Abstract
Among patients with chronic lymphocytic leukemia (CLL) with deletion 17p (del[17p]), evidence from clinical trials for the effectiveness of single-agent ibrutinib as first-line therapy is limited. This retrospective analysis compared real-world clinical outcomes among patients with CLL, with and without del(17p), treated with first-line ibrutinib monotherapy. Overall survival, time to next treatment, time to treatment discontinuation, and reasons for ibrutinib discontinuation were evaluated. Using data from a real-world database, patients included were aged ≥18 years, had been diagnosed with CLL between January 1, 2011 and December 31, 2019, had undergone cytogenetic testing, and had received first-line ibrutinib monotherapy. A total of 1,069 patients were included in the analysis (62.7% male; median age 69 years); 23.8% (n=254) had del(17p). The median overall survival was significantly shorter in patients with del(17p) than in patients without (57.7 months vs. not reached; P=0.0006). Similar results were observed for median time to next treatment (49.4 months vs. not reached, P=0.0330). The median time to treatment discontinuation was non-significantly shorter in the group of patients with del(17p) (32.5 months vs. 42.9 months, P=0.3370). Results of an adjusted Cox proportional hazards model showed that the group with del(17p) was at significantly higher risk of death than was the group without del(17p) (hazard ratio=1.70, P=0.0031). Event rates for switching to new treatment and discontinuation were higher but not statistically significantly so. The most common reason for discontinuing ibrutinib treatment in both groups was toxicity, but discontinuation due to progression was significantly more frequent among patients with del(17p) (20% vs. 6%; P<0.0001). This study identifies an unmet need for more effective first-line therapeutic options in patients with CLL/small lymphocytic lymphoma and del(17p), despite the advent of ibrutinib.
Introduction
In patients with chronic lymphocytic leukemia (CLL), the presence of molecular-cytogenetic lesions such as deletion 17p (del[17p]), TP53 mutations, and/or expression of unmutated immunoglobulin heavy chain variable region (IGHV) confers a negative prognostic outcome.1-3 Among these, del(17p) and TP53 mutations are the least favorable, with patients considered at very high risk of having a poor response to initial chemoimmunotherapy or earlier relapse after achieving remission.1,2 This unfavorable prognosis has been attributed to the del(17p)-mediated loss of one allele of TP53, a tumor suppressor that plays a crucial role in apoptosis, cell cycle arrest, and DNA repair in response to genotoxic insults.4 Del(17p) is more common in relapsed/refractory CLL, but it does occur in treatmentnaïve patients, albeit at lower rates.5,6
Overall, the prognosis for patients with CLL and small lymphocytic lymphoma (SLL) has improved with the advent of Bruton tyrosine kinase (BTK) inhibitors. The first drug in this class, ibrutinib, was approved by the US Food and Drug Administration (FDA) in February 2014 for the treatment of patients with relapsed/refractory CLL. This approval was based on results from the pivotal RESONATE trial, which showed that ibrutinib had efficacy in a heavily pretreated cohort of patients. However, this study included only patients with relapsed/refractory disease, and found that overall survival at 18 months was 86% and progression-free survival was 76% in the ibrutinib arm, while in patients with del(17p) the corresponding values were 83% and 71%, respectively.7, 8 Following this, the RESONATE-2 trial was performed in the front-line setting and showed that ibrutinib was effective for treatment-naïve patients. Based on these data, FDA approval for ibrutinib was extended to first-line therapy.9 However, RESONATE-2 excluded two key groups of patients: individuals younger than 65 years and those with del(17p).10 Therefore, findings from this trial do not directly reflect and may not be generalizable to these high-risk patients.11,12
Additional research has shown favorable outcomes for patients with del(17p) CLL/SLL treated with ibrutinib, but has largely focused on patients with relapsed/refractory disease. The phase II RESONATE-17 trial was a single-arm open-label study including 144 patients with del(17p) CLL/SLL; it showed an estimated 24-month overall survival of 75% with ibrutinib monotherapy for this population of patients.13 Likewise, an integrated analysis of three studies of ibrutinib in patients with relapsed/refractory del(17p) CLL/SLL found an estimated 12-month overall survival of 85%.14 The Alliance trial provided some insight into frontline ibrutinib use in older patients.15 However, the trial was not powered to compare results between patients with and without del(17p). Meanwhile, the phase II National Heart, Lung, and Blood Institute (NHLBI) trial investigating ibrutinib use in front-line treatment of CLL patients with TP53 alterations is ongoing.16 As such, options for front-line treatment of del(17p) CLL/SLL are largely extrapolated from experience in the relapsed/refractory setting. While ibrutinib has shown convincing data in this population to date, gaps still exist within the available data.
The aim of this observational retrospective study was to compare outcomes of patients with CLL/SLL, with and without del(17p), who received first-line ibrutinib mono-therapy, in terms of the following outcomes: overall survival, time to next treatment, time to treatment discontinuation, and reasons for ibrutinib discontinuation. The study used information from the Flatiron Health electronic health record-derived database, one of the largest electronic datasets of community oncology practices in the USA.
Methods
Objectives
Study objectives were to (i) describe and compare baseline characteristics among patients with CLL/SLL with and without del(17p) receiving first-line ibrutinib mono-therapy, (ii) compare real-world overall survival, time to next treatment, and time to treatment discontinuation, and (iii) evaluate the reasons for ibrutinib discontinuation in the two groups.
Study design and cohort selection
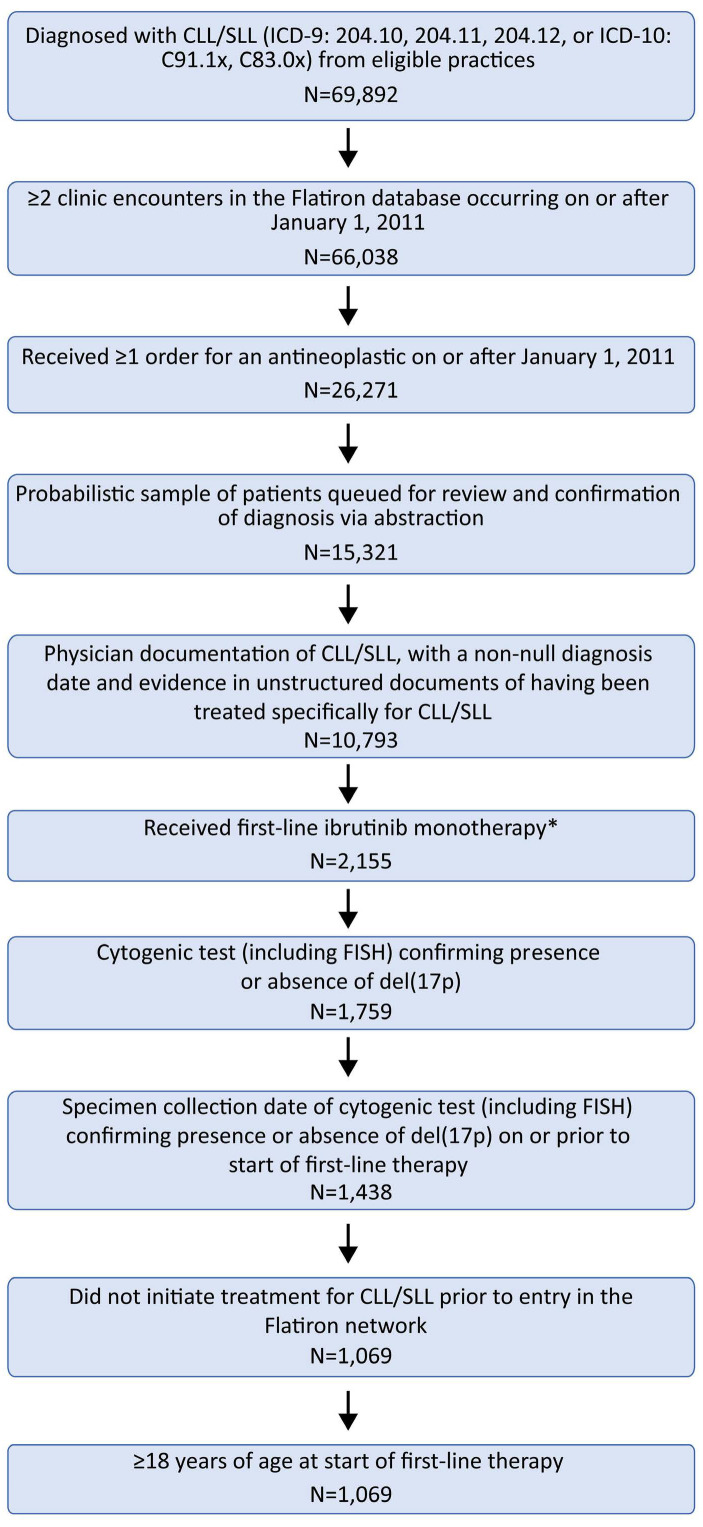
De-identified patients’ data were obtained from the Flati-ron Health electronic health record-derived database. The initial cohort of patients was selected based on CLL/SLL codes (ICD-9: 204.10-12, or ICD-10: C91.1x, C83.0x). Patients also had to have had two or more clinic encounters and one or more antineoplastic therapy order between January 1, 2011 and December 31, 2019, along with cytogenetic testing confirming del(17p) status at or before initiation of first-line ibrutinib therapy. Patients were included in the final analysis cohort only after manual chart review, including physician confirmation of diagnosis. Patients who initiated CLL/SLL treatment before entering the Flatiron Health network were excluded. Figure 1 illustrates the steps for selecting the patients.
Definitions
The index date was defined as the start of first-line ibrutinib monotherapy. Patients’ characteristics of interest were: disease subtype; Rai stage at diagnosis; Eastern Cooperative Oncology Group (ECOG) status at index date; del(17p), del(11q), del(13q), trisomy 12, and IGHV mutation status; and time from diagnosis to, and follow-up from, index date.
Overall survival was defined as the time between the index date and death, with patients otherwise censored at their last date of confirmed electronic health record activity. Time to next treatment was the time from the index date to initiation of a subsequent non-ibrutinib line of therapy, or to date of death (patients with no subsequent line of therapy). Time to treatment discontinuation was the time from the index date to discontinuation of ibrutinib for any reason; discontinuation was defined as an abstraction-confirmed discontinuation episode, death, or lack of prescription despite structured activity within the Flatiron Health network in ≥120 days after the last ibrutinib treatment. Additional details regarding the definition of a discontinuation event are provided in the Online Supplementary Material (section 1). Reasons for ibrutinib discontinuation were classified as due to disease progression, toxicity, patient’s request, financial reasons, disease-related symptoms not due to therapy, treatment completion, or other (including death).
Ethics review
Institutional Review Board approval of the study protocol was obtained prior to conducting the study and included a waiver of informed consent.
Statistical analysis
Descriptive statistics were used to summarize patients’ characteristics. Between-group comparisons were based on the Kruskal-Wallis test for medians and the t-test for means.
Overall survival and time to next treatment were estimated using Kaplan-Meier curves, along with median durations and 95% confidence intervals (95% CI). Time to treatment discontinuation was modeled using non-parametric maximum likelihood estimator due to interval censoring. Outcomes were compared using log-rank testing or the Sun log-rank test (2-sided significance level, 0.05). The Online Supplementary Material (section 2) contains additional details regarding these statistical tests. Cox proportional hazards model comparisons (unadjusted and adjusted) were also conducted, with adjustments made for index year; sex; age; ECOG status; Rai stage; practice type; 11q, 13q, trisomy 12 deletion; and IGHV status. Descriptive statistics were used to summarize reasons for ibrutinib discontinuation, with comparisons made using the χ2 or Fisher exact test (2-sided significance level, 0.05).
Results
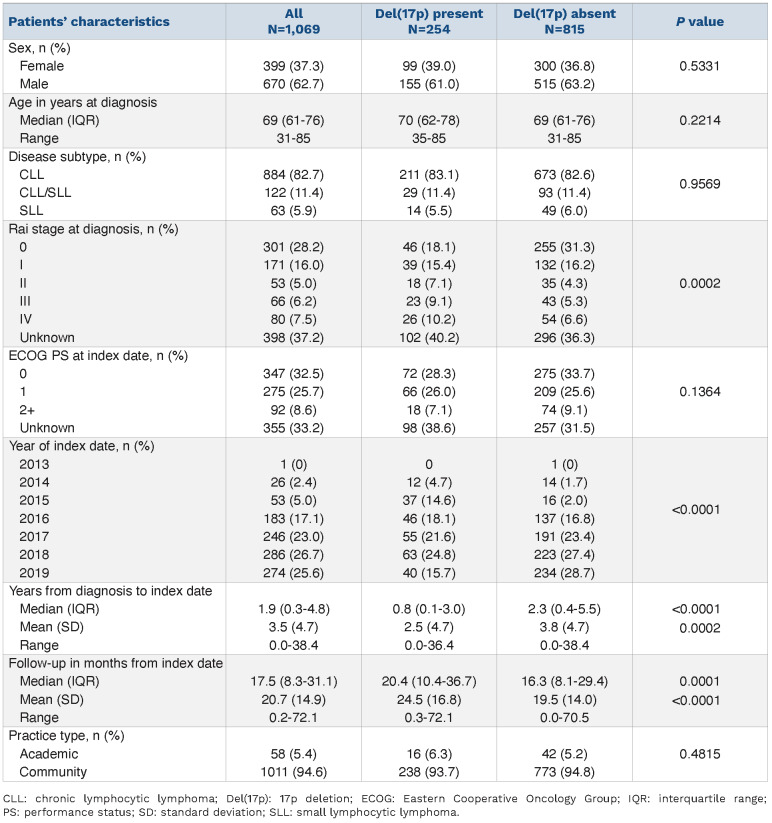
The analysis cohort consisted of 1,069 patients; of these, 254 (23.8%) had a del(17p) and 815 (76.2%) patients did not have del(17p). The baseline characteristics of the patients, categorized by the presence or absence of del(17p), are shown in Table 1. The median age at diagnosis was similar for both groups of patients (70 years for those with del[17p]; 69 years for those without del[17p]). In terms of index year, only one patient entered the study in 2013, while the largest proportion of study patients (26.7%) enetered the study in 2018. Most patients were treated in community practices (94.6%), with the remainder (5.4%) treated in an academic setting.
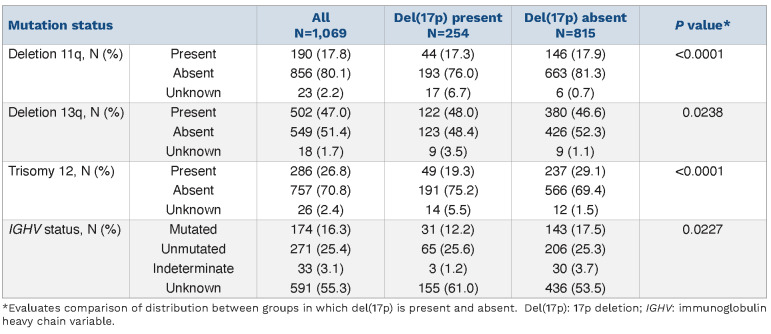
At diagnosis, patients with del(17p) were more likely to be at a later Rai stage (P=0.0002); 9.1% and 10.2% of patients with del(17p) were at Rai stage 3 and 4, respectively, compared to 5.3% and 6.6% of patients without del(17p). Patients with del(17p) also received first-line ibrutinib sooner after diagnosis, with a median 0.8 years from diagnosis to index date, compared to 2.3 years for patients without del(17p) (P<0.0001). Overall, the median follow-up was 17.5 months. Meanwhile, the median follow-up was longer in the group with del(17p) (20.4 months) than in the group without del(17p) (16.3 months, P=0.0001). A majority of patients (58.2%) had ECOG performance status ≤1 at entry into the study, with no significant differences between the two groups. In terms of other genetic prognostic factors, as shown in Table 2, there was a statistically significant difference between the percentages of patients with and without del(17p) who also had deletion 11q (P<0.0001), deletion 13q (P=0.0238), trisomy 12 (P<0.0001), or IGHV mutated status (P=0.0227).
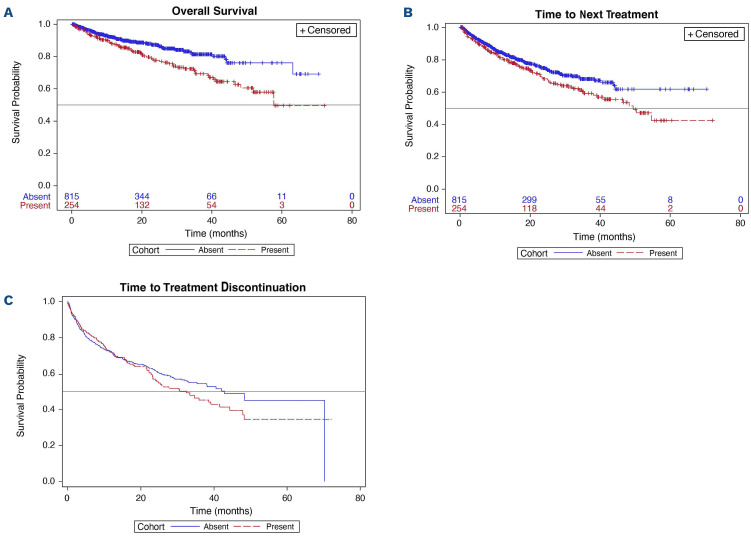
Outcomes of interest, including median overall survival, time to next treatment, and time to treatment discontinuation, are summarized in Table 3. For the overall cohort, median overall survival and median time to next treatment were not reached, while the median time to treatment discontinuation was 38.6 months (95% CI: 33.4, 42.9). The median overall survival was shorter in the group with del(17p) (57.7 months) than in the group without del(17p), in which the median was not reached (P=0.0006) (Table 3; Figure 2A). The median time to next treatment was also significantly shorter in the group with del(17p) than in the group without del(17p) (49.4 months vs. not reached; P=0.0330). The median time to treatment discontinuation was shorter in the group with del(17p) than in the group without del(17p); however, this difference was not statistically significant (32.5 months vs. 42.9; P=0.3370).
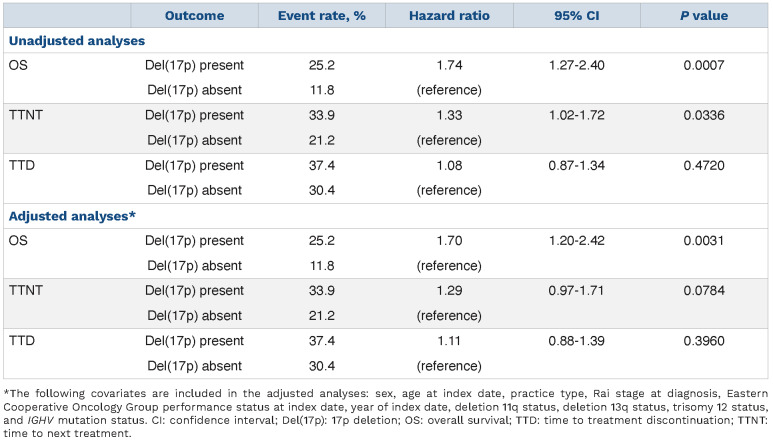
Figure 1.
Selection of the study population. *One patient started ibrutinib in 2013, prior to its approval; the remainder started ibrutinib therapy between 2014-2019. CLL: chronic lymphocytic lymphoma; Del(17p): 17p deletion; FISH: fluorescence in situ hybridization; SLL: small lymphocytic lymphoma.
In terms of event rates in the overall cohort, the rates of death, switching to a new treatment, and treatment discontinuation were 15.0%, 24.2%, and 32.1%, respectively (data not shown). As shown in Table 4, the rate of death among patients with del(17p) was higher at 25.2% versus 11.8%, as were rates of starting a new treatment and discontinuation (33.9% vs. 21.2% and 37.4% vs. 30.4%, respectively). Cox proportional hazard models showed that the hazard ratios (HR) for overall survival, time to next treatment, and time to treatment discontinuation were consistent with these findings. In the adjusted analysis, patients in the group with del(17p) were significantly more likely to die than those without del(17p) (HR=1.7; 95% CI: 1.20, 2.42; P=0.0031). Additionally, although not statistically significant, patients with del(17p) were 1.3 times more likely to receive subsequent therapy (95% CI: 0.97, 1.71; P=0.0784). At 1 year of treatment, patients with and without del(17p) had overall survival rates of 88% (95% CI: 83.1, 91.6) and 92% (95% CI: 89.7, 93.8), respectively (data not shown).
Table 1.
Description and comparison of patients’ characteristics.
Table 2.
Mutation status based on fluorescence in situ hybridization and classical cytogenetic testing.
Table 3.
Median overall survival, time to next treatment, and time to treatment discontinuation.
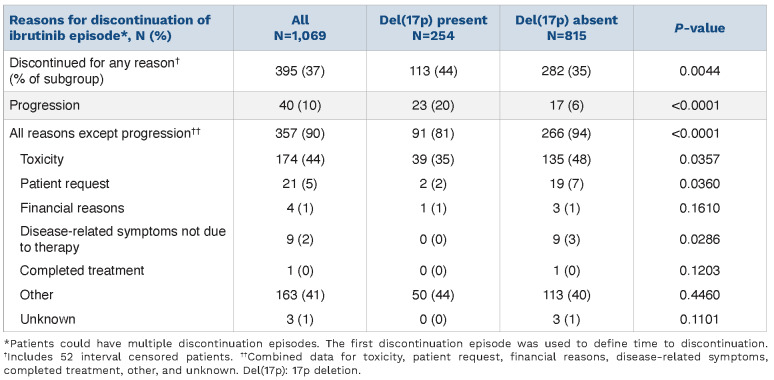
As shown in Table 5, out of 1,069 patients, 37% (n=395, including 52 with interval censorings) discontinued ibrutinib treatment for any reason. A significantly higher proportion of patients with del(17p) than without del(17p) discontinued ibrutinib treatment due to disease progression (20% vs. 6%; P<0.0001). When considering all other reasons for discontinuation (except for disease progression) overall discontinuation rates were higher in the group without del(17p) (81% vs. 94%; P<0.0001). Among all individual reasons given for discontinuation, toxicity was the most common (44% in the overall group). Meanwhile, toxicity rates in patients with del(17p) were significantly lower than in those without del(17p) (35% vs. 48%; P=0.0357). Although numbers were small, our results indicate that patients without del(17p) were also more likely to discontinue ibrutinib treatment based on patients’ request or disease-related symptoms not due to therapy. Both interval- and right-censored data effects existed in the discontinuation analysis. The Online Supplementary Material provides detailed outputs for the discontinuation analysis conducted using non-parametric maximum likelihood estimator. Online Supplementary Table S1 shows sample distributions by censoring status, while Online Supplementary Table S2 details quantile estimates for the population, stratified by del(17p) status.
Discussion
The initial management of patients with CLL/SLL with del(17p) requires special attention as they are at higher risk of rapid disease progression. Chemotherapy is no longer the standard of care in this population of patients, and the optimal treatment strategy for them is still evolving.12 While BTK inhibitors are currently considered a standard of care for these patients in the first-line setting, there is still room for improvement in defining the best approach.13,17 This retrospective cohort analysis was conducted to improve clinical understanding of the impact of first-line ibrutinib monotherapy for patients with CLL/SLL, particularly those with del(17p), in the real-world setting. As expected, our study showed that patients with del(17p) had poorer outcomes than those without del(17p). Specifically, the median overall survival and time to next treatment were significantly shorter for patients with del(17p) than for those without, while time to treatment discontinuation was non-significantly shorter with discontinuation mostly due to progression and toxicity. Furthermore, while the median overall survival and time to next treatment were not reached among patients without del(17p), among patients with the deletion, median survival following discontinuation of ibrutinib was relatively short (approximately 8 months) inferred from a median time to next treatment in this group of 49.4 months while the overall survival was 57.7 months.
Figure 2.
Kaplan-Meier curves for study outcomes. (A) Overall survival, (B) time to next treatment, and (C*) time to treatment discontinuation. *Censor marks are not shown as the non-parametric maximum likelihood estimator survival function and interval censoring method were used. With interval censoring, the event is assumed to occur within a time interval rather than at a specific time.
Table 4.
Cox proportional hazards model results: del(17p) present vs. del(17p) absent.
The current results support findings from a 2018 retrospective cohort analysis of treatment-naïve patients with CLL/SLL (n=391) who received first-line ibrutinib. The study utilized the eligibility criteria of the RESONATE-2 trial while expanding the inclusion criteria to two key groups: patients with del(17p) and individuals younger than 65 years. Overall, at 1 year, the overall survival and progression-free survival were 95% and 92%, respectively, and 81% of surviving patients remained on ibrutinib. However, patients with del(17p) (n=110; 28% of the study population) had inferior 1-year overall survival (89%, HR=3.9; P=0.001) and progression-free survival (87%, HR=1.9; P=0.04) compared to those without the deletion.11 The study reported a 1-year overall survival that was very similar to the current results at 1 year (88%). The consistency of this finding is noteworthy for several reasons. First, the Flatiron Health electronic health record-derived database primarily represents patients from community practice settings, as opposed to academic centers, which were predominant in the earlier study, thus reflecting a consistency between both types of practices. In addition, these results may be somewhat more representative of the US population with CLL/SLL, a majority of whom would likely be treated in community practice.18,19 This analysis also includes data that extend through 5 years of follow-up and is current through to the end of 2019. Finally, this research was based on real-world clinical practice patterns and was not limited by eligibility criteria applied in prior clinical trials. Thus, compared with the findings of previous research, these results are representative of a broader patient experience with first-line ibrutinib.
Among all patients, toxicity was reported as the most common reason for ibrutinib discontinuation in 35% of patients with del(17p) and 48% without the deletion, which is comparable to findings in previously published studies.20,21 It was notable that patients without del(17p) were more likely to discontinue due to toxicity than those with del(17p). On the other hand, patients with del(17p) were more likely to discontinue because of disease progression than were patients without the deletion. This could be explained by the fact that patients with del(17p) progress more quickly than those without, as demonstrated by the time to next treatment, a surrogate for progression-free survival, which was shorter in patients with del(17p). It may also reflect an unmet need as it is unlikely that patients with del(17p) experienced fewer toxicities than those without. Rather, this may reflect a choice by clinicians and patients to maintain treatment due to limited viable options within the timeframe of the study.
Reducing discontinuations due to toxicity could help to improve overall outcomes. In the ongoing ELEVATE-RR head-to-head trial, acalabrutinib appears to cause lower rates of cardiac adverse events than ibrutinib.22 In an interim analysis of the head-to-head phase III ALPINE study in patients with relapsed/refractory CLL/SLL, zanubrutinib-treated patients had a lower rate of atrial fibrillation/flutter compared to those treated with ibrutinib, as well as a superior response rate and an improved progression-free survival.23 Despite improved tolerability with next-generation BTK inhibitors, we believe there is still a need to define the best therapeutic approach to treating patients with del(17p).
Table 5.
Reasons for discontinuation of ibrutinib treatment.
Ongoing trials investigating combination therapy with targeted agents, including BTK inhibitors, B-cell lymphoma 2 (Bcl-2) antagonists, and/or anti-CD20 monoclonal antibodies may provide insight into improved therapeutic approaches for patients with del(17p). The SEQUOIA study (arm D - zanubrutinib plus venetoclax), the phase II AVO study (cohort 2 - acalabrutinib, venetoclax, and obinutuzumab), the BOVen trial (zanubrutinib, venetoclax, obinutuzumab), and the German CLL Study Group trial CLL-2 GIVe (ibrutinib, venetoclax, obinutuzumab) are directed at or have dedicated del(17p) cohorts.24-27 These studies could help to distinguish the potential for overlapping toxicities while better defining effective first-line treatments for the management of high-risk CLL, but more time is needed to obtain follow-up data.28
Single-agent ibrutinib is currently the most frequently utilized BTK inhibitor as initial treatment for del(17p)-positive patients with CLL/SLL,2,17 despite a paucity of data specific to this population of patients. In the past year, there has been an increasing number of patients treated with acalabrutinib following its approval in CLL although there are less data available for this agent in patients with del(17p).29 Nevertheless, we believe that the differences in outcomes of patients with and without del(17p) described in this study may be applicable to BTK inhibitors as a class and suggest that future randomized clinical studies limited to patients with del(17p) are needed to best define the ideal treatment for this high-risk population. As more treatments and combination regimens appear on the horizon to treat CLL, such as zanubrutinib and other novel agents,30 opportunities to leverage data from real-world experience and ongoing clinical studies should continue to be taken to answer some of these key questions.
The question remains as to what regimen may work best in the del(17p) population.3 Anti-CD20 monoclonal antibodies, including rituximab, ublituximab, and obinutuzumab, are under investigation for use in combination with BTK inhibitors, and have shown mixed results. In a randomized trial, the addition of rituximab to ibrutinib did not improve outcomes in previously untreated patients with del(17p).15,31 However, in a single-arm study, the addition of ublituximab to ibrutinib was shown to safely produce high overall response rates among patients with relapsed/refractory del(17p) CLL/SLL.32 The subsequent phase III GENUINE trial evaluated ublituximab plus ibrutinib versus ibrutinib alone in patients with high-risk relapsed/refractory CLL, defined as the presence of del(17p) and/or del(11q) deletions and/or the TP53 mutation. Although this study was not powered to evaluate endpoints specific to individual mutation profiles, the authors affirmed that patients with del(17p) treated with ibrutinib alone showed inferior progression-free survival as compared to that of patients without the mutation, and that the addition of ublituximab to the treatment regimen improved these patients’ outcomes.33 In the phase III ELEVATE-TN trial, improvements in efficacy (assessed as progression-free survival) with the second-generation BTK inhibitor acalabrutinib, used with or without obinutuzumab, were observed in previously untreated patients with del(17p) compared to those receiving chemoimmunotherapy. In the del(17p) subset, there was no progression-free survival advantage from acalabrutinib-obinutuzumab versus acalabrutinib alone.29 Combining ibrutinib and venetoclax in patients with del(17p) may address these inferior outcomes, while second-generation BTK inhibitors may be associated with fewer toxicity-related treatment discontinuations.
Some limitations of this study must be acknowledged. A key limitation is that this was a real-world study and results are not easily comparable to those of prospective clinical trials. Endpoints that are standard in trials may be lacking in real-world data. For example, our dataset did not contain information on progression in the same way as it is defined in clinical trials. Because progression-free survival data were not available, time to next treatment was used as an imperfect proxy. As mentioned above, our results were in line with those of an earlier retrospective study;11 however, we note that the survival time after next treatment of 8 months in our study may seem poorer than anticipated based on the ongoing NHLBI phase II trial (n=34 patients with TP53 alterations),16 which reported a median post-progression survival of 25 months. In addition to differences in study approach, other factors, such as age (median 69 years in this study vs. 63 years in the NHBLI trial), may have led to differences in survival. Population differences in comorbid conditions and presence of other cytogenetic markers may also have shortened tail-end survival.
Other limitations of this study are the possibilities of inaccurate or incomplete data, similar to any retrospective database analysis,34 and, potentially, the inclusion of patients with index dates that preceded the approval of ibrutinib for CLL. The possibility of inaccurate data is mitigated by the Flatiron Health cohort selection method, which combines structured and abstracted data to ensure accurate selection of patients. Our study included patients diagnosed from 2011 onward, while ibrutinib was not approved to treat CLL until 2014. This choice was made to allow the capture of community practice patterns while also ensuring the inclusion of all patients and maximum follow-up duration. Despite this, the analysis cohort contained only one patient who began treatment in 2013 while the others had index dates from 2014 onwards.
The rate of del(17p) has been reported as being between 5% to 7% in most populations of patients with previously untreated CLL/SLL.18,35,36 Since our current study was focused on patients with del(17p) we note that the proportion of patients in this group may appear relatively high at 23.8%. This was due to selection criteria into the analysis cohort requiring availability of information on cytogenetic testing (including fluorescence in situ hybridization) (Figure 1). This was necessary in order to enable a meaningful comparison between patients with and without del(17p) and address the research question of interest. A similar rate of del(17p) positivity (29%) was observed in the prospective, USA-based, InformCLL Registry among previously untreated patients who had undergone fluorescence in situ hybridization testing.19 A final study limitation is that this study did not assess presence or absence of the TP53 mutation as a risk factor. TP53 and del(17p) are closely linked (treatment resistance in patients with del[17p] has been attributed to the presence of a variant in a TP53 al-lele),6 and high concordance exists between these measures.37 However, the Flatiron Health database does not routinely capture TP53 mutation status and an effort to obtain TP53 data would have been prohibitive. Similarly, there are several variables that have a high level of unknown status or missing data, such as IGHV status (>50%), ECOG score at index date (>33%), and Rai stage at diagnosis (>37%). Although these rates may seem relatively high, we believe this is consistent and not unexpected from data captured directly from day-to-day clinical practice patterns in the community as opposed to academic centers.18,19 Despite these limitations, we believe these results are highly meaningful.
In conclusion, this real-world retrospective analysis of first-line treatment with ibrutinib suggests that patients with CLL/SLL with del(17p) had inferior survival and were more likely to discontinue treatment due to disease progression than were patients without del(17p) in this study population. Despite the advent of ibrutinib, which has changed the overall outlook for patients with CLL/SLL, there remains an unmet need among those with del(17p). The ideal treatment regimen for this group of high-risk patients is yet to be determined. Randomized clinical trials comparing novel agent-based therapies designed specifically for this high-risk patient population are needed.
Supplementary Material
Acknowledgments
Medical writing and editorial assistance were provided by Caitlin Rothermel, MA, MPH, of MedVal Scientific Information Services, LLC, and were funded by BeiGene, Ltd.
Funding Statement
Funding: This study was designed, funded, and conducted by Bei-Gene, Ltd.
References
- 1.Tausch E, Schneider C, Robrecht S, et al. Prognostic and predictive impact of genetic markers in patients with CLL treated with obinutuzumab and venetoclax. Blood. 2020;135(26):2402-2412. [DOI] [PubMed] [Google Scholar]
- 2.Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23-33. [DOI] [PubMed] [Google Scholar]
- 3.Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol. 2019;94(11):1266-1287. [DOI] [PubMed] [Google Scholar]
- 4.Yu L, Kim HT, Kasar S, et al. Survival of del17p CLL depends on genomic complexity and somatic mutation. Clin Cancer Res. 2017;23(3):735-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768-778. [DOI] [PubMed] [Google Scholar]
- 6.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28(29):4473-4479. [DOI] [PubMed] [Google Scholar]
- 7.Brown JR, Hillmen P, O'Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32(1):83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koffman B. Ibrutinib’s approval history and its use as frontline therapy for CLL. https://cllsociety.org/2018/08/ibrutinibs-approval-history-and-its-use-as-frontline-therapy-for-cll/. Accessed 2 February 2022. [Google Scholar]
- 10.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mato AR, Roeker LE, Allan JN, et al. Outcomes of front-line ibrutinib treated CLL patients excluded from landmark clinical trial. Am J Hematol. 2018;93(11):1394-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhodes JM, Barrientos JC. Chemotherapy-free frontline therapy for CLL: is it worth it? Hematology Am Soc Hematol Educ Program. 2020;2020(1):24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409-1418. [DOI] [PubMed] [Google Scholar]
- 14.Jones J, Mato A, Coutre S, et al. Evaluation of 230 patients with relapsed/refractory deletion 17p chronic lymphocytic leukaemia treated with ibrutinib from 3 clinical trials. Br J Haematol. 2018;182(4):504-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn IE, Tian X, Wiestner A. Ibrutinib for chronic lymphocytic leukemia with TP53 alterations. N Engl J Med. 2020;383(5):498-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NCCN clinical practice guidelines: chronic lymphocytic leukemia/small lymphocytic lymphoma. Version 1. 2020. https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf. Accessed 2 February 2022. [Google Scholar]
- 18.Mato A, Nabhan C, Kay NE, et al. Real-world clinical experience in the Connect® chronic lymphocytic leukaemia registry: a prospective cohort study of 1494 patients across 199 US centres. Br J Haematol. 2016;175(5):892-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mato AR, Barrientos JC, Ghosh N, et al. Prognostic testing and treatment patterns in chronic lymphocytic leukemia in the era of novel targeted therapies: results from the informCLL registry. Clin Lymphoma Myeloma Leuk. 2020;20(3):174-183.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mato AR, Allan JN, Pagel JM, et al. Front-line ibrutinib therapy for chronic lymphocytic leukemia (CLL) in the real world: responses, toxicity, outcomes and subsequent therapies. Blood. 2017;130(Suppl 1):3011. [Google Scholar]
- 22.Byrd JC, Hillmen P, Ghia P, et al. First results of a head-to-head trial of acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia [abstract]. J Clin Oncol. 2021;39(Suppl 15):7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillmen P, Eichhorst B, Brown JR, et al. First interim analysis of alpine study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma [abstract]. Presented at the Congress of the European Hematology Association (EHA), June 9-17,. 2021, virtual session. [Google Scholar]
- 24.Tam CS, Robak T, Ghia P, et al. Zanubrutinib monotherapy for patients with treatment naïve chronic lymphocytic leukemia and 17p deletion. Haematologica. 2021;106(9):2354-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davids MS, Lampson BL, Tyekucheva S, et al. Acalabrutinib, venetoclax, and obinutuzumab as frontline treatment for chronic lymphocytic leukaemia: a single-arm, open-label, phase 2 study. Lancet Oncol. 2021;22(10):1391-1402. [DOI] [PubMed] [Google Scholar]
- 26.Soumerai JD, Mato AR, Dogan A, et al. Zanubrutinib, obinutuzumab, and venetoclax with minimal residual disease-driven discontinuation in previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2021;8(12):e879-e890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber H, Edenhofer S, von Tresckow J, et al. Phase 2 study of obinutuzumab (GA-101), ibrutinib and venetoclax (CLL2-GIVe) in patients with untreated high-risk chronic lymphocytic leukemia. Blood. 2022;139(9):1318-1329. [DOI] [PubMed] [Google Scholar]
- 28.Lipsky A, Lamanna N. Managing toxicities of Bruton tyrosine kinase inhibitors. Hematology Am Soc Hematol Educ Program. 2020;2020(1):336-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson MC, Roeker LE, Mato AR. All in the family: back-to-back kinase inhibitors for the treatment of chronic lymphocytic leukemia. Haematologica. 2021;106(9):2300-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burger JA, Sivina M, Jain N, et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood. 2019;133(10):1011-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharman JP, Farber CM, Mahadevan D, et al. Ublituximab (TG-1101), a novel glycoengineered anti-CD20 antibody, in combination with ibrutinib is safe and highly active in patients with relapsed and/or refractory chronic lymphocytic leukaemia: results of a phase 2 trial. Br J Haematol. 2017;176(3):412-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharman JP, Brander DM, Mato AR, et al. Ublituximab plus ibrutinib versus ibrutinib alone for patients with relapsed or refractory high-risk chronic lymphocytic leukaemia (GENUINE): a phase 3, multicentre, open-label, randomised trial. Lancet Haematol. 2021;8(4):e254-e266. [DOI] [PubMed] [Google Scholar]
- 34.Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126(6):2234-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zenz T, Gribben JG, Hallek M, Döhner H, Keating MJ, Stilgenbauer S. Risk categories and refractory CLL in the era of chemoimmunotherapy. Blood. 2012;119(18):4101-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910-1916. [DOI] [PubMed] [Google Scholar]
- 37.Dicker F, Herholz H, Schnittger S, et al. The detection of TP53 mutations in chronic lymphocytic leukemia independently predicts rapid disease progression and is highly correlated with a complex aberrant karyotype. Leukemia. 2009;23(1):117-1124. [DOI] [PubMed] [Google Scholar]
- 38.Zhao X, Zhao Q, Sun J, Kim JS. Generalized log-rank tests for partly interval-censored failure time data. Biom J. 2008;50(3):375-385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.