Abstract
CD6 is a co-stimulatory receptor expressed on T cells that binds activated leukocyte cell adhesion molecule (ALCAM), expressed on antigen presenting cells, epithelial and endothelial tissues. The CD6-ALCAM pathway plays an integral role in modulating T-cell activation, proliferation, and trafficking. In this study we examined expression of CD6 by reconstituting T cells in 95 patients after allogeneic cell transplantation and evaluated the effects of itolizumab, an anti-CD6 monoclonal antibody, on T-cell activation. CD6 T cells reconstituted early after transplant with CD4 regulatory T cells (Treg)-expressing lower levels of CD6 compared to conventional CD4 T cells (Tcon) and CD8 T cells. After onset of acute graft-versus-host disease (aGvHD), CD6 expression was further reduced in Treg and CD8 T cells compared to healthy donors, while no difference was observed for Tcon. ALCAM expression was highest in plasmacytoid dendritic cells (pDC), lowest in myeloid dendritic cells (mDC) and intermediate in monocytes and was generally increased after aGvHD onset. Itolizumab inhibited CD4 and CD8 T-cell activation and proliferation in preGvHD samples, but inhibition was less prominent in samples collected after aGvHD onset, especially for CD8 T cells. Functional studies showed that itolizumab did not mediate direct cytolytic activity or antibody-dependent cytotoxicity in vitro. However, itolizumab efficiently abrogated the costimulatory activity of ALCAM on T-cell proliferation, activation and maturation. Our results identify the CD6-ALCAM pathway as a potential target for aGvHD control and a phase I/II study using itolizumab as first line treatment in combination with steroids for patients with aGvHD is currently ongoing (clinicaltrials gov. Identifier: NCT03763318).
Introduction
Acute graft-versus-host disease (aGvHD) continues to be an important cause of morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT).1 Steroids provide effective treatment, but most patients with severe aGvHD do not achieve a complete response.2,3 Moreover, steroid treatment is often associated with severe toxicities. Novel therapeutic options are needed and different strategies to selectively modulate alloreactive T cells and antigen-presenting cells (APC) have been studied for the treatment of steroid refractory GvHD. Various approaches are currently being evaluated in clinical trials including: i) targeting key inflammatory mediators (IL-6, siltuximab or tocilizumab), ii) selective depletion of alloreactive T cells (ricin-conjugated anti-CD3/CD7, anti-CD30 brentuximab, post-transplant cyclophosphamide), iii) modulation of cytokine-driven signal transduction (ruxolitinib), iv) inhibition of target organ homing (anti-integrin α4β7, vedolizumab) and v) inhibition of costimulatory signals (abatacept).4 Despite these advances, treatment of steroid refractory GvHD remains a challenge.5
The co-stimulatory receptor CD6 is a 105-130 kDa type I transmembrane glycoprotein belonging to the highly conserved scavenger receptor cysteine-rich superfamily (SRCR-SF).6 CD6 is expressed on the majority of T cells and minor populations of B and natural killer (NK) cells.7,8 CD166, activated leukocyte cell adhesion molecule (ALCAM) is the primary ligand for CD6.9 ALCAM is expressed on APC and various epithelial and endothelial cells.10,11 Upon ligation, the CD6-ALCAM complex helps stabilize the immunological synapse between the T cell and the APC. In this context, CD6-ALCAM binding promotes T-cell activation, proliferation, maturation, and trafficking from the intravascular space into tissues, including the central nervous system.12–17 Early studies by Soiffer and colleagues demonstrated that ex vivo depletion of CD6+ donor T cells prior to transplantation markedly decreased the incidence of aGvHD, highlighting the importance of CD6+ T cells in pathogenesis of GvHD.18–21
Itolizumab, a humanized IgG1 anti-CD6 monoclonal antibody, has been shown to block CD6 signaling, leading to a reduction in T-cell activation and proliferation.22 Itolizumab therapy has been evaluated for treatment of different autoimmune disorders,9 such as severe chronic plaque psoriasis23 and COVID-19 cytokine-release syndrome with promising results.24 However, it is not known whether blocking the CD6-ALCAM pathway with itolizumab can modulate T-cell responses after allogeneic HCT in the setting of aGvHD.
In the present study, we characterized the expression of CD6 and ALCAM by reconstituting immune cells in a cohort of 95 patients who underwent allogeneic HCT and examined the effects of itolizumab on T-cell responses in vitro in the setting of aGvHD. Our results show that T cells and dendritic cells (DC) expressed CD6 and ALCAM, respectively early after HCT and surface expression of both structures is maintained during aGvHD. Then, we demonstrated the ability of itolizumab to inhibit in vitro T-cell proliferation and activation in peripheral blood (PB) obtained from patients with aGvHD, in an ALCAM-dependent manner without causing T-cell depletion. Our results provide new insights into the mechanisms of action of itolizumab and suggest that targeting the CD6-ALCAM co-stimulatory pathway represents a novel approach for prevention and treatment of aGvHD.
Methods
Patients and sample collection
This study included 95 patients who underwent allogeneic HCT at the Dana-Farber Cancer Institute and Brigham and Woman’s Hospital (Boston, MA) between September 2018 and January 2020. Blood samples were obtained at 1, 2, 3 and 6 months after transplant for analysis of CD6 and ALCAM expression. Samples from nine healthy donors (HD) were used as controls. Samples from nine additional patients and nine HD were used for in vitro functional assays (Online Supplementary Table S1). Written informed consent was obtained from all patients and HD prior to sample collection, in accordance with the Declaration of Helsinki. Protocol approval was obtained from the Human Subjects Protection Committee of the Dana-Farber/Har-vard Cancer Center.
Monitoring CD6 and ALCAM immune reconstitution
Immune reconstitution was evaluated by flow cytometry using fresh whole blood samples. For analysis, both percentages of positive cells and median fluorescence intensity (MFI) were considered. Two panels of directly conjugated monoclonal antibodies (Online Supplementary Table S2) were used to define the expression of CD6 and ALCAM on functionally distinct T-cell and APC subsets, respectively. After staining, cells were acquired on a Fortessa LSR flow cytometer (BD Biosciences) and analyzed using FlowJo and Cytobank software. Cell gating strategy and markers used for cell subset definition are described in the Online Supplementary Figure S1 and S2.
Functional activity of itolizumab in vitro
Frozen peripheral blood mononuclear cells (PBMC) obtained from patients who developed aGvHD after transplant (Online Supplementary Table S1) were stimulated using anti-CD3/CD2/CD28 beads in the presence of itolizumab or isotype control cetuximab. T-cell proliferation, activation and maturation were evaluated after 72 hours of culture using the flow cytometry panel in Online Supplementary Table S3. An example of the gating strategy used in this analysis is shown in the Online Supplementary Figure S3. Detailed description of the protocol is provided in the Online Supplementary Appendix.
Complement-dependent cytotoxicity, antibody direct cytotoxicity and antibody-dependent cellular cytotoxicity assays
The ability of itolizumab to induce complement-dependent cytotoxicity (CDC), antibody direct cytotoxicity (ADC) or antibody-dependant cytotoxicity (ADCC) was measured as the percentage of cell lysis in the presence of itolizumab compared to alemtuzumab as positive control or cetuximab as negative control. For ADCC evaluation we also measured the percent CD107a+ cells on NK cells after 6 hours of culture.25 Detailed descriptions of CDC, ADC and ADCC protocols are provided in the Online Supplementary Appendix.
T-cell stimulation using ALCAM-Fc and anti-CD3 antibody
Recombinant human ALCAM Fc chimera (ALCAM-Fc) and anti-CD3 antibody were resuspended in phospahte-buffered saline overnight at 4°C in flat bottom 96-well plate. The day after, HD CD3+ T cells were added and cultured in media with itolizumab or cetuximab for 96 hours. Cells were analyzed using flow cytometry (Online Supplementary Table S4). Detailed description of the protocol is provided in the Online Supplementary Appendix and Online Supplementary Figure S3.
Statistical analysis
Statistical methods are provided in the Online Supplementary Appendix.
Results
Patient characteristics
In order to investigate the expression of CD6 and its ligand ALCAM during immune reconstitution after allogeneic HCT, we prospectively studied a homogeneous population of 95 adult patients with hematological malignancies. Clinical characteristics of these patients are summarized in Table 1. Median patient age at the time of transplant was 61 years (range, 19-76 years). All patients received pretransplant conditioning with busulfan and fludarabine and
Table 1.
Patient characteristics.
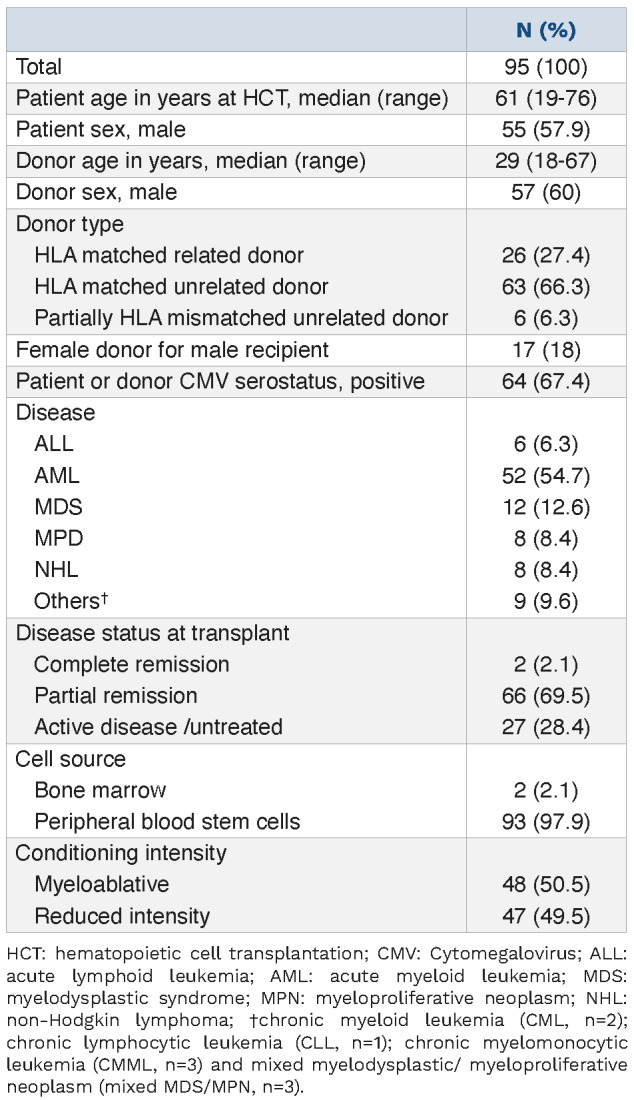
GvHD prophylaxis with tacrolimus and methotrexate. Half of the patients received a myeloablative conditioning regimen (50.5%) and almost all patients received PB stem cell (PBSC) grafts (97.9%). A matched unrelated donor (MUD) was used in 63 patients (66.3%). Median follow-up among survivors was 12 months (range, 4-20 month). Median donor T-cell chimerism was 76% 1 month after HCT and 96% at 6 months (Table 2). Acute GvHD occurred in 42 patients (44.2%) at a median of 50 days after transplant (range, 20-294 days).
CD6 is expressed on T cells early afer transplant but expression levels vary in different T-cell populations
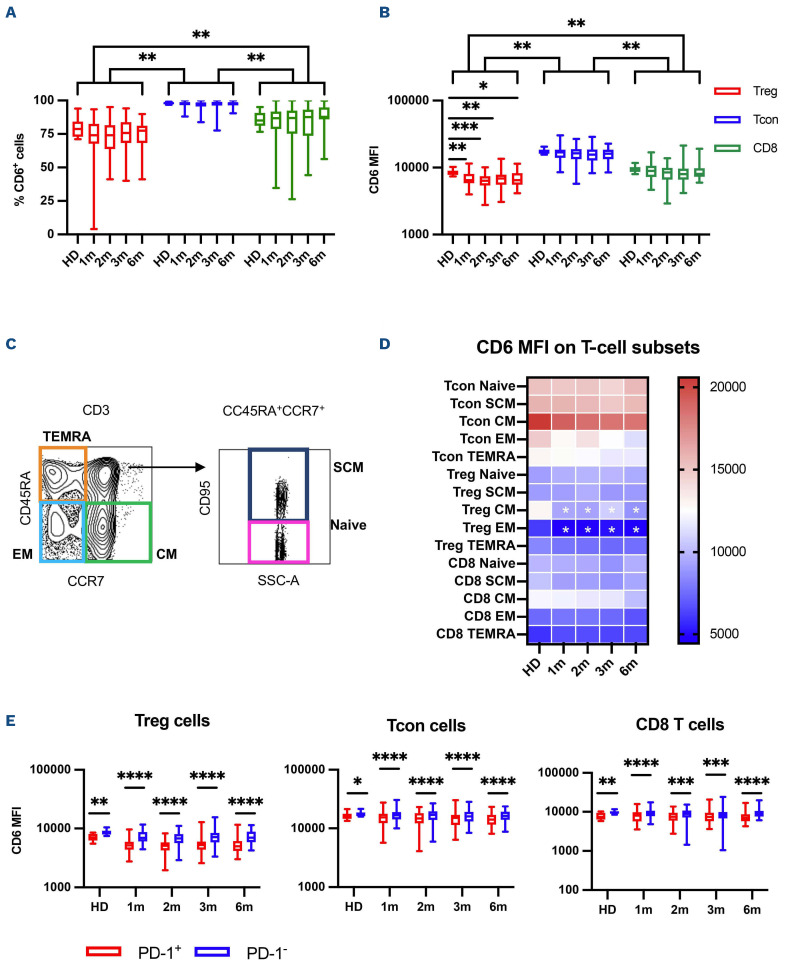
We first examined the percent of T cells expressing CD6 as well as the level of CD6 expression on different T-cell populations measured by MFI. While almost all T cells expressed CD6, expression level was highest in conventional CD4 T cells (Tcon) and lowest in CD4 regulatory T cells (Treg) while CD8 T cells displayed intermediate levels of CD6 expression (Figure 1A and B). CD6 expression was maintained at all time points after HCT and both percentage of CD6+ cells and CD6 MFI were comparable between HD and patients at the different time points analyzed. The only exception was a reduction of CD6 MFI in Treg cells after transplant compared to HD. We also divided T cells into five maturation stages based on the expression of CD45RA, CCR7 and CD95: naïve (CD45RA+, CCR7+, CD95-), stem cell memory (SCM, CD45RA+, CCR7+, CD95+), central memory (CM, CD45RA-, CCR7+), effector memory (EM, CD45RA-, CCR7-) and terminally differentiated effector memory (TEMRA, CD45RA+, CCR7-) (Figure 1C). As shown in the heat map in Figure 1D, Tcon subsets displayed the highest CD6 MFI. In both Tcon, Treg and CD8 T cells, CM T cells have the highest expression of CD6, while EM T cells express the lowest levels. CD6 expression was maintained after transplant at levels similar to HD, with the exception of lower CD6 MFI in CM and EM Treg in patients compared to HD. Finally, we examined whether CD6 was differentially expressed on PD-1 positive T cells, since PD-1 represents a marker of T-cell activation and exhaustion that is up-regulated after T-cell receptor (TCR) engagement.26 For all three T-cell populations, we observed that PD-1-positive T cells expressed significantly lower levels of CD6 compared to PD-1-negative T cells in both HD and patients after transplant (Figure 1E).
Table 2.
Engraftment, chimerism and acute graft-versus-host disease outcomes.
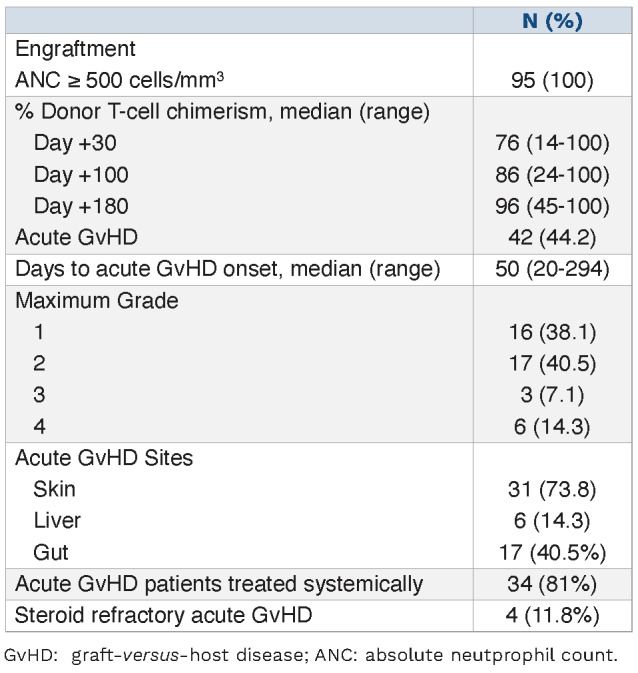
Figure 1.
CD6 expression on T cells afer hematopoietic cell transplantation. (A) Percentage of CD6-positive cells and (B) CD6 median fluorescence intensity (MFI) on regulatory CD4 T cells (Treg, red boxes), conventional CD4 T cells (Tcon, blue boxes) and CD8 T cells (CD8, green boxes) in healthy donors (HD) and in patients at 1, 2, 3 and 6 months after hematopoietic cell transplantation (HCT). Box plots indicate median, Q1 and Q3 and min and max. (C) Representative gating strategy used to define T-cell subsets, based on the expression of CD45RA, CCR7 and CD95 markers. (D) Heat map summarizes the median CD6 MFI values in the different T-cell subsets in HD and in patients at 1, 2, 3 and 6 months after HCT. White stars show statistically significant differences between HD and samples after transplant (any P<0.05). (E) CD6 MFI on Treg, Tcon and CD8 T cells based on the expression of PD-1 in HD and in patients at 1, 2, 3 and 6 months after HCT. Statistically significant differences are noted: ****P<0.0001; ***P< 0.001; **P<0.01; *P<0.05; Wilcoxon rank-sum test.
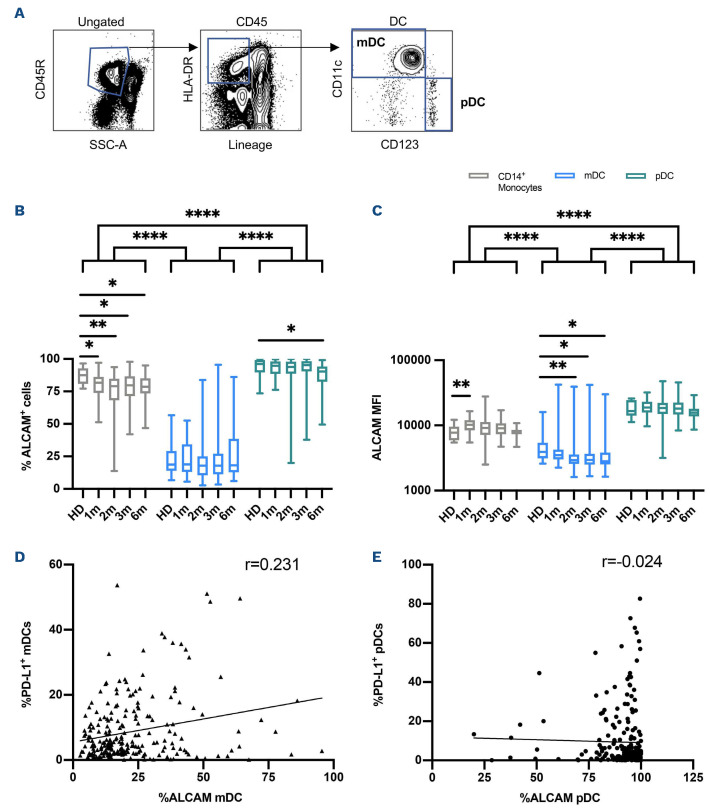
Figure 2.
ALCAM expression on monocytes and dendritic cells afer hematopoietic cell transplantation. (A) Representative gating strategy used to define dentritic cell (DC) subsets, based on the expression of CD11c and CD123 in flow cytometry. (B) Percentage of ALCAM-positive cells and (C) levels of ALCAM expression (MFI) on CD14+ monocytes (grey boxes), myeloid DC (mDC, light-blue boxes) and plasmacytoid DC (pDC, green boxes) in healthy donors (HD) and in patients at 1, 2, 3 and 6 months after hematopoietic cell transplantation (HCT). Correlation of PD-L1 and ALCAM expression on (D) mDC and (E) pDC. Statistically significant differences are noted: ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05; Wilcoxon rank-sum test.
ALCAM expression on monocytes and dendritic cells afer hematopoietic cell transplantation
In order to examine whether the CD6-ALCAM pathway represents a suitable target for aGvHD treatment, we also analyzed ALCAM expression on monocytes and DC. DC were divided into two major populations based on the expression of CD11c and CD123: myeloid DC (mDCs, CD11c+ and CD123-) and plasmacytoid DCs (pDs, CD11c- and CD123+, Figure 2A). In HD, almost all monocytes and pDC expressed ALCAM, but significantly fewer mDC expressed ALCAM. After transplant, significantly fewer monocytes and pDC expressed ALCAM compared to HD but the magnitude of this difference was relatively small (Figure 2B). ALCAM expression (MFI) was highest on pDC, relatively low on mDC and intermediate on monocytes (Figure 2C). ALCAM MFI on pDC after transplant was similar to HD controls whereas ALCAM MFI on monocytes was significantly elevated compared to HD in the first month after transplant. In contrast, ALCAM MFI was lower in mDC after transplant compared to HD. We also examined whether ALCAM expression on mDC and pDC was correlated with expression of PD-L1. As shown in Figure 2D and E, there was a weak positive correlation between ALCAM and PD-L1 expression on mDC, while no correlation was observed for pDC, where almost all cells uniformly express high levels of ALCAM.
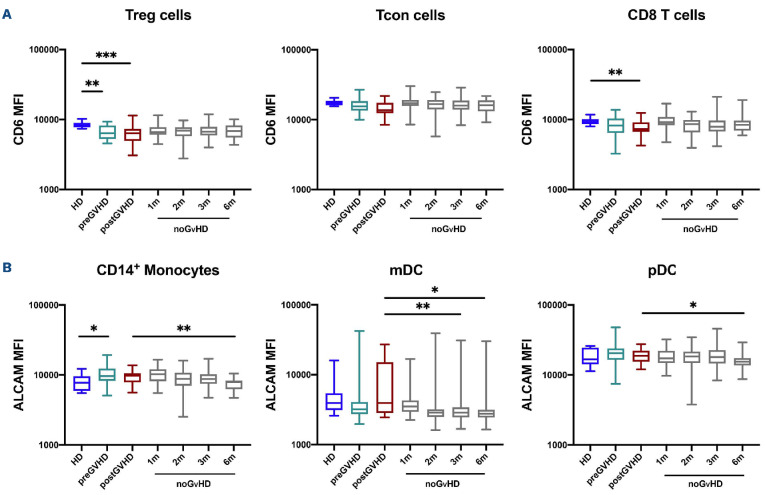
CD6 and ALCAM expression are maintained during acute graf-versus-host disease
In order to examine whether CD6 and ALCAM expression were affected by the development of aGvHD, we compared CD6 and ALCAM MFI in samples obtained before (preGvHD) and after aGvHD onset (postGvHD) with samples from HD and from patients that did not develop aGvHD (noGvHD). Based on the median time of aGvHD onset, preGvHD samples were compared to noGvHD samples collected at 1 and 2 months after transplant while postGvHD samples were compared with noGvHD samples collected at 3 and 6 months after transplant. As shown in Figure 3A, Tcon expressed high levels of CD6 in both pre- and postGvHD samples that were similar to HD and patients who did not develop aGvHD. CD6 expression by Treg and CD8 T cells in aGvHD samples was significantly decreased compared to HD but was similar to noGvHD samples. When compared to HD, ALCAM expression was increased on CD14 monocytes preGvHD but there were no differences in expression by mDC and pDC (Figure 3B). Despite small differences, ALCAM expression was generally maintained at similar levels in monocytes, mDC and pDC in patients with and without aGvHD. We only observed a slightly increased ALCAM MFI on mDC in postGvHD samples compared to noGvHD samples (Figure 3B).
We also compared samples from patients who developed aGvHD with patients who did not at specific time points after transplant. We observed a small decrease of CD6 MFI in Tcon and CD8 T cells in the GvHD cohort at 1 month, compared with the noGvHD group. For ALCAM we observed a higher ALCAM MFI and percentage of ALCAM-positive cells on mDC in the GvHD cohort compared to noGvHD at 3 months after transplant (Online Supplementary Figures S4 and S5). In order to address the impact of systemic aGvHD treatment (steroids) on CD6 and ALCAM expression, we compared samples from patients with low grade aGvHD who only received topical therapy with patients with higher grade aGvHD who received systemic steroids. With the limitation of a low number of samples, we did not observe significant differences for CD6 expression on T cells and no clear trend was observed for ALCAM expression on monocytes and DC (data not shown).
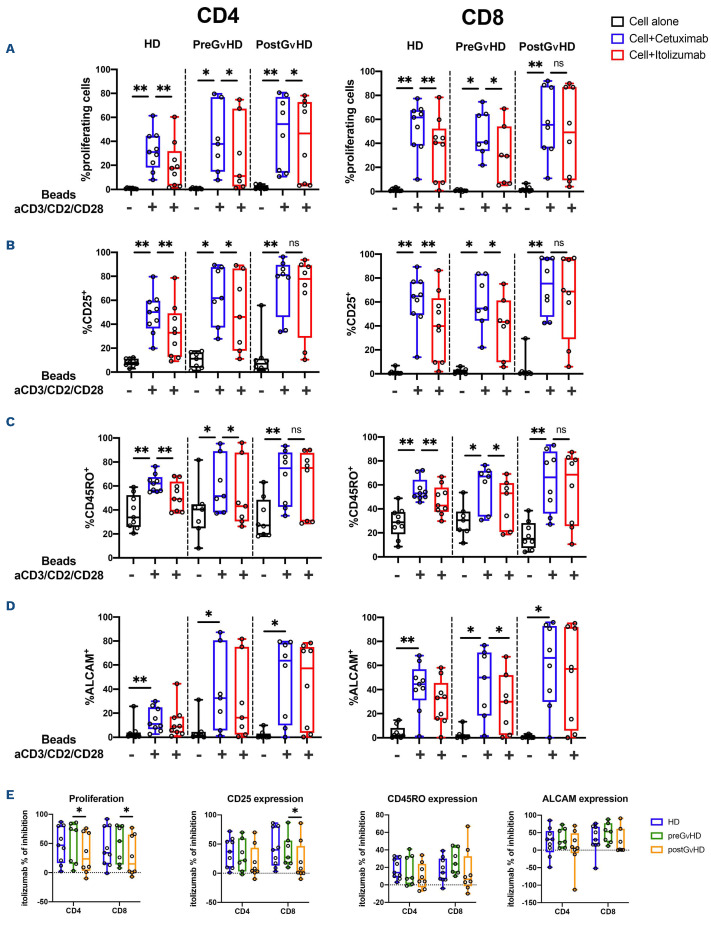
Itolizumab inhibits in vitro T-cell proliferation, activation and maturation afer T-cell receptor engagement
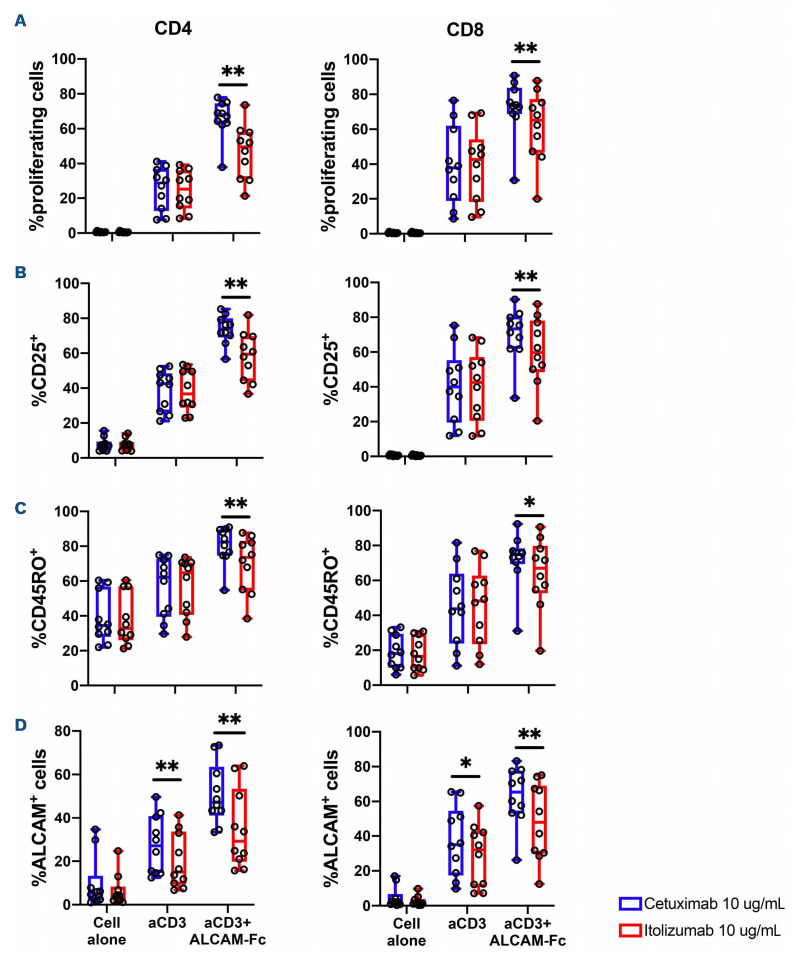
In order to test the ability of itolizumab to modulate T-cell responses after TCR stimulation, cryopreserved PBMC obtained from nine patients before (preGvHD, median time between GvHD and sample collection -24; range, -32 to -7 days) and after GvHD onset (postGvHD, median time between GvHD and sample collection 20; range, 0-64 days) were stimulated with antiCD3/CD2/CD28 in the presence of itolizumab or isotype control antibody (cetuximab). Clinical characteristics of these patients are summarized in the Online Supplementary Table S1. Of note, postGvHD samples were collected from patients who were receiving aGvHD treatment (steroids) and one patient was also receiving ruxolitinib at the time of sample collection. Only one sample was collected at aGvHD onset prior to starting treatment for aGvHD. T-cell proliferation, activation and maturation were evaluated 72 hours after stimulation using flow cytometry. Prior to GvHD onset, itolizumab inhibited CD4 and CD8 T-cell proliferation in a similar fashion to HD control. This effect was less prominent in CD8 T cells collected after GvHD onset (Figure 4A). Similar results were observed for CD25 expression, as a marker of T-cell activation and CD45RO expression, as a marker of T-cell maturation into memory subsets (Figure 4B and C). Itolizumab inhibited these other measures of T-cell activation in both CD4 and CD8 T cells from HD and from patients prior to aGvHD. However, increased expression of CD25 and CD45RO on T cells in postGvHD samples were no longer inhibited by itolizumab. After TCR stimulation, we also observed increased ALCAM expression on both CD4 and CD8 T cells from HD and transplant patients. However, except for CD8 T cells collected preGvHD, no statistically significant inhibition was observed using itolizumab (Figure 4D).
Figure 3.
Expression of CD6 and ALCAM in patients afer hematopoietic cell transplantation with and without acute graf-versus-host disease. (A) Levels of CD6 expression (median fluorescence intensity [MFI]) in Treg, Tcon and CD8 T cells. (B) Levels of ALCAM expression (MFI) in CD4 regulatory T cells (Treg), conventional CD4 T cells (Tcon) and CD8 T cells. Before graft-versus-host disease (preGvHD) samples (green boxes) and after GvHD (postGvHD) samples (red boxes) from patients who developed acute GvHD are compared with noGvHD samples obtained at different times after transplant (grey boxes) and healthy donors (HD) (blue boxes). Statistically significant differences are noted: ***P<0.001; **P<0.01; *P<0.05; Wilcoxon rank-sum test. HD n=9, preGVHD n=30, postGVHD n=20, noGVHD 1 month (1m) n=38, 2 months (2m) n=40, 3 months (3m) n=43 and 6 months (6m) n=21.
Comparisons of inhibition achieved by itolizumab for each of these assays in CD4 and CD8 T cells from HD and patients after transplant are shown in Figure 4E. In all assays, the level of inhibition induced by itolizumab in preGvHD T cells was similar to that achieved in HD. Itolizumab induced less inhibition of proliferation in both CD4 and CD8 T cells collected after GvHD onset. Less inhibition was also noted for CD25 expression in CD8 T cells postGvHD but there were no differences in the ability of itolizumab to inhibit CD45RO and ALCAM expression in both CD4 and CD8 T cells in any groups.
Itolizumab does not induce complement-dependent cytotoxicity, antibody-dependent cytotoxicity or antibody direct cellular cytotoxicity
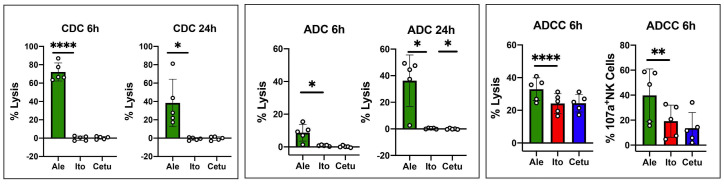
In order to test the ability of itolizumab to induce direct or indirect killing of target cells we evaluated the effects of itolizumab in CDC, ADC and ADCC assays. Cell lysis in the presence of itolizumab was compared to alemtuzumab as positive control or cetuximab as negative control. The gating strategy used to measure target cell lysis is shown in the Online Supplementary Figure S6. Itolizumab did not mediate CDC or ADC at 6 or 24 hours. After 6 hours of incubation, itolizumab did not induce ADCC measured either by direct lysis of target cells or degranulation of CD107a on NK cells (Figure 5).
Itolizumab activity is dependent on the presence of ALCAM
In order to test whether the inhibitory activity of itolizumab was dependent on the presence of ALCAM, T cells from HD were stimulated with anti-CD3 antibody with or without ALCAM-Fc for 96 hours. Itolizumab or isotype control cetuximab were added at the start of each culture. As shown in Figure 6A, the addition of cetuximab or itolizumab did not induce proliferation of CD4 or CD8 T cells alone, and anti-CD3 induced proliferation of CD4 or CD8 T cells was not inhibited by the addition of either antibody. The addition of ALCAM-Fc to anti-CD3 further enhanced CD4 and CD8 T-cell proliferation. The addition of itolizumab significantly impaired this increased proliferation when compared to the addition of cetuximab. When T cells were monitored for activation (expression of CD25, Figure 6B) and maturation (expression of CD45RO, Figure 6C), itolizumab had similar effects on both CD4 and CD8 T cells and inhibitory activity was only detected in the presence of both anti-CD3 and ALCAM-Fc. As shown Figure 6D, activation of CD4 and CD8 T cells with anti-CD3 also induces expression of ALCAM and this is further enhanced in the presence of ALCAM-Fc. In this setting, the presence of itolizumab inhibits increased expression of ALCAM even if ALCAM-Fc is not present.
Figure 4.
Inhibition of T-cell proliferation, activation and differentiation by itolizumab. Cryopreserved peripheral blood mononuclear cells (PBMC) obtained from healthy donors (HD) and patients before or after acute graft-versus-host disease (aGvHD) onset were stimulated with anti-CD3/CD2/CD28 beads in the presence of itolizumab or isotype control (cetuximab). (A) Proliferation of CD4 and CD8 T cells measured by CFSE dye dilution; (B) Activation of CD4 and CD8 T cells measured by expression of CD25; (C) maturation of CD4 and CD8 T cells measured by expression of CD45RO; (D) ALCAM expression is absent in resting T cells and is an additional marker of T-cell activation. (E) Percent inhibition induced by itolizumab, comparing activity against HD, pre- and postGvHD samples in CD4 and CD8 T cells. Percentage inhibition was calculated using the following formula: (% cells in isotype control - % cells in itolizumab)/% cells in isotype control. If no difference was observed between isotype control and itolizumab the percentage of itolizumab inhibition equals 0%. Statistically significant differences are noted: **P<0.01; *P<0.05; Wilcoxon rank-sum test. HD n=9, preGvHD n=7, postGvHD n=8.
Discussion
Acute GvHD remains a frequent cause of non-relapse mortality and contributes to poor quality of life in patients who have undergone allogeneic HCT. Corticosteroids are administered as first line therapy, but steroid treatment fails to achieve complete responses in up to 50% of cases and is also associated with short and long term toxicities.5 Ideally, new treatment approaches for GvHD should be aimed at specific modulation of donor T-cell alloreactivity and induction of tolerance rather than nonspecific T-cell depletion or broad inhibition of T-cell functions which can increase risk of tumor immune escape as well as opportunistic infections.27 In this study we demonstrate that blocking the CD6-ALCAM co-stimulatory pathway inhibits T-cell activation and proliferation thus representing a new potential target for GvHD prevention and treatment. Indeed, CD6 is highly expressed on Tcon after HCT, and its expression is maintained at the onset of aGvHD. Moreover, expression of ALCAM, the primary ligand for CD6, is increased in DC and monocytes in patients who develop aGvHD. In contrast, Treg express lower levels of CD6,28–30 and targeting CD6 with itolizumab could preferentially affect Tcon while sparing Treg, thus promoting a more tolerogenic state. CD6 expression also varies as T cells undergo maturation.31 Naïve and central memory T cells express high levels of CD6 compared to more mature effector memory T cells. Previous studies have suggested that GvHD is primarily mediated by naïve T cells and these cells may also be preferentially affected by itolizumab.32– 39 Similarly, we observed that PD-1 positive T cells, that are already exposed to TCR activation,26 express lower levels of CD6 compared to PD-1 negative cells. Therefore, targeting the CD6-ALCAM pathway could potentially spare tolerogenic T-cell subsets (Treg) and more mature (EM) pathogen specific T cells, leading to a more favorable balance between GvHD control and maintenances of graftversus-leukemia (GvL) and anti-infection capabilities.
Figure 5.
Testing itolizumab for complement-dependent cytotoxicity, antibody-dependent cytotoxicity and antibody direct cellular cytotoxicity. For complement-dependent cytotoxicity (CDC) and antibody-dependent cytotoxicity (ADC), CD3+ T cells were isolated from cryopreserved peripheral blood mononuclear cells (PBMC) from healthy donors (HD) and cultured in the presence of medium + antibody + 25% of human serum (HS) and medium + antibody, respectively. Percentage cell lysis was calculated by combining the percentage of positive cells for 7-AAD or Annexin V or both. CDC activity was calculated by subtracting the values obtained in medium + antibody from the values obtained in the culture with medium + antibody + HS. ADC activity was calculated by subtracting the values obtained in the culture with medium alone from the values obtained in the culture with medium + antibody. CDC and ADC were assessed after 6 and 24 hours of culture. For antibody direct cellular cytotoxicity (ADCC) PBMC from HD were cultured in the presence of antibody for 6 hours. Both percentage cell lysis and CD107a expression on NK cells were evaluated after 6 hours of culture. The effects of itolizumab (red boxes) were compared to alemtuzumab (green boxes - positive control) and cetuximab (blue boxes - negative control). Values are expressed as mean and standard deviation (SD), paired t-test, 2 tails was used. Statistically significant differences are noted: ****P<0.0001; ***P<0.001; **P<0.01; *P<0.05; paired t-test. HD n=5.
Figure 6.
Itolizumab activity is dependent on the presence of ALCAM. T cells from healthy donors (HD) were stimulated with anti-CD3 antibody with or without recombinant human ALCAM Fc chimera (ALCAM-Fc) for 96 hours. Itolizumab or isotype control cetuximab were added at the start of each culture. (A) Proliferation of CD4 and CD8 T cells; (B) expression of CD25; (C) expression of CD45RO; (D) expression of ALCAM after stimulation with anti-CD3 antibody alone or in combination with ALCAM-Fc, in the presence of cetuximab (blue boxes) or itolizumab (red boxes). Statistically significant differences are noted: **P<0.01; *P<0.05; Wilcoxon rank-sum test. HD n=10.
This hypothesis is supported by previous clinical studies using T12, the first anti-CD6 monoclonal antibody used in the transplant setting. T12, an IgM antibody, induced profound ex vivo depletion of CD6+ T cells when donor grafts were manipulated ex vivo in the presence of complement.18 Patients in these trials received myeloablative conditioning and did not receive any prophylactic immune suppressive medications. CD3+CD6- T cells recovered rapidly after transplantation with CD6-depleted allogeneic stem cell products with very low incidence of acute or chronic GvHD.19 Functional immune recovery was not delayed and there was no increase in viral reactivation after transplant. There was also a very low incidence of graft failure and GvL activity appeared to be preserved.20,21,40 Taken together, these previous clinical trials suggested that in vivo blockade of CD6 function might also be an effective approach for selective modulation of alloreactive T cells without affecting other critical T-cell functions.
As expected, in our cohort of HCT patients, we observed mixed donor-recipient T-cell chimerism in the early post-transplant period with a median donor T-cell chimerism of 76% at 1 month. We are not able to distinguish CD6 expression on individual donor versus recipient T cells, but we found that CD6 expression on T cells in patients with >95% T-cell donor chimerism was similar to levels of expression in patients with <95% T-cell donor chimerism. Similarly, no difference was observed for ALCAM expression on monocytes, mDC and pDC (Online Supplementary Table S5). This suggests that CD6 and ALCAM are equally expressed on recipient and donor cells after transplant.
Our studies also found that ALCAM expression was increased on both DC and monocytes in patients with aGVHD compared to patients without aGVHD. In myeloid DC, ALCAM expression was positively correlated with PD-L1 expression. ALCAM is expressed at high levels on all pDC, and this did not change when aGVHD developed. These observations support the concept that ALCAM is upregulated when DC are activated.41 ALCAM expression on DC is important for the promotion and maintenance of the immunological synapse and this is one of the mechanisms whereby ALCAM promotes T-cell activation.13 Since immune reconstitution varies depending on the transplant setting, the expression of CD6 and ALCAM should also be explored in patients with haploidentical donors and with different GvHD prophylaxis regimens such as ATG or post-transplant cyclophosphamide.
Itolizumab, an IgG1 anti-CD6 monoclonal antibody has been evaluated in different autoimmune disorders,9 including psoriasis,23 rheumatoid arthritis,42 Sjogren syndrome43 and in COVID-19 infection.24,44 Using PBMC from patients with aGvHD, itolizumab inhibited T-cell proliferation, activation, and maturation of both CD4 and CD8 T cells. This was most evident with T cells obtained just prior to development of aGvHD and less inhibition was observed in postGvHD T cells obtained from patients receiving immune suppressive medications. These findings suggest that itolizumab may be more effective in early stages of GvHD in combination with steroids or as GvHD prophylaxis. Even though these ex vivo experiments lack GvHD target tissues and do not perfectly recapitulate aGvHD in vivo, using whole PBMC, we were able to mimic the alloreactive activation of T cells in the presence of ALCAM expressed on circulating APC (monocytes and B cells) and demonstrate the inhibitory activity of itolizu-mab.
Infusion of itolizumab in patients with autoimmune diseases has resulted in only transient reduction of CD6+ circulating T cells, suggesting that this antibody does not induce profound T-cell depletion.45 This is consistent with our results demonstrating that itolizumab does not directly eliminate T cells in vitro in CDC, ADC or ADCC assays. Thus, the mechanism by which itolizumab inhibits T-cell function in vivo is likely through its ability to prevent the interaction between CD6 on T cells and ALCAM on APC.22 This is consistent with our in vitro results showing that the addition of itolizumab to activated CD3 T cells in the absence of ALCAM did not inhibit T-cell proliferation, activation, or maturation. However, in the presence of ALCAM, itolizumab efficiently abrogates the co-stimulatory effects of this interaction and is able to suppress T-cell proliferation, activation, and maturation. In contrast to other pan-T-cell antibodies that either activate T cells (anti-CD3) or deplete T cells (ATG, anti-CD52), itolizumab only inhibits T-cell activation in the presence of ALCAM and has no direct or indirect depleting functions. While ruxolitinib, a JAK1/2 inhibitor, is currently the only drug approved for the treatment of steroid refractory aGvHD,46 several drugs that target different T-cell costimulatory molecules are currently being tested in preclinical models and in patients with aGvHD.4,27 In this setting, itolizumab may be a promising agent for either prevention or treatment of aGvHD, due to higher expression of CD6 on naïve and central memory Tcon cells, while sparing more tolerogenic Treg and effector memory T cells, important for control of both tumor cells and opportunistic infections after transplant.37 Moreover, itolizumab may not inhibit responses to viral infections or reactivation. Indeed, different NK- and T-cell populations are involved in responses to Cytomegalovirus infection (NKG2C+CD8+ T cells) and these cells have low expression of CD6.47
Recently, Ruth and colleagues demonstrated that targeting CD6 may also be a novel approach to enhance cancer immunotherapy.48 This study utilized UMCD6, an anti-CD6 monoclonal antibody that binds the same CD6 domain (Domain 1) as itolizumab. UMCD6 upregulated the expression of the activating receptor NKG2D and downregulated expression of the inhibitory receptor NKG2A on both NK cells and CD8 T cells, with concurrent increased expression of perforin and granzyme-B.48 The combined capabilities of an anti-CD6 monoclonal antibody to control autoimmunity through effects on CD4 T cells and the enhanced killing of cancer cells through distinct effects on CD8 T cells and NK cells, may represent a new approach to induce tolerance without suppressing GvL activity after HCT.
In conclusion, our studies demonstrate that functional inhibition of the CD6-ALCAM co-stimulatory pathway may be a novel therapeutic strategy for prevention or treatment of aGvHD. On the basis of these biological properties, a phase I/II study using itolizumab as first line treatment in combination with steroids for patients with aGvHD is currently ongoing (clinicaltrials gov. Identifier: NCT03763318). Future clinical studies may also test the feasibility and clinical efficacy of itolizumab for prevention of GvHD.
Supplementary Material
Acknowledgments
We thank the staf of the Pasquarello Tissue Bank in Hematologic Malignancies for processing all of the clinical samples analyzed in this study.
Funding Statement
Funding: This work was supported by Equillium Inc and NIH grant PO1CA229092 .
References
- 1.Zeiser R, Blazar BR. Acute Graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med. 2017;377(22):2167-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: Comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387-394. [DOI] [PubMed] [Google Scholar]
- 3.Levine JE, Braun TM, Harris AC, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2015;2(1):e21-e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watkins B, Qayed M, McCracken C, et al. Phase II trial of costimulation blockade with abatacept for prevention of acute GVHD. J Clin Oncol. 2021;39(17):1865-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toubai T, Magenau J. Immunopathology and biology-based treatment of steroid-refractory graft-versus-host disease. Blood. 2020;136(4):429-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martínez VG, Moestrup SK, Holmskov U, Mollenhauer J, Lozano F. The conserved scavenger receptor cysteine-rich superfamily in therapy and diagnosis. Pharmacol Rev. 2011;63(4):967-1000. [DOI] [PubMed] [Google Scholar]
- 7.Aruffo A, Melnick MB, Linsley PS, Seed B. The lymphocyte glycoprotein CD6 contains a repeated domain structure characteristic of a new family of cell surface and secreted proteins. J Exp Med. 1991;174(4):949-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun M, Müller B, ter Meer D, et al. The CD6 scavenger receptor is differentially expressed on a CD56dim natural killer cell subpopulation and contributes to natural killer-derived cytokine and chemokine secretion. J Innate Immun. 2011;3(4):420-434. [DOI] [PubMed] [Google Scholar]
- 9.Consuegra-Fernández M, Lin F, Fox DA, Lozano F. Clinical and experimental evidence for targeting CD6 in immune-based disorders. Autoimmun Rev. 2018;17(5):493-503. [DOI] [PubMed] [Google Scholar]
- 10.Bowen MA, Patel DD, Li X, et al. Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med. 1995;181(6):2213-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappell PE, Garner LI, Yan J, et al. Structures of CD6 and its ligand CD166 give insight into their interaction. Structure. 2015;23(8):1426-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cayrol R, Wosik K, Berard JL, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9(2):137-145. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman AW, Joosten B, Torensma R, Parnes JR, van Leeuwen FN, Figdor CG. Long-term engagement of CD6 and ALCAM is essential for T-cell proliferation induced by dendritic cells. Blood. 2006;107(8):3212-3220. [DOI] [PubMed] [Google Scholar]
- 14.Hassan NJ, Barclay AN, Brown MH. Frontline: optimal T cell activation requires the engagement of CD6 and CD166. Eur J Immunol. 2004;34(4):930-940. [DOI] [PubMed] [Google Scholar]
- 15.Hassan NJ, Simmonds SJ, Clarkson NG, et al. CD6 regulates T-cell responses through activation-dependent recruitment of the positive regulator SLP-76. Mol Cell Biol. 2006;26(17):6727-6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nair P, Melarkode R, Rajkumar D, Montero E. CD6 synergistic co-stimulation promoting proinflammatory response is modulated without interfering with the activated leucocyte cell adhesion molecule interaction. Clin Exp Immunol. 2010;162(1):116-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira MI, Gonçalves CM, Pinto M, et al. CD6 attenuates early and late signaling events, setting thresholds for T-cell activation. Eur J Immunol. 2012;42(1):195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohatiner A, Gelber R, Schlossman SF, Ritz J. Depletion of T cells from human bone marrow using monoclonal antibodies and rabbit complement. A quantitative and functional analysis. Transplantation. 1986;42(1):73-80. [DOI] [PubMed] [Google Scholar]
- 19.Soiffer RJ, Bosserman L, Murray C, Cochran K, Daley J, Ritz J. Reconstitution of T-cell function after CD6-depleted allogeneic bone marrow transplantation. Blood. 1990;75(10):2076-2084. [PubMed] [Google Scholar]
- 20.Soiffer RJ, Murray C, Mauch P, et al. Prevention of graft-versus-host disease by selective depletion of CD6-positive T lymphocytes from donor bone marrow. J Clin Oncol. 1992;10(7):1191-1200. [DOI] [PubMed] [Google Scholar]
- 21.Soiffer RJ, Weller E, Alyea EP, et al. CD6+ donor marrow T-cell depletion as the sole form of graft-versus-host disease prophylaxis in patients undergoing allogeneic bone marrow transplant from unrelated donors. J Clin Oncol. 2001;19(4):1152-1159. [DOI] [PubMed] [Google Scholar]
- 22.Bughani U, Saha A, Kuriakose A, et al. T cell activation and differentiation is modulated by a CD6 domain 1 antibody Itolizumab. PLoS One. 2017;12(7):e0180088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krupashankar DS, Dogra S, Kura M, et al. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study. J Am Acad Dermatol. 2014;71(3):484-492. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, De Souza R, Nadkar M, et al. A two-arm, randomized, controlled, multi-centric, open-label phase-2 study to evaluate the efficacy and safety of Itolizumab in moderate to severe ARDS patients due to COVID-19. Expert Opin Biol Ther. 2021;21(5):675-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bologna L, Gotti E, Manganini M, et al. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol. 2011;186(6):3762-3769. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura H, Agata Y, Kawasaki A, et al. Developmentally regulated expression of the PD-1 protein on the surface of double-negative(CD4–CD8–) thymocytes. Int Immunol. 1996;8(5):773-780. [DOI] [PubMed] [Google Scholar]
- 27.Hill GR, Koyama M. Cytokines and costimulation in acute graft-versus-host disease. Blood. 2020;136(4):418-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierini A, Ruggeri L, Carotti A, et al. Haploidentical age-adapted myeloablative transplant and regulatory and effector T cells for acute myeloid leukemia. Blood Adv. 2021;5(5):1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia Santana CA, Tung JW, Gulnik S. Human treg cells are characterized by low/negative CD6 expression. Cytom A. 2014;85(10):901-908. [DOI] [PubMed] [Google Scholar]
- 31.Carrasco E, Escoda-Ferran C, Climent N, et al. Human CD6 down-modulation following T-cell activation compromises lymphocyte survival and proliferative responses. Front Immunol. 2017;8:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112(1):101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Joe G, Zhu J, et al. Dendritic cell–activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103(10):3970-3978. [DOI] [PubMed] [Google Scholar]
- 34.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L– memory T cells without graft-versus-host disease. Blood. 2004;103(4):1534-1541. [DOI] [PubMed] [Google Scholar]
- 35.Dutt S, Tseng D, Ermann J, et al. Naive and memory T cells induce different types of graft-versus-host disease. J Immunol. 2007;179(10):6547-6554. [DOI] [PubMed] [Google Scholar]
- 36.Chen BJ, Deoliveira D, Cui X, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2006;109(7):3115-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H, Matte-Martone C, Li H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111(4):2476-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng H, Matte-Martone C, Jain D, McNiff J, Shlomchik WD. Central memory CD8+ T cells induce graft-versus-host disease and mediate graft-versus-leukemia. J Immunol. 2009;182(10):5938-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bleakley M, Heimfeld S, Loeb KR, et al. Outcomes of acute leukemia patients transplanted with naive T cell–depleted stem cell grafts. J Clin Invest. 2015;125(7):2677-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soiffer RJ, Fairclough D, Robertson M, et al. CD6-depleted allogeneic bone marrow transplantation for acute leukemia in first complete remission. Blood. 1997;89(8):3039-3047. [PubMed] [Google Scholar]
- 41.Figdor CG. Molecular characterization of dendritic cells operating at the interface of innate of acquired immunity. Pathol Biol. 2003;51(2):61-63. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez PC, Torres-Moya R, Reyes G, et al. A clinical exploratory study with itolizumab, an anti-CD6 monoclonal antibody, in patients with rheumatoid arthritis. Results Immunol. 2012;2:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Dantec C, Alonso R, Fali T, et al. Rationale for treating primary Sjögren’s syndrome patients with an anti-CD6 monoclonal antibody (Itolizumab). Immunol Res. 2013;56(2):341-347. [DOI] [PubMed] [Google Scholar]
- 44.Loganathan S, Athalye SN, Joshi SR. Itolizumab, an anti-CD6 monoclonal antibody, as a potential treatment for COVID-19 complications. Expert Opin Biol Ther. 2020;20(9):1025-1031. [DOI] [PubMed] [Google Scholar]
- 45.Aira LE, López-Requena A, Fuentes D, et al. Immunological and histological evaluation of clinical samples from psoriasis patients treated with anti-CD6 itolizumab. MAbs. 2014;6(3):782-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800-1810. [DOI] [PubMed] [Google Scholar]
- 47.Sottile R, Panjwani MK, Lau CM, et al. Human cytomegalovirus expands a CD8+ T cell population with loss of BCL11B expression and gain of NK cell identity. Sci Immunol. 2021;6(63):eabe6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruth JH, Gurrea-Rubio M, Athukorala KS, et al. CD6 is a target for cancer immunotherapy. JCI Insight. 2021;6(5):e145662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.