategy was adopted to resolve the RHO gene mutation in human cells successfully. Collectively, CRISPR/Cas9 technology has shown to be effective at targeting gene/alleles in an efficient and specific manner in this study, demonstrating that it could be used in the treatment of RP and other genetic disorders, including dominant human conditions.
3.3. Age-related macular degeneration
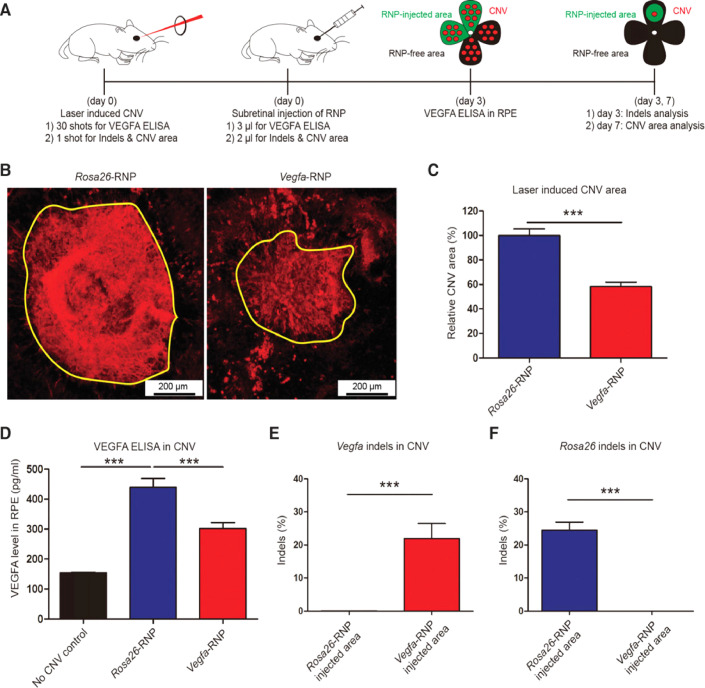
Age-related macular degeneration (AMD) is a multi genetic disorder [29]. Wet AMD, which is the neovascular form of AMD, is marked by abnormal growth of the choroidal vessels in the macula region of the retina, resulting in a loss of central vision. Macula is enriched with cone photoreceptors and is responsible for the bright light activities and color vision [44]. Neovascularization in wAMD occurs due to overproduction of vascular endothelial growth factor (VEGF); hence anti-VEGF agents becomes the therapy of choice [45]. Currently, wet AMD patients are treated with intravitreal injection of anti-VEGF agents such as ranibizumab, aflibercept and bevacizumab [46]. For the treatment of AMD, AAV-CRISPR systems have also been developed based on CjCas9 (Campylobacter jejuni) [47] and LbCpcf1 nucleases (nucleases which are a member of the type-V CRISPR-Cas systems). In the study, authors packaged the CjCas9 gene, its corresponding sgRNA sequence, along with a marker gene into an AAV vector. Being highly specific, CjCas9 can cleave only a restricted number of sites in the human or mouse genome. Hence, when delivered using AAV, CjCas9 lead to targeted mutations in the RPE cells. CjCas9 can be specifically targeted to the Vegfa or Hif1a gene in RPE cells thereby, decreasing the size of laser-induced choroidal neovascularization, making in vivo genome editing with CjCas9 a new advancement in the therapy of AMD. Further, the results indicated an Indel efficiency of 22±3 and 31±2%, for Vegfa and Hifla genes, respectively at 6-weeks post injection of AAV-CjCas9 intravitreally. Moreover, the effect of Indel was seen at protein level as well where significant decrease in VEGF-A protein was observed in RPE cells with respect to control group. In another study, sgRNA/Cas9 expressing plasmid and Cas9 RNPs were delivered using lipofectamine 2000, wherein Cas9 RNPs showed 82%±5% and 57%±3% of indel in NIH3T3 and ARPE-19 cells, respectively. Comparative study indicated that the Cas9 RNPs were more effective w.r.t plasmid at 2nd day of transfection. Further, it was observed that level of VEGF A mRNA and protein reduced to 40%±8% and 52%±9%, respectively in adult retinal pigment epithelial cells (ARPE) after Cas9 RNPs treatment. For in vivo efficacy evaluation, Cy3 labelled RNPs were delivered via intravitreal injection. The results indicated the accumulation of Cy3 dye into RPE cells after 3 days post injection and 25%±3% of indel was also detected in RPE cells at delivery site. Moreover, CNV model was also developed in mice using laser (to mimic wAMD) followed by subretinal injection of Cas9 RNPs. After 3 d, 22%±5% indel was observed in RPE cells for Vegfa gene. Additionally, Cas9 RNPs treatment significantly reduced CNV area by 8%±4%, and VEGF A protein level as well (Fig. 3) [48].
Fig. 3.
VEGF A gene editing efficiency of Cas9 RNPs in retinal dystrophy in mice. (A) The overall study outline, herein, CNV model was developed in mice using laser followed by subretinal injection of VEGF A targeting Cas9 RNP. After 7 d injection of RPE complexes were analyzed for CNV area and deep sequencing was performed to evaluate gene editing in the targeted cells/tissues. Meanwhile, after 3 d injection of VEGFA ELISA was also performed. (B) Representative laser-induced CNV stained with isolectin B4 (IB4) in C57BL/6 J mice injected with the Rosa26-specific Cas9 RNP (as a control) or the VEGFA-RNP. The area of CNV is demonstrated as yellow line. (C) CNV area. (D) level of VEGF A in the CNV area. (E) Gene editing in terms of indel in RPE cells at VEGFA targeted site. Reprinted from [48], licensed under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/legalcode). Copyright © 2017 the authors. Published by Cold Spring Harbor Laboratory Press.
3.4. Retinitis pigmentosa
Retinitis pigmentosa (RP), affecting 1 in 4000 people, has become the leading cause of progressive blindness [49]. Classical RP, also known as the rod-cone dystrophy, is identified by a “tunnel vision”, which is a progressive loss of peripheral vision. The first signs include nyctalopia, which is the development of night blindness and difficulties in adapting to the dark that occurred through the loss of rod function in the early years of life [50]. The RPE starts losing its pigment due to loss of photoreceptor that ultimately leads to the accumulation of intraretinal melanin deposits, which look like a “bone spicule” conformation. However, the central vision remains intact until the last stages. It can be inherited through different transmission modes such as autosomal dominant, autosomal recessive or X-linked and is heterogeneously related with mutations in at least 79 genes [49]. RP is mainly of two types, MERTK associated and RPGR X-linked. The RPE apical membrane contains photoreceptors that are light sensitive and their turns over is enabled by MERTK (Mer tyrosine kinase), the receptor involved in phagocytosis of the rods and cones [51]. These photoreceptors must be continuously recycled for efficient working, which is disrupted by mutations in MERTK, leading to degradation and loss of photoreceptors [52]. Meta analyses have revealed that only 3% of MERTK type RP are due to autosomal recessive transmission [53,54] and causes macular atrophy and early-age photoreceptor abnormality [55,56]. Mutations in RP GTPase regulator (RPGR), an X-linked RP (XLRP) is seen in 1 in 3500 people. RPGR, along with the delta subunit of rod cGMP phosphodiesterase, regulates the proteins, and its dysfunction leads to progressive loss of central vision and night blindness [57], [58], [59], [60]. Some in vitro and in vivo studies have been reported where RP has been treated using CRISPR/Cas9 technology. For example, CRISPR/Cas9 tool was used in rat model of adRP by Bakondi et al., in 2016 to ablate mutation in rhodopsin gene (RhoS334). In this study, sgRNA/Cas9 plasmid (targeting exon 1 immediately upstream of a PAM unique to the RhoS334 locus) was administered intravitrealy in S334ter-3 rats. Genome analysis of transfected retinal cells confirmed a cleavage efficiency of 33% and 36% in two different rats. Also, improved visual acuity and extensive preservation of retina was observed via immunohistology following sgRNA/Cas9 plasmid injection [61]. In addition, a CRISPR/Cas based strategy was developed for editing RHO gene mutations. In this study, a plasmid was designed which contained an insert for two sgRNAs targeting RHO gene to cause DSB followed by NHEJ. The outcome of the study dictated successful editing of RHO gene, which further downregulated the expression of RHO protein. Further, Bassuk et al. treated XLRP by correcting RPGR point mutation using CRISPR/Cas9 in iPSCs. In this report CRISPR was used to treat the pathogenic mutation in iPSCs obtained from a patient with photoreceptor degeneration. The authors screened 21 different sgRNAs for editing where g58 was found most effective. Therefore, g58/Cas9 expressing plasmid was designed and transfected into iPSCs along with a RPGR single-stranded oligo deoxy ribonucleotide (ssODN), which acts as a donor during HDR pathway. Further, deep sequencing was performed, and the data revealed the successful correction of mutation in 13% of transfected cells [62]. Moreover, the results showed that TAG (premature stop codon) gets replaced by GAG (wild type codon), which encode glutamate at residue 1024. On the other hand, no changes in the mutation were seen in the untransfected iPSCs. Further it was concluded that the correction rate of 13% was significantly fruitful and can be improved by minimizing error-prone NHEJ by inhibiting DNA ligase IV at the DNA cleavage site.
In addition, a CRISPR/Cas-based strategy was developed for editing RHO gene mutations in a mouse model of ADRP. In this study, a plasmid was designed that contained an insert for two sgRNAs, targeting RHO gene (exon 1) having P23H dominant mutation. Firstly, the gene editing was performed in vitro in HeLa cells, where 70%, 76% and 82% of indel frequency was observed with sgRNA1, sgRNA3 and 2sgRNA, respectively, Additional, the RHO expression was also observed using Real time Taqman PCR wherein 35%, 25% and 20% of reduction in expression was seen with cells treated with sgRNA1 sgRNA3, and 2sgRNA, respectively. Later, the electroporation of CRISPR/Cas plasmid containing 2sgRNA along with green fluorescence protein (GFP) expression was performed subretinally in P23 RHO transgenic mice. The GFP expressing section of the retina was isolated and evaluated for the Cas9 expression wherein the Cas9 expression was limited to the cells expressing GFP along with 84 edited sequences [63].
3.5. Choroideremia
Choroideremia (CHM) is named from the complete loss of the choroid, retina and RPE, exposing the underlying white sclera, which is unique to the disease [64]. CHM is an X-linked recessive degenerative retinal dystrophy, affecting 1 in 50,000 individuals and is only associated with males. Due to mutations in CHM gene, which encodes for Rab escort protein 1 (REP1) and its dysfunction leads to progressive loss of vision and choroid atrophy. It starts with night blindness in the early years of life with a gradual decline in peripheral vision and legal blindness by 50–70 years of age [65]. The CHM disease is characterized by retinal thickening, resulting from Müller cell activation and photoreceptor layer hypertrophy. This further causes RPE depigmentation, degeneration of photoreceptors and retinal remodeling. Hence, retinal remodeling is being considered as a possible strategy for in vivo studies [66].
3.6. Stargardt disease
Stargardt disease is an autosomal recessive genetic disorder majorly caused by mutations in the ABCA4 (ATP-binding cassette, subfamily A, member 4) gene and is the most common form of juvenile-onset macular degeneration. It is characterized by the loss in central vision due to the gradual accumulation of cytotoxic lipofuscin within the RPE [67]. This disease affects at least 1 in 10,000 people, with approximately 31,000 cases in the United States. The disorder consists of a quite fast degeneration of the macula resulting from the deposition of lipid enriched deposits called lipofuscin (comprised mainly of A2E, a vitamin A derivative) in the RPE cell layer. Due to this, the interaction between photoreceptors and RPE is affected, causing the death of photoreceptors by hampering with their ability to uptake nutrients and perform the visual cycle [68].
3.7. Usher syndrome
With a prevalence of 1 in 20 000, usher syndrome is one of the common forms of syndromic IRD. Its unique features include RP and hearing loss [69]. The heterogenous syndrome is classified into three subtypes depending on the progression and severity of the hearing loss and the age of onset of the RP. Usher syndrome type 1 (USH1) is the most critical; usher syndrome type 2 (USH2) presents moderate to severe symptoms and is most frequently observed. Lastly, usher syndrome type 3 (USH3) is characterized by a moderate phenotype, and the onset of the disease and its progression could vary on a case by case basis [70]. USH1 is the most common cause of deaf-blindness in humans, characterized by vestibular dysfunction, profound congenital deafness and RP and is inherited in an autosomal recessive manner. USH1 is caused due to mutations in myosin VIIA, which encodes for an organelle transport protein within the RPE [45]. In 2017, Fuster-Garca et al., proposed the use of CRISPR/Cas9 gene editing to restore c.2299delG mutation in the USH2A gene. Human dermal fibroblasts (HDFs) cells were isolated from an USH2 patient with c.2299delG mutation and used for gene editing. Briefly, using nucleofection, a Cas9 RNPs (comprising Cas9 (15 µg) and sgRNA (20 µg)) was transfected into HDFs of the normal patient, yielding 18% indel frequency. Subsequently, RNPs were co-delivered with ssODN-2299, which yielded HDR efficiency of 5%. Similarly, HDFs of the patient with c.2299delG mutation were transfected with ssODN with a WT sequence and the PAM sequence ablated. As per the results, 6% indel frequency and a 2.5% HDR were detected [71].
3.8. Best disease
Bestrophin, encoded by the BEST1 (VMD2) gene, is a transmembrane protein expressed on the basolateral aspect of the RPE cells and is responsible for the conduction of chlorine across the RPE. Mutations in the BEST1 (VMD2) gene, and hence bestrophin, hampered the fluid transport across the RPE thereby causing debris to build up between the RPE and photoreceptors. Consequently, atrophic macula scar and central visual loss occur in a short span of time, leading to best disease or Vitelliform macular dystrophy. It affects between 1 and 9/100 000 people and is inherited in an autosomal dominant manner. Many other retinal dystrophies can also occur due to BEST1 mutations, including RP and ADVIRC (Autosomal Dominant Vitreo Retino Choroidopathy). Biallelic mutations lead to multifocal small egg yolk deposits leading to Recessive Best Disease. Neovascularization in the choroid, along with hemorrhage and leak into the retina, further aggravates the disease condition and intravitreal anti-VEGF agents can be used for successful therapy [72]. In the year 2020, Sinha et al. demonstrated the effectiveness of gene augmentation in the treatment of the Best disease. Induced pluripotent stem cell-derived RPE (iPSC-RPE) was used as an in vitro Best disease model for this objective. Gene augmentation restored BEST1 gene activity and improve rhodopsin degradation. Meanwhile, some of the mutations did not respond to the gene augmentation, therefore CRISPR/Cas9 was used to investigate the efficiency of site-specific gene editing in iPSCs RPE models. The findings revealed that CRISPR/Cas9 edit the mutant BEST1 gene while leaving the wild-type BEST1 gene intact. Off-target indels were also tested, however, no evidence of off-target gene editing was reported. The study overall revealed the application of CRISPR/Cas based precise and specific gene editing for the management of retinal dystrophies [73].
3.9. X-linked juvenile retinoschisis
RS1 is a retinoschisin gene that encodes a protein responsible for the cell adhesion. Mainly observed in males, RS1 mutations cause the development of cystic cavities in the center of the retina that enlarge gradually with age, along with decreased visual acuity. As the dystrophy progresses in the retinal periphery, the condition of the split retina worsens with large atrophic holes; hence, the residual retinal blood vessels left hanging in the vitreous cavity above the retina which may result in vitreous hemorrhage. It has been observed that people with this disease usually have a refractive error of long-sightedness. Worldwide, about 1 in every 5000 to 25,000 suffer from the condition. Children who suffer slowly lose out on central vision; however, most children can complete a fully sighted education, with the help of magnified texts and teachers for visual support. The main therapy for the retinal cysts is the carbonic anhydrase inhibitors, although a significant improvement in symptoms is rare. Huang et al. developed a base editing strategy to cure X-linked juvenile retinoschisis (XLRS) in 2019. Using human induced pluripotent stem cells (hiPSCs) from patients, a 3D retinal organoid model with XLRS characteristics was created in vitro. To evaluate the model, CRISPR/Cas9 targeting the C625T mutation in the RS1 gene was introduced as a plasmid using a viral vector. According to the findings, CRISPR effectively repairs gene mutations while also correcting the phenotypes by up to 50%. The findings also revealed the existence of off-target indel, which is a CRISPR constraint [74].
3.10. Congenital stationary night blindness
Congenital stationary night blindness (CSNB), also called nyctalopia, is a non-progressive type of night blindness. Patients suffering from this condition have difficulty observing in low light. The symptoms start early in children along with low amplitude nystagmus, strabismus and reduced visual acuity [75]. Associated with 17 genes, CSNB is a polygenic disease, and diagnosis involves electroretinography to measure photoreceptor function. The ERG results may show lack of rod functioning or incomplete functioning of both cone and rod as well as abnormal fundus upon examination. The state of complete CSNB is caused when bipolar cell signaling is disrupted, leaving a single intact alternate pathway [76]. CSNB is of four subtypes - Schubert Bornstein (branched into complete and incomplete), Riggs, Fundus Albipunctatus and Oguchi Disease, of which the last two types show abnormal fundus. Myopia and photophobia are two of the prominent features observed in patients. Children with ‘incomplete’ CSNB may not be aware of the condition as the symptoms are mild and central vision is reduced from normal [72].
3.11. Achromatopsia
Affecting only 1 in 30 000 to 40 000 people, achromatopsia is a rare autosomal recessive disease. Mutations in six different genes have been identified that are responsible for this disease. 75% cases are due to CNGB3 and CNGA3, while the rest are accounted by GNAT2, PDE6C, PDE6H and ATF6. There is complete color blindness and central vision is diminished. In the early months, patients have to deal with profound photophobia and nystagmus. However, the nystagmus in achromatopsia patients is pendular or horizontal unlike the roving nystagmus of LCA. The diagnosis involves electrophysiology where it is observed that the function of cone photoreceptors is absent, and rods functions normally. Usually, the complete form is seen, and the incomplete form with a milder phenotype tends to be much rare. The symptoms of the disease are mostly constant, and the glare and photophobia can be managed by incorporating red/brown shade glasses.
3.12. Progressive cone and cone-rod dystrophies
As the name suggests, cone cell degeneration (COD) or cone followed by rod degeneration (CORD) are progressive retinal dystrophies and are seen from a young age. The major difference between them is that rod involvement increases the severity of the disease and by age 40, these people reach the stage of legal blindness [93]. Examination of the fundus and macula reveals an atrophic appearance or deposits of retinal pigments seen variably in different patients. Mutations in over 30 genes have been found as well as molecular causes identified in around 20% and 74% of autosomal dominant and X-linked COD/CORD respectively, while 23%−25% of autosomal recessive types have been worked out [94].
A number of ongoing clinical trials are living proof that gene therapy has made retinal dystrophies curable [29]. Furthermore, constraints such as multiple intravitreal injections resulting in physical retinal damage and resistance render the current therapy ineffective. Fortunately, the eye, and specifically the retina, is accessible to therapeutic gene editing due to its unique anatomical position, immune-privileged nature, presence of the blood-retinal barrier, and known underlying mutations [12]. As a result, the eye has been extensively studied for gene editing. However, just a few RDs in terms of preclinical evidence related to effective gene editing utilizing CRISPR/Cas have been published, and more research in this field is needed. Interestingly, some preclinical studies have been published wherein wAMD was treated by employing a CRISPR/Cas-based tool to knock out the VEGF A gene in RPE cells. Off-target effects and the deletion of some uncleared functions of the concerned gene are two key pitfalls that could be encountered with CRISPR. On the other hand, several groups are working to integrate data and screen for off-target effects [95], [96], [97]. It will be intriguing to observe if a CRISPR/Cas-based gene editing method can prevent angiogenesis in wAMD in clinical trials as it directly eliminates the fundamental cause of RDs. Further, the CRISPR/Cas-based therapy could also treat RDs with a single dose injection. We have discussed various factors that should be considered while adopting CRISPR/Cas9 for the treatment of RDs (Table 3).
Table 3.
Factors affecting the use of CRISPR/Cas for gene editing in RDs.
| Factor | Description |
|---|---|
| Ethical Issue [98] | While using CRISPR/Cas9, the ethical issues will be there, since CRISPR/Cas based gene editing could result in serious off-target gene manipulations. |
| Selection of gene [99] | For example, in LCA there are 14 genes that have mutations. Therefore, one should be clear about the gene that needed to be edited for improvement of the complications associated with the RD. |
| Knock out [100] | Knock out is not always beneficial, until and unless the role of the gene has been vastly understood. For instance, knockout of Vegfa gene is shown in wAMD to stop angiogenesis. But it cannot be applicable for every gene because one gene can be involved in various cellular functions and knockout could cause the loss of some important cellular functions. |
| Knock in [101] | Some of the RDs require HDR. Since NHEJ is more prominent w.r.t HDR, therefore, one should consider this factor while utilizing the CRISPR/Cas technique. |
| Vitreal barrier [102] | The presence of vitreous fluid may retard the diffusion of the CRISPR/Cas components toward the posterior segment of the eye. Additionally, the nucleases/proteases present in the vitreous fluid could degrade the CRISPR components. |
| Targeted Delivery | RDs requires editing of the gene in retinal cells only, therefore, delivering the CRISPR/Cas component specifically to retinal cells could be challenging. |
| Off-target effect | While designing sgRNA for a specific gene, one should ensure the specificity of the sgRNA toward the gene of interest. Else, it could lead to undesired gene editing. |
| PAM sequence [100] | As it is known that the CRISPR/Cas perform DSB near the PAM sequences (NGG, GGG), and however, it is not always possible to have a PAM sequence at the desired gene editing site. |
| Limited delivery route | Blood retinal barrier limits the distribution of therapeutic agents to the eye tissue, therefore localized injection is the only potential approach left. Localized injection such as intravitreal (IVT) injection has the risk of eye damage and requires trained healthcare personnel. |
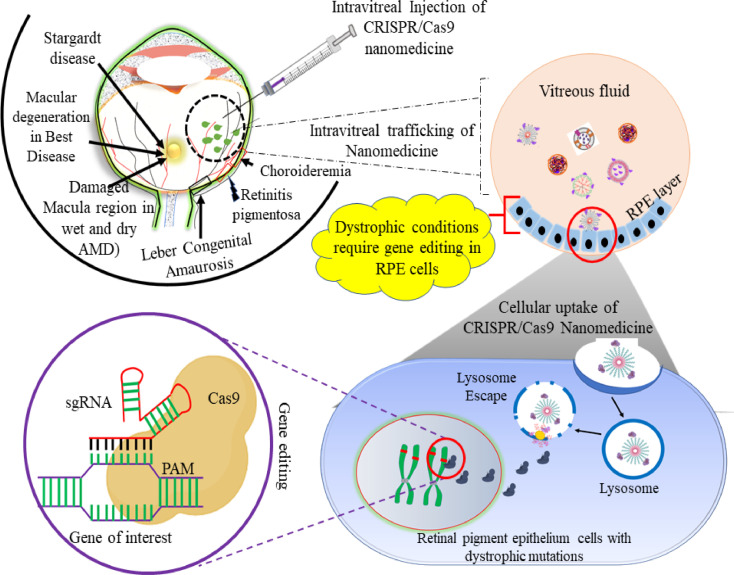
4. Use of CRISPR for genome editing in RDs
The three main approaches for CRISPR based genome editing include the use of (a) plasmid DNA (pDNA) that expresses the Cas9 protein and sgRNA, (b) mRNA that encodes the Cas protein, and (c) RNP which is a complex of Cas protein and sgRNA. Among reported approaches, the plasmid-based approach is the simplest while the RNP based approach showed minimal off-target effects. Nevertheless, these CRISPR components ought to be delivered to the target cells followed by their translocation to the nucleus. Owing to the nature of the cargo, different challenges including packaging, immunogenicity, mutagenesis, extra- and intra-cellular barrier, etc., needs to be overcome to achieve efficient genome editing (Fig. 4).
Fig. 4.
Schematic representation of nanomedicine trafficking in the treatment of retinal dystrophic conditions starting from (1) intravitreal injection, (2) diffusion through vitreous fluid, (3) cellular uptake, (4) endosomal escape and (5) interest specific gene editing.
4.1. Vector packaging
The major challenge faced in the delivery of CRISPR components for therapy is their packaging into a single vector system. Through the AAV approach, the maximum possible size for the cargo gene is ∼4.7 kb, whereas that of the SpCas9 gene alone is ∼4.3 kb. Hence, for AAV method, it becomes a hurdle to insert additional CRISPR components like sgRNAs, or extra genes. To solve this issue, various techniques such as using a smaller sized Cas9 (SaCas9) or splitting Cas9 into two vectors, have been propagated, but their feasibility for therapeutic applications have to be evaluated [103]. For the packaging of RNPs, viral vectors cannot be used. The use of non-viral vectors possesses a multitude of problems, be it the high molecular weight of the protein, highly negative charge and/or the stability of the RNPs.
4.2. Immunogenicity
CRISPR/Cas9 is a bacterial immune system, and therefore, being bacteria-derived they could lead to the immune response in the host. Specifically, if the gene-based approach is used, it could lead to the integration of Cas9 protein into the cells of the host. The expression of ectopic Cas9 protein in the individual could cause an MHC class I mediated immune response thereby, eliminating Cas9 expressing cells [104].. A study by Chew et al. showed that the in vivo delivery of AAV vector prompted immunogenicity not against the viral antigens but against the Cas9 protein. According to this result, immunogenicity of AAV-CRISPR-Cas9 has been considered as a key property that destabilizes the host system and will negatively impact its application in vivo [105]. The best results have been found with a protein-based delivery of the CRISPR-Cas system, which has shown the least potential immunogenicity, as the ectopic Cas9 protein is present only transiently in the host cells [103].
4.3. Insertional mutagenesis
A lot of times, the vectors may get inserted in random sites within the genome thereby causing mutagenesis of essential genes. When trials were conducted using a retroviral vector based gene-therapy approach to treat Severe combined immunodeficiency (SCID), it caused leukemic transformation, as the virus got integrated into the host DNA [106] and triggered abnormal expression of the targeted gene. Tumorigenesis can result from vector insertions, when the integration occurs near a protooncogene, thus posing a greater risk for integrating viral vector based CRISPR delivery systems. This issue has been circumvented using non-integrating viral vectors such as AAV-based systems and by protein/RNA based CRISPR delivery systems [103].
4.4. Systemic delivery
CRISPR/Cas system is required to be delivered in cells or tissue of interest without any off-target effects. Therefore, localized delivery route advantageous than systemic delivery of CRISPR/Cas, especially to reduce immunogenicity, avoid off target effects and to improve the target cell edit efficiencies. In the case of retinal dystrophies, localized injections are given via intravitreal or subretinal administrations.
4.5. Targeted delivery
Targeting may be defined as a preferential accumulation of the active agent at a predetermined site which could be a tissue or organ (first order targeting), a specific cell type (second order targeting) or an intracellular site of targeted cells (third order targeting). Targeting is important because the therapeutic product can cause many adverse effects and damage the non-target cells. It also decreases the concentration of drug required to produce desired effect at the site of action. One of the advantages that viral vectors provide is tissue tropism, which will be beneficial for targeted CRISPR/Cas9 delivery [107] . But, if non-viral vectors are employed, specific moieties such as peptides and antibodies will be required for targeting [108]. However, such targeting is quite difficult to achieve due to complications in packaging that may arise due to the insertion of extra biomolecules to a delivery vector along with the CRISPR components.
4.6. Transfection and editing efficiency
CRISPR/Cas9 components need to be successfully delivered in sufficient quantity into the target cells by transfection, a prerequisite for efficient genome editing. Transfection methods are of three types, viral, chemical, and physical. Among these, the most used non-viral method is electroporation. But, due to the high electric field strength and accompanying electrochemical reactions, electroporation often causes high post-transfection mortality [109]. The editing efficiency for CRISPR/Cas9 obtained in vivo is much lower than that of those achieved in vitro in cell lines. In another study, when Cas9-RNP was delivered locally into the mouse inner ear, it caused 20% GFP fluorescence loss. This small percentage may work in some diseases such as liver tyrosinemia and muscular dystrophy. The editing efficiency is also linked to delivery efficiency. Recently Cas9-RNP delivery efficiency up to ∼95% in cultured cells has been attained although the in vivo delivery efficiency requires further investigation [110].
4.7. Off-target effects
One of the biggest setbacks for genome editing technology is the off-target effects. Off-target effects are occurring when the specially engineered sgRNA, apart from targeting the gene of interest, targets the non-specific genes [111,112]. TALENs usually have lesser toxicity and greater specificity than ZFNs. Also, with CRISPRs, different cells may carry different edits even if they get edited by a single gRNA. It has been shown that even a single mismatch between base pairs can decrease binding to a great extent. A mismatch occurring at the 5′ end of the target is much more destructive than the 3′ end. In case of CRISPR therapy as well, off-target effects have been a significant problem. Secondary targets of the sgRNA, which have multiple mismatches with respect to the sgRNA undergo mutations at rate similar to the desired target [113]. The CRISPR/Cas off-target effects are further amplified when viral gene delivery method is employed, possibly due to the long-term constitutive expression of Cas9/sgRNA that leads to continued exposure of Cas9/sgRNA to non-specific genes. Various techniques are being developed to eliminate off-target effects such as designing sgRNA of high specificity [114,115]. In vivo effects for these systems have not been fully developed for the off-target effects. The best technique in this regard remains protein-based delivery of CRISPR since there is only a transient exposure of the host genome to the Cas9/sgRNA, thus decreasing off-target events [116].
5. Ocular delivery: Challenges and opportunities for nanomedicine
Anatomically, anterior and posterior segments of the eye are affected by vision threatening disorders. Most of the currently available ophthalmic preparations are eye drops possessing poor bioavailability through conjunctival route [117]. There are ample of physiological and anatomical barrier which impede the delivery of active pharmaceutical ingredient (API) to affected areas of the eye. Tear film, eye blinking, efflux pump, nasolacrimal drainage, are some of the barrier to drug absorption [118]. Most of the dystrophic conditions require delivery of the therapeutic agent to the posterior portion of the eye and is limited by static barriers including blood-retinal barrier, Bruch's membrane, sclera choroid, and the dynamic barriers i.e. lymph and choroidal blood flow [119]. Intravitreal route is the most common mode of administration of drugs to the posterior chamber of the eye [120]. On the same note there are some limitations such as patient compliance, need expertise, risk of retinal detachment, risk of cataract and hemorrhage [121]. Attempts have been made to overcome existing problems related to the delivery of molecules towards the posterior portion of the eye using nanotechnology-based delivery carriers. Nano size and surface charge of nanoparticle helps target specific retention and conjugation in vivo. Also, nanoparticles with higher zeta potential are supposed to have higher stability. Additionally, cationic nanoparticles are considered more applicable for topical ophthalmic delivery, as conjunctiva and cornea have negative charge on the surface [122]. Therefore, electrostatic interaction helps in the internalization of nanoparticle into eye. For intravitreal injection, anionic nanoparticle diffuses more effectively through vitreous as it is composed of anionic hyaluronic acid which helps anionic particle to reach posterior chamber of the eye without any interaction [123]. On the other hand, cationic nanoparticles interact with anionic hyaluronic species and remain undiffused and are entrapped in the vitreous. Therefore, anionic charge of nanoparticles ease the intravitreal delivery of cargo to posterior part of eye [124]. Till now, several nanocarrier systems have been explored for ocular delivery viz., polymeric micelles, liposome, polymeric nanoparticles, nano-emulsion/suspension, solid lipid nanoparticles etc. These nanocarrier systems provide advantages such as targeted delivery, enhanced bioavailability, sustained release, improving residence time in ocular space etc. [125]. Additionally, for ocular retinal delivery, intraocular route has significant benefits over systemic route. Systemic route poses hurdles such as blood retinal barrier, systemic toxicity of the drug administered, poor target specificity, rapid clearance, off target effects, and only1%−2% drug reaches to the eye via systemic route. Hence intravitreal route can provide distinct advantages and nanomedicines could serve as potential therapeutics for the treatment of retinal dystrophic conditions. On the same note, there are some clinical complications with intravitreal injection such as patient compliance, infectious endophthalmitis, intraocular inflammation, rhegmatogenous retinal detachment, intraocular pressure elevation, ocular hemorrhage, glaucoma, cataract, non-infectious uveitis etc. These complications can be minimized by reviewing patient medical history, appropriate ocular examination, ancillary diagnostic testing, individualized medical decision-making, and proper follow-up by a clinician.
6. Delivery strategies used for CRISPR/Cas9 components
Many genome engineering applications have been developed for CRISPR/Cas systems for in vitro studies in cell lines; however, achieving an efficient in vivo delivery of CRISPR/Cas system is a major challenge as multiple components need to be delivered to the target cell to produce the desired effect. Nucleofection, electroporation and lipid-based deliveries have been tried for plasmid DNA (that encodes for the Cas9-gRNA) through the cell membrane [126]. Electroporation is a technique in which high voltage is applied to create pores in the cell membrane so that, direct transfection can occur into the cells both in vitro and in vivo [127]. Electroporation can be extremely toxic as it disturbs the cell membrane and may even lead to cell death [128]. Microinjection is preferred in rapidly dividing single cells, specifically in larger cells such as fertilized embryos, wherein the CRISPR components are directly injected into the single cell to create varieties of knockout and transgenic animals. However, this is a technically demanding procedure [129]. These mechanical methods are preferred for in vitro editing as they are reproducible, simple and have high levels of gene expression. Further, delivery through these direct methods has been used ex vivo in cells harvested from patients and then reintroduced into their body. Collectively, existing literature revealed the accuracy and efficiency of direct methods, but these methods are limited to in vitro or ex vivo applications. However, efficient in vivo delivery systems need further research and technical advancements.
Delivering payload specifically to any organ in the body should have some common factors that need to be considered, such as immune response, hematological toxicity, targeted delivery, distribution, etc. However, there are additional factors to be considered for retinal delivery of the therapeutic agent. The blood-retinal barrier limits the amount of payload that reaches the eye after intravenous injection thus localized injections (such as subretinal, intravitreal, corneal permeation, etc.) are mostly preferred for a retinal delivery route. Fortunately, the anatomical location also makes the eye more feasible to the localized injection. Vitreous fluid is one of the major barriers to retinal delivery. The viscosity and anionic charge of the vitreous fluid must be considered while developing a nano carrier-based delivery system [130]. As reported earlier, the high positive charge nanoparticle gets accumulated within the vitreous due to electrostatic interaction with anionic hyaluronic acid present in the vitreous [131]. Similarly, particle size is also having a considerable impact on vitreous diffusibility [132]; reports say that the particle size below 50 nm showed rapid clearance from the vitreous. Since the volume of the vitreous is very less, the injection volume is limited to a certain amount (25–100 ul). Therefore, the nanocarrier should have sufficient payload capacity so that the desired concentration of the payload could be delivered in that limited injection volume. Being a sense organ, the eye is more sensitive to toxicity, and therefore while selecting a nano-carrier, the toxicity issue should be considered. Although the immune-privileged nature of the eye provides opportunities to use distinct biomaterial used in delivery, despite that, toxicity may lead to the loss of eye integrity and could cause vision loss. Chitosan is the best example, which is known to cause retinal toxicity by inducing an immune response in the eye [133]. An increase in intraocular pressure is also a major challenge in intravitreal delivery and certain measures must be taken to resolve this issue. The intravitreal injection dose must be given by a trained professional because any mistake could lead to a serious eye injury. Additionally, multiple frequent injections need to be avoided in case of retinal delivery.
6.1. Viral vectors
CRISPR therapy had been developed for the primary purpose of treating inherited genetic diseases. Thus, the carrier package needs to be structured with a high degree of specificity, with no toxicity and rapid elimination after payload delivery [134]. The most widely used method for the efficient delivery of nucleic acid that encodes for the required protein, has been the viral vectors. But, as we have seen in the earlier section, even the viral delivery of CRISPR/Cas components causes undesirable effects such as immunogenicity and insertional mutations, hence restricting their clinical application [135].
Broadly, three types of viral vectors i.e., adenoviral, lentiviral, and AAV have been used to deliver genes that encode Cas9 into cells of interest. While the adenoviral vectors can elicit severe immunogenic reactions against the complex capsid proteins, the lenti and retroviral vectors have the risk of host gene integration and insertional mutations. AAVs are known for their low immunogenicity and target specificity based on their serotypes, and are preferred gene delivery vectors. They also show good transduction efficiencies and long-term transgene expression, without genome integration [136]. Various changes such as removing the endogenous Rep protein and encoding double self-complementary replicase of the viral genome (scAAVs) have led to reduced integration of the vector and improved their transduction efficiency by about 140 times [136,137]. However, the AAV-based viral vectors have limited packaging capacity and this limits the packaging of large gene cargos such as Cas9 and make it difficult to accommodate other regulatory elements such as promoters, polyadenylation signals and selection markers. Splitting the spCas9 into two parts will make the genes fit into the vector, but it reduces the delivery and edit efficiencies [138].
AAVs have been successfully used for in vivo gene delivery and shown long-term therapeutic effects for up to six years in LCA patients administered with AAV2 vector encoding RPE65 [77,139]. AAV and adenovirus delivered RPE65 in the rd12 mouse model of LCA2 have been shown to restore vision significantly [140,141]. The efficacy of AAV gene therapy was convincingly demonstrated in 2008 for the treatment of Leber congenital amaurosis. Many phase I and phase IIa trials for the subretinal delivery of AAV2-RPE65 cDNA have shown no serious adverse effects, along with improved pupillary reflexes, visual acuity and mobility in few of the treated patients [77,142,143]. The first AAV-based gene therapy drug, Glybera, was approved by the European Medicines Agency (EMA) in 2012 for the delivery of LPLD gene, with Luxturna becoming the first AAV gene therapy product to receive US FDA approval five years later, for the delivery of RPE65 gene.
Since AAVs pose a significant problem of packaging, many smaller Cas9 orthologs have been isolated from Streptococcus thermophilus (StCas9) [144], Staphylococcus aureus (SaCas9) [145], Campylobacter jejuni (CjCas9) and Neisseria meningitidis (NmCas9) [146]. Kim et al. used a combination of SaCas9 and CjCas9 together along with gRNAs incorporated into a single AAV. Their results showed that the cleaving action was as efficient as SpCas9 in vitro applications. In another study, Lachnospiraceae bacterium (LbCpf1) was used to prepare Cpf1 nuclease which was put together with the crRNA into a single AAV vector [81] showcasing excellent prospects for its use as an in vivo genome editing tool in the therapy of angiogenesis-related disorders.
Adenoviral vectors (AVs) are not the most used delivery vectors due to immunological concerns; however, their bigger genomes, episomal nature of intracellular maintenance and efficient transduction are advantages for in vivo delivery systems. The have a high packaging capacity (∼30–40 kb pairs), which can fit all the required elements. Thus, a single virus vector can express the Cas protein and one or many sgRNAs. To facilitate homology-directed repair, large donor DNA sequences can also be co-delivered. Hence, the Cas proteins and sgRNA are expressed proportionately within cells and the episomes may get lost in dividing cells thereby allowing only transient Cas9 expressions and reduced off target risks. AVs have been successfully used for in vivo genome editing in mice, although immune-related toxicities were observed [147]. However, immunogenicity is not a concern for in vitro editing of cell lines and stem cells. One of the first studies on AV was conducted in 1996 by Bennett et al. to study retinal disease in an animal model. They delivered the cDNA encoding phosphodiesterase β subunit into photoreceptors of the rd1 mouse model and shown successful delay of photoreceptor degeneration by six weeks. The disadvantage in AV therapy is their relatively high immunogenicity and the existence of neutralizing antibodies in humans against certain serotypes such as Ad5, renders the vector ineffective in most patients [148]. Studies have shown that subretinal delivery causes a lower T cell-mediated immune response than that of intravitreal injections [149,150]. AVs are being used to inhibit retinoblastoma growth in a mouse model and reduce retinal and choroidal neovascularization in rat and rabbit models [151], [152], [153].
Lentiviral vectors (LVs) are currently one of the most used vectors in the clinical application where long term effects are desired. Lentiviruses belong to the family of viruses known as Retroviridae; they are RNA viruses, which integrate into the host DNA using reverse transcriptase and integrase enzymes. Studies have shown that the LVs are safe and effective for gene delivery into photoreceptor cells of humans [154], [155], [156], [157]. LV packaging capacity is higher than AAVs in the range of approximately 8 to 9 kb. LVs can transduce both non-dividing and dividing cells with very high efficiency and can integrate into the host cell genomes to enable long-term transgene expression. However, long-lasting expression of Cas proteins may increase the risk of unwanted off-target edits [158]. To address this concern, self-inactivating constructs were engineered with two sgRNAs: one against the Cas9 gene and one against the target sequence of interest, thus allowing only transient expression of Cas9 to achieve the desired target site edits.
Among the viral delivery methods, AAV vectors are most preferred because of their mild immune response and absence of pathogenicity. AAVs can target non-dividing cells but have a limited packaging size. The development of the shorter dCas9 of 1 kb size has overcome this limitation to some extent [14]. Newer approaches are now being explored to decrease cytotoxicity, and to escape neutralizing antibodies for an overall improvement in vivo transduction efficiencies. As a results, some of the AAV based gene therapy products are already in clinical trials for the treatment of RDs (Table 4).
Table 4.
List of therapeutic molecules in clinical trials for the treatment RDs.
| Retinal dystrophy | Targeted gene | Therapeutic approach | Phase | Year | NCT ID |
|---|---|---|---|---|---|
| LCA | CEP290 Intron 26(IVS26) | subretinal EDIT-101 (CRISPR/Cas9) | I/II | 2019–24 | NCT03872479 |
| CEP290 p.Cys998X | Intravitreal QR-110(Antisense oligonucleotides) | I/II | 2017–19 | NCT03140969 | |
| I/II | 2019–21 | NCT03913130 | |||
| II/III | 2019–21 | NCT03913143 | |||
| RPE65 | Subretinal rAAV2-CBSB-hRPE65 | I | 2007–26 | NCT00481546 | |
| Subretinal tgAAG76(rAAV2/2.hRPE65p.hRPE65) | I/II | 2007–14 | NCT00643747 | ||
| rAAV2-CBSB-hRPE65 | I/II | 2009–17 | NCT00749957 | ||
| Subretinal AAV2-hRPE65v2 | I | 2007–18 | NCT00516477 | ||
| III | 2012–29 | NCT01208389 | |||
| I/II | 2010–26 | NCT00999609 | |||
| Subretinal rAAV2-hRPE65 | I | 2009–17 | NCT00821340 | ||
| Subretinal rAAV2-CBSB-hRPE65 Applied | I/II | 2009–17 | NCT00749957 | ||
| Subretinal rAAV-2/4.hRPE65 | I/II | 2011–14 | NCT01496040 | ||
| Subretinal AAV2/5-OPTIRPE65 | I/II | 2016–18 | NCT02781480 | ||
| I/II | 2016–23 | NCT02946879 | |||
| CHM | REP1 | Subretinal rAAV2.REP1 | I/II | 2015–25 | NCT02077361 |
| Subretinal rAAV2.REP1 | I/II | 2011–17 | NCT01461213 | ||
| Subretinal AAV2.REP1 | II | 2016–21 | NCT02407678 | ||
| Subretinal rAAV2.REP1 | II | 2016–18 | NCT02671539 | ||
| Subretinal rAAV2.REP1 | II | 2015–18 | NCT02553135 | ||
| Subretinal BIIB111(AAV2-REP1) | II | 2018–22 | NCT03507686 | ||
| Subretinal AAV2-REP1 | III | 2017–20 | NCT03496012 | ||
| CHM | Intravitreal 4D-100 | I | 2020–23 | NCT04483440 | |
| Subretinal AAV2-hCHM | I/II | 2015–22 | NCT02341807 | ||
| RP | PDE6B | Subretinal AAV2/5-hPDE6B | I/II | 2017–24 | NCT03328130 |
| RLBP1 | Subretinal CPK850 | I/II | 2018–26 | NCT03374657 | |
| PDE6A | Subretinal rAAV.hPDE6A | I/II | 2019–25 | NCT04611503 | |
| USH2A | Intravitreal QR-421a | I/II | 2019–22 | NCT03780257 | |
| Advanced RP | ChR2 | Intravitreal RST-001 | I/II | 2015–35 | NCT02556736 |
| adRP | RHO | unilateral IVT injection QR-1123 | I/II | 2019–21 | NCT04123626 |
| XLRS | RS1 | Intravitreal rAAV2tYF-CB-hRS1 | I/II | 2015–23 | NCT02416622 |
| Intravitreal AAV8-scRS/IRBPhRS | I/II | 2015–23 | NCT02317887 | ||
| XLRP | RPGR | sub-retinal BIIB112 | I/II | 2017–20 | NCT03116113 |
| Intravitrea 4D-125 | I/II | 2020–23 | NCT04517149 | ||
| Subretinal AAV2/5-RPGR | I/II | 2017–20 | NCT03252847 | ||
| Subretinal AGTC-501 (rAAV2tYF-GRK1-RPGR) | I/II | 2018–26 | NCT03316560 | ||
| Subretinal AAV5-RPRG | III | 2021–22 | NCT04671433 | ||
| Achromatopsia | CNGB3 | Subretinal rAAV2tYF-PR1.7-hCNGB3 | I/II | 2016–25 | NCT02599922 |
| Subretinal rAAV.hCNGA3 | I/II | 2015–27 | NCT02610582 | ||
| Leber Hereditary Optic Neuropathy |
ND4 | GS010; Sham intravitreal injection | III | 2016–19 | NCT02652767 |
| III | 2016–18 | NCT02652780 | |||
| Intravitreal scAAV2-P1ND4v2 | I | 2014–23 | NCT02161380 | ||
| Intravitreal rAAV2-ND4 | — | 2011–15 | NCT01267422 | ||
| USH1B | — | Subretinal SAR422459 | I/II | 2012–19 | NCT01505062 |
| — | Blood draw for the laboratory assessment | I/II | 2013–32 | NCT02065011 | |
| Stargardt's Macular Degeneration | — | Long term follows up in all patients who received SAR422459 in previous study TDU13583 | I/II | 2012–34 | NCT01736592 |
| — | SAR422459 | I/II | 2011–19 | NCT01367444 | |
| Neovascular AMD | — | Subretinal RetinoStat | I | 2011–15 | NCT01301443 |
| — | I | 2012–29 | NCT01678872 | ||
| — | Intravitreal AAV2-sFLT01 | I | 2010–18 | NCT01024998 | |
| — | Subretinal rAAV.sFlt-1; Control (ranibizumab alone) | I/II | 2011–17 | NCT01494805 |
Abbreviations: RPE65: Retinal pigmented epithelium-specific protein with molecular mass 65 kDa; CEP290: Centrosomal Protein 290; ChR2: Channelrhodopsin-2; PDE6B: Phosphodiesterase 6B; RLBP1: Retinaldehyde-binding protein 1; USH2A: Usherin; PDE6A: Phosphodiesterase 6A; RPGR: Retinitis pigmentosa GTPase regulator; REP1: Rab escort protein 1; RS1: Retinoschisin.
6.2. Non-viral vectors
The problems associated with viral vectors such as immunogenicity and packaging issues have paved way for the development of non-viral systems that are usually better characterized and can be modified chemically to meet the delivery requirements [159]. However, non-viral vectors have concerns related to toxicity, biocompatibility, adverse immunological reactions, risk of release of therapeutic material into non-targeted sites. They also suffer from low in vivo delivery efficiency, although this problem is being solved with the advancements in material sciences. Nanotechnology-based formulations are expected to provide ample benefits over existing approaches [160]. These include (i) sustained release of payload, (ii) improved uptake in retinal cells, (iii) better vitreous penetrability, (iv) could be tailored to achieve cell-specific delivery and (v) reduce vitreal clearance leading to improved exposure time. Improved success has been achieved with newer polymer- and lipid-based complexes that have the required properties for effective transport of their genetic cargo across multiple physiological barriers. As a results, ample of nanobased products are approved by FDA or under investigation (Table 5).
Table 5.
List of nanomedicines approved or under clinical investigation for the treatment of RDs.
| RD | Status | Nanomedicine | Product name | Molecule | NCT ID |
|---|---|---|---|---|---|
| AMD | Approved by FDA | Liposome | Visudyne® | Verteporfin | NCT00121407 |
| wAMD | Approved by FDA | Aptamer–polymer nanoparticle | Macugen® | Pegaptanib sodium | NCT00549055 |
| Macular edema | Approved by FDA | Suspension | Kenalog | Triamcinolone acetonide | NCT00101764 |
| Phase II | Lipid-based nanoparticle | TLC399 (ProDex) | Dexamethasone sodium phosphate | NCT03093701 |
For the delivery of CRISPR/Cas components via non-viral vectors, mechanisms of direct conjugation of the active molecule to the excipient has been adopted, such as gRNA or Cas protein conjugation with cell-penetrating peptides (CPPs). Studies by Ramakrishna et al. in HEK293T cells have demonstrated that the conjugation has led to 72% and 62% editing efficiencies with plasmids and RNPs, respectively. But, these CPP systems have not proven efficient to cross all delivery barriers [116]. The following are the current non-viral delivery systems that are being used for CRISPR/Cas9 delivery (Fig. 5).
Fig. 5.
Non-viral vectors being explored for the delivery of CRISPR/Cas RNPs.
6.3. Lipoplexes
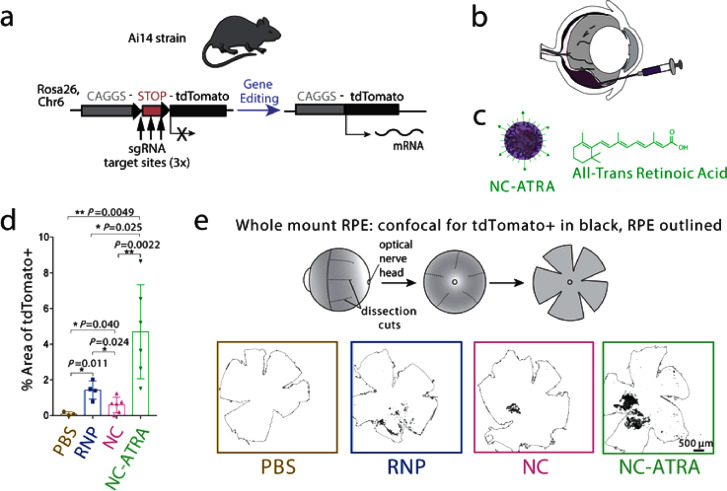
In 1987, the scientific expression ‘‘lipofection’’ was first used to describe a lipidic system used for gene transfection [161]. It is one of the oldest and widely used techniques for gene transfer. Lipids have been extensively studied for their characteristics as nanocarriers and to electrostatically complex with a negatively charged gene, a positively charged cationic lipid is incorporated in the carrier. Commercially available cationic lipids include N-[1-(2,3-dioleyloxy) propyl]-N,N,N-trimethyl-ammonium chloride (DOTMA), 1,2-dioleoyl-3-trimethylammoniumpropane (DOTAP), 1,2-dimyristyloxypropyl-3-dimethyl-hydroxyethylammonium bromide (DMRIE). and 2,3-dioleyloxy-N-[2(sperminecarboxamido)ethyl]-N,N-dimethyl-1-propanaminium trifluoroacetate (DOSPA) [162]. Wang et al. in their experiments showed that CRISPR/Cas RNP can be administered into the cell using biodegradable cationic lipid nanoparticles, leading to effective knockout of genes [163]. The delivery of supercharged Cre protein and Cas9:sgRNA complexed with bio-reducible lipids into cultured human cells (HeLa-DsRed cells) enabled gene recombination and genome editing with efficiencies greater than 70%. Further, disulfide linkages in the lipid material can be used to trigger release by the degradation of endosomal particles leading to endosomal release. In addition, the authors demonstrated that these lipids are effective for functional protein delivery into mouse brain for gene recombination in vivo [164]. In 2020, Wei et al. used mixture of lipid (5A2-SC8, DOTAP, DMG-PEG, Chol, DOPE) to prepare a lipidic system i.e. 5A2-DOT with different concentrations of DOTAP (10 - 50 mol%). The nanoformulation showed sufficient payload for Cas9 RNPs and showed efficient, precise gene editing (for TdTomato gene) in mice brain, muscle, when administered locally. Further, formulation given intravenously also showed significant gene editing in liver and lungs tissues as well [165].
6.4. Polyplexes
There are various types of polymer complexes that are being used for CRISPR/Cas delivery. Cationic polymers pose lesser immunogenicity problems as well as are much easier to synthesize on a large scale. Those which have gained attraction include polyethylenimine (PEI), poly(L-lysine) (PLL), poly[2-(dimethylamino) ethyl methacrylate] (PDMAEMA), and polyamidoamine (PAMAM) dendrimers [166]. Among polymeric vectors, polyethylenimine (PEI) has been extensively researched upon. Scientists in 1995 carried out the first successful transfection using PEI, after which it is regarded as a gold standard in the study of polymeric non-viral carriers due to their high transfection efficiency. The characteristics of PEI which make it suitable for gene transfer are its “proton sponge” nature and high charge density [167]. Like cationic lipids, PEI can be complexed with nucleic acids, induce endosomal uptake and release in the cytoplasm of the cell. A study by Zhang et al. shows a formulation made up of PEI-β-cyclodextrin to deliver plasmids coding for sgRNA and Cas9 in HeLa cells, achieving gene knockout [168]. PEI has also been used in a formulation by Sun et al., in which they have used DNA as a nanomaterial for coating of CRISPR/Cas. These particles were encapsulated by PEI to enhance endosomal release. These particles were directly injected in mice which had EGFP tumors, and the resulting phenotypes showed knockout of EGFP [169]. Apart for PEI, the high charge density PLL, a synthetic polypeptide, has been explored for gene delivery. However, due to the absence of buffering capacity (required for endosomal escape), PLL displays lower transfection efficiency than other polyplex systems [170]. Further, the high-molecular-weight PLL leads to a high level of cytotoxicity as it interacts with serum proteins, causing rapid elimination of the complexes from the system. Studies have shown a PLL-PEG copolymer can help solve these issues and facilitates its duration in the system [171]. The success of PLL-PEG copolymer in gene delivery has been proved in both in vitro and in vivo studies. In a study conducted in 2004 on treating cystic fibrosis (CF), PLL-PEG was explored as a gene delivery construct carrying the cystic fibrosis transmembrane regulator encoding gene [172]. PDMAEMA is another non-viral delivery method. A water-soluble cationic polymer, polysaccharide modified PDMAEMAs are gaining significance in CRISPR/Cas delivery [135]. In 2020, Carlos et al., synthesized magnetite/silver-PDMAEMA conjugate and evaluated it for the delivery of CRISPR plasmid. It showed 16.4% loading efficiency with minimum toxicity. Further, colocalization studies were performed in SH-Y5Y cells with lysotracker and data indicated, the Pearson's correlation coefficient approached 0.240 ± 0.024 after 0.5 h and decreased to 0.215 ± 0.029 in a statistically significant manner after 4 h. Collectively, the magnetite/silver-pDMAEMA showed endosomal escape after 4 h of internalization and showed effective delivery potential in vitro [173]. In 2019, Chen et al. developed Cas9 RNPs loaded nanocapsule (NCs) composed of cationic polymer and liposome components along with a glutathione cleavable linker in between. NCs carry 40% of RNPs content with a particle size of 25 nm. The research focused on in vivo gene editing performed in eyes and muscles of transgenic Ai14 mice having three sv40 polyA transcription terminators as stop cassette for TdTomato gene. NCs were decorated with all-trans-retinoic acid (NTRA, binds to inter-photoreceptor retinoid-binding protein) to form NTRA-NCs, and evaluated for gene editing in RPE cells in the eyes of Ai14 mice (Fig. 6). Further, mice were injected with PBS, naked RNPs, NCs and all trans retinoic acid NCs (ATRA-NCs) subretinally and eyes tissue were excised after 12 dpost injection. NCs showed considerable and ATRA-NCs showed significantly higher gene editing in RPE cells in terms of TdTomato fluorescence. Moreover, the NCs were injected intramuscular and NCs showed gene editing and a strong TdTomato fluorescence was observed in muscle tissues. Collectively, the study explored the potential role of non-viral biodegradable NCs in the delivery of high molecular weight Cas9 RNPs for in vivo gene editing application [174].
Fig. 6.
In vivo gene editing efficiency of RNP loaded nanocapsule in mice.Graphicalillustration of the (a) TdTomato gene, (b) site of intravitreal injection in mice eye, (c) ATRA targeted nanocapsule. (d) post injection in vivo gene editing in RPE layer quantified in terms of TdTomato positive area (%) in different treatment groups, (e) mounted RPE layer of the mouse treated with PBS, RNP, NCs and NC-ATRA, herein black area represents TdTomato signals after 12 d treatment. Reprinted by permission from [174]. Copyright ©2019 the authors under exclusive licence to Springer Nature Limited.
6.5. Dendriplexes
Polyamidoamine dendrimers (PAMAM) dendrimers are commercially available for gene transfer and one of the most frequently used systems [175]. The structure is made up of a core surrounded by polymeric branches with the surface expressing cationic primary amines that helps them complex to nucleic acids. PAMAM dendrimers are known to be generation-dependent; low-generation (G0–G3) dendriplexes have poor gene transfection efficiencies and are less cytotoxic, but higher generation (G4–G8) PAMAMs have improved gene transfection efficiencies and are more cytotoxic [176]. Yu et al. in their research, prepared a formulation made up of a lipid and dendrimer hybrid. The structure consisted of a long hydrophobic alkyl chain and a low-generation hydrophilic PAMAM dendron. In this way, the system exhibited the pros of both lipid and polymers in delivering siRNA and achieved the efficient gene-silencing effect in vitro and in vivo studies [177]. In another study, l-arginine was used to transform the surface of PAMAM enhancing gene transfection efficiency due to ease of complexation [178]. In 2019, Liu et al., synthesized boric acid rich 5 (G5) amine-terminated PAMAM (P4) for CRISPR/Cas9 RNPs delivery. The P4 dendrimer nanoparticle loaded with RNPs showed particle size of 300 nm. The functionalized dendrimer showed efficient delivery along with 45% of EGFP gene knock out in EGFP-HEK293T cells, analyzed via flow cytometry. Further, another set of experiments were performed to evaluate gene editing efficiency, and CRISPRMax was taken as standard along with P4 dendrimer. CRISPR/Cas9 RNPs were designed for targeting adeno-associated virus integration site 1 (AAVS1) and hemoglobin subunit beta (HBB) and transfection assay was performed followed by T7 endonuclease (T7E) assay, where results indicate indel frequency of 23.1% and 17.5% for AAVS gene with P4 dendrimer/RNPs and CRISPRMax/RNPs, respectively. Likewise, indel frequency of 1.1% and 9.7% was observed for HBB gene with P4 dendrimer/RNPs and CRISPRMax/RNPs, respectively. Collectively, this study explored the potential role of dendrimers as a delivery vehicle for CRISPR/Cas9 [179].
7. CRISPR/Cas based RNA editing approach in retinal degeneration diseases
Theoretically, G>A or T>C single-base mutations in any gene related to inherited retinal degeneration could be corrected by using base editing [180]. Nevertheless, all the diseases are not equally responsive to RNA editing. AAV mediated gene replacement provides effective treatment for small gene deliveries. On the other hand, higher rate of mutant allele-specific editing is required for dominant diseases where the mutant alleles have to be efficiently knocked out. Interestingly, larger recessive genes (>4.2 kb) are difficult to correct by gene replacement using AAV and requires in-situ editing strategy either in vitro or in vivo. Stone et al. have listed the major recessive genes such as ABCA4, USH2A, CEP290, MYO7A, EYS, CDH23 along with their relative frequency in the patients with inherited retinal degeneration [181]. The most common single nucleotide mutation is G>A, amenable to currently available base editing techniques. The proportion of G>A or T>C mutation in CEP290 and ABCA4 gene was 9% and 32%, respectively [181]. Further, 6% of the mutations observed in USH2A gene results in the creation of premature stop codon, while missense and donor/acceptor slice mutations were predominantly seen in ABCA4 gene. Collectively, the data suggested the dominancy of G>A mutation (∼75 in number) in both the genes, that leads to premature stop codons. Humans have adenosine deaminase acting on RNA (ADAR) enzymes, which play a key role in adenosine to inosine conversion (A→ I) [182]. Since inosine is read as guanosine by the splicing and translation apparatus, ADARs can also be used to alter RNA splicing to restore normal reading frames and modify amino acid sequences.
In 2017, Cox et al. explored a new system called ‘REPAIR’ (RNA Editing for Programmable A to I Replacement), which aimed to cleave RNA at a specific site by the means of a novel protein Cas13a, isolated form the Leptotrichia wadei. Cas13a binds to RNA-RNA hybrid unlike the DNA-RNA hybrid bound by Cas9 and is also a part of the bacterial CRISPR mediated RNA interference system [183]. The Cas13-based RNA editing technique may be advantageous over the conventional DNA editing, as DNA manipulations are permanent in cellular genomes and may have unanticipated long-term side effects. Currently, a synthetic RNA targeting system has been developed with a human protein, with Cas13 like ssRNA recognizing properties. This system termed as CIRTS (CRISPR/Cas inspired RNA targeting system) is an engineered modular RNA-guided RNA-targeting effector system, synthesized totally from human proteins and provide a new tool set to overcome the size and immunogenicity limitations of bacterial CRISPR-Cas systems. This system consists of an engineered gRNA, that carries sequences complementary to the target RNA and also carries a sequence to recruit an engineered hairpin binding protein and a non-specific ssRNA binding protein for complex stabilization and an ADAR2 like effector protein to enable base editing (Fig. 7). CIRTS8 system, when delivered in the form of a plasmid construct, could achieve 47% edit efficiency in HEK293T cells without significant off-target edits [180]. RNA editing thus provides immense potential in repairing pathogenic mutations in many diseases such as Duchenne's muscular dystrophy, cystic fibrosis, hurler's syndrome etc. RNA edits are also reversible and therefore offers improved safety in terms of therapeutic considerations. Inducible RNA editors with automatic switch off systems and external stimuli triggered switch on systems further improves the safety profiles of RNA editing [184].
Fig. 7.
Schematic representation of mRNA editing using Cas13b in retinal dystrophic conditions.
8. Conclusions and future perspective
CRISPR/Cas system has emerged as a rapidly evolving therapeutic tool in genomic engineering. Genome therapy using CRISPR/Cas in ophthalmic diseases is a boon for the society considering the impact it has on the lives of thousands of people. It offers newer hopes of developing promising therapeutics for the treatment of inherited retinal disorders. In the past 20 years, the eye and ocular diseases have caught the limelight of gene therapeutic and cell therapeutic efforts, mainly for the unique anatomical location that enables easy interventions, detailed imaging and documentations; the immune privileged status of the eye; the existence of blood-retinal barrier safety that ensures long-term ocular retention and containment of therapeutics; and finally for the huge societal impact of even marginal improvements in vision to patient beneficiaries. Gene therapeutics either alone or when combined with gene editing strategies can enable delivery of normal gene copies; or in situ mutation editing for normal protein and RNA expression; and targeted disruption of dominant mutant alleles or their transcripts for the reversal of disease phenotypes. Several pre-clinical studies have been initiated using both the viral and non-viral vectors for delivering CRISPR components for therapeutic applications. While it is exciting to watch the fast pace of developments in this field, it is prudent to move forward responsibly, considering both the ethical and societal implications. It is important to exercise well-informed caution and consider both the intended and unintended outcomes, while designing gene editing based therapeutic strategies.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We sincerely acknowledge the Indian Council of Medical Research (ICMR) for financial support through senior research fellowship (SRF) to DKS (file no. 45/66/2019-Nan/BMS) and junior research fellow to MS (file no. 3/1/3/JRF-2019/HRD(LS)). The funding support from the Department of Biotechnology, Ministry of Science and Technology (DBT), Government of India to DC through project grant (BT/PR26897/NNT/28/1489/2017) is duly acknowledged.
References
- 1.Samson J.E., Magadan A.H., Sabri M., Moineau S. Revenge of the phages: defeating bacterial defences. Nat Rev Microbiol. 2013;11:675–687. doi: 10.1038/nrmicro3096. [DOI] [PubMed] [Google Scholar]
- 2.Sahel D.K., Mittal A., Chitkara D. CRISPR/Cas system for genome editing: progress and prospects as a therapeutic tool. J Pharmacol Exp Ther. 2019;370:725–735. doi: 10.1124/jpet.119.257287. [DOI] [PubMed] [Google Scholar]
- 3.Mojica F.J.M., Diez-Villasenor C., Garcia-Martinez J., Almendros C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology (Reading, Engl.) 2009;155:733–740. doi: 10.1099/mic.0.023960-0. [DOI] [PubMed] [Google Scholar]
- 4.Zhan T., Rindtorff N., Betge J., Ebert M.P., Boutros M. CRISPR/Cas9 for cancer research and therapy. Semin Cancer Biol. 2019;55:106–119. doi: 10.1016/j.semcancer.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Makarova K.S., Haft D.H., Barrangou R., Brouns S.J., Charpentier E., Horvath P., et al. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9:467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burmistrz M., Pyrc K. CRISPR-Cas systems in prokaryotes. Pol J Microbiol. 2015;64:193–202. [PubMed] [Google Scholar]
- 7.Alkan F., Wenzel A., Anthon C., Havgaard J.H., Gorodkin J. CRISPR-Cas9 off-targeting assessment with nucleic acid duplex energy parameters. Genome Biol. 2018;19:177. doi: 10.1186/s13059-018-1534-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Y., Zhang L., Huang X. Genome modification by CRISPR/Cas9. FEBS J. 2014;281:5186–5193. doi: 10.1111/febs.13110. [DOI] [PubMed] [Google Scholar]
- 9.Javed M.R., Sadaf M., Ahmed T., Jamil A., Nawaz M., Abbas H., et al. CRISPR-Cas system: history and prospects as a genome editing tool in microorganisms. Curr Microbiol. 2018;75:1675–1683. doi: 10.1007/s00284-018-1547-4. [DOI] [PubMed] [Google Scholar]
- 10.Haeussler M., Schonig K., Eckert H., Eschstruth A., Mianne J., Renaud J.B., et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016;17:148. doi: 10.1186/s13059-016-1012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vázquez-Domínguez I., Garanto A., Collin R.W.J. Molecular therapies for inherited retinal diseases-current standing, opportunities and challenges. Genes (Basel) 2019;10:654. doi: 10.3390/genes10090654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnight E.R., Giacalone J.C., Cooke J.A., Thompson J.R., Bohrer L.R., Chirco K.R., et al. CRISPR-Cas9 genome engineering: treating inherited retinal degeneration. Prog Retin Eye Res. 2018;65:28–49. doi: 10.1016/j.preteyeres.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulcha J.T., Wang Y., Ma H., Tai P.W.L., Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. 2021;6:53. doi: 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lino C.A., Harper J.C., Carney J.P., Timlin J.A. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018;25:1234–1257. doi: 10.1080/10717544.2018.1474964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim E., Koo T., Park S.W., Kim D., Kim K., Cho H.Y., et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat Commun. 2017;8:14500. doi: 10.1038/ncomms14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abudayyeh O.O., Gootenberg J.S., Essletzbichler P., Han S., Joung J., Belanto J.J., et al. RNA targeting with CRISPR–Cas13. Nature. 2017;550:280–284. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi L.S., Larson M.H., Gilbert L.A., Doudna J.A., Weissman J.S., Arkin A.P., et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–1183. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert L.A., Larson M.H., Morsut L., Liu Z., Brar G.A., Torres S.E., et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442–451. doi: 10.1016/j.cell.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavez A., Scheiman J., Vora S., Pruitt B.W., Tuttle M., Iyer E.P., et al. Highly efficient Cas9-mediated transcriptional programming. Nat Methods. 2015;12:326–328. doi: 10.1038/nmeth.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaudelli N.M., Komor A.C., Rees H.A., Packer M.S., Badran A.H., Bryson D.I., et al. Programmable base editing of A• T to G• C in genomic DNA without DNA cleavage. Nature. 2017;551:464–471. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei Y., Zhang X., Su J., Jeong M., Gundry M.C., Huang Y.H., et al. Targeted DNA methylation in vivo using an engineered dCas9-MQ1 fusion protein. Nat Commun. 2017;8:1–10. doi: 10.1038/ncomms16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng W., Shi X., Tjian R., Lionnet T., Singer R.H. CASFISH: cRISPR/Cas9-mediated in situ labeling of genomic loci in fixed cells. Proc Natl Acad Sci. 2015;112:11870–11875. doi: 10.1073/pnas.1515692112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azhar M., Phutela R., Kumar M., Ansari A.H., Rauthan R., Gulati S., et al. Rapid and accurate nucleobase detection using FnCas9 and its application in COVID-19 diagnosis. Biosens. Bioelectron. 2021;183 doi: 10.1016/j.bios.2021.113207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis P.J. Genetics of inherited retinal disease. J R Soc Med. 2006;99:189–191. doi: 10.1258/jrsm.99.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacione L.R., Szego M.J., Ikeda S., Nishina P.M., McInnes R.R. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu Rev Neurosci. 2003;26:657–700. doi: 10.1146/annurev.neuro.26.041002.131416. [DOI] [PubMed] [Google Scholar]
- 26.Sundaramurthi H., Moran A., Perpetuini A.C., Reynolds A., Kennedy B. Emerging drug therapies for inherited retinal dystrophies. Adv Exp Med Biol. 2019;1185:263–267. doi: 10.1007/978-3-030-27378-1_43. [DOI] [PubMed] [Google Scholar]
- 27.Berger W., Kloeckener-Gruissem B., Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res. 2010;29:335–375. doi: 10.1016/j.preteyeres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Dias M.F., Joo K., Kemp J.A., Fialho S.L., da Silva, Cunha A., Jr, Woo S.J., et al. Molecular genetics and emerging therapies for retinitis pigmentosa: basic research and clinical perspectives. Prog Retin Eye Res. 2018;63:107–131. doi: 10.1016/j.preteyeres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 29.DiCarlo J.E., Mahajan V.B., Tsang S.H. Gene therapy and genome surgery in the retina. J Clin Invest. 2018;128:2177–2188. doi: 10.1172/JCI120429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sengillo J.D., Justus S., Cabral T., Tsang S.H. Correction of monogenic and common retinal disorders with gene therapy. Genes (Basel) 2017;8(2):53. doi: 10.3390/genes8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanik M., Muller B., Song F., Gall J., Wagner F., Wende W., et al. In vivo genome editing as a potential treatment strategy for inherited retinal dystrophies. Prog Retin Eye Res. 2017;56:1–18. doi: 10.1016/j.preteyeres.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Colella P., Auricchio A. Gene therapy of inherited retinopathies: a long and successful road from viral vectors to patients. Hum Gene Ther. 2012;23:796–807. doi: 10.1089/hum.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colella P., Auricchio A. Gene therapy of inherited retinopathies: a long and successful road from viral vectors to patients. Human gene therapy. 2012;23:796–807. doi: 10.1089/hum.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang F.Q., Anand V., Maguire A.M., Bennett J. Intraocular delivery of recombinant virus. Methods Mol Med. 2001;47:125–139. doi: 10.1385/1-59259-085-3:125. [DOI] [PubMed] [Google Scholar]
- 35.Schimmer J., Breazzano S. Investor outlook: focus on upcoming LCA2 gene therapy phase III results. Hum Gene Ther Clin Dev. 2015;26:144–149. doi: 10.1089/humc.2015.29001.sch. [DOI] [PubMed] [Google Scholar]
- 36.Cong L., Ran F.A., Cox D., Lin S., Barretto R., Habib N., et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrangou R., Doudna J.A. Applications of CRISPR technologies in research and beyond. Nat Biotechnol. 2016;34:933–941. doi: 10.1038/nbt.3659. [DOI] [PubMed] [Google Scholar]
- 38.Saha S.K., Saikot F.K., Rahman M.S., Jamal M.A., Rahman S.K., Islam S.R., Kim K.H. Programmable molecular scissors: applications of a new tool for genome editing in biotech. Mol Ther Nucleic Acids. 2019;14:212–238. doi: 10.1016/j.omtn.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.den Hollander A.I., Roepman R., Koenekoop R.K., Cremers F.P. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Aleman T.S., Jacobson S.G., Chico J.D., Scott M.L., Cheung A.Y., Windsor E.A., et al. Impairment of the transient pupillary light reflex in Rpe65(-/-) mice and humans with leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2004;45:1259–1271. doi: 10.1167/iovs.03-1230. [DOI] [PubMed] [Google Scholar]
- 41.Simonelli F., Ziviello C., Testa F., Rossi S., Fazzi E., Bianchi P.E., et al. Clinical and molecular genetics of Leber's congenital amaurosis: a multicenter study of Italian patients. Invest Ophthalmol Vis Sci. 2007;48:4284–4290. doi: 10.1167/iovs.07-0068. [DOI] [PubMed] [Google Scholar]
- 42.Giannelli S.G., Luoni M., Castoldi V., Massimino L., Cabassi T., Angeloni D., et al. Cas9/sgRNA selective targeting of the P23H Rhodopsin mutant allele for treating retinitis pigmentosa by intravitreal AAV9.PHP.B-based delivery. Hum Mol Genet. 2018;27:761–779. doi: 10.1093/hmg/ddx438. [DOI] [PubMed] [Google Scholar]
- 43.Tsai Y.T., Wu W.H., Lee T.T., Wu W.P., Xu C.L., Park K.S., et al. Clustered regularly interspaced short palindromic repeats-based genome surgery for the treatment of autosomal dominant retinitis pigmentosa. Ophthalmology. 2018;125:1421–1430. doi: 10.1016/j.ophtha.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman D.S., O'Colmain B.J., Munoz B., Tomany S.C., McCarty C., de Jong P.T., et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 45.Chan L., Mahajan V.B., Tsang S.H. Genome surgery and gene therapy in retinal disorders. Yale J Biol Med. 2017;90:523–532. [PMC free article] [PubMed] [Google Scholar]
- 46.Fogli S., Del Re M., Rofi E., Posarelli C., Figus M., Danesi R. Clinical pharmacology of intravitreal anti-VEGF drugs. Eye (Lond) 2018;32:1010–1020. doi: 10.1038/s41433-018-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jo D.H., Koo T., Cho C.S., Kim J.H., Kim J.S., Kim J.H. Long-term effects of in vivo genome editing in the mouse retina using campylobacter jejuni Cas9 expressed via adeno-associated virus. Mol Ther. 2019;27:130–136. doi: 10.1016/j.ymthe.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim K., Park S.W., Kim J.H., Lee S.H., Kim D., Koo T., et al. Genome surgery using Cas9 ribonucleoproteins for the treatment of age-related macular degeneration. Genome Res. 2017;27:419–426. doi: 10.1101/gr.219089.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q. Retinitis pigmentosa: progress and perspective. Asia Pac J Ophthalmol (Phila) 2016;5:265–271. doi: 10.1097/APO.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 50.Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet (Lond) 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 51.Nandrot E.F., Kim Y., Brodie S.E., Huang X., Sheppard D., Finnemann S.C. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nandrot E.F., Anand M., Almeida D., Atabai K., Sheppard D., Finnemann S.C. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci USA. 2007;104:12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abu-Safieh L., Alrashed M., Anazi S., Alkuraya H., Khan A.O., Al-Owain M., et al. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013;23:236–247. doi: 10.1101/gr.144105.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel N., Aldahmesh M.A., Alkuraya H., Anazi S., Alsharif H., Khan A.O., et al. Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies. Genet Med. 2016;18:554–562. doi: 10.1038/gim.2015.127. [DOI] [PubMed] [Google Scholar]
- 55.Mackay D.S., Henderson R.H., Sergouniotis P.I., Li Z., Moradi P., Holder G.E., et al. Novel mutations in MERTK associated with childhood onset rod-cone dystrophy. Mol Vis. 2010;16:369–377. [PMC free article] [PubMed] [Google Scholar]
- 56.Ksantini M., Lafont E., Bocquet B., Meunier I., Hamel C.P. Homozygous mutation in MERTK causes severe autosomal recessive retinitis pigmentosa. Eur J Ophthalmol. 2012;22:647–653. doi: 10.5301/ejo.5000096. [DOI] [PubMed] [Google Scholar]
- 57.Churchill J.D., Bowne S.J., Sullivan L.S., Lewis R.A., Wheaton D.K., Birch D.G., et al. Mutations in the X-linked retinitis pigmentosa genes RPGR and RP2 found in 8.5% of families with a provisional diagnosis of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2013;54:1411–1416. doi: 10.1167/iovs.12-11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meindl A., Dry K., Herrmann K., Manson F., Ciccodicola A., Edgar A., et al. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3) Nat Genet. 1996;13:35–42. doi: 10.1038/ng0596-35. [DOI] [PubMed] [Google Scholar]
- 59.Hong D.H., Li T. Complex expression pattern of RPGR reveals a role for purine-rich exonic splicing enhancers. Invest Ophthalmol Vis Sci. 2002;43:3373–3382. [PubMed] [Google Scholar]
- 60.Linari M., Ueffing M., Manson F., Wright A., Meitinger T., Becker J. The retinitis pigmentosa GTPase regulator, RPGR, interacts with the delta subunit of rod cyclic GMP phosphodiesterase. Proc Nat Acad Sci USA. 1999;96:1315–1320. doi: 10.1073/pnas.96.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bakondi B., Lv W., Lu B., Jones M.K., Tsai Y., Kim K.J., et al. In vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. Mol Ther. 2016;24:556–563. doi: 10.1038/mt.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bassuk A.G., Zheng A., Li Y., Tsang S.H., Mahajan V.B. Precision medicine: genetic repair of retinitis pigmentosa in patient-derived stem cells. Sci Rep. 2016;6:19969. doi: 10.1038/srep19969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Latella M.C., Di Salvo M.T., Cocchiarella F., Benati D., Grisendi G., Comitato A., et al. In vivo editing of the human mutant rhodopsin gene by electroporation of plasmid-based CRISPR/Cas9 in the mouse retina. Mol Ther Nucl Acids. 2016;5:e389. doi: 10.1038/mtna.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacLaren R.E., Groppe M., Barnard A.R., Cottriall C.L., Tolmachova T., Seymour L., et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet (Lond) 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zinkernagel M.S., MacLaren R.E. Recent advances and future prospects in choroideremia. Clin Ophthalmol. 2015;9:2195–2200. doi: 10.2147/OPTH.S65732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobson S.G., Cideciyan A.V., Sumaroka A., Aleman T.S., Schwartz S.B., Windsor E.A., et al. Remodeling of the human retina in choroideremia: rab escort protein 1 (REP-1) mutations. Invest Ophthalmol Vis Sci. 2006;47:4113–4120. doi: 10.1167/iovs.06-0424. [DOI] [PubMed] [Google Scholar]
- 67.Charbel Issa P., Barnard A.R., Herrmann P., Washington I., MacLaren R.E. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc Nat Acad Sci USA. 2015;112:8415–8420. doi: 10.1073/pnas.1506960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zaneveld J., Wang F., Wang X., Chen R. Dawn of ocular gene therapy: implications for molecular diagnosis in retinal disease. Sci China Life Sci. 2013;56:125–133. doi: 10.1007/s11427-013-4443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kremer H., van Wijk E., Marker T., Wolfrum U., Roepman R. Usher syndrome: molecular links of pathogenesis, proteins and pathways. Hum Mol Genet. 2006:R262–R270. doi: 10.1093/hmg/ddl205. 15 Spec No 2. [DOI] [PubMed] [Google Scholar]
- 70.Sanjurjo-Soriano C., Kalatzis V. Guiding lights in genome editing for inherited retinal disorders: implications for gene and cell therapy. Neural Plast. 2018;2018 doi: 10.1155/2018/5056279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fuster-García C., García-García G., González-Romero E., Jaijo T., Sequedo M.D., Ayuso C., et al. USH2A Gene editing using the CRISPR system. Mol Ther Nucl Acids. 2017;8:529–541. doi: 10.1016/j.omtn.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Celea C., Mihai P.O., Avidis-Zamfiroiu N., Celea C. Evolution of choroidal neovascular membrane in best disease after single intravitreal bevacizumab. Case report. Maedica. 2015;10:61. [PMC free article] [PubMed] [Google Scholar]
- 73.Sinha D., Steyer B., Shahi P.K., Mueller K.P., Valiauga R., Edwards K.L., et al. Human iPSC modeling reveals mutation-specific responses to gene therapy in a genotypically diverse dominant maculopathy. Am J Hum Genet. 2020;107:278–292. doi: 10.1016/j.ajhg.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang K.C., Wang M.L., Chen S.J., Kuo J.C., Wang W.J., Nhi Nguyen P.N., et al. Morphological and molecular defects in human three-dimensional retinal organoid model of X-linked juvenile retinoschisis. Stem Cell Rep. 2019;13:906–923. doi: 10.1016/j.stemcr.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boycott K.M., Maybaum T.A., Naylor M.J., Weleber R.G., Robitaille J., Miyake Y., et al. A summary of 20 CACNA1F mutations identified in 36 families with incomplete X-linked congenital stationary night blindness, and characterization of splice variants. Hum Genet. 2001;108:91–97. doi: 10.1007/s004390100461. [DOI] [PubMed] [Google Scholar]
- 76.van Genderen M.M., Bijveld M.M., Claassen Y.B., Florijn R.J., Pearring J.N., Meire F.M., et al. Mutations in TRPM1 are a common cause of complete congenital stationary night blindness. Am J Hum Genet. 2009;85:730–736. doi: 10.1016/j.ajhg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bainbridge J.W., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 78.Jo D.H., Song D.W., Cho C.S., Kim U.G., Lee K.J., Lee K., et al. CRISPR-Cas9-mediated therapeutic editing of Rpe65 ameliorates the disease phenotypes in a mouse model of Leber congenital amaurosis. Sci Adv. 2019;5:eaax1210. doi: 10.1126/sciadv.aax1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burnight E.R., Gupta M., Wiley L.A., Anfinson K.R., Tran A., Triboulet R., et al. Using CRISPR-Cas9 to generate gene-corrected autologous iPSCs for the treatment of inherited retinal degeneration. Mol Ther. 2017;25:1999–2013. doi: 10.1016/j.ymthe.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holmgaard A., Askou A.L., Benckendorff J.N.E., Thomsen E.A., Cai Y., Bek T., et al. In vivo knockout of the vegfa gene by Lentiviral delivery of CRISPR/Cas9 in mouse retinal pigment epithelium cells. Mol Ther Nucl acids. 2017;9:89–99. doi: 10.1016/j.omtn.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koo T., Park S.W., Jo D.H., Kim D., Kim J.H., Cho H.Y., et al. CRISPR-LbCpf1 prevents choroidal neovascularization in a mouse model of age-related macular degeneration. Nat Commun. 2018;9 doi: 10.1038/s41467-018-04175-y. 1855-018-04175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noel N.C.L., Nadolski N.J., Hocking J.C., MacDonald I.M., Allison W.T. Progressive photoreceptor dysfunction and age-related macular degeneration-like features in rp1l1 mutant zebrafish. Cells. 2020;9(10):2214. doi: 10.3390/cells9102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu S., Du J., Chen N., Jia R., Zhang J., Liu X., et al. In vivo CRISPR/Cas9-mediated genome editing mitigates photoreceptor degeneration in a mouse model of X-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2020;61:31. doi: 10.1167/iovs.61.4.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li P., Kleinstiver B.P., Leon M.Y., Prew M.S., Navarro-Gomez D., Greenwald S.H., et al. Allele-specific CRISPR-Cas9 genome editing of the single-base P23H mutation for rhodopsin-associated dominant retinitis pigmentosa. CRISPR J. 2018;1:55–64. doi: 10.1089/crispr.2017.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu W.H., Tsai Y.T., Justus S., Lee T.T., Zhang L., Lin C.S., et al. CRISPR repair reveals causative mutation in a preclinical model of retinitis pigmentosa. Mol Ther. 2016;24:1388–1394. doi: 10.1038/mt.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feehan J.M., Chiu C.N., Stanar P., Tam B.M., Ahmed S.N., Moritz O.L. Modeling dominant and recessive forms of retinitis pigmentosa by editing three rhodopsin-encoding genes in xenopus laevis using CRISPR/Cas9. Sci Rep. 2017;7(1):6920. doi: 10.1038/s41598-017-07153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu W., Mookherjee S., Chaitankar V., Hiriyanna S., Kim J.W., Brooks M., et al. Nrl knockdown by AAV-delivered CRISPR/Cas9 prevents retinal degeneration in mice. Nat Commun. 2017;8:14716. doi: 10.1038/ncomms14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fuster-Garcia C., Garcia-Garcia G., Gonzalez-Romero E., Jaijo T., Sequedo M.D., Ayuso C., et al. USH2A gene editing using the CRISPR system. Mol Ther Nucl acids. 2017;8:529–541. doi: 10.1016/j.omtn.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sanjurjo-Soriano C., Erkilic N., Baux D., Mamaeva D., Hamel C.P., Meunier I., et al. Genome editing in patient iPSCs corrects the most prevalent USH2A mutations and reveals intriguing mutant mRNA expression profiles. Mol Ther Methods Clin Dev. 2019;17:156–173. doi: 10.1016/j.omtm.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen D., Xu T., Tu M., Xu J., Zhou C., Cheng L., et al. Recapitulating X-linked juvenile retinoschisis in mouse model by knock-in patient-specific novel mutation. Fron Mol Neurosci. 2018;10:453. doi: 10.3389/fnmol.2017.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ye G.J., Budzynski E., Sonnentag P., Nork T.M., Sheibani N., Gurel Z., et al. Cone-specific promoters for gene therapy of achromatopsia and other retinal diseases. Hum Gene Ther. 2016;27:72–82. doi: 10.1089/hum.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lheriteau E., Petit L., Weber M., Le Meur G., Deschamps J.Y., Libeau L., et al. Successful gene therapy in the RPGRIP1-deficient dog: a large model of cone-rod dystrophy. Mol Ther. 2014;22:265–277. doi: 10.1038/mt.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thiadens A.A., Phan T.M., Zekveld-Vroon R.C., Leroy B.P., van den Born L.I., Hoyng C.B., et al. Clinical course, genetic etiology, and visual outcome in cone and cone-rod dystrophy. Ophthalmology. 2012;119:819–826. doi: 10.1016/j.ophtha.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 94.Roosing S., Thiadens A.A., Hoyng C.B., Klaver C.C., den Hollander A.I., Cremers F.P. Causes and consequences of inherited cone disorders. Prog Retin Eye Res. 2014;42:1–26. doi: 10.1016/j.preteyeres.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 95.Naeem M., Majeed S., Hoque M.Z., Ahmad I. Latest developed strategies to minimize the off-target effects in CRISPR-Cas-mediated genome editing. Cells. 2020;9:1608. doi: 10.3390/cells9071608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garrood W.T., Kranjc N., Petri K., Kim D.Y., Guo J.A., Hammond A.M., et al. Analysis of off-target effects in CRISPR-based gene drives in the human malaria mosquito. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2004838117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Modrzejewski D., Hartung F., Lehnert H., Sprink T., Kohl C., Keilwagen J., et al. Which factors affect the occurrence of off-target effects caused by the use of CRISPR/Cas: a systematic review in plants. Front Plant Sci. 2020;11 doi: 10.3389/fpls.2020.574959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonzalez-Avila L.U., Vega-López J.M., Pelcastre-Rodríguez L.I., Cabrero-Martínez O.A., Hernández-Cortez C., Castro-Escarpulli G. The challenge of CRISPR-Cas toward bioethics. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hejtmancik J.F., Daiger S.P. Understanding the genetic architecture of human retinal degenerations. Proc Natl Acad Sci USA. 2020;117:3904–3906. doi: 10.1073/pnas.1922925117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uddin F., Rudin C.M., Sen T. CRISPR gene therapy: applications, limitations, and implications for the future. Front Oncol. 2020;10:1387. doi: 10.3389/fonc.2020.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu M., Rehman S., Tang X., Gu K., Fan Q., Chen D., et al. Methodologies for improving HDR efficiency. Front Genet. 2019;9:691. doi: 10.3389/fgene.2018.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith D.W., Lee C.J., Gardiner B.S. No flow through the vitreous humor: how strong is the evidence? Prog Retin Eye Res. 2020;78 doi: 10.1016/j.preteyeres.2020.100845. [DOI] [PubMed] [Google Scholar]
- 103.Mout R., Ray M., Lee Y.W., Scaletti F., Rotello V.M. In vivo delivery of CRISPR/Cas9 for therapeutic gene editing: progress and challenges. Bioconjug Chem. 2017;28:880–884. doi: 10.1021/acs.bioconjchem.7b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Neefjes J., Jongsma M.L., Paul P., Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 105.Chew W.L., Tabebordbar M., Cheng J.K., Mali P., Wu E.Y., Ng A.H., et al. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat Methods. 2016;13:868–874. doi: 10.1038/nmeth.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hacein-Bey-Abina S., Garrigue A., Wang G.P., Soulier J., Lim A., Morillon E., et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zincarelli C., Soltys S., Rengo G., Rabinowitz J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 108.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Na Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 109.Xu X., Gao D., Wang P., Chen J., Ruan J., Xu J., et al. Efficient homology-directed gene editing by CRISPR/Cas9 in human stem and primary cells using tube electroporation. Sci Rep. 2018;8 doi: 10.1038/s41598-018-30227-w. 11649-018-30227-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mout R., Ray M., Yesilbag Tonga G., Lee Y.W., Tay T., Sasaki K., et al. Direct cytosolic delivery of CRISPR/Cas9-ribonucleoprotein for efficient gene editing. ACS Nano. 2017;11:2452–2458. doi: 10.1021/acsnano.6b07600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cho S.W., Kim S., Kim Y., Kweon J., Kim H.S., Bae S., et al. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014;24:132–141. doi: 10.1101/gr.162339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fu Y., Foden J.A., Khayter C., Maeder M.L., Reyon D., Joung J.K., et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carroll D. Genome engineering with targetable nucleases. Annu Rev Biochem. 2014;83:409–439. doi: 10.1146/annurev-biochem-060713-035418. [DOI] [PubMed] [Google Scholar]
- 114.Kleinstiver B.P., Pattanayak V., Prew M.S., Tsai S.Q., Nguyen N.T., Zheng Z., et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature. 2016;529:490–495. doi: 10.1038/nature16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Slaymaker I.M., Gao L., Zetsche B., Scott D.A., Yan W.X., Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. 2016;351:84–88. doi: 10.1126/science.aad5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ramakrishna S., Kwaku Dad A.B., Beloor J., Gopalappa R., Lee S.K., Kim H. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 2014;24:1020–1027. doi: 10.1101/gr.171264.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agrahari V., Mandal A., Agrahari V., Trinh H.M., Joseph M., Ray A., et al. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016;6:735–754. doi: 10.1007/s13346-016-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cholkar K., Patel S.P., Vadlapudi A.D., Mitra A.K. Novel strategies for anterior segment ocular drug delivery. J Ocul Pharmacol Ther. 2013;29:106–123. doi: 10.1089/jop.2012.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nickla D.L., Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Edelhauser H.F., Rowe-Rendleman C.L., Robinson M.R., Dawson D.G., Chader G.J., Grossniklaus H.E., et al. Ophthalmic drug delivery systems for the treatment of retinal diseases: basic research to clinical applications. Invest Ophthalmol Vis Sci. 2010;51:5403–5420. doi: 10.1167/iovs.10-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shikari H., Silva P.S., Sun J.K. Complications of intravitreal injections in patients with diabetes. Semin Ophthalmol. 2014;29:276–289. doi: 10.3109/08820538.2014.962167. [DOI] [PubMed] [Google Scholar]
- 122.Tsai C.H., Wang P.Y., Lin I.C., Huang H., Liu G.S., Tseng C.L. Ocular drug delivery: role of degradable polymeric nanocarriers for ophthalmic application. Int J Mol Sci. 2018;19(9):2830. doi: 10.3390/ijms19092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Varela-Fernandez R., Diaz-Tome V., Luaces-Rodriguez A., Conde-Penedo A., Garcia-Otero X., Luzardo-Alvarez A., et al. Drug delivery to the posterior segment of the eye: biopharmaceutic and pharmacokinetic considerations. Pharmaceutics. 2020;12(3):269. doi: 10.3390/pharmaceutics12030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Patel A., Cholkar K., Agrahari V., Mitra A.K. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2:47–64. doi: 10.5497/wjp.v2.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Souto E.B., Dias-Ferreira J., Lopez-Machado A., Ettcheto M., Cano A., Camins Espuny A., et al. Advanced formulation approaches for ocular drug delivery: state-of-the-art and recent patents. Pharmaceutics. 2019;11(9):460. doi: 10.3390/pharmaceutics11090460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fu Y., Foden J.A., Khayter C., Maeder M.L., Reyon D., Joung J.K., Sander J.D. Hgh-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol. 2013;31:822–826. doi: 10.1038/nbt.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hung K.L., Meitlis I., Hale M., Chen C.Y., Singh S., Jackson S.W., et al. Engineering protein-secreting plasma cells by homology-directed repair in primary human B cells. Mol Ther. 2018;26:456–467. doi: 10.1016/j.ymthe.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hui S.W. Overview of drug delivery and alternative methods to electroporation. Methods Mol Biol. 2008;423:91–107. doi: 10.1007/978-1-59745-194-9_6. [DOI] [PubMed] [Google Scholar]
- 129.Xu W. Microinjection and micromanipulation: a historical perspective. Methods Mol Biol. 2019;1874:1–16. doi: 10.1007/978-1-4939-8831-0_1. [DOI] [PubMed] [Google Scholar]
- 130.Subrizi A., del Amo E.M., Korzhikov-Vlakh V., Tennikova T., Ruponen M., Urtti A. Design principles of ocular drug delivery systems: importance of drug payload, release rate, and material properties. Drug Discov Today. 2019;24:1446–1457. doi: 10.1016/j.drudis.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 131.Käsdorf B.T., Arends F., Lieleg O. Diffusion regulation in the vitreous humor. Biophys J. 2015;109:2171–2181. doi: 10.1016/j.bpj.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tavakoli S., Kari O.K., Turunen T., Lajunen T., Schmitt M., Lehtinen J., et al. Diffusion and protein corona formation of lipid-based nanoparticles in the vitreous humor: profiling and pharmacokinetic considerations. Mol Pharm. 2020;18:699–713. doi: 10.1021/acs.molpharmaceut.0c00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yu H., Wu W., Lin X., Feng Y. Polysaccharide-based nanomaterials for ocular drug delivery: a perspective. Fron Bioeng Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.601246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wilbie D., Walther J., Mastrobattista E. Delivery aspects of CRISPR/Cas for in vivo genome editing. Acc Chem Res. 2019;52:1555–1564. doi: 10.1021/acs.accounts.9b00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li L., He Z.Y., Wei X.W., Gao G.P., Wei Y.Q. Challenges in CRISPR/CAS9 delivery: potential roles of nonviral vectors. Hum Gene Ther. 2015;26:452–462. doi: 10.1089/hum.2015.069. [DOI] [PubMed] [Google Scholar]
- 136.Nakai H., Yant S.R., Storm T.A., Fuess S., Meuse L., Kay M.A. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McCarty D.M., Monahan P.E., Samulski R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 138.Zetsche B., Volz S.E., Zhang F. A split-Cas9 architecture for inducible genome editing and transcription modulation. Nat Biotechnol. 2015;33:139–142. doi: 10.1038/nbt.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bainbridge J.W., Mehat M.S., Sundaram V., Robbie S.J., Barker S.E., Ripamonti C., et al. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med. 2015;372:1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen Y., Moiseyev G., Takahashi Y., Ma J.X. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65-/- mice. Invest Ophthalmol Vis Sci. 2006;47:1177–1184. doi: 10.1167/iovs.05-0965. [DOI] [PubMed] [Google Scholar]
- 141.Pang J.J., Chang B., Kumar A., Nusinowitz S., Noorwez S.M., Li J., et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13:565–572. doi: 10.1016/j.ymthe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 142.Hauswirth W.W., Aleman T.S., Kaushal S., Cideciyan A.V., Schwartz S.B., Wang L., et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Human Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennicelli J., et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kleinstiver B.P., Prew M.S., Tsai S.Q., Topkar V.V., Nguyen N.T., Zheng Z., et al. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 2015;523:481–485. doi: 10.1038/nature14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ran F.A., Cong L., Yan W.X., Scott D.A., Gootenberg J.S., Kriz A.J., et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–191. doi: 10.1038/nature14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Esvelt K.M., Mali P., Braff J.L., Moosburner M., Yaung S.J., Church G.M. Orthogonal Cas9 proteins for RNA-guided gene regulation and editing. Nat Methods. 2013;10:1116–1121. doi: 10.1038/nmeth.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang D., Mou H., Li S., Li Y., Hough S., Tran K., et al. Adenovirus-mediated somatic genome editing of Pten by CRISPR/Cas9 in mouse liver in spite of Cas9-specific immune responses. Hum Gene Ther. 2015;26:432–442. doi: 10.1089/hum.2015.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang Y., Jooss K.U., Su Q., Ertl H.C., Wilson J.M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 149.Hoffman L.M., Maguire A.M., Bennett J. Cell-mediated immune response and stability of intraocular transgene expression after adenovirus-mediated delivery. Invest Ophthalmol Vis Sci. 1997;38:2224–2233. [PubMed] [Google Scholar]
- 150.Ueyama K., Mori K., Shoji T., Omata H., Gehlbach P.L., Brough D.E., et al. Ocular localization and transduction by adenoviral vectors are serotype-dependent and can be modified by inclusion of RGD fiber modifications. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bainbridge J.W., Mistry A., De Alwis M., Paleolog E., Baker A., Thrasher A.J., et al. Inhibition of retinal neovascularisation by gene transfer of soluble VEGF receptor sFlt-1. Gene Ther. 2002;9:320–326. doi: 10.1038/sj.gt.3301680. [DOI] [PubMed] [Google Scholar]
- 152.Julien S., Kreppel F., Beck S., Heiduschka P., Brito V., Schnichels S., et al. A reproducible and quantifiable model of choroidal neovascularization induced by VEGF A165 after subretinal adenoviral gene transfer in the rabbit. Mol Vis. 2008;14:1358–1372. [PMC free article] [PubMed] [Google Scholar]
- 153.Wang H., Wei F., Li H., Ji X., Li S., Chen X. Combination of oncolytic adenovirus and endostatin inhibits human retinoblastoma in an in vivo mouse model. Int J Mol Med. 2013;31:377–385. doi: 10.3892/ijmm.2012.1197. [DOI] [PubMed] [Google Scholar]
- 154.Sellon D.C., Perry S.T., Coggins L., Fuller F.J. Wild-type equine infectious anemia virus replicates in vivo predominantly in tissue macrophages, not in peripheral blood monocytes. J Virol. 1992;66:5906–5913. doi: 10.1128/jvi.66.10.5906-5913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Palfi S., Gurruchaga J.M., Ralph G.S., Lepetit H., Lavisse S., Buttery P.C., et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson's disease: a dose escalation, open-label, phase 1/2 trial. Lancet (Lond) 2014;383:1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 156.Hashimoto T., Gibbs D., Lillo C., Azarian S.M., Legacki E., Zhang X.M., et al. Lentiviral gene replacement therapy of retinas in a mouse model for Usher syndrome type 1B. Gene Ther. 2007;14:584–594. doi: 10.1038/sj.gt.3302897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Balaggan K.S., Binley K., Esapa M., Iqball S., Askham Z., Kan O., et al. Stable and efficient intraocular gene transfer using pseudotyped EIAV lentiviral vectors. J Gene Med. 2006;8:275–285. doi: 10.1002/jgm.845. [DOI] [PubMed] [Google Scholar]
- 158.Shin J., Lee N., Cho S., Cho B.K. Targeted genome editing using DNA-free RNA-guided Cas9 ribonucleoprotein for CHO cell engineering. Methods Mol Biol. 2018;1772:151–169. doi: 10.1007/978-1-4939-7795-6_8. [DOI] [PubMed] [Google Scholar]
- 159.Lee M., Kim S.W. Polyethylene glycol-conjugated copolymers for plasmid DNA delivery. Pharm Res. 2005;22:1–10. doi: 10.1007/s11095-004-9003-5. [DOI] [PubMed] [Google Scholar]
- 160.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Felgner P.L., Gadek T.R., Holm M., Roman R., Chan H.W., Wenz M., et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kariko K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang M., Zuris J.A., Meng F., Rees H., Sun S., Deng P., et al. Efficient delivery of genome-editing proteins using bioreducible lipid nanoparticles. Proc Natl Acad Sci USA. 2016;113:2868–2873. doi: 10.1073/pnas.1520244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chang J., Chen X., Glass Z., Gao F., Mao L., Wang M., et al. Integrating combinatorial lipid nanoparticle and chemically modified protein for intracellular delivery and genome editing. Acc Chem Res. 2019;52:665–675. doi: 10.1021/acs.accounts.8b00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wei T., Cheng Q., Min Y.L., Olson E.N., Siegwart D.J. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17029-3. 3232-020-17029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li L., Hu S., Chen X. Non-viral delivery systems for CRISPR/Cas9-based genome editing: challenges and opportunities. Biomaterials. 2018;171:207–218. doi: 10.1016/j.biomaterials.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Akinc A., Thomas M., Klibanov A.M., Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Z., Wan T., Chen Y., Chen Y., Sun H., Cao T., et al. Cationic Polymer-mediated CRISPR/Cas9 plasmid delivery for genome editing. Macromol Rapid Commun. 2019;40 doi: 10.1002/marc.201800068. [DOI] [PubMed] [Google Scholar]
- 169.Sun W., Ji W., Hall J.M., Hu Q., Wang C., Beisel C.L., et al. Self-assembled DNA nanoclews for the efficient delivery of CRISPR-Cas9 for genome editing. Angew Chem Int Ed Engl. 2015;54:12029–12033. doi: 10.1002/anie.201506030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li L., Wei Y., Gong C. Polymeric nanocarriers for non-viral gene delivery. J Bio med Nanotechnol. 2015;11:739–770. doi: 10.1166/jbn.2015.2069. [DOI] [PubMed] [Google Scholar]
- 171.Maruyama A., Watanabe H., Ferdous A., Katoh M., Ishihara T., Akaike T. Characterization of interpolyelectrolyte complexes between double-stranded DNA and polylysine comb-type copolymers having hydrophilic side chains. Bioconjug Chem. 1998;9:292–299. doi: 10.1021/bc9701510. [DOI] [PubMed] [Google Scholar]
- 172.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 173.Ramirez-Acosta C.M., Cifuentes J., Castellanos M.C., Moreno R.J., Munoz-Camargo C., Cruz J.C., et al. pH-responsive, cell-penetrating, core/shell magnetite/silver nanoparticles for the delivery of plasmids: preparation, characterization, and preliminary in vitro evaluation. Pharmaceutics. 2020;12(6):561. doi: 10.3390/pharmaceutics12060561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen G., Abdeen A.A., Wang Y., Shahi P.K., Robertson S., Xie R., et al. A biodegradable nanocapsule delivers a Cas9 ribonucleoprotein complex for in vivo genome editing. Nat Nanotechnol. 2019;14:974–980. doi: 10.1038/s41565-019-0539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Madaan K., Kumar S., Poonia N., Lather V., Pandita D. Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6:139–150. doi: 10.4103/0975-7406.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shah N., Steptoe R.J., Parekh H.S. Low-generation asymmetric dendrimers exhibit minimal toxicity and effectively complex DNA. J Pept Sci. 2011;17:470–478. doi: 10.1002/psc.1347. [DOI] [PubMed] [Google Scholar]
- 177.Yu T., Liu X., Bolcato-Bellemin A.L., Wang Y., Liu C., Erbacher P., et al. An amphiphilic dendrimer for effective delivery of small interfering RNA and gene silencing in vitro and in vivo. Angew Chem Int Ed Engl. 2012;51:8478–8484. doi: 10.1002/anie.201203920. [DOI] [PubMed] [Google Scholar]
- 178.Choi J.S., Nam K., Park J.Y., Kim J.B., Lee J.K., Park J.S. Enhanced transfection efficiency of PAMAM dendrimer by surface modification with l-arginine. J Control Rel. 2004;99:445–456. doi: 10.1016/j.jconrel.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 179.Liu C., Wan T., Wang H., Zhang S., Ping Y., Cheng Y. A boronic acid-rich dendrimer with robust and unprecedented efficiency for cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci Adv. 2019;5:eaaw8922. doi: 10.1126/sciadv.aaw8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fry L.E., Peddle C.F., Barnard A.R., McClements M.E., MacLaren R.E. RNA editing as a therapeutic approach for retinal gene therapy requiring long coding sequences. Int J Mol Sci. 2020;21(3):777. doi: 10.3390/ijms21030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stone E.M., Andorf J.L., Whitmore S.S., DeLuca A.P., Giacalone J.C., Streb L.M., et al. Clinically focused molecular investigation of 1000 consecutive families with inherited retinal disease. Ophthalmology. 2017;124:1314–1331. doi: 10.1016/j.ophtha.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zinshteyn B., Nishikura K. Adenosine-to-inosine RNA editing. Wiley Interdiscip Rev Syst Biol Med. 2009;1:202–209. doi: 10.1002/wsbm.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Matsoukas I.G. Commentary: RNA editing with CRISPR-Cas13. Front Genet. 2018;9:134. doi: 10.3389/fgene.2018.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Walters B.J., Azam A.B., Gillon C.J., Josselyn S.A., Zovkic I.B. Advanced in vivo use of CRISPR/Cas9 and anti-sense DNA inhibition for gene manipulation in the brain. Front Genet. 2016;6:362. doi: 10.3389/fgene.2015.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]