Abstract
Sera from cattle naturally infected with Mycobacterium avium subsp. paratuberculosis (n = 56) and naturally (n = 4) and experimentally (n = 8) infected with Mycobacterium bovis were tested for the presence of antibodies against paratuberculosis antigens. An enzyme-linked immunosorbent assay (ELISA) was established based on absorption of M. avium subsp. paratuberculosis antigens on a hyperimmune antiserum against M. avium subsp. avium proteins in order to remove cross-reacting antigens. This absorbed-antigen ELISA recognized 66% of animals with paratuberculosis (37 of 56), while none of the animals with naturally occurring bovine tuberculosis (TB) had detectable antibodies. However, the animals with experimental bovine TB also responded in this ELISA. Similar results were found in a commercial ELISA, showing that neither of these tests was able to distinguish between paratuberculosis and bovine TB. The sera were further tested for antibody activities against purified AhpC and AhpD, which are proteins constitutively expressed by M. avium subsp. paratuberculosis, and against a secreted 14-kDa protein present in culture filtrates from the M. avium complex. Elevated antibody levels to AhpC, AhpD, and the 14-kDa antigen were found in 27% (13 of 48), 15% (7 of 48), and 27% (13 of 48), respectively, of the cattle with paratuberculosis. Together these ELISAs were positive with 35% (17 of 48) of the animals. None of the animals with bovine TB had detectable antibodies against any of the purified proteins despite their high levels of cross-reacting antibodies. These results show that purified specific antigens are needed to differentiate between paratuberculosis and bovine TB in ELISA.
Mycobacterium avium subsp. paratuberculosis causes chronic granulomatous enteritis, characterized by persistent diarrhea and emaciation, in domestic and wild ruminants. The incubation time is long, and only 10 to 15% of infected animals develop overt paratuberculosis (also called Johne's disease). However, subclinical infections result in decreased production, with substantial financial consequences for farmers. Another mycobacterial disease in animals, of even greater importance, is bovine tuberculosis (TB), which continues to cause problems in several countries. The diagnosis of bovine TB is usually based on the single or comparative intradermal skin test, measuring delayed-type hypersensitivity reactions after injection of antigen. This test has been largely successful in the attempt to eradicate bovine TB in countries without a wildlife reservoir. One of the problems with the skin test, in addition to a moderate sensitivity, is that the injection of antigens into the animal may influence subsequent testing. There is also a need to gather the animals twice, which is costly, especially in large cattle farms. These problems have led to the development of an in vitro-based assay measuring gamma interferon (IFN-γ) responses after stimulation with antigen (17, 23). The IFN-γ test seems to have a higher sensitivity but a lower specificity than the skin test.
The diagnosis of paratuberculosis is even more difficult than that of bovine TB, especially in subclinically infected animals. Several tests, such as antibody enzyme-linked immunosorbent assay (ELISA), cultivation, and PCR on feces, are available, but these tests are all hampered by a low sensitivity in the early stage of the infection (14). The immune responses in paratuberculosis resemble the immune responses against other mycobacteria. such as Mycobacterium bovis, Mycobacterium leprae, and Mycobacterium tuberculosis (1, 3, 9). Protective immunity is characterized by strong Th1-cell responses, while animals with fulminant disease usually have antibody responses and weak cellular responses. The skin test and the IFN-γ test can therefore be used in subclinically infected animals (2, 19). However, the specificity of these tests for paratuberculosis is very low, and the feasibility and cost of using such assays in national surveillance or screening programs make serological assays still the most widely used tests for paratuberculosis. Several ELISA kits are commercially available, and the specificities of these tests are claimed to be high (14). Most of these ELISAs are based on preabsorption of the test sera on Mycobacterium phlei in order to remove cross-reacting antibodies to increase the specificity. However, the abilities of these tests to differentiate between paratuberculosis and bovine TB have not been evaluated.
The need for improved diagnostic testing for bovine and human TB has led to an extensive search for antigens specific for the M. tuberculosis complex, and several candidate antigens have been identified and characterized (6, 10, 16, 21, 22). In contrast, relatively few specific antigens from M. avium subsp. paratuberculosis have been characterized and evaluated in immunological assays (20). The use of purified antigens usually leads to a lower sensitivity, and it is therefore preferable to include a panel of such antigens to get an optimal test.
We have previously reported that alkyl hydroperoxide reductases C and D (AhpC and AhpD) are constitutively expressed by M. avium subsp. paratuberculosis, and not by other mycobacterial species, and that these proteins are immunodominant antigens in immunized rabbits (11). A deletion in the regulator gene oxyR, controlling expression of AhpC and AhpD, has led to lack of expression of these proteins in M. tuberculosis and M. bovis (4), whereas the expression of these proteins can be induced by oxidative stress in M. avium subsp. avium (18). We have also recently shown that the major differences in secreted proteins between M. avium complex and M. tuberculosis were in the low-molecular-mass area, and one secreted 14-kDa protein specific for the M. avium complex was purified (12). The aim of the present study was to test these three proteins in serological assays to see if they potentially can differentiate between paratuberculosis and bovine TB.
MATERIALS AND METHODS
Strains and antisera.
M. avium subsp. paratuberculosis strain 2E was obtained from the National Veterinary Institute, Oslo, Norway. Polyclonal, polyvalent rabbit antisera against M. avium subsp. paratuberculosis strain 2E (batch B312) and M. avium subsp. avium strain D4 were obtained from Dako, Glostrup, Denmark.
Bacterial culture and antigen preparation.
M. avium subsp. paratuberculosis strains were cultivated as surface pellicles on liquid synthetic Reid's medium with mycobactin J (2 μg/ml) (Allied Monitor, Fayette, Mo.) for 8 weeks at 37°C. Harvested bacteria were washed three times in phosphate-buffered saline (PBS) and suspended in PBS at a concentration of 200 mg/ml. The bacteria were kept on ice and sonicated 20 times for 1 min each. Sonicated samples were cleared by centrifugation at 20,000 × g for 15 min followed by filtration of the supernatant (0.22-μm-pore-size filter) to remove residual particulate material.
Absorption of M. avium subsp. paratuberculosis antigens.
A partly purified antigen preparation was made as described previously (11). Briefly, M. avium subsp. avium antiserum (5 ml; 45 mg of immunoglobulin/ml) was adsorbed to a HiTrap protein G column (Pharmacia Fine Chemicals, Uppsala, Sweden). Approximately 10 mg of sonicated proteins of M. avium subsp. paratuberculosis was subsequently applied on the column. The primary effluent fractions were collected, and bound immunoglobulin G-antigen complexes were eluted with glycine-HCl buffer, pH 2.7. After each step the column was washed with 0.2 mM Na-phosphate buffer, pH 7.0. The protein content in the output was monitored by measuring the optical density (OD) at 280 nm.
Animal sera.
Sera from cattle naturally infected with M. avium subsp. paratuberculosis were from three different countries (Sweden, n = 8; Denmark, n = 25; and Holland, n = 23), and the infection was confirmed by cultivation of feces by the laboratories that provided the sera. Sera from 4 cattle naturally infected with bovine TB, sera from 8 cattle experimentally infected with bovine TB (intratracheal inoculation of 5 μg of M. bovis BM228), and 10 sera from a herd with minimal disease were provided by the Animal Disease Research Institute, Agriculture Canada, Nepean, Ontario, Canada, and have been described previously (7). The 10 sera from healthy cattle came from a Norwegian herd with no history of paratuberculosis or bovine TB. The positive control serum used in the established absorbed-antigen ELISA was from a cow with naturally occurring M. avium subsp. paratuberculosis infection confirmed by cultivation. Monospecific rabbit antisera (11, 12) against AhpC, AhpD, and the 14-kDa antigen were used as positive controls in their respective ELISAs.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis with immunoblotting.
The antigens were separated under reducing and nonreducing conditions by horizontal sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a precast 8 to 18% gradient Excel gel (Pharmacia) using a Multiphor II unit 2117 (Pharmacia). After separation the proteins were transferred to a nitrocellulose membrane (pore size, 0.2 μm) by diffusion blotting (13). The membranes were blocked with PBS containing 2% bovine serum albumin and 1% gelatin and were incubated with antiserum overnight. Bound antibodies were recognized by biotinylated protein G (Sigma-Aldrich Norway AS, Oslo, Norway) followed by streptavidin-peroxidase (Boehringer Mannheim, GmbH, Mannheim, Germany) As a substrate, 3,3′-diaminobenzidine was added to visualize the bound antibodies.
Commercial ELISA for paratuberculosis.
A commercial ELISA for detection of paratuberculosis in cattle (IDEXX Laboratories, Inc, Westbrook, Maine) was used according to the manufacturer's instructions. The 12 sera from animals with bovine TB were tested for the presence of cross-reacting antibodies.
Antigen-absorbed ELISA.
Flat-bottomed 96-well microtiter plates (Nunc Immuno Polysorp, batch no. 044214; Nunc A/S, Roskilde, Denmark) were coated with absorbed M. avium subsp. paratuberculosis antigen (0.5 μg/ml) in 100 μl of coating buffer (0.06 M Na-carbonate, pH 9.6) for at least 48 h at 4°C. In all of the following steps, mixtures were incubated at 20°C for 1 h except as noted, and the plates were washed five times with PBS with 0.1% Tween 20, pH 7.2 (PBS-T), after each step. The plates were blocked with 150 μl of PBS-T containing 1% bovine serum albumin. Serum samples were diluted twofold from 1/512 to 1/16,384 in PBS-T, and 100 μl of diluted serum was added, followed by biotinylated protein G (Sigma-Aldrich) diluted 1:15,000 in PBS-T. Streptavidin-peroxidase (Boehringer Mannheim) diluted 1:10,000 in PBS-T was added and incubated for 30 min. As a substrate, ortho-phenylenediamine (Dako) diluted in citric acid phosphate buffer (pH 5.0) was added, and the plates were incubated in the dark for 10 min. The enzyme reaction was stopped by adding 50 μl of 2 M H2SO4, and the color reaction was measured in a spectrophotometer (Multiskan EX version 1.0; Labsystems Oy, Helsinki, Finland) at 492 nm.
Purified-protein ELISA.
Polysorp microtiter plates were coated with AhpC, AhpD, or the 14-kDa antigen (0.1 μg/ml) in PBS, and the plates were blocked with fetal calf serum diluted 1/10 in PBS-T. The sera were diluted 1/100 in PBS-T before testing. All of the other steps were performed as described above. A test was considered positive at an OD ratio (test OD/positive control OD) of above 0.1. The cutoff and optimal serum dilution were set by taking the mean OD plus three times the standard error of the mean when testing sera from 10 healthy animals from a farm with no history of paratuberculosis or TB.
Statistical analyses.
The Student t test was used to compare the OD ratios between the sera from the herd with minimal disease and from the animals with paratuberculosis. The serological responses against AhpC, AhpD, and the 14-kDa antigen in animals with paratuberculosis were compared using the Pearson correlation coefficient.
RESULTS
Serological reactivity in absorbed-antigen ELISA.
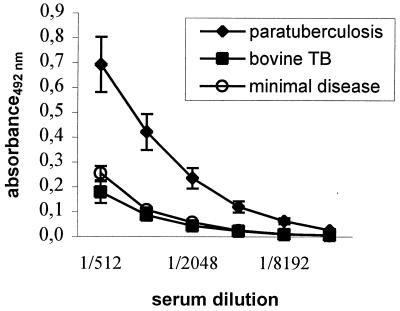
The established ELISA was based on preabsorption of the coating antigen on hyperimmune rabbit M. avium subsp. avium antiserum. Twofold dilutions of sera from a herd with minimal disease (n = 11) and cattle naturally infected with paratuberculosis (n = 25) gave significantly (P < 0.01) elevated antibody levels in the animals with paratuberculosis when the sera were diluted from 1/1,024 through 1/8,192 (Fig. 1). Based on these results, a standard serum dilution of 1/2,000 and an OD ratio cutoff of >0.10 (mean plus three times the standard error of the mean) were chosen for the screening of a larger number of sera. This absorbed-antigen ELISA subsequently recognized 37 of the 56 animals with paratuberculosis (66%) (Table 1). The mean OD ratio of the responding individuals in the absorbed-antigen ELISA was 0.44 (range, 0.10 to 1.14). None of the animals from the healthy herd or the animals with natural bovine TB infection reacted in this ELISA, whereas all of the animals with experimental bovine TB were positive, with OD ratios ranging from 0.50 to 3.17.
FIG. 1.
Antibody responses in the established absorbed-antigen ELISA. The absorbances at 492 nm of serial dilutions of sera from animals infected with paratuberculosis (n = 25), sera from a herd with minimal disease (n = 11), and sera from animals with bovine TB (n = 4) are shown. The results are given as means and standard errors of the means. The differences between the herd with minimal disease and paratuberculosis-infected animals were significant when sera were diluted from 1/1,024 through 1/8,192 (P < 0.01).
TABLE 1.
Sera from cattle infected with M. avium subsp. paratuberculosis or M. bovis and noninfected controls with detectable antibodies in various ELISAs
| Animal group (n) | No. (%) with detectable antibodies in the following ELISA:
|
||||
|---|---|---|---|---|---|
| Commercial | Antigen absorbed | AhpC | AhpD | 14-kDa protein | |
| Paratuberculosis infection (56) | 30 (54) | 37 (66) | 13 (27)b | 7 (15)b | 13 (27)b |
| Experimental bovine TB infection (8) | 8 (100) | 7 (100)a | 0 | 0 | 0 |
| Natural bovine TB infection (4) | 1 (25) | 0 | 0 | 0 | 0 |
| Herd with minimal disease (11) | NDc | 0 | ND | ND | ND |
| Healthy (10) | ND | 0 | 0 | 0 | 1 (10) |
n = 7.
n = 48.
ND, not done.
Responses in the commercial ELISA.
The paratuberculosis and bovine TB sera were also tested in a commercially available ELISA for paratuberculosis, which is based on preabsorption of the sera on M. phlei. In this commercial ELISA, 30 of 56 of the animals with paratuberculosis (54%) were positive (Table 1). The eight cattle experimentally infected with bovine TB all had a strong positive reaction with this kit, with a mean OD ratio of 1.04 (range, 0.41 to 1.49). One of the cattle naturally infected with TB was also positive in the commercial paratuberculosis ELISA (OD ratio, 0.17). These results showed that several of the bovine TB sera had high levels of cross-reactive antibodies and that this ELISA was unable to distinguish between paratuberculosis and bovine TB.
Serological responses against purified antigens.
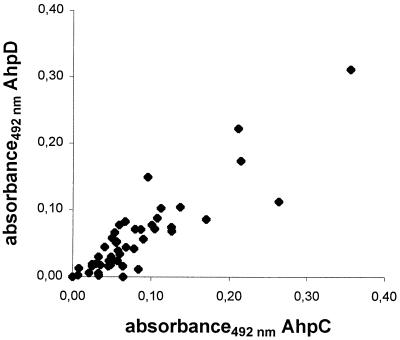
Protein G-based ELISAs using AhpC, AhpD, and the 14-kDa antigen were established and used to test sera from animals infected with M. avium subsp. paratuberculosis and M. bovis (Table 1). Detectable amounts of antibodies to AhpC in the animals with paratuberculosis were present in 13 of 48 animals (27%), while 7 animals (15%) reacted with AhpD and 13 of 48 animals (27%) reacted with the 14-kDa antigen. The OD ratios were generally low in the responding animals, ranging from 0.10 to 0.36 for AhpC, 0.10 to 0.31 for AhpD, and 0.10 to 0.80 for the 14-kDa antigen. All of the animals with antibodies against AhpD also had detectable antibodies against AhpC. A total of 17 animals (35%) had detectable antibodies against either AhpC or the 14-kDa antigen or both. Two animals with paratuberculosis that tested negative in the absorbed-antigen ELISA or commercial ELISA were positive in at least one of the ELISAs for purified antigens. None of the animals with TB reacted with any of the purified antigens; however, one animal from the healthy herd had an OD ratio above the cutoff for the 14-kDa antigen. In animals with paratuberculosis, there were strong positive correlations of the antibody responses against AhpC and AhpD (r = 0.89; P < 0.001) (Fig. 2), AhpC and the 14-kDa antigen (r = 0.78; P < 0.001), and AhpD and the 14-kDa antigen (r = 0.80; P < 0.001).
FIG. 2.
Correlation plot of antibody responses against AhpC and AhpD in animals infected with M. avium subsp. paratuberculosis. Absorbance in the AhpC ELISA was plotted against absorbance in the AhpD ELISA, giving a correlation coefficient (r) of 0.89 and a P value of <0.001. Each point represents one animal.
DISCUSSION
Serological assays are still being used routinely for the diagnosis of paratuberculosis, although the overall sensitivity is low, especially in subclinically infected animals. The specificities of the available ELISAs have usually been considered high since the method of preabsorbing the sera on M. phlei was introduced (15, 24). This method is believed to remove most of the cross-reactive antibodies due to infection with related mycobacteria, hence increasing the specificity of the test. However, no reports on the ability of this method to distinguish between paratuberculosis and bovine TB have been published. In the present study, we show that a commercial ELISA kit using preabsorption of sera on M. phlei clearly does not differentiate between these two infections, since all of the animals experimentally infected with M. bovis had high antibody levels in this test.
Another approach to increase the specificity of serological assays was applied in the present study. The method included a step in which cross-reactive antigens were removed by preabsorption of the coating antigen on a polyclonal and polyvalent M. avium subsp. avium antiserum. This absorbed-antigen ELISA had a sensitivity equal to or slightly better than that of the commercial ELISA, but neither of the tests could distinguish between experimental M. bovis infection and infection with M. avium subsp. paratuberculosis.
The two main proteins in the antigen preparation used in the established ELISA were AhpC and AhpD, as previously shown; however, other bands could also be detected by Western blotting, including a characteristic smear around 32 to 42 kDa that probably represents lipoarabinomannan (LAM) (11, 14). It is likely that the sera also reacted with some of those antigens in addition to AhpC and AhpD, since the M. bovis-infected animals did not have any detectable antibodies against purified AhpC or AhpD despite their strong reaction in the absorbed-antigen ELISA. A likely candidate for the observed cross-reactions is LAM, which has been shown to be a potent B-cell antigen (8, 14). To investigate this possibility further, one paratuberculosis serum was tested in Western blotting, and this serum showed a reaction against a smear around 32 to 42 kDa, probably representing LAM, in addition to a 24-kDa band (results not shown).
The results in this study, using both the ELISA based on absorption of sera on M. phlei and the presently established method of absorption of the antigen, demonstrated the need to use purified specific proteins to be able to distinguish between bovine TB and paratuberculosis. The use of such proteins will, however, usually give a lower sensitivity. It has been demonstrated, for instance, that antibodies against purified MPB70 from M. bovis are present in approximately one-third of animals with bovine TB (7). A natural approach, therefore, is to use a less specific ELISA as a screening method, followed by further testing of the responders against a panel of purified proteins. Alternatively, a panel of specific proteins can be used directly if a sufficient number of specific proteins are to be included.
In the present study we have shown that the 14-kDa antigen, AhpC, and AhpD seem to be able to differentiate between bovine TB and bovine paratuberculosis. Even though the antigens did not elicit strong antibody responses in animals infected with paratuberculosis, the AhpC and 14-kDa ELISAs together were positive in 35% of the animals with confirmed paratuberculosis. None of the animals with bovine TB had detectable antibodies against any of these proteins despite the high levels of cross-reacting antibodies to M. avium subsp. paratuberculosis antigens present in these sera as demonstrated in the commercial ELISA. AhpC and AhpD are usually not expressed in the M. tuberculosis complex due to a deletion in the regulatory oxyR gene. However, some isoniazid-resistant strains have been shown to overproduce these proteins, and infections with such strains can potentially induce cross-reactive antibodies. Elsaghier et al. have previously shown that only M. avium subsp. paratuberculosis-infected mice and not M. avium subsp. avium-infected mice had elevated antibodies against a protein that was later identified as AhpC (5). It is therefore a distinct possibility that AhpC and AhpD can be used to distinguish infections with different M. avium complex organisms. However, further verification is necessary, because AhpC expression can be induced in M. avium subsp. avium when the bacteria are subjected to oxidative stress (18).
The present study also showed that even though a homologue to the 14-kDa antigen is present in the M. tuberculosis complex, cross-reacting antibodies against this antigen were not present in cattle with TB. These findings are in agreement with previously published in vitro results, where a polyclonal antiserum against the 14-kDa antigen did not cross-react with any proteins in M. tuberculosis or M. bovis by using Western blotting (12). The 14-kDa secreted protein is, however, present in all of the species of the M. avium complex. Animals sensitized to these bacteria may therefore have antibodies against this protein. Despite these considerations, it is clear that the specificity of single-antigen ELISAs is significantly higher than that of the tested commercial ELISA for paratuberculosis. Further studies will focus on the applicability of these proteins in serological assays and the ability to distinguish between infections with different M. avium subspecies.
ACKNOWLEDGMENTS
The sera used in this study were kindly provided by Gøran Bølske, Søren Nielsen, Douwe Bakker, Morten Harboe, and Tore Tollersrud. We also thank Øivind Ødegaard for the collection of the sera and for letting us use the results from the commercial ELISA kit for paratuberculosis. We thank Inger Austrheim Heffernan and Karen Bækken Soleim for excellent technical assistance.
This work was supported by the Norwegian Research Council (project number 116 086).
REFERENCES
- 1.Bendixen P H. Immunological reactions caused by infection with Mycobacterium paratuberculosis. A review. Nord Vet Med. 1978;30:163–168. [PubMed] [Google Scholar]
- 2.Billman-Jacobe H, Carrigan M, Cockram F, Corner L A, Gill I J, Hill J F, Jessep T, Milner A R, Wood P R. A comparison of the interferon gamma assay with the absorbed ELISA for the diagnosis of Johne's disease in cattle. Aust Vet J. 1992;69:25–28. doi: 10.1111/j.1751-0813.1992.tb07426.x. [DOI] [PubMed] [Google Scholar]
- 3.de Lisle G W, Duncan J R. Bovine paratuberculosis. III. An evaluation of a whole blood lymphocyte transformation test. Can J Comp Med. 1981;45:304–309. [PMC free article] [PubMed] [Google Scholar]
- 4.Deretic V, Philipp W, Dhandayuthapani S, Mudd M H, Curcic R, Garbe T, Heym B, Via L E, Cole S T. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol Microbiol. 1995;17:889–900. doi: 10.1111/j.1365-2958.1995.mmi_17050889.x. [DOI] [PubMed] [Google Scholar]
- 5.Elsaghier A, Nolan A, Allen B, Ivanyi J. Distinctive western blot antibody patterns induced by infection of mice with individual strains of the Mycobacterium avium complex. Immunology. 1992;76:355–361. [PMC free article] [PubMed] [Google Scholar]
- 6.Harboe M, Nagai S, Patarroyo M E, Torres M L, Ramirez C, Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986;52:293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harboe M, Wiker H G, Duncan J R, Garcia M M, Dukes T W, Brooks B W, Turcotte C, Nagai S. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J Clin Microbiol. 1990;28:913–921. doi: 10.1128/jcm.28.5.913-921.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jark U, Ringena I, Franz B, Gerlach G F, Beyerbach M. Development of an ELISA technique for serodiagnosis of bovine paratuberculosis. Vet Microbiol. 1997;57:189–198. doi: 10.1016/s0378-1135(97)00125-9. [DOI] [PubMed] [Google Scholar]
- 9.Merkal R S, Kopecky K E, Larsen A B. Immunologic mechanisms in bovine paratuberculosis. Am J Vet Res. 1970;31:475–485. [PubMed] [Google Scholar]
- 10.Nagai S, Wiker H G, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olsen I, Reitan L J, Holstad G, Wiker H G. Alkyl hydroperoxide reductases C and D are major antigens constitutively expressed by Mycobacterium avium subsp. paratuberculosis. Infect Immun. 2000;68:801–808. doi: 10.1128/iai.68.2.801-808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen I, Reitan L J, Wiker H G. Distinct differences in repertoires of low-molecular-mass secreted antigens of Mycobacterium avium complex and Mycobacterium tuberculosis. J Clin Microbiol. 2000;38:4453–4458. doi: 10.1128/jcm.38.12.4453-4458.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen I, Wiker H G. Diffusion blotting for rapid production of multiple identical imprints from sodium dodecyl sulfate polyacrylamide gel electrophoresis on a solid support. J Immunol Methods. 1998;220:77–84. doi: 10.1016/s0022-1759(98)00147-1. [DOI] [PubMed] [Google Scholar]
- 14.Reichel M P, Kittelberger R, Penrose M E, Meynell R M, Cousins D, Ellis T, Mutharia L M, Sugden E A, Johns A H, de Lisle G W. Comparison of serological tests and faecal culture for the detection of Mycobacterium avium subsp. paratuberculosis infection in cattle and analysis of the antigens involved. Vet Microbiol. 1999;66:135–150. doi: 10.1016/s0378-1135(98)00311-3. [DOI] [PubMed] [Google Scholar]
- 15.Ridge S E, Morgan I R, Sockett D C, Collins M T, Condron R J, Skilbeck N W, Webber J J. Comparison of the Johne's absorbed EIA and the complement-fixation test for the diagnosis of Johne's disease in cattle. Aust Vet J. 1991;68:253–257. doi: 10.1111/j.1751-0813.1991.tb03230.x. [DOI] [PubMed] [Google Scholar]
- 16.Romain F, Augier J, Pescher P, Marchal G. Isolation of a proline-rich mycobacterial protein eliciting delayed-type hypersensitivity reactions only in guinea pigs immunized with living mycobacteria. Proc Natl Acad Sci USA. 1993;90:5322–5326. doi: 10.1073/pnas.90.11.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothel J S, Jones S L, Corner L A, Cox J C, Wood P R. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust Vet J. 1990;67:134–137. doi: 10.1111/j.1751-0813.1990.tb07730.x. [DOI] [PubMed] [Google Scholar]
- 18.Sherman D R, Sabo P J, Hickey M J, Arain T M, Mahairas G G, Yuan Y, Barry C E, Stover C K. Disparate responses to oxidative stress in saprophytic and pathogenic mycobacteria. Proc Natl Acad Sci USA. 1995;92:6625–6629. doi: 10.1073/pnas.92.14.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stabel J R. Production of gamma-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J Vet Diagn Investig. 1996;8:345–350. doi: 10.1177/104063879600800311. [DOI] [PubMed] [Google Scholar]
- 20.Vannuffel P, Gilot P, Limbourg B, Naerhuyzen B, Dieterich C, Coene M, Machtelinckx L, Cocito C. Development of species-specific enzyme-linked immunosorbent assay for diagnosis of Johne's disease in cattle. J Clin Microbiol. 1994;32:1211–1216. doi: 10.1128/jcm.32.5.1211-1216.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weldingh K, Rosenkrands I, Jacobsen S, Rasmussen P B, Elhay M J, Andersen P. Two-dimensional electrophoresis for analysis of Mycobacterium tuberculosis culture filtrate and purification and characterization of six novel proteins. Infect Immun. 1998;66:3492–3500. doi: 10.1128/iai.66.8.3492-3500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiker H G, Harboe M, Nagai S, Patarroyo M E, Ramirez C, Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81:307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]
- 23.Wood P R, Corner L A, Plackett P. Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of gamma interferon. Res Vet Sci. 1990;49:46–49. [PubMed] [Google Scholar]
- 24.Yokomizo Y, Kishima M, Mori Y, Nishimori K. Evaluation of enzyme-linked immunosorbent assay in comparison with complement fixation test for the diagnosis of subclinical paratuberculosis in cattle. J Vet Med Sci. 1991;53:577–584. doi: 10.1292/jvms.53.577. [DOI] [PubMed] [Google Scholar]