Abstract
Objectives
Heartburn and constipation are common gastrointestinal symptoms during pregnancy. High fiber and liquid intake have beneficial effects on these symptoms in non- pregnant population. Our aim was to evaluate the association of dietary fiber, fluid intake and lifestyle characteristics with constipation, heartburn, and pregnancy outcome.
Study design
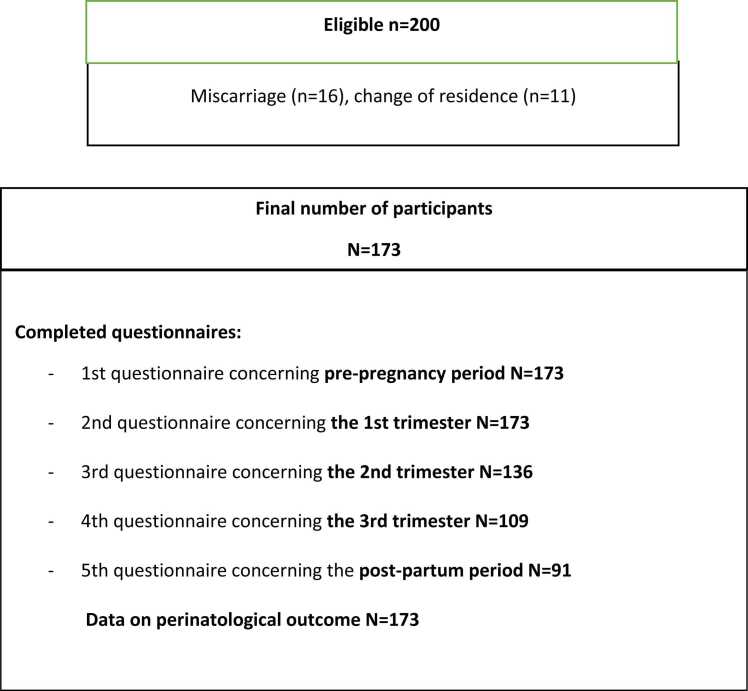
Two hundred pregnant women were enrolled in this prospective cohort study during the 1st trimester of pregnancy. Participants completed a self- administered questionnaire concerning bowel symptoms, dietary fiber, liquid intake, and lifestyle characteristics before pregnancy, during pregnancy and post-partum. After exclusions 173 pregnant women participated the study and 173, 173, 136, 109 and 91 completed pre-pregnancy, and 1st, 2nd, and 3rd trimester, and post-partum questionnaires, respectively. Data on deliveries and perinatal outcome (n = 173) were collected from hospital records. In trajectory analysis, the women were clustered in groups based on the intake of fiber and liquids. Generalized linear mixed models and logistic regression analyses were used to find associations of fiber and fluid intake with constipation, heartburn and pregnancy outcome.
Results and conclusions
Heartburn increased significantly during pregnancy and the highest prevalence (33%) was during the third trimester. A combination of low fiber and low fluid intake increased the risk of constipation during pregnancy (OR 5.9, 95% CI 2.00–17.4). Low fiber intake increased the risk of combined adverse outcome (cesarean section, premature delivery and/or small for gestational age; OR 3.4, 95% CI 1.2–9.6). Sufficient fiber and liquid intake may be protective against pregnancy-associated constipation and may be associated with improved pregnancy outcome.
Abbreviations: CS, Cesarean Section; BMI, Body Mass Index; SGA, Small for Gestational Age; LGA, Large for Gestational Age; AGA, Appropriate for Gestational Age
Keywords: Heartburn and constipation in pregnancy, Dietary fiber, Pregnancy outcome
Highlights
-
•
Participants completed a self- administered questionnaire.
-
•
Women with low fiber and fluid intake had a higher risk of constipation.
-
•
Women with low fiber intake had a higher risk of combined adverse pregnancy outcome.
-
•
Sufficient fiber and liquid intake may be protective against constipation.
-
•
Sufficient fiber and liquid intake may improve pregnancy outcome.
1. Introduction
Heartburn and constipation are common symptoms in pregnancy. The incidence of heartburn has been reported to vary between 17% and 80% [1], [2] and the prevalence has been found to steadily increase from the first trimester throughout the pregnancy [3]. Although serious complications are rare, symptoms may be frequent, severe, and distressing [1], [2]. Pregnancy-related risk factors of heartburn have been found to be multiparity, pre-existing heartburn, and advanced gestational age [1], [4]. Typically, symptoms of constipation are most prevalent in the first and second trimesters, and they decrease in the third [4]. The prevalence rate has been reported to vary between 11% and 40% [5]. Risk factors include multiparity, lack of exercise, low fiber intake, inadequate fluid intake, iron supplementation and previous cesarean section (CS) [5], [6], [7], [8]. Women who consume more fruit and vegetables and who take regular exercise may be protected from constipation [9]. The formation of a hard stool may be caused by aldosterone-mediated increased colonic water absorption [4], [8]. Medications taken during pregnancy, such as iron salts and magnesium sulfate, have been linked to constipation.
Dietary fiber has various favorable functions in the human digestive tract. Insoluble fiber adds bulk to the stool and speeds the passage of foods through the digestive system, alleviating constipation [10], [11]. In the nonpregnant population, intake of two liters of water daily will enhance the positive effects of dietary fiber [12], while epidemiological evidence indicates an association between lower fluid intake and constipation [13]. Bile acids can provoke heartburn. Soluble fiber and bile have great affinity toward one another. Fiber cannot cross the intestinal barrier and all the bile that has been bound together with soluble fiber will exit the body. This means that bile will not be part of the enterohepatic cycle and thus heartburn might be relieved.
Because of various beneficial effects of fiber and liquids on the digestive system, we hypothesized that a high fiber intake might alleviate the common symptoms of heartburn and constipation in pregnancy. The aim of the present study was to investigate the association of dietary fiber consumption with heartburn and constipation in pregnancy.
There are only a few studies concerning dietary characteristics and pregnancy outcome. Our secondary aim was to see whether high intakes of dietary fiber and fluid are associated with improved pregnancy outcome. In earlier studies, infants born to women with heartburn have had significantly higher birthweights [14]. So far, there have been no substantial studies on the association between dietary fiber and liquid intake, and pregnancy outcome.
2. Materials and methods
Consecutive healthy pregnant women were asked to participate in this study at the first visit to the maternal outpatient ward, at a gestational age of eight weeks. Only singleton pregnancies were accepted. Further exclusion criteria included a history of any bowel disease. Completion of food diaries was requested. After exclusions, 200 women were eligible. Of these, 27 (13.5%) were dropped because of miscarriage or moving to another district, and finally 173, 173, 136, 109 and 91 women completed the questionnaires at pre-pregnancy, and 1st, 2nd, and 3rd trimesters, and post-partum stages, respectively (Fig. 1). Data on deliveries and perinatal outcome were collected from hospital records. A combined adverse pregnancy outcome was defined as a combination of CS, preterm delivery (< 37 weeks), and/or being born small for gestational age (SGA).
Fig. 1.
Participant flow.
The first questionnaire included the pre-pregnancy period. The second, third and fourth questionnaires covered the 1st, 2nd and 3rd trimesters, respectively. The fifth questionnaire concerned the post-partum period till two months after delivery. The questionnaires contained items concerning demographic data, obstetric and medical history, and gastrointestinal symptoms (nausea and vomiting, constipation, heartburn, diarrhea). To estimate fiber intake, the questionnaires included a section where the participants were asked to report their typical daily frequency of consumption of ten different kinds of fiber-containing food items of a given portion size (Table 1). The questions referred to the usual consumption of these food items during the month before the current pregnancy, during pregnancy and post-partum.
Table 1.
Assessment table. Daily intake of cereal-derived and fruit/vegetable-derived fiber. In the questionnaire the patients estimated their typical daily consumption of ten different kinds of fiber-containing food items.
| Fiber-containing food items | Portion | Number of portions per day | Fiber coefficient for each food item |
|---|---|---|---|
| Cereal-derived fiber | |||
| Rye bread/crisp bread/multigrain bread with grains | 1 slice/piece | x 3 | |
| Other multigrain bread/oat bread/barley bread/graham bread/rolls | 1 slice/piece | x 2 | |
| French bread, baguette or other white bread | 1 slice/piece | x 1 | |
| Breakfast cereals, what? …………………………………. | 1 dL | x 6 (All-Bran PLUS) x 3 (All-Bran REG) x 0.5 (regular) |
|
| Muesli or piece of Weetabix | 1 dL/piece | x 0.5 | |
| Flake porridge | 1 dL | x 4 | |
| Brans, | 1 tablespoon | x 1 | |
| Fruit/vegetable-derived fiber | |||
| Plums or raisins | 1 plum/ 1 tablespoon of raisins |
x 0.5 | |
| Fruit or berries | 1 piece of fruit/ 1 dL of berries | x 2 | |
| Fresh or cooked vegetables | 1 dL | x 2 |
In the questionnaire the participants were asked how many glasses or cups of five different kinds of liquid they had drunk daily on average. Here, a glass is about 2 dL, a cup 1.5 dL. The total daily liquid intake (dL/day) was calculated by summing the amounts of each liquid.
Water ………. glasses,
Milk or buttermilk ………. glasses,
Juice ………. glasses,
Soft drinks ………. glasses,
Coffee ………. cups,
Tea ………. cups.
Questions on heartburn and constipation were translated and modified from the Rome III criteria. Body weight, height, body mass index (BMI) and change of body weight during pregnancy were measured routinely as part of the maternity healthcare program. If heartburn occurred at least once a week, the women were considered to suffer from it. Constipation was defined by having at least two of the following symptoms: fewer than three defecations per week, lumpy or hard stools, a sensation of incomplete evacuation or straining during defecation.
We asked how often the women exercised efficiently for at least 20 min at a time. We then divided the women into two groups: those who actively exercised before pregnancy, during pregnancy and in the post-partum period, and those who did not. In the active group, the women exercised briskly at least twice a week. Questions concerning exercise were based on national recommendations. A BMI of > 30 kg/m2 was used as a cut-off point for obesity, based on the WHO definition. An SGA infant was defined as one with a birth weight below the 10th percentile for gestational age and an infant large for gestational age (LGA) was defined as one with a birth weight above the 90th percentile for gestational age.
Daily fiber intake was calculated by multiplying the amount of the specific food item by its fiber coefficient. Table 1 includes fiber coefficients for each food item in the questionnaire. These coefficients were determined by applying information from the Finnish Bread Information website and the Finnish Food Composition Database [10]. Participants were asked how many glasses or cups of five different kinds of liquid they drank daily. The total daily liquid intake (dL/day) was calculated by summing the amount of each liquid (Table 1).
3. Theory/calculation
In statistical analyses of characteristics, the Mann–Whitney test, the Chi-square test or Fisher's exact test were used in group comparisons, as appropriate.
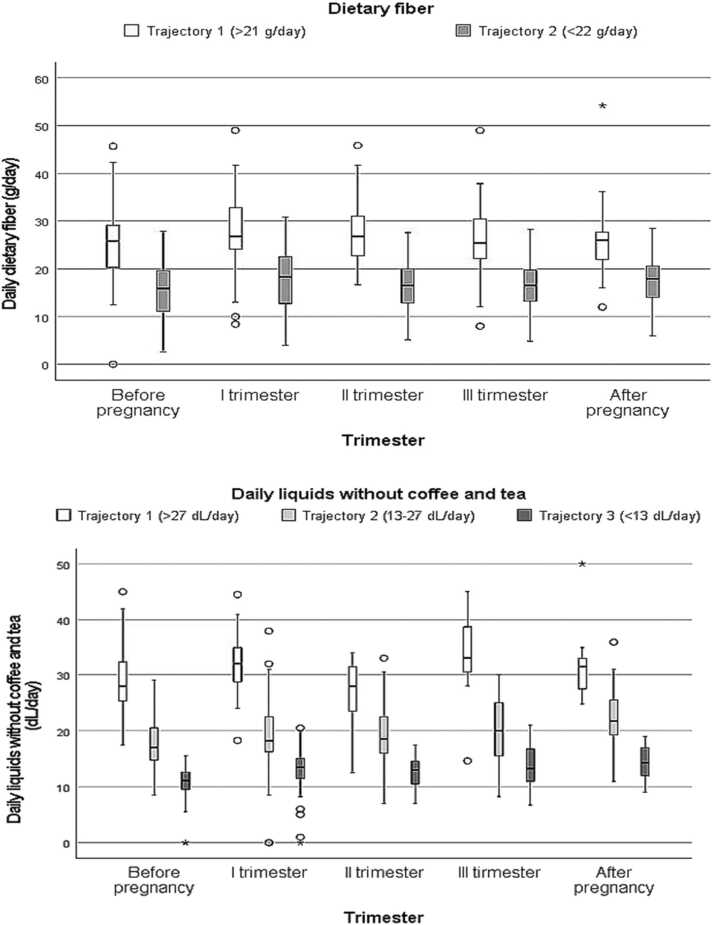
Fiber and liquid values were clustered by trajectory analysis, as originally presented by Nagin (2005). Trajectory groups are clusters of individuals following similar trajectories in outcome over time [15]. The trajectories were created according to all measurements of fiber and liquid per parturient as a continuous outcome measure. Two trajectory groups were created according to total fiber consumption, covering all trimesters: those who consumed a fiber average of < 22 g/day vs. ≥ 22 g/day during pregnancy. Similarly, the amounts of daily liquid were clustered into three trajectory groups: averages of < 13, 13–27 and > 27 dL/day during pregnancy. The trajectories are presented in Fig. 2. The analyses undertaken were latent class mixture models of quadratic trajectories including a random intercept and concomitant variables. Models were fitted by using the flexmix package [16] of the statistical program R, version 3.3.0, from the R Foundation for Statistical Computing [17]. Relative goodness of fit was assessed using Bayesian information Criteria (BIC).
Fig. 2.
Distributions of dietary fiber and liquids (excluding coffee and tea) according to trajectory analysis. Median is depicted as a black line inside the box. The box represents interquartile range and lines indicate the ranges. Dots indicate outliers and stars extreme outliers.
Heartburn and constipation during pregnancy were investigated by using a Generalized Linear Mixed Model (GLMM) with a glmer function, because their appearance varied during pregnancy. A binary response (heartburn or constipation, yes vs. no) was used as a dependent variable, and the unadjusted and multivariable adjusted results of analysis were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). The fiber and liquid trajectories and their interaction were the main explanatory factors for heartburn and constipation. Additionally, GLMM models were adjusted simultaneously for age, BMI, smoking, primparity, physical activity, and drinking of coffee and/or tea. Other explanatory variables included in the analyses were categorized as follows: age ≤ 35/> 35, BMI < 30/≥ 30 kg/m2, smoking, no/yes, primipara, yes/no, drink coffee or tea, not at all/at least 1 cup per day, vigorous physical activity at least once a week, no/yes. All included explanatory variables shown in Table 2 were modeled as fixed variables. The trimesters of any one mother constituted a potential source of variation and therefore this subject-specific effect was included as a random effect in the models. Generalized linear mixed model analyses were performed by using Statistical Package R, described above.
Table 2.
Use of fiber and liquids (excluding coffee and tea) with other predictors as risk factors of constipation and heartburn during pregnancy.
| Constipation |
Heartburn |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Multivariable adjusted | Unadjusted | Multivariable adjusted | |||||||
| N | n | (%) | OR (95% CI | OR (95% CI) | N | n | (%) | OR (95% CI) | OR (95% CI) | |
| Total | 673 | 171 | (25.4) | 646 | 95 | (14.7) | ||||
| Trimesters | ||||||||||
| Before pregnancy | 171 | 38 | (22.2) | 1.00 | 1.00 | 170 | 4 | (3.4) | 1.00 | 1.00 |
| I trimester | 170 | 45 | (26.5) | 1.26 (0.77–2.07) | 1.09 (0.64–1.87) | 169 | 28 | (16.6) | 8.24 (2.82–24.1) | 7.96 (2.65–23.9) |
| II trimester | 134 | 39 | (29.1) | 1.44 (0.85–2.41) | 1.28 (0.73–2.23) | 109 | 26 | (23.9) | 13.0 (4.39–38.5) | 12.8 (4.20–38.8) |
| III trimester | 109 | 22 | (20.2) | 0.89 (0.49–1.60) | 0.76 (0.40–1.44) | 108 | 36 | (33.3) | 20.8 (7.12–60.5) | 22.1 (7.29–66.7) |
| After pregnancy | 89 | 27 | (30.3) | 1.52 (0.85–2.72) | 1.41 (0.76–2.58) | 90 | 1 | (1.1) | 0.47 (0.05–4.24) | 0.47 (0.05–4.30) |
| Use of fibers, g/day | ||||||||||
| Trajectory 1 (≥22 g/day) | 244 | 61 | (25.0) | 1.00 | 1.00 | 237 | 43 | (18.1) | 1.00 | 1.00 |
| Trajectory 2 (<22 g/day) | 428 | 109 | (25.4) | 1.24 (0.84–1.82) | 0.77 (0.45–1.30) | 408 | 51 | (12.5) | 0.75 (0.46–1.21) | 1.14 (0.59–2.21) |
| Use of liquids, dl/day | ||||||||||
| Trajectory 2 (13–27 dL/day) | 425 | 105 | (24.7) | 1.00 | 1.00 | 407 | 56 | (13.8) | 1.00 | 1.00 |
| Trajectory 1 (>27 dL/day) | 60 | 14 | (23.3) | 1.24 (0.70–2.21) | 1.17 (0.40–3.44) | 57 | 13 | (22.8) | 1.06 (0.50–2.22) | 2.42 (0.70–8.35) |
| Trajectory 3 (<13 dL/day) | 188 | 52 | (27.7) | 1.52 (1.04–2.21) | 0.37 (0.14–0.98) | 182 | 26 | (14.3) | 0.80 (0.48–1.34) | 1.40 (0.53–3.68) |
| Use of fibers*liquids | ||||||||||
| Trajectory 1 (≥22 g/day) *Trajectory 2 (13–27 dL/day) | 1.00 | 1.00 | ||||||||
| Trajectory 2 (<22 g/day) *Trajectory 1 (>27 dL/day) | 1.17 (0.32–4.26) | 0.23 (0.04–1.18) | ||||||||
| Trajectory 2 (<22 g/day) *Trajectory 3 (<13 dL/day) | 5.89 (2.00–17.4) | 0.40 (0.12–1.29) | ||||||||
| Age | ||||||||||
| ≤35 | 580 | 141 | (24.3) | 1.00 | 1.00 | 558 | 85 | (15.2) | 1.00 | 1.00 |
| > 35 | 91 | 30 | (33.0) | 1.53 (0.95–2.47) | 1.95 (1.17–3.27) | 87 | 10 | (11.5) | 0.76 (0.37–1.57) | 0.74 (0.34–1.60) |
| BMI | ||||||||||
| ≤30 | 601 | 151 | (25.1) | 1.00 | 1.00 | 577 | 82 | (14.2) | 1.00 | 1.00 |
| > 30 | 39 | 5 | (12.8) | 0.44 (0.17–1.14) | 0.35 (0.13–0.95) | 40 | 6 | (15.0) | 0.97 (0.38–2.48) | 0.83 (0.30–2.25) |
| Smoking | ||||||||||
| No | 533 | 130 | (24.4) | 1.00 | 1.00 | 510 | 70 | (13.7) | 1.00 | 1.00 |
| Yes | 130 | 39 | (30.0) | 1.33 (0.87–2.03) | 1.38 (0.87–2.18) | 127 | 25 | (19.7) | 1.61 (0.94–2.76) | 1.94 (1.08–3.48) |
| Primipara | ||||||||||
| No | 184 | 42 | (22.8) | 1.00 | 1.00 | 178 | 26 | (14.6) | 1.00 | 1.00 |
| Yes | 489 | 129 | (26.4) | 1.21 (0.81–1.81) | 1.46 (0.94–2.26) | 468 | 69 | (14.7) | 1.01 (0.60–1.69) | 0.95 (0.55–1.66) |
| Vigorous physical activity | ||||||||||
| No | 413 | 107 | (25.9) | 1.00 | 1.00 | 395 | 68 | (17.2) | 1.00 | 1.00 |
| Yes | 260 | 64 | (24.6) | 0.93 (0.65–1.34) | 0.83 (0.55–1.24) | 251 | 27 | (10.8) | 0.97 (0.58–1.62) | 1.11 (0.64–1.94) |
| Drink coffee | ||||||||||
| No | 271 | 79 | (29.2) | 1.00 | 1.00 | 263 | 47 | (17.9) | 1.00 | 1.00 |
| Yes | 402 | 92 | (22.9) | 0.72 (0.51–1.02) | 0.66 (0.45–0.98) | 383 | 48 | (12.5) | 0.79 (0.50–1.26) | 0.70 (0.42–1.16) |
| Drink tea | ||||||||||
| No | 398 | 102 | (25.6) | 1.00 | 1.00 | 380 | 56 | (14.7) | 1.00 | 1.00 |
| Yes | 275 | 69 | (25.1) | 0.97 (0.68–1.38) | 0.80 (0.54–1.18) | 266 | 39 | (14.7) | 0.92 (0.58–1.47) | 0.84 (0.51–1.38) |
N = total number of times when all mothers have participated in the study during whole follow-up in 5 timepoints: before pregnancy- 1–111 trimesters, after pregnancy; n = number of times when mothers had constipation or heartburn. A Generalized Linear Mixed Model was used, with results given as odds ratios (ORs) with 95% confidence intervals (CIs).
The risk factors of combined adverse outcomes of pregnancy were investigated by using logistic regression. A binary response (yes vs. no) was used as a dependent variable, and the univariable and multivariable (fiber and liquid trajectories, drink of coffee or tea during trimesters, age, BMI, smoking, constipation and heartburn during trimesters) adjusted results of analysis were expressed as odds ratios (ORs) with 95% confidence intervals (CIs).
The study was approved by the Ethics Committee of Tampere University Hospital, Finland (R05025). Each subject signed an informed consent form before participation. The participants were recruited from four public maternity outpatient wards in the city of Tampere.
4. Results
Table 3 presents the characteristics of women with low or high fiber intake. Baseline characteristics of the women did not differ between the high and low fiber intake groups. Fig. 2 shows the intake of dietary fiber and liquids (without coffee and tea) during the study period according to trajectory analysis. As regards fiber there were two separate groups of women with similar intake (low use < 22 g/day and high use ≥ 22 g/day) and when considering fluid intake there were three similarly acting groups (averages of >27, 13–27 and <13 dL/day). Intake of liquids and fiber did not change significantly during pregnancy.
Table 3.
Baseline characteristics of mothers according to intake of fiber (N = 173).
| Intake of fiber |
|||||
|---|---|---|---|---|---|
| Trajectory 2 (< 22 g/day, n = 125) | Trajectory 1 (≥ 22 g/day, n = 48) | p | |||
| Age, Md (Range) | 29 | (18–40) | 28.5 | (22–41) | 0.484 |
| Age > 35, n (%) | 15 | (12) | 8 | (17) | 0.418 |
| BMI, Md (Range) | 22.8 | (18.4–39.1) | 22.8 | (18.6–33.3) | 0.892 |
| BMI ≥ 30 | 7 | (6) | 3 | (6) | 1.000 |
| Primiparas, n (%) | 91 | (73) | 36 | (75) | 0.769 |
| Smoking, n (%) | 30 | (24) | 8 | (17) | 0.297 |
| Use of alcohol, n (%) | 105 | (84) | 43 | (90) | 0.350 |
| Use of liquids, n (%) | 0.548 | ||||
| Trajectory 1 (> 27 dL/day) | 5 | (10) | 14 | (11) | |
| Trajectory 2 (13–27 dL/day) | 30 | (63) | 67 | (54) | |
| Trajectory 3 (< 13 dL/day) | 13 | (27) | 44 | (35) | |
| Vigorous physical activity, n (%) | 73 | (58) | 33 | (69) | 0.211 |
| Drink coffee, n (%) | 89 | (71) | 37 | (77) | 0.436 |
| Drink tea, n (%) | 50 | (40) | 22 | (46) | 0.486 |
The prevalence of heartburn increased significantly from the first trimester (8%) to the third trimester (33%) and was twenty-times higher in the end of pregnancy compared to pre-pregnancy (OR 20.8, 95% CI 7.12–60.5). After delivery, heartburn was rare. In contrast, the prevalence of constipation remained relatively stable (20–30%) during the whole study period (Table 2).
Risk factors of constipation and heartburn during pregnancy are presented in Table 2. Due to some missing data, the number of mothers differs from numbers as seen in Fig. 1. High intake of fiber alone did not protect the women from constipation, but a combination of low fiber and low fluid intake increased the risk of constipation during pregnancy. Age was a risk factor of constipation, while intake of coffee was protective. There was no clear association between fluid or fiber intake and heartburn, but smoking increased its prevalence.
Pregnancy outcomes according to intake of fiber during pregnancy are presented in Table 4. Combined adverse pregnancy outcome was significantly more common among women with low fiber intake. Table 5 shows risk factors of combined adverse outcome of pregnancy. In addition to low fiber intake, being obese (BMI > 30 kg/m2) was also a significant risk factor.
Table 4.
Pregnancy outcome according to intake of fiber (N = 173).
| Intake of fiber |
|||||
|---|---|---|---|---|---|
| Trajectory 2 (< 22 g/day, n = 125) |
Trajectory 1 (≥ 22 g/day, n = 48) |
||||
| n | (%) | n | (%) | p | |
| Delivery | 0.094 | ||||
| Vaginal | 99 | (79) | 42 | (70) | |
| Cesarean section | 26 | (21) | 6 | (13) | |
| Pregnancy duration | 0.217 | ||||
| Preterm < 37 +0 | 8 | (6) | 0 | (0) | |
| Term 37 + 1–41 + 6 | 105 | (84) | 43 | (90) | |
| Post-term > 41 + 6 | 12 | (10) | 5 | (10) | |
| Newborn`s weight | 0.808 | ||||
| SGA | 7 | (6) | 0 | (0) | 0.192 |
| AGA | 116 | (93) | 47 | (98) | |
| LGA | 2 | (2) | 1 | (2) | |
| Combined outcome*, n (%) | 35 | (28) | 6 | (13) | 0.032 |
*Cesarean section, gestational weeks < 37 and/or Small for Gestational Age (≤ 10%).
Table 5.
Dietary risk factor analysis for combined outcome (N = 173).
| Combined adverse outcome* (n = 41; 24%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Multivariable adjusted |
||||||||
| N | n | OR | (95% CI) | OR | (95% CI) | p | |||
| Total fibers g/day | |||||||||
| Trajectory 1 (≥22 g/day) | 48 | 6 | 1.00 | 1.00 | |||||
| Trajectory 2 (<22 g/day) | 125 | 35 | 2.72 | (1.06–6.97) | 3.10 | (1.10–8.70) | 0.032 | ||
| Liquid without coffee and tea trajectory dl/day | |||||||||
| Trajectory 2 (13–27 dL/day) | 97 | 19 | 1.00 | 1.00 | |||||
| Trajectory 1 (>27 dL/day) | 19 | 4 | 1.10 | (0.33–3.68) | 1.66 | (0.45–6.14) | 0.444 | ||
| Trajectory 3 (<13 dL/day) | 57 | 18 | 1.90 | (0.89–4.01) | 2.01 | (0.87–4.64) | 0.100 | ||
| Drink coffee or tea during I-III trimesters | |||||||||
| No | 26 | 9 | 1.00 | 1.00 | |||||
| Yes | 147 | 32 | 0.53 | (0.21–1.29) | 0.44 | (0.16–1.22) | 0.115 | ||
| Age | |||||||||
| ≤ 35 | 150 | 34 | 1.00 | 1.00 | |||||
| > 35 | 23 | 7 | 1.49 | (0.57–3.93) | 2.29 | (0.78–6.67) | 0.130 | ||
| BMI | |||||||||
| < 30 | 160 | 34 | 1.00 | 1.00 | |||||
| ≥ 30 | 10 | 6 | 5.56 | (1.48–20.8) | 7.89 | (1.80–34.4) | 0.006 | ||
| Smoking | |||||||||
| No | 135 | 33 | 1.00 | 1.00 | |||||
| Yes | 38 | 8 | 0.84 | (0.34–1.94) | 0.66 | (0.24–1.77) | 0.406 | ||
| Constipation during I-III trimesters | |||||||||
| No | 106 | 25 | 1.00 | 1.00 | |||||
| Yes | 67 | 16 | 1.02 | (0.49–2.09) | 0.99 | (0.44–2.19) | 0.977 | ||
| Heartburn during I-III trimesters | |||||||||
| No | 115 | 31 | 1.00 | 1.00 | |||||
| Yes | 58 | 10 | 0.57 | (0.25–1.25) | 0.63 | (0.26–1.52) | 0.303 | ||
N = total number of mothers; n = incident number of mothers. Logistic Regression Model was used, with results given as odds ratios (ORs) with 95% confidence intervals (CIs).
5. Discussion
In our study low fiber intake alone was not associated with constipation during pregnancy, which is in contrast to reports concerning the nonpregnant population [3]. However, together with low fluid intake it increased the risk of constipation. It is possible that during pregnancy anatomic and hormonal changes along with lack of exercise are so predominant that it is harder to relieve symptoms of constipation by means of intake of dietary fiber than in the nonpregnant population [4], [6], [7], [8]. It is also possible that even among the higher fiber-intake group in our population the consumption of fiber was not optimal, as according to Findiet data reported in 2007, daily fiber intake in adult Finnish women was only 21 g at that time, while recommendations for daily intake of fiber are substantially higher [18]. Thus, an even greater intake of fiber might have resulted in better prevention of constipation. As in earlier studies, we found that age was a risk factor of constipation, while intake of coffee was protective [19], [20]. In our study obesity decreased the risk of constipation, which is in contrast to earlier reports [6].
The risk of heartburn increased in each successive trimester, and it was clearly resolved after labor. This is likely to be a result of increased intra-abdominal pressure during pregnancy and relief of the pressure after delivery. We did not find a clear association between liquid or fiber intake and heartburn, but smoking increased it. This is not surprising, as tobacco smoking seems to be a risk factor of gastro-esophageal reflux symptoms [21].
Our secondary aim was to seek for possible associations between fiber intake and pregnancy outcome. We noticed a significant connection between fiber intake and combined adverse pregnancy outcome, as low fiber intake was associated with a more than threefold risk of combined CS, prematurity and/or fetal growth restriction. While fiber mainly works to promote the wellbeing of the intestines, it has several positive effects on the whole body [18]. Thus, there may be associations between fiber, the intestines and pregnancy, albeit the mechanisms may still be obscure. Studies concerning dietary fiber have demonstrated promising regulatory effects on the gut, and microbial effects have important implications also for the whole body. Dietary fiber has been reported to reduce the risk of cardiovascular disease and lower the risk of type 2 diabetes [22]. Likewise, it may lower blood pressure and levels of LDL-cholesterol [22], [23]. Cesarean section, prematurity, and fetal growth restriction may to some extent be reflections of adverse metabolic balance or suboptimal general condition of the mother, and dietary fiber might have beneficial effects on pregnancy through various mechanisms. In one study a dietary pattern characterized by high intake of vegetables, other plant foods and vegetable oils resulted in a lower risk of preeclampsia, whereas a dietary pattern characterized by high-level consumption of meat, sweet drinks and snacks increased the risk [24]. In earlier studies a Mediterranean-style diet has been associated with a lower risk of hypertensive disorders of pregnancy [25], preterm birth [26] and SGA infants [27], while other studies have revealed that a Western diet leads to significantly higher risks of having an SGA infant and lower birth weight [28], and an increased risk of preterm birth [29].
Constipation or heartburn were not associated with pregnancy outcome, but obesity increased the risk of combined adverse outcome. This is in line with the results of earlier studies [30].
Our study has several strengths and limitations. This is the first study on the relationship between fiber and liquid intake along with other lifestyle factors during pregnancy, and heartburn, constipation, and pregnancy outcome. As part of our national maternal outpatient care program the women had regular documented midwife visits, so there were only a few dropouts, and bias was unlikely. Pregnancy outcome was well documented and reliable. By examining food diaries, typical daily meals were revealed, and the amount of fiber ingested calculated according to nourishment tables. However, no direct measurements of food fiber content were performed, so the calculated fiber intakes reported in this study do not represent exact measures of fiber intake. As a result of the retrospective nature of describing the women`s pre-pregnancy nourishment, some inaccuracies cannot be excluded.
6. Conclusion
Low fiber intake during pregnancy may increase the risk of adverse pregnancy outcome. The occurrence of heartburn is related to advancing weeks of gestation. The risk of constipation may be modulated by diet, as low fiber and liquid intake increases the rate of pregnancy-related constipation.
Author contributions
All authors have read through, commented on, and accepted the final manuscript. JR was the principal investigator. JU and KT planned the study and guided its progress. TL was responsible for statistical analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This study was financially supported by the Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital.
Biographies

Johanna Reijonen is a gynecologist. She works mainly in maternity outpatient clinic and labor room.

Kati Tihtonen is a senior consultant in perinatology and obstetrics. She is a member of the board in The Finnish Perinatological Association. Special scientific interest are pre-eclampsia, patient safety and general perinatology.

Tiina Luukkaala is a statistician. She has over twenty years of experience in medical research.

Jukka Uotila is an obstetrician and perinatologist. Special scientific interests in general obstetrics and preeclampsia.
References
- 1.Neilson James P. Interventions for heartburn in pregnancy. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD007065.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vazquez Juan C. Heartburn in pregnancy. BMJ Clin Evid. 2015;09:1411. [PMC free article] [PubMed] [Google Scholar]
- 3.Tytgat G.N., Heading R.C., Muller- Lissne S., et al. Contemporary understanding and management of reflux and constipation in the general population and pregnancy: consensus meeting. Aliment Pharmacol Ther. 2003;18:291–301. doi: 10.1046/j.1365-2036.2003.01679.x. [DOI] [PubMed] [Google Scholar]
- 4.Body Cameron, Christie Jennifer A. Gastrointestinal diseases in pregnancy. nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin N Am. 2016;45:267–283. doi: 10.1016/j.gtc.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Cullen G., O´Donoghue D. Constipation and pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):807–818. doi: 10.1016/j.bpg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Vazquez Juan C. Constipation, haemorrhoids, and heartburn in pregnancy. BMJ Clin Evid. 2010;08:1411. [PMC free article] [PubMed] [Google Scholar]
- 7.Derbyshire E., Davies J., Costarelli V., et al. Diet, physical inactivity and the prevalence of constipation throughout and after pregnancy. Mater Child Nutr. 2006;2:127–134. doi: 10.1111/j.1740-8709.2006.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longo Sherri A., Moore Robert C., Canzoneri Bernard J., et al. Gastrointestinal conditions during pregnancy. Clin Colon Rectal Surg. 2010;23(Number 2) doi: 10.1055/s-0030-1254294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Wenjun, Xu Xiaohang, Zhang Yi, Guo Sa, et al. Epidemiology and risk factors of functional constipation in pregnant women. PLoS One. 2015 doi: 10.1371/journal.pone.0133521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finnish Food Composition Database, National Institute for Health and Welfare, 2003–2011. Available online at: 〈http://www.fineli.fi/〉 Fineli®.
- 11.Hasunen K., Kalavainen M., Keinonen H., Lagström H., Lyytikäinen A., Nurttila A., Peltola T., Talvia S. The Child, Family and Food. Nutrition recommendations for infants and children as well as pregnant and breastfeeding mothers. Hels Soc Health Minist. 2004 [Google Scholar]
- 12.Krogh K., Chiarioni G., Whitehead W. Management of choric constipation in adults. Review Article. U Eur Gastroenterol J. 2017;5(4):465–472. doi: 10.1177/2050640616663439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boilesen Sabine N., Tahan Soraia, Dias Francine C., et al. Water and fluid intake in the prevention and treatment of functional constipation in children and adolescents: is there evidence? J Pediatr. 2017;93(4):320–327. doi: 10.1016/j.jped.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Naumann C.R., Zelig C., Napolitano P.G., Ko C.W. Nausea, vomiting and heartburn in pregnancy: a prospective look at risk, treatment, and outcome. J Matern Fetal Neonatal Med. 2012;25(8):1488–1493. doi: 10.3109/14767058.2011.644363. [DOI] [PubMed] [Google Scholar]
- 15.Nagin D.S., Odgers C.L. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 16.Leisch F. FlexMix: a general framework for finite mixture models and latent class regression in R. J Stat Soft. 2004;11(8) [Google Scholar]
- 17.R Development Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2008, ISBN 3-900051-07-0, URL. Available online at: 〈http://www.R-project.org〉.
- 18.Marlett Judith A., McBurney Michael I., Slavin Joanne L. American Dietetic Association Position of the American Dietetic Association: health implications of dietary fiber review. J Pain Symptom Mana. 2009;37(4):737–745. doi: 10.1016/s0002-8223(02)90228-2. [DOI] [PubMed] [Google Scholar]
- 19.Murad-Regadas Sthela Maria, Vilarinho Adjra da Silva, Borges Livia, et al. Correlation between pelvic floor dysfunction on dynamic 3D ultrasound and vaginal delivery, parity, and age in women with obstructed defecation symptoms. Gut. 2004;53(12):1730–1735. doi: 10.1590/S0004-2803.202100000-52. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson M., Johnsen R., Ye W., et al. Lifestyle related risk factors in the aetiology of gastro-oesophageal reflux. Proc Nutr Soc Rev. 2020;79(1):61–67. doi: 10.1136/gut.2004.043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maret-Ouda John, Markar Sheraz R., Lagergren Jesper. Gastroesophageal reflux disease: a review. JAMA. 2020;324(24):2536–2547. doi: 10.1001/jama.2020.21360. [DOI] [PubMed] [Google Scholar]
- 22.Louise Evans Charlotte Elizabeth. Dietary fibre and cardiovascular health: a review of current evidence and policy review. Nutrients. 2013;5(4):1417–1435. [Google Scholar]
- 23.Slavin Joanne. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brantsaeter A.L., Haugen M., Samuelsen S.O., et al. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J Nutr. 2009;139:1162–1168. doi: 10.3945/jn.109.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoenaker D.A., Soedamah-Muthu S.S., Callaway L.K., et al. Prepregnancy dietary patterns and risk of developing hypertensive disorders of pregnancy: results from the Australian longitudinal study on women’s health. Am J Clin Nutr. 2015;102:94–101. doi: 10.3945/ajcn.114.102475. [DOI] [PubMed] [Google Scholar]
- 26.Mikkelsen T.B., Osterdal M.L., Knudsen V.K., et al. Association between a mediterranean-type diet and risk of preterm birth among Danish women: a prospective cohort study. Acta Obstet Gynecol Scand. 2008;87:325–330. doi: 10.1080/00016340801899347. [DOI] [PubMed] [Google Scholar]
- 27.Timmermans S., Steegers-Theunissen R.P., Vujkovic M., et al. The mediterranean diet and fetal size parameters: the generation R study. Br J Nutr. 2012;108:1399–1409. doi: 10.1017/S000711451100691X. [DOI] [PubMed] [Google Scholar]
- 28.Knudsen V.K., Orozova-Bekkevold I.M., Mikkelsen T.B., et al. Major dietary patterns in pregnancy and fetal growth. Eur J Clin Nutr. 2008;62:463–470. doi: 10.1038/sj.ejcn.1602745. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen M.A., Maslova E., Halldorsson T.I., et al. Characterization of dietary patterns in the Danish national birth cohort in relation to preterm birth. PLoS One. 2014;9:351. doi: 10.1371/journal.pone.0093644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catalano Patrick M., Shankar Kartik. Obesity and pregnancy: mechanisms of short term and long-term adverse consequences for mother and child. Review article. BMJ. 2017:356. doi: 10.1136/bmj.j1. [DOI] [PMC free article] [PubMed] [Google Scholar]