Abstract
A wild-type allele of TaHRC suppresses the calcium-mediated immune response to Fusarium graminearum infection and facilitates the spread of Fusarium head blight disease symptoms within a wheat spike.
Dear Editor,
Fusarium head blight (FHB) caused by Fusarium graminearum is a destructive disease in wheat (Triticum aestivum) worldwide (Bai et al., 2018). Previously, we have cloned a histidine-rich calcium-binding protein gene (TaHRC) as the causal gene for Fhb1, a major quantitative locus for FHB resistance, and demonstrated that the TaHRC wild-type allele conditions FHB susceptibility (Su et al., 2019). However, the molecular mechanisms on how TaHRC regulates FHB susceptibility remain unknown.
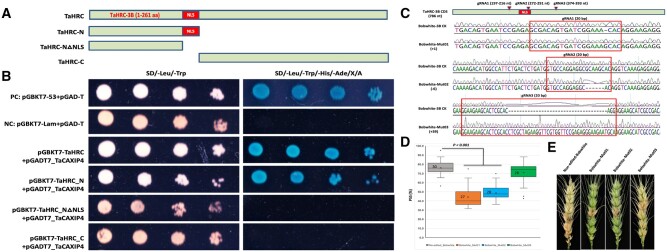
To identify TaHRC interacting proteins, we conducted yeast two-hybrid (Y2H) screening against the wheat cDNA libraries using TaHRC as a bait. After screening 130 million clones, we identified 25 high confidence candidate interacting proteins (HCIPs) (Supplemental Table S1). Since previous studies showed that HRC regulates Ca2+-uptake and -release to maintain Ca2+-homeostasis and a cation exchanger (CAX)-interacting protein 4 (TaCAXIP4) is the only one that associated with calcium transport activity among the 25 HCIPs (Cheng et al., 2002), we selected TaCAXIP4 to investigate its interaction with TaHRC in yeast. We first cloned the full-length coding sequences (CDSs) of TaCXIP4 and TaHRC from “Clark” (an Fhb1 susceptible wheat cultivar) and then constructed the CDSs into a prey vector (pGADT7-TaCAXIP4 with a leucine report gene) and a bait vector (pGBKT7-TaHRC with a tryptophan report gene), respectively. We also constructed additional bait vectors for the TaHRC N-terminal fragment containing/without a nuclear localization signal (NLS) domain (pGBKT7-TaHRC-N and pGBKT7-TaHRC-N△NLS) and the C-terminal fragment without NLS domain (pGBKT7-TaHRC-C) (Figure 1A) and then co-transformed them into a Y2HGold yeast strain. All of the yeast cells grew well on the medium lacking leucine and tryptophan, however, only the yeast cells co-expressing the full-length or N-terminus of TaHRC with TaCXIP4 grew on the selective medium (Figure 1B), suggesting a strong interaction between the two proteins in yeast and that the NLS domain is essential for the interaction.
Figure 1.
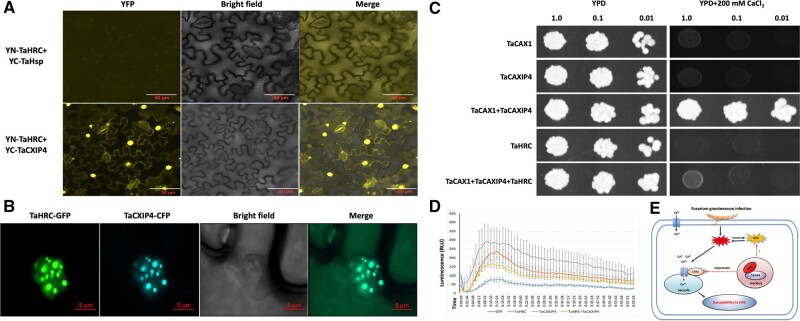
Confirmation of the interaction between TaHRC and TaCAXIP4 in yeast and determination of the functional role of the N-terminus carrying the NLS domain of TaHRC on FHB susceptibility using the CRISPR/Cas9 gene editing technology. A, Schematic presentation of different TaHRC constructs used for yeast transformation. aa, amino acids; NLS, nuclear localization signal domain. B, Co-transformation assays to validate the TaHRC–TaCAXIP4 interaction in yeast. Five microliters of serial dilutions of yeast cells were spotted onto the synthetic dextrose (SD) medium. SD/-Leu/-Trp indicates SD medium lacking leucine (Leu) and tryptophan (Trp). SD/-Leu/-Trp/-His/-Ade/X/A indicates SD medium lacking Leu, Trp, adenine (Ade), and histidine (His), but contains 20 ng/μL of X-alpha-Gal and 125 ng/mL of Aureobasidin A (AbA). PC is the positive control by co-transformation of pGADT7 and pGBKT7-53. NC is the negative control by co-transformation of pGADT7 and pGBKT7-lam. C, Edited sequences at three different target sites of TaHRC as identified from the three Bobwhite mutant lines by Sanger sequencing. Bobwhite-Mut01 has one nucleotide insertion (+1), Bobwhite-Mut02 has six nucleotide deletion (−6) and Bobwhite-Mut03 has 39 nucleotide insertion (+39). The bar within the CDS of TaHRC-3B is a nuclear localization domain. The arrows point to the targeted sites of three different gRNAs. D, Comparison of mean percentages of FHB symptomatic spikelets (PSS) between the three mutant lines and non-edited control plants. Boxes indicate the 25th–75th percentile, whiskers indicate the full data range, center lines indicate medians, crosses indicate means, and the numbers inside boxes indicate sample size, data points outside of the whiskers are treated as potential outliers. P-values were generated from two-sided unpaired Student’s t tests of the mean PSS of the mutant lines versus the mean PSS of the non-edited line. E, FHB symptoms in the inoculated spikes from the three mutant lines and non-edited Bobwhite control plants.
To confirm the critical role of N-terminus carrying NLS domain of TaHRC on FHB susceptibility, we edited three different sites (one before and one within and one after the NLS domain) of TaHRC in a susceptible wheat cultivar “Bobwhite” using the CRISPR/Cas9 gene editing technology (Chen et al., 2022) and identified one mutant each at the three different target sites, respectively, with two insertion mutations and one deletion mutation (Figure 1C). The homozygous M2 plants were inoculated with F. graminearum as described previously (Su et al., 2019). The percentage of symptomatic spikelets (PSS) in a spike in the Mut01 and Mut02 with disrupted N-terminus carrying NLS domain was significantly reduced at 14 days after inoculation, whereas PSS of the Mut03 with the complete N-terminus carrying NLS domain did not change (Figure 1, D and E), suggesting that reduced FHB susceptibility in the mutants Mut01 and Mut02 with the disrupted N-terminus is likely due to abolished TaHRC function and the NLS domain is critical for FHB susceptibility.
To validate the interaction between TaHRC and TaCXIP4 in planta, we fused the full-length CDS of TaHRC and TaCAXIP4 into the N-terminus and C-terminus of a split yellow fluorescent protein (YFP) in the expression vectors as YN-TaHRC and YC-TaCAXIP4, respectively (Lu et al., 2010), and infiltrated the mixed Agrobacterium tumefaciens cultures harboring YN-TaHRC and YC-TaCAXIP4 vectors into 6-week-old epidermal Nicotiana benthamiana leaves for bimolecular fluorescence complementation (BiFC) assays. We observed strong signals of the reconstituted YFP fluorescence in the nuclei of cells at 48 h after infiltration (hai) (Figure 2A), indicating a very strong interaction between TaHRC and TaCXIP4 in planta.
Figure 2.
Confirmation of the interaction between TaHRC and TaCAXIP4 in planta and effect of the HRC–CXIP4 interaction on CAX1-mediated Ca2+ transport activity and chitin-triggered plant immune responses during Fusarium infection. A, BiFC assays to show the protein interaction between TaHRC and TaCAXIP4 in planta. The reconstituted YFP signals were observed under a Zeiss LSM 880 confocal microscope (Carl Zeiss, Germany). An unrelated heat shock protein of YFP fusion (YC-TaHsp) was used as the negative control. Scale bars = 50 μm. B, Subcellular colocalization of TaHRC and TaCXIP4 in the nuclei of plant cells. After infiltration with the mixed Agrobacterium cultures harboring equal concentrations of TaHRC-GFP and TaCAXIP4-CFP vectors into 6-week-old epidermal N. benthamiana leaves at 48 h, the fluorescence signal of GFP and CFP was imaged under a Zeiss LSM 880 confocal microscope in two channels and merged using a lookup table with raw data in green and cyan colors, respectively. Scale bars = 5 μm. C, Suppression of K667 yeast Ca2+ sensitivity in cells coexpressing various combinations with three plasmids, TaCAXIP4, TaCAX1, and TaHRC as indicated. Yeast cells were grown to A600 = 1.0 in selection medium at 30°C. Five microliters of serial dilutions were spotted onto YPD medium supplemented with/without 200 mM CaCl2. Photographs were taken after 3 days of culture. D, Chitin-induced ROS assay via transient expression of TaHRC-GFP and TaCAXIP4-GFP in N. benthamiana leaves. Luminescence was measured in 200 μL of the assay solution (17 mM lumino, 1 μM horseradish peroxidase, and crab shell chitin at 200 μg/mL) for 60 min. Leaves expressing GFP served as the positive control and the assay solution without chitin served as the negative control. Lines are means and standard error with n = 12. Assays were repeated four times with similar results. RLU, relative light unit. E, A proposed model of the calcium-mediated plant immune response suppressed by wild-type allele of TaHRC to enhance wheat FHB susceptibility. TaHRC hijacks TaCAXIP4 to suppress the Ca2+ transporting activity of TaCAX1 and disrupts the Ca2+ signal transduction during the immune response to F. graminearum infection.
To determine where the interaction occurs in plant cells, we fused the CDS of TaHRC and TaCAXIP4 into the N-terminus of an intact green fluorescent protein (GFP) and a cyan fluorescent protein (CFP) in the expression vectors as TaHRC-GFP and TaCXIP4-CFP, respectively, and agroinfiltrated the vectors into tobacco leaves as mentioned above for subcellular colocalization assays. At 48 hai, the strong GFP and CFP fluorescence signals from the co-expression of TaHRC-GFP and TaCXIP4-CFP fusion proteins were observed in the nucleus speckles (Figure 2B), confirming their interaction in the nuclei.
The Arabidopsis (Arabidopsis thaliana) CAX1 is a H+/Ca2+ antiporter that plays an important role in maintaining cellular Ca2+ homeostasis, whereas CAX1 activity is activated by CAXIP4 (Cheng et al., 2004). To investigate whether the HRC–CXIP4 interaction affects the ability of CXIP4 to activate CAX1 Ca2+ transport activity, we cloned the full-length CDS of TaCAX1 and TaCAXIP4 from Clark into pGBKT7 vector and transformed/cotransformed the constructs (pGBKT7-TaHRC, pGBKT7-TaCAXIP4, and pGBKT7-TaCAX1) into a Ca2+ sensitive yeast strain K667 (hypersensitive to high concentrations of Ca2+). Yeast cells expressing/coexpressing TaHRC, TaCAXIP4, and TaCAX1 were assayed on a yeast extract/peptone/dextrose (YPD) medium supplemented with and without 200 mM CaCl2, respectively. The K667 cells expressing TaCXIP4 or TaCAX1 or TaHRC alone did not grow on the medium containing 200 mM CaCl2, whereas the K667 cells coexpressing both TaCXIP4 and TaCAX1 grew well on the same medium (Figure 2C), indicating TaCXIP4 activated TaCAX1 to mediate the Ca2+ transport activity in yeast. However, the K667 cells coexpressing TaCAX4, TaCAX1, and TaHRC did not grow on the medium with 200 mM CaCl2, indicating TaHRC sequestered TaCAXIP4 to suppress the Ca2+ transporting activity of TaCAX1.
Production of reactive oxygen species (ROS) is critical for successful activation of plant immune responses against pathogens (Hao et al., 2019). To determine the role of TaHRC in regulating plant immunity, we infiltrated N. benthamiana leaves with Agrobacterium cultures harboring the full-length CDSs of TaHRC-GFP and TaCAXIP4-GFP vectors, and observed a high level of chitin-trigged ROS in the plants expressing TaHRC or TaCAXIP4 alone, but a low level of ROS in the plants coexpressing both TaCAXIP4-GFP and TaHRC-GFP (Figure 2D), which suggested that TaHRC might suppress the chitin-triggered plant immune responses during Fusarium infection by sequestering TaCAXIP4 to trigger FHB susceptibility.
In summary, we demonstrated that TaCAXIP4 interacts with TaHRC in the nuclei of cells to trigger wheat FHB susceptibility and the functional NLS domain in TaHRC N-terminus is essential for the interaction. TaHRC may hijack TaCAXIP4 to suppress calcium-mediated plant immune responses and facilitate the pathogen spread within a wheat spike (Figure 2E).
Supplementary Material
Acknowledgments
We would like to extend our thanks to Dr. Kendal D. Hirschi and Dr. Jian Yang at Baylor College of Medicine for providing the yeast strain K667 and to the confocal core facility in the College of Veterinary Medicine at Kansas State University for the assistance and usage of the confocal microscopes.
Funding
This project was partially supported by the U.S. Wheat and Barley Scab Initiative and the National Research Initiative Competitive Grants 2017-67007-25939 and 2022-68013-36439 from the National Institute of Food and Agriculture, U.S. Department of Agriculture. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer. This is contribution number 22-311-J from the Kansas Agricultural Experiment Station.
Conflict of interest statement. The authors declare that they have no conflict of interest.
Contributor Information
Hui Chen, Department of Agronomy, Kansas State University, Manhattan, Kansas 66506, USA.
Zhenqi Su, Department of Agronomy, Kansas State University, Manhattan, Kansas 66506, USA; College of Agriculture and Biotechnology, China Agricultural University, Beijing 100193, China.
Bin Tian, Department of Plant Pathology, Kansas State University, Manhattan, Kansas 66506, USA.
Guixia Hao, Mycotoxin Prevention and Applied Microbiology Research Unit, NCAUR, USDA-ARS, Peoria, Illinois 61604, USA.
Harold N Trick, Department of Plant Pathology, Kansas State University, Manhattan, Kansas 66506, USA.
Guihua Bai, Department of Agronomy, Kansas State University, Manhattan, Kansas 66506, USA; Hard Winter Wheat Genetics Research Unit, USDA-ARS, Manhattan, Kansas 66506, USA.
Data Availability
Data that support the findings of this work are included in the article and its Supplemental Information files.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Materials and Methods.
Supplemental Table S1. The results of ULTImate Y2H screen against wheat cDNA libraries using TaHRC as a bait.
Supplemental Table S2. Primer sequences used in this study.
H.C. and G.B. designed the project. H.C., Z.S., G.H., and B.T. performed the experiments. H.C., H.N.T., and G.B. wrote the manuscript and all authors read and approved the final version of the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Guihua Bai (guihua.bai@ars.usda.gov).
References
- Bai GH, Su ZQ, Cai J (2018) Wheat resistance to Fusarium head blight. Can J Plant Pathol 40: 336–346 [Google Scholar]
- Chen H, Su ZQ, Tian B, Liu Y, Pang YH, Kavetskyi V, Trick HN, Bai GH (2022) Development and optimization of a Barley stripe mosaic virus (BSMV)-mediated gene editing system to improve Fusarium head blight (FHB) resistance in wheat. Plant Biotechnol J 20: 1018–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Liu J Z, Nelson RS, Hirschi KD (2004) Characterization of CXIP4, a novel Arabidopsis protein that activates the H+/Ca2+ antiporter, CAX1. FEBS Lett 559: 99–106 [DOI] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Hirschi KD (2002) Characterization of CAX4, an Arabidopsis H+/cation antiporter. Plant Physiol 128: 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao G, McCormick S, Vaughan MM, Naumann TA, Kim HS, Proctor R, Kelly A, Ward TJ (2019) Fusarium graminearum arabinanase (Arb93B) enhances wheat head blight susceptibility by suppressing plant immunity. Mol Plant Microbe Interact 32: 888–898 [DOI] [PubMed] [Google Scholar]
- Lu Q, Tang X, Tian G, Wang F, Liu K, Nguyen V, Kohalmi SE, Keller WA, Tsang EW, Harada JJ, et al. (2010) Arabidopsis homolog of the yeast TREX‐2 mRNA export complex: components and anchoring nucleoporin. Plant J 61: 259–270 [DOI] [PubMed] [Google Scholar]
- Su ZQ, Bernardo A, Tian B, Chen H, Wang S, Ma H, Cai S, Liu D, Zhang D, Li T, et al. (2019) A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat Genet 51: 1099–1105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this work are included in the article and its Supplemental Information files.