Key Points
Question
What is the genetic architecture of maximum habitual alcohol intake (MaxAlc), and how does it compare with other alcohol consumption measures?
Findings
This genetic association study of MaxAlc in 247 455 European- and African-ancestry individuals identified 15 genome-wide significant loci, including multiple novel associations. MaxAlc was genetically correlated with measures of alcohol-related problems, demonstrated significantly different correlations with psychiatric traits compared with other alcohol consumption traits, and loaded on a factor with alcohol problem traits, while alcohol consumption measures loaded on a separate factor.
Meaning
These findings suggest that MaxAlc is genetically different from consumption measures in relation to problematic alcohol use.
This genetic association study identifies genetic loci associated with maximum habitual alcohol intake and assesses the genetic architecture across alcoholic traits.
Abstract
Importance
Alcohol genome-wide association studies (GWASs) have generally focused on alcohol consumption and alcohol use disorder (AUD); few have examined habitual drinking behaviors like maximum habitual alcohol intake (MaxAlc).
Objectives
To identify genetic loci associated with MaxAlc and to elucidate the genetic architecture across alcohol traits.
Design, Setting, and Participants
This MaxAlc genetic association study was performed among Million Veteran Program participants enrolled from January 10, 2011, to September 30, 2020. Ancestry-specific GWASs were conducted in participants with European (n = 218 623) and African (n = 29 132) ancestry, then meta-analyzed (N = 247 755). Linkage-disequilibrium score regression was used to estimate single nucleotide variant (SNV)–heritability and genetic correlations (rg) with other alcohol and psychiatric traits. Genomic structural equation modeling (gSEM) was used to evaluate genetic associations between MaxAlc and other alcohol traits. Mendelian randomization was used to examine potential causal relationships between MaxAlc and liver enzyme levels. MTAG (multitrait analysis of GWAS) was used to analyze MaxAlc and problematic alcohol use (PAU) jointly.
Exposures
Genetic associations.
Main Outcomes and Measures
MaxAlc was defined from the following survey item: “in a typical month, what is/was the largest number of drinks of alcohol you may have had in one day?” with ordinal responses from 0 to 15 or more drinks.
Results
GWASs were conducted on sample sizes of as many as 247 455 US veterans. Participants were 92.68% male and had mean (SD) age of 65.92 (11.70) years. The MaxAlc GWAS resulted in 15 genome-wide significant loci. Top associations in European-ancestry and African-ancestry participants were with known functional variants in the ADH1B gene, namely rs1229984 (P = 3.12 × 10−101) and rs2066702 (P = 6.30 × 10−17), respectively. Novel associations were also found. SNV-heritability was 6.65% (SE, 0.41) in European-ancestry participants and 3.42% (SE, 1.46) in African-ancestry participants. MaxAlc was positively correlated with PAU (rg = 0.79; P = 3.95 × 10−149) and AUD (rg = 0.76; P = 1.26 × 10−127) and had negative rg with the UK Biobank “alcohol usually taken with meals” (rg = −0.53; P = 1.40 × 10−50). For psychiatric traits, MaxAlc had the strongest genetic correlation with suicide attempt (rg = 0.40; P = 3.02 × 10−21). gSEM supported a 2-factor model with MaxAlc loading on a factor with PAU and AUD and other alcohol consumption measures loading on a separate factor. Mendelian randomization supported an association between MaxAlc and the liver enzyme gamma-glutamyltransferase (β = 0.012; P = 2.66 × 10−10). MaxAlc MTAG resulted in 31 genome-wide significant loci.
Conclusions and Relevance
The findings suggest that MaxAlc closely aligns genetically with PAU traits. This study improves understanding of the mechanisms associated with normative alcohol consumption vs problematic habitual use and AUD as well as how MaxAlc relates to psychiatric and medical conditions genetically and biologically.
Introduction
Progress has been made in understanding the genetic architecture of many alcohol-related traits,1,2 including alcohol use disorder (AUD),3,4 forms of problematic alcohol use (PAU),4,5 and measures of alcohol consumption.3,5,6 A major contribution that genome-wide association studies (GWASs) have made to understanding the genetics of alcohol traits is the finding that the genetic architecture of AUD differs from alcohol use measures.2 Genetic correlation analyses have overturned the idea that alcohol use traits differ from AUD only in degree of use by showing consistently that they differ in nature too.3,4,5 Quantity-frequency measures like the Alcohol Use Disorders Identification Test–Consumption (AUDIT-C) score correlate genetically with positive metabolic outcomes but not psychiatric disorders, while AUD correlates genetically with psychiatric disorders.3,4 Although these findings have been replicated, they are not well understood and warrant further study.7 Few well-powered efforts have examined the genetic influences across levels of habitual alcohol consumption—an important area of investigation given that higher levels of alcohol intake often lead to increased negative alcohol outcomes.
We aim to improve understanding of the genetic architecture of habitual alcohol consumption by greatly increasing the sample size of GWAS of maximum habitual alcohol intake (MaxAlc)8 in European and African ancestry participants in the Million Veteran Program (MVP) and examining the similarities and differences between MaxAlc and other alcohol traits. MaxAlc can be distinguished from similar phenotypes that measure the maximum number of drinks consumed in a lifetime drinking episode, in that MaxAlc captures habitual maximum alcohol intake (ie, “in a typical month”), while lifetime maximum drinks may capture consumption on only a single occasion, often being influenced by milestone drinking events (eg, 21st birthday celebrations).9 Because MaxAlc is more representative of routine behaviors, it is likely more clinically meaningful and representative of behavior that could result in more negative alcohol-related outcomes than occasional binge-drinking or lower levels of alcohol consumption.10
To our knowledge, the current study is the largest GWAS of MaxAlc to date. The larger sample enables us to estimate the heritability of MaxAlc and examine its genetic correlations with traits from large studies of alcohol consumption and alcohol-related problems. We used genomic structural equation modeling (gSEM) to evaluate the overarching genetic relationships between MaxAlc and other alcohol traits. To investigate the association of MaxAlc with long-term alcohol-related disease, we conducted mendelian randomization (MR) to examine potential relationships between MaxAlc and liver enzyme levels. Together, these analyses provide a better understanding of the genetic basis of regular heavy drinking. We hypothesized that MaxAlc would be more genetically similar to AUD than to other alcohol consumption measures.
Method
Participants and Phenotyping
MVP participants are US military veterans enrolled through the Department of Veteran Affairs (VA) health care system and studied in the United States.11 MaxAlc was defined using information from the MVP Lifestyle Survey, a self-report survey composed of questions from validated instruments,12 question: “in a typical month, what is/was the largest number of drinks of alcohol you may have had in one day?” with ordinal response options ranging from 0 to 15 or more drinks (eFigure 1 in Supplement 1). We removed individuals with second-degree or closer relatives pairwise based on a kinship coefficient of greater than 0.0884, prioritizing the retention of the individual with the higher reported MaxAlc value (the heavier drinking member of the pair of related individuals). The study was approved by the Central VA institutional review board (IRB) and site-specific IRBs, including Yale University School of Medicine and VA Connecticut, and was conducted in accordance with all relevant ethical regulations. Written informed consent was obtained from all participants. This study followed the Strengthening the Reporting of Genetic Association Studies (STREGA) reporting guideline.13 Genotyping and imputation for MVP are described in the eAppendix in Supplement 1.
Ancestry-Specific GWAS and Cross-Ancestry GWAS Meta-analysis
Individual GWASs were conducted on the ordinal trait in the respective MVP samples of participants with European and African ancestry using a linear regression model implemented in PLINK 2.0.14 Age, sex, and the first 10 within-ancestry genetic principal components were included as covariates. Ancestry-specific GWASs were then meta-analyzed across ancestries using inverse-variance weighting in METAL.15 1KG Phase 3 was used as a reference panel to determine European and African ancestry linkage disequilibrium (LD) structure.16 Independent GWAS loci were identified using an LD threshold of r2 = 0.1 to determine lead single nucleotide variant (SNVs) at each genome-wide significant locus.17 Variants were assigned to the nearest gene based on physical position (<10 kilobase from the assigned gene). Additional gene-mapping techniques using expression quantitative trait locus and 3-dimensional chromatin interactions data were also performed and are described in the eAppendix in Supplement 1.
Gene, Gene Set, and Tissue-Specific Gene Expression Analysis
We conducted gene, gene set, and gene expression analyses. These are described in the eAppendix in Supplement 1.
Statistical Analysis
SNV-Heritability and Genetic Correlations
LD score regression (LDSC)18 was used to estimate SNV-heritability (h2SNV) and to characterize genetic correlations (rg) with a set of alcohol, psychiatric, and medical traits. Genetic correlations were examined for MaxAlc with GWASs of AUD,4 PAU,4 drinks per week (DPW),6 a UK Biobank (UKB) measure of “when you drink alcohol, is it usually with meals?” (UKB Field ID 1618), and the AUDIT total (AUDIT-T), problems (AUDIT-P), and consumption (AUDIT-C) scales3,5 in European-ancestry participants. The AUDIT is a 10-item screening-tool for AUD, with the first 3 items assessing alcohol consumption (AUDIT-C) and the final 7 items assessing alcohol-related problems (AUDIT-P).19 Included traits are described in greater detail in the eAppendix in Supplement 1. Measures of AUDIT-C from 2 samples that have demonstrated differences in substance use problems and demographic composition, MVP and UKB, were included to examine distinctions between the measure across these populations. COV-LDSC20 was used to estimate MaxAlc h2SNV in African-ancestry participants.
Large-scale GWASs of AUD and PAU, unlike alcohol consumption measures, have shown positive genetic correlations with psychiatric disorders.3,4 GWASs have not yet been used to examine patterns of genetic correlation between psychopathology and heavier alcohol consumption (eg, MaxAlc) in comparison with AUD and other consumption measures. Thus, we performed genetic correlation analyses between the included alcohol traits and GWASs of anxiety,21 depression,22 posttraumatic stress disorder (PTSD),23 schizophrenia,24 and suicide attempt.25
gSEM of Alcohol Traits
gSEM26 was used to evaluate the shared polygenic architecture of MaxAlc, alcohol use problems, and other alcohol consumption measures using GWAS summary statistics. Exploratory factor analysis (EFA) was used to evaluate 1-, 2-, and 3-factor model fit. Confirmatory factor analysis (CFA) was then performed to evaluate model convergence and factor loadings using conventional model fit indices.26 CFA models were estimated using diagonally weighted least squares estimation and a smoothed genetic covariance matrix to guard against nonpositive definite scenarios. The 1KG Phase 3 European ancestry reference panel was used to calculate LD.16
MR Analyses
We used MR to examine potential relationships between MaxAlc and alcohol-related liver enzyme levels with available GWAS data (ALP [alkaline phosphatase], ALT [alanine transaminase], and GGT [gamma-glutamyl transferase])27 using inverse-variance weighted (IVW), weighted median, and MR-Egger methods in TwoSampleMR28 and MR-PRESSO.29 MR analysis was limited to liver enzymes demonstrating a significant genetic correlation with MaxAlc. To ensure sufficient genetic instruments to test for causal relationships, we included LD-independent MaxAlc loci reaching a suggestive significance threshold of P < 5.0 × 10 −5 (171 SNVs). Multiple methods were used to evaluate method-specific biases to determine consistent effect estimates. Sensitivity analyses, leave-one-out analyses, and outlier removal were all evaluated. The MR-Egger intercept test was used to evaluate the presence of horizontal pleiotropy. MR-PRESSO29 was used to identify genetic instrument outliers that show horizontal pleiotropy for removal. MR–Robust Adjusted Profile Score (MR-RAPS)30 was used to test for biased effect estimates due to the inclusion of suggestive significance SNVs as genetic instruments.
Multitrait Analysis of MaxAlc and PAU
A joint analysis of European ancestry MaxAlc and PAU4 GWAS summary statistics was performed in multitrait analysis of GWAS (MTAG).31 The MTAG analysis is described in the eAppendix in Supplement 1.
Results
MVP participants of European and African ancestry with MaxAlc phenotypic and genotypic data (European ancestry, 218 623; African ancestry, 29 132) were included in the GWAS analyses, resulting in a total sample of 247 755 participants. The total sample was 92.68% male and had a mean (SD) age of 65.92 (11.70) years. Overall, 36.92% reported MaxAlc at the binge-drinking threshold or greater32 (female participants, ≥4 drinks; male participants, ≥5 drinks) (eTable 1 in Supplement 2 and eFigure 2 in Supplement 1).
Ancestry-Specific GWAS and Cross-Ancestry GWAS Meta-analysis
The MaxAlc GWAS resulted in 15 LD-independent genome-wide significant (P ≤ 5.0 × 10−08) loci across the respective analyses (Figure 1 and Table 1). There were 10 genome-wide significant loci in the European-ancestry analysis, 2 genome-wide significant loci in the African-ancestry analysis, and 3 additional genome-wide significant loci in the cross-ancestry meta-analysis that did not reach genome-wide significance in the ancestry-specific analyses.
Figure 1. Manhattan Plot of Maximum Habitual Alcohol Intake GWAS in 247 755 Individuals of African and European Ancestry.
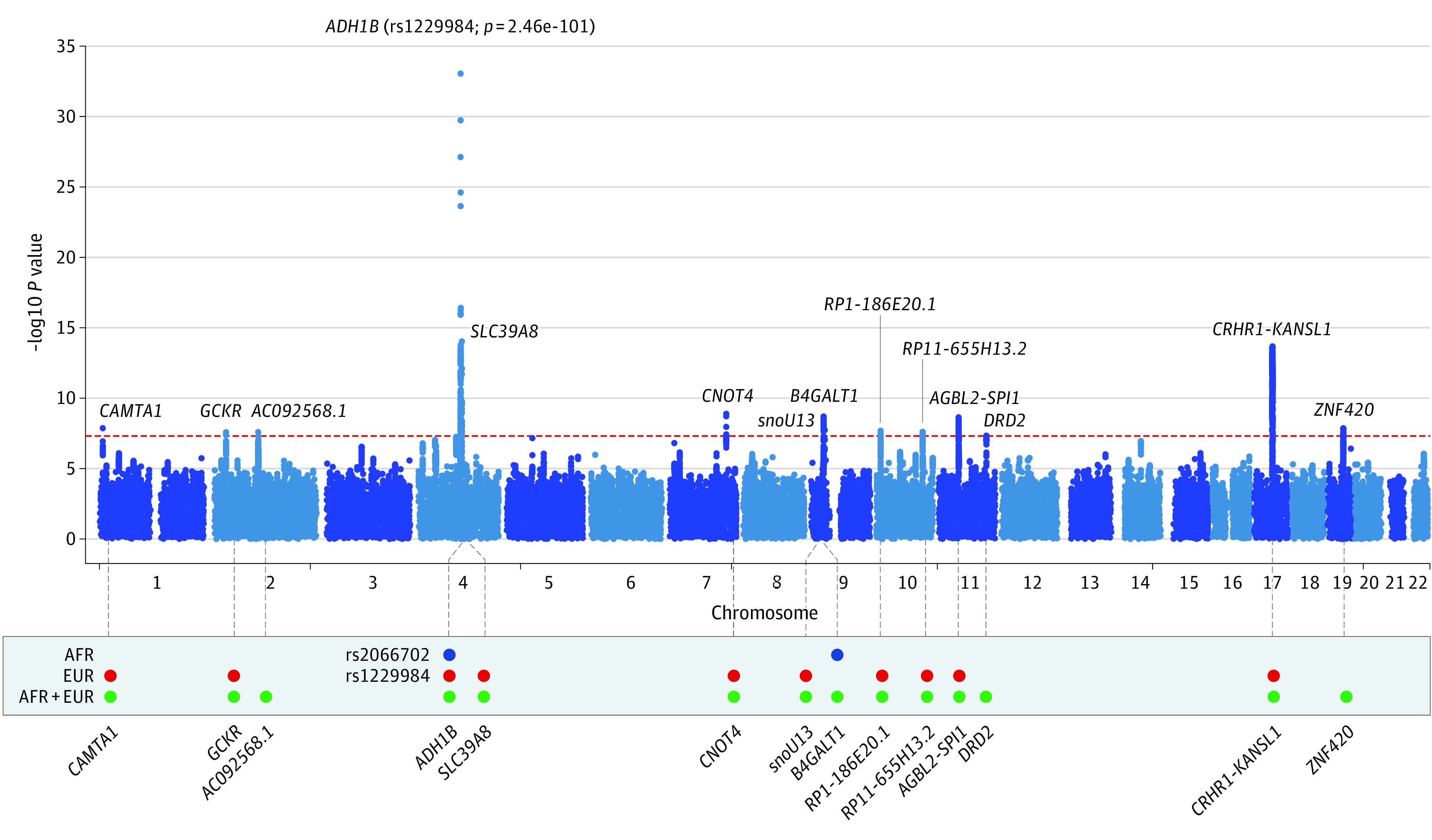
Red-dashed line indicates genome-wide significance (P = 5.00 × 10−8). In the lower panel, blue circles indicate genome-wide significance in the African-ancestry analysis (AFR); red circles indicate genome-wide significance in the European-ancestry analysis (EUR); and green circles indicate genome-wide significance in the cross-ancestry meta-analysis (AFR+EUR).
Table 1. Genome-Wide Significant Maximum Habitual Alcohol Intake Loci in the Cross-Ancestry GWAS, European GWAS, African Ancestry GWAS, and MaxAlc MTAG GWAS.
| Chr | Position | Marker name | A1 | A2 | Nearest gene | Direction or MAFa | P value | SNVs, No. | GWAS SNVs, No. | IS SNVs, No. | Lead SNVs, No. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross-ancestry GWAS | |||||||||||
| 4 | 100239319 | rs1229984 | C | T | ADH1B | -? | 2.46 × 10−101 | 529 | 393 | 22 | 9 |
| 4 | 103188709 | rs13107325 | T | C | SLC39A8 | – | 9.40 × 10−15 | 23 | 23 | 4 | 1 |
| 17 | 44305199 | rs570285046 | C | T | CRHR1 | ++ | 2.12 × 10−14 | 3590 | 3538 | 29 | 3 |
| 7 | 135162897 | rs2551763 | T | C | CNOT4 | -? | 1.29 × 10−9 | 29 | 18 | 1 | 1 |
| 9 | 30359634 | rs13297433 | A | G | snoU13 | ++ | 2.01 × 10−9 | 76 | 73 | 2 | 1 |
| 11 | 47392114 | rs7928419 | A | G | SPI1 | ++ | 2.29 × 10−9 | 196 | 172 | 3 | 1 |
| 1 | 7830262 | rs2153733 | C | T | CAMTA1 | +? | 1.36 × 10−8 | 1 | 1 | 1 | 1 |
| 19 | 37504953 | rs826323 | A | G | ZNF420 | ++ | 1.39 × 10−8 | 162 | 153 | 1 | 1 |
| 9 | 33094361 | rs56175925 | A | G | B4GALT1 | ?+ | 1.82 × 10−8 | 5 | 5 | 1 | 1 |
| 10 | 10704659 | rs34165302 | C | CA | RP1-186E20.1 | -? | 2.08 × 10−8 | 87 | 35 | 1 | 1 |
| 10 | 110572259 | rs1577857 | G | T | RP11-655H13.2 | +? | 2.47 × 10−8 | 100 | 82 | 1 | 1 |
| 2 | 104056769 | rs6752675 | A | G | AC092568.1 | – | 2.57 × 10−8 | 196 | 170 | 1 | 1 |
| 2 | 27730940 | rs1260326 | C | T | GCKR | – | 2.63 × 10−8 | 9 | 9 | 1 | 1 |
| 11 | 113392994 | rs2514218 | T | C | DRD2 | – | 4.59 × 10−8 | 25 | 23 | 1 | 1 |
| European-ancestry GWAS | |||||||||||
| 4 | 100239319 | rs1229984 | C | T | ADH1B | 0.04 | 3.12 × 10−101 | 420 | 275 | 14 | 8 |
| 4 | 103188709 | rs13107325 | T | C | SLC39A8 | 0.08 | 6.78 × 10−15 | 24 | 24 | 2 | 1 |
| 17 | 43810896 | rs77804065 | T | C | CRHR1 | 0.23 | 6.04 × 10−14 | 3839 | 3533 | 4 | 2 |
| 11 | 47676170 | rs7107356 | G | A | AGBL2 | 0.5 | 1.43 × 10−10 | 422 | 313 | 3 | 1 |
| 7 | 135162897 | rs2551763 | T | C | CNOT4 | 0.41 | 1.30 × 10−9 | 43 | 19 | 1 | 1 |
| 1 | 7830262 | rs2153733 | C | T | CAMTA1 | 0.19 | 1.36 × 10−8 | 35 | 31 | 1 | 1 |
| 10 | 10714452 | rs145961009 | C | CTT | RP1-186E20.1 | 0.22 | 1.73 × 10−8 | 133 | 42 | 1 | 1 |
| 9 | 30346448 | rs4879424 | C | T | snoU13 | 0.15 | 2.18 × 10−8 | 190 | 169 | 1 | 1 |
| 10 | 110572259 | rs1577857 | G | T | RP11-655H13.2 | 0.26 | 2.47 × 10−8 | 122 | 102 | 1 | 1 |
| 2 | 27730940 | rs1260326 | C | T | GCKR | 0.42 | 4.53 × 10−8 | 18 | 18 | 1 | 1 |
| African-ancestry GWAS | |||||||||||
| 4 | 100229017 | rs2066702 | A | G | ADH1B | 0.19 | 6.30 × 10−17 | 109 | 103 | 7 | 1 |
| 9 | 33094361 | rs56175925 | A | G | B4GALT1 | 0.02 | 1.83 × 10−8 | 6 | 5 | 1 | 1 |
| MTAG | |||||||||||
| 4 | 100239319 | rs1229984 | T | C | ADH1B | 0.04 | 3.35 × 10−176 | 1291 | 646 | 26 | 9 |
| 4 | 103188709 | rs13107325 | T | C | SLC39A8 | 0.08 | 6.01 × 10−36 | 75 | 26 | 6 | 1 |
| 2 | 27730940 | rs1260326 | T | C | GCKR | 0.41 | 2.72 × 10−21 | 261 | 98 | 4 | 1 |
| 4 | 39423789 | rs4975013 | A | G | KLB | 0.39 | 1.44 × 10−19 | 64 | 27 | 4 | 1 |
| 11 | 113364691 | rs17602038 | T | C | DRD2 | 0.37 | 2.62 × 10−16 | 70 | 36 | 3 | 2 |
| 11 | 47406592 | rs11039216 | T | C | RP11-750H9.5 | 0.47 | 1.07 × 10−15 | 519 | 245 | 5 | 1 |
| 17 | 44083402 | rs1991556 | A | G | MAPT | 0.23 | 2.25 × 10−15 | 3854 | 2089 | 2 | 1 |
| 10 | 110572259 | rs1577857 | T | G | RP11-655H13.2 | 0.26 | 6.44 × 10−15 | 494 | 304 | 2 | 1 |
| 2 | 104361420 | rs9308868 | A | G | AC013727.1 | 0.49 | 4.88 × 10−13 | 518 | 329 | 3 | 1 |
| 14 | 58765903 | rs61974485 | T | C | ARID4A | 0.28 | 1.02 × 10−12 | 117 | 5 | 1 | 1 |
| 2 | 45139904 | rs472140 | T | C | RP11-89K21.1 | 0.42 | 1.11 × 10−11 | 50 | 5 | 2 | 1 |
| 7 | 135119417 | rs2551774 | A | G | CNOT4 | 0.41 | 1.50 × 10−11 | 43 | 14 | 1 | 1 |
| 10 | 10688361 | rs7910201 | A | G | RP1-186E20.1 | 0.25 | 6.36 × 10−11 | 168 | 69 | 2 | 1 |
| 16 | 53834607 | rs7188250 | T | C | FTO | 0.41 | 8.06 × 10−10 | 106 | 82 | 1 | 1 |
| 7 | 153487944 | rs2098112 | A | G | DPP6 | 0.47 | 8.99 × 10−10 | 112 | 2 | 1 | 1 |
| 19 | 49244219 | rs2307018 | A | C | IZUMO1 | 0.43 | 9.98 × 10−10 | 59 | 21 | 1 | 1 |
| 1 | 66503517 | rs1354063 | T | C | PDE4B | 0.47 | 1.21 × 10−9 | 224 | 81 | 3 | 1 |
| 15 | 47681384 | rs8033799 | A | C | CTD-2050N2.1 | 0.21 | 1.86 × 10−9 | 157 | 65 | 2 | 1 |
| 5 | 153364650 | rs2245405 | A | G | FAM114A2 | 0.45 | 2.24 × 10−9 | 70 | 32 | 1 | 1 |
| 22 | 41789408 | rs4822028 | A | G | TEF | 0.23 | 7.97 × 10−9 | 762 | 503 | 2 | 2 |
| 12 | 81602449 | rs1921035 | A | G | ACSS3 | 0.48 | 8.44 × 10−9 | 100 | 55 | 1 | 1 |
| 2 | 144225215 | rs13024996 | A | C | RP11-570L15.2 | 0.36 | 8.56 × 10−9 | 29 | 7 | 1 | 1 |
| 13 | 97019647 | rs1925104 | T | G | HS6ST3 | 0.33 | 1.37 × 10−8 | 100 | 56 | 1 | 1 |
| 8 | 21831385 | rs1484162 | A | G | XPO7 | 0.41 | 1.49 × 10−8 | 56 | 15 | 1 | 1 |
| 2 | 138197269 | rs10496771 | T | C | THSD7B | 0.25 | 1.56 × 10−8 | 222 | 84 | 1 | 1 |
| 20 | 18546199 | rs6045466 | T | C | LINC00493 | 0.47 | 1.92 × 10−8 | 150 | 102 | 1 | 1 |
| 2 | 58042241 | rs1402398 | A | G | CTD-2026C7.1 | 0.39 | 2.00 × 10−8 | 268 | 55 | 2 | 1 |
| 7 | 117576675 | rs6466637 | A | G | AC007568.1 | 0.28 | 2.00 × 10−8 | 39 | 22 | 1 | 1 |
| 7 | 73015369 | rs13240065 | A | G | MLXIPL | 0.12 | 2.95 × 10−8 | 66 | 46 | 1 | 1 |
| 2 | 204123832 | rs56059523 | T | C | CYP20A1 | 0.11 | 3.17 × 10−8 | 18 | 5 | 1 | 1 |
| 3 | 18793340 | rs9835977 | T | C | AC144521.1 | 0.30 | 3.57 × 10−8 | 79 | 44 | 1 | 1 |
Abbreviations: +, positive; –, negative; ?, not present; A, allele; Chr, chromosome; GWAS, genome-wide association studies; IS, independently significant; MAF, minor allele frequency; MTAG, multitrait analysis of genome-wide association studies; SNV, single nucleotide variant.
For the cross-ancestry GWAS, direction is shown; for European-ancestry GWAS, African-ancestry GWAS, and MTAG, MAF is shown.
The top association in the European-ancestry analysis was with the well-replicated functional ADH1B (OMIM 103720) rs1229984 variant (P = 3.12 × 10−101) (Table 1; eFigure 3 in Supplement 1). Additional genome-wide significant loci in the European ancestry analysis included SLC39A8 (OMIM 608732; rs13107325; P = 6.78 × 10−15), CRHR1 (OMIM 122561; rs77804065; P = 6.04 × 10−14), AGBL2 (OMIM 617345; rs7107356; P = 1.43 × 10−10), and GCKR (OMIM 600842; rs1260326; P = 4.53 × 10−8). All loci in the European ancestry analysis remained genome-wide significant in the cross-ancestry meta-analysis, although there were some differences in the lead SNVs identified (Figure 1 and Table 1).
The top association in the African-ancestry analysis was with the known African-ancestry–specific functional locus at ADH1B (rs2066702; P = 6.30 × 10−17) (Table 1; eFigure 4 in Supplement 1). A second genome-wide significant association was found on chromosome 9 nearest B4GALT1 (OMIM 137060; rs56175925; P = 1.83 × 10−8) (Figure 1 and Table 1).
In the cross-ancestry meta-analysis, 3 loci not identified in the ancestry-specific GWAS were genome-wide significant: rs826323, which maps to ZNF420 (OMIM 617216; P = 1.39 × 10−8) on chromosome 19; rs6752675 on chromosome 2 nearest AC092568.1 (P = 2.57 × 10−8); and the DRD2 (OMIM 126450) rs2514218 variant on chromosome 11 (P = 4.59 × 10−8) (Figure 1 and Table 1).
Gene, Gene Set, and Tissue-Specific Gene Expression Analysis
Gene, gene set, and gene expression results are reported in the Supplement. Results can be found in eFigures 5 to 8 in Supplement 1 and eTables 2 to 7 in Supplement 2.
SNV-Heritability and Genetic Correlations
LDSC estimated an observed-scale MaxAlc h2SNV of 6.65% (SE, 0.41) in European-ancestry participants. LDSC estimates indicated that genome-wide inflation (λGC = 1.26) was likely indicative of the polygenic architecture of MaxAlc, as supported by an LDSC intercept of 0.99 (SE, 0.004) and attenuation ratio less than 0. For African-ancestry participants, COV-LDSC estimated h2SNV of 3.42% (SE, 1.46), a λGC of 1.03, LDSC intercept of 0.99 (SE, 0.007), and attenuation ratio less than 0.
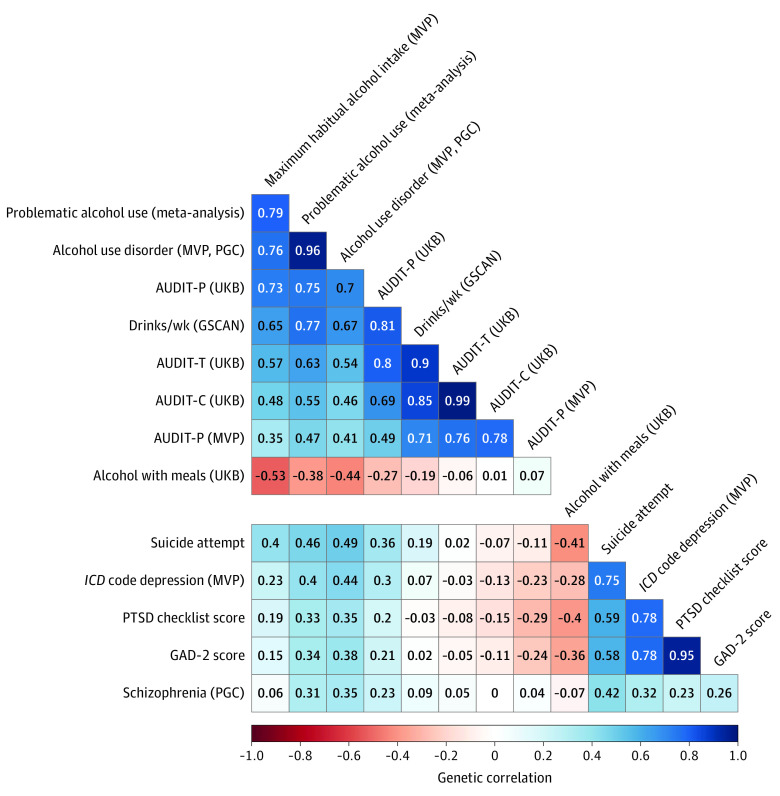
In European-ancestry participants, LDSC estimated a high rg for MaxAlc with PAU (rg = 0.79; P = 3.95 × 10−149) and AUD (rg = 0.76; P = 1.26 × 10−127), a lower rg with consumption traits (MVP AUDIT-C, rg = 0.35; P = 8.49 × 10−15; DPW, rg = 0.65; P = 1.26 × 10−103), and a negative rg with “alcohol usually taken with meals” (rg = −0.53; P = 1.40 × 10−50) (Figure 2; eTable 8 in Supplement 2). Among the psychiatric traits examined, MaxAlc had the highest rg with suicide attempt (rg = 0.40; P = 3.02 × 10−21) (Figure 2; eTable 9 in Supplement 2). The MaxAlc rg with suicide attempt was significantly higher than the rg between suicide attempt and all other consumption measures but was not significantly different than the rg between suicide attempt and AUD or PAU (eTable 9 in Supplement 2). The genetic correlations of MaxAlc with depression (rg = 0.23; P = 8.40 × 10−16), PTSD (rg = 0.19; P = 4.75 × 10−5), and anxiety (rg = 0.15; P = 5.60 × 10−3) were also significantly different from those observed for alcohol consumption traits. No significant differences between MaxAlc and other consumption measures were observed for schizophrenia. Alcohol trait correlations with the core psychiatric traits included in the current study are reported in eTable 9 in Supplement 2.
Figure 2. Genetic Correlations Between Million Veteran Program (MVP) Maximum Habitual Alcohol Intake, Alcohol Traits, and Psychiatric Outcomes.
AUDIT-C indicates Alcohol Use Disorders Identification Test–Consumption; AUDIT-P, Alcohol Use Disorders Identification Test–Problems; AUDIT-T, Alcohol Use Disorders Identification Test–Total; GAD-2, 2-item Generalized Anxiety Disorder; GSCAN, GWAS and Sequencing Consortium of Alcohol and Nicotine use; ICD, International Classification of Diseases; PGC, Psychiatric Genomics Consortium; PTSD, posttraumatic stress disorder; and UKB, UK Biobank.
gSEM
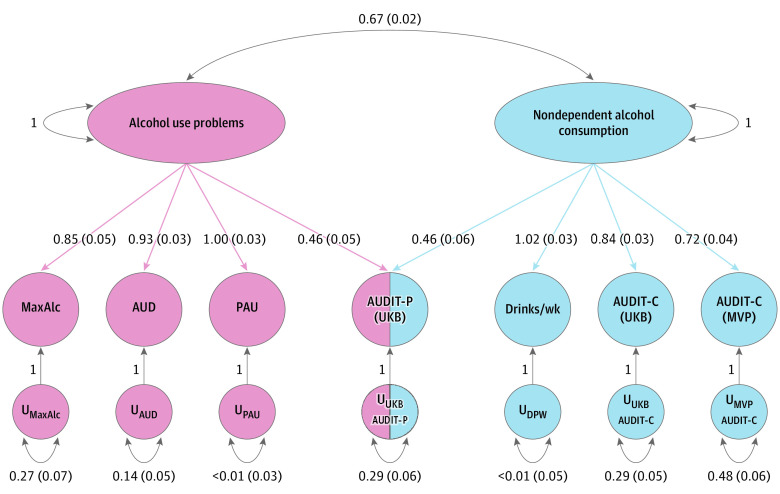
EFA and CFA were conducted using gSEM for MaxAlc and a range of previously published alcohol traits ranging from AUD to alcohol consumption (AUDIT-C and DPW). Using EFA to examine different models showed that a common factor model did not adequately capture the associations across all alcohol traits (cumulative variance explained, 0.59) (eTable 10 in Supplement 2). The cumulative variance explained by the 2-factor model was 0.82 (factor 1, 0.42; factor 2, 0.40), with high sum of squared (SS) loadings for both factor 1 (SS, 2.91) and factor 2 (SS, 2.83). The 3-factor model explained less variance than the 2-factor model (cumulative variance, 0.80; factor 1, 0.35; factor 2, 0.32; factor 3, 0.14) and had SS loadings of 2.42 (factor 1), 2.23 (factor 2), and 0.98 (factor 3). Based on these findings, factor 3 was not worth retaining given that the 3-factor model accounted for less variance and the SS loading of factor 3 was less than 1.0. The 2-factor model best fit the data and was evaluated further using CFA (eTable 10 in Supplement 2).
CFA converged on a 2-factor model (correlation, 0.67) and demonstrated strong fit across the included alcohol traits (comparative fit index [CFI], 0.98; χ2 = 238.38; Aikake information criterion [AIC], 270.38; standardized root mean square residual [SRMR], 0.05) (Figure 3; eTable 11 in Supplement 2). MaxAlc loaded most strongly on factor 1 (loading, 0.85 [SE, 0.05]) along with AUD (loading, 0.93 [SE, 0.03]) and PAU (loading, 1.00 [SE, 0.03]). MVP AUDIT-C (loading, 0.72 [SE, 0.04]), UKB AUDIT-C (loading, 0.84 [SE, 0.03]), DPW (loading, 1.02 [SE, 0.03]), and AUDIT-P (loading, 0.46 [SE, 0.06]) loaded most strongly on factor 2, although AUDIT-P also loaded equally well on factor 1 (loading, 0.46 [SE, 0.05]).
Figure 3. Genomic Structural Equation Modeling of Million Veteran Program (MVP) Maximum Habitual Alcohol Intake (MaxAlc) and Other Alcohol Use Traits.
Factor 1 (pink) was interpreted to capture alcohol use problems and factor 2 (blue), nondependent alcohol consumption. Values within the figure are loadings with SEs. AUD indicates alcohol use disorder; AUDIT-C, Alcohol Use Disorders Identification Test–Consumption; AUDIT-P, Alcohol Use Disorders Identification Test–Problems; PAU, problematic alcohol use; U, residual variance; UKB, UK Biobank.
MR Analyses
Of the 3 liver enzyme levels examined (ALP, ALT, and GGT), only GGT was significantly genetically correlated with MaxAlc (rg = 0.18; SE, 0.03; P = 1.17 × 10−7) (eTable 12 in Supplement 2). We thus focused MR analyses on the potential relationship between MaxAlc and GGT. Following the removal of 13 genetic variant outliers identified by MR-PRESSO, IVW methods indicated a small but statistically significant association between MaxAlc and GGT liver levels (β = 0.012; SE, 0.002; P = 2.66 × 10−10) (eFigures 9, 10, 11, and 12 in Supplement 1 and eTable 13 in Supplement 2). MR-RAPS results indicated that the effect estimates were not biased by the inclusion of SNVs of suggestive significance (eTable 14 in Supplement 2). Even after accounting for all SNV outliers, unexplained heterogeneity remained, potentially due to demographic differences between the 2 data sets used for the MR analysis (eTable 13 in Supplement 2).
Multitrait Analysis of MaxAlc and PAU
The MaxAlc MTAG analysis provided an increase in the European ancestry MaxAlc sample size from 218 623 (GWAS mean χ2 = 1.29) to an equivalent sample size of 353 981 (MTAG mean χ2 = 1.47), resulting in 31 independent genome-wide significant MaxAlc loci. The top association was with ADH1B rs1229984 variant (P = 3.35 × 10−176)(Table 1; eFigure 13 in Supplement 1). Results for the MTAG analysis of PAU also showed increased locus discovery (eFigure 14 in Supplement 1) and are reported in eTables 15, 16, and 17 in Supplement 1). Circos plots for chromosomes containing identified loci in the respective MaxAlc MTAG and MaxAlc GWAS are reported in eFigures 15 and 16 in Supplement 1.
Discussion
These findings highlight the additional information that can be gleaned from GWAS of habitual alcohol consumption traits that augment prior conclusions from GWAS of other alcohol consumption measures. We identified genome-wide significant associations with both novel and well-replicated alcohol use loci of relevance for heavy alcohol use (Table 2), observed differences in the genetic architecture of AUD and MaxAlc from that of other alcohol consumption traits, and provided insight into the genetic architecture of MaxAlc and its association to other psychiatric and medical conditions.
Table 2. Million Veteran Program MaxAlc Loci Summary Tablea.
| Position | MaxAlc wave 2 GWS | Ancestry | Nearest gene | Novel locus | MaxAlc wave 1 GWS | Alcohol use | PAU | AUDIT-P | AUDIT-C | DPW | Locus previously GWS for other related outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4:100239319 | rs1229984 | E | ADH1B | No | Yes | Yes | Yes | Yes | Yes | Yes | NA |
| 4:100229017 | rs2066702 | A | ADH1B | No | Yes | Yes | No | No | No | No | NA |
| 4:103188709 | rs13107325 | E | SLC39A8 | No | No | Yes | Yes | Yes | Yes | Yes | Sleep, schizophrenia |
| 17:43810896 | rs77804065 | E | CRHR1 | N | Yes | No | No | No | No | Yes | Neuroticism, feeling guilty |
| 11:47676170 | rs7107356 | E | AGBL2 | Yes | No | No | No | No | No | No | Neuroticism, well-being, prefers bitter alcohol beverage |
| 7:135162897 | rs2551763 | E | CNOT4 | Yes | No | No | No | No | No | No | Educational attainment |
| 1:7830262 | rs2153733 | E | CAMTA1 | Yes | No | No | No | No | No | No | Morningness |
| 19: 37504953 | rs826323 | C | ZNF420 | Yes | No | No | No | No | No | No | Body shape, body mass index |
| 10:10714452 | rs145961009 | E | RP1-186E20.1 | Yes | No | No | No | No | No | No | NA |
| 9:33094361 | rs56175925 | A | B4GALT1 | Yes | No | No | No | No | No | No | Aspartate aminotransferase levels |
| 9:30346448 | rs4879424 | E | snoU13 | Yes | No | No | No | No | No | No | NA |
| 10:110572259 | rs1577857 | E | RP11-655H13.2 | No | Yes | No | Yes | No | No | No | NA |
| 2:104056769 | rs6752675 | C | AC092568.1 | Yes | No | No | No | No | No | No | NA |
| 2:27730940 | rs1260326 | E | GCKR | No | No | Yes | Yes | No | Yes | Yes | NA |
| 11:113392994 | rs2514218 | C | DRD2 | No | No | Yes | Yes | No | No | No | Depression, neuroticism, well-being |
Abbreviations: A, African ancestry; AUDIT-C, Alcohol Use Disorders Identification Test–Consumption; AUDIT-P, Alcohol Use Disorders Identification Test–Problems; C, cross-ancestry; DPW, drinks per week; E, European ancestry; GWS, genome-wide significance; MaxAlc, maximum habitual alcohol intake; NA, not applicable; PAU, problematic alcohol use.
GWS set at P = 5.0 × 10−8.
The strongest MaxAlc associations were with the 2 functional ADH1B loci—rs1229984 in the European-ancestry analysis and rs2066702 in the African-ancestry analysis. These ADH1B loci, among the few that survived from the candidate gene era,1,33,34 were also identified as risk loci for alcohol dependence in the respective ancestry groups in a genome-wide significant context.35 ADH1B has been associated with many alcohol traits studied using GWAS, including our previous MaxAlc study.8
MaxAlc loci at ADH1B, CRHR1 (rs77804065), and RP11-655H13.2 (rs1577857), previously identified in European-ancestry or African-ancestry participants, were confirmed in the present study, but the locus at XPO7 (rs7821592), previously identified for MaxAlc in European-ancestry participants,8 fell below genome-wide significance; although XPO7 was genome-wide significant in the MaxAlc MTAG analysis. Additional genome-wide significant associations included loci previously associated with alcohol consumption,3,5,6 PAU,4 and AUD3 in SLC39A8 (rs13107325), GCKR (rs1260326), and DRD2 (rs2514218). A summary of genome-wide significant associations from the MaxAlc GWAS, including previous associations with other alcohol traits and relevant outcomes, is presented.
We also identified novel associations (Table 2). For example, the MaxAlc European-ancestry GWAS identified a genome-wide significant association (rs7107356) near AGBL2 (AGBL carboxypeptidase 2) on chromosome 11. AGBL2 was previously identified as a locus for bitter alcoholic beverage taste preference.36 Decreased perception of bitterness can result in an increase in alcohol consumption,37 and genetic influences for bitter taste preference may influence alcoholic beverage intake.38
A novel finding in the MaxAlc African-ancestry GWAS was the genome-wide significant association near B4GALT1 (BETA-1,4, galactrotransferase 1) on chromosome 9. B4GALT1 is a member of the galactosyltransferase gene family and encodes an enzyme related to both glycoconjugate and lactose biosynthesis.39 Glycoconjugate metabolism occurring in the liver is often altered in the presence of chronic alcohol use, and glycoconjugate-related biomarkers are often considered markers of excessive alcohol use (differentiating alcohol-related vs non–alcohol-related tissue damage).40 These findings show how MaxAlc GWAS can provide novel insight into the biology of alcohol use, including in non–European-ancestry populations.
LDSC and gSEM results provided insight into differences and similarities in genetic architecture across a broad spectrum of alcohol consumption measures and AUD. Based on genetic correlations, MaxAlc is genetically more similar to AUD and PAU than to measures of alcohol consumption and is distinct from less problematic drinking styles such as the UK Biobank measure of “having alcohol with meals.” MaxAlc, similar to AUD and PAU, demonstrated significant differences in genetic correlation with many psychiatric outcomes compared with the largely negative or near zero genetic correlations between psychopathology and other alcohol consumption measures. The strongest genetic correlation between MaxAlc and psychiatric traits was with suicide attempt, which is consistent with the positive phenotypic association between heavy alcohol consumption and suicide behaviors.41
Our gSEM showed that a 2-factor solution best fit the data, including 1 factor that we suggest captures alcohol use problems and a second factor that captures nondependent alcohol consumption. MaxAlc loaded most strongly on the alcohol use problems factor, along with AUD, PAU, and AUDIT-P (which loaded equally on both factors). Thus, MaxAlc, a measure of habitual alcohol consumption, aligns with traits that capture alcohol use problems rather than episode-limited measures of quantity or frequency of alcohol use. The genetic association between alcohol consumption and AUD thus appears dependent on the steadiness and heaviness of alcohol consumption, which in turn appears to reflect dependent use.
The genetic study of MaxAlc is also positioned to study the impact of habitual alcohol use on alcohol-related disease, such as liver function. Understanding the causal role of problematic alcohol use on alcohol-related medical conditions is important. Previous genetic studies have focused primarily on examining the associations of AUD diagnosis with disease,42 although we know that harmful levels of drinking can negatively impact health outcomes even if not meeting AUD diagnostic criteria.43 The MR findings from the current study suggested significant genetic overlap and a small causal role of habitual drinking on GGT levels. Clinically, GGT elevations can be pronounced in individuals with a history of heavy drinking and those with alcohol-related cirrhosis and can be valuable for detecting alcohol-induced liver disease.44 Genetically informed studies of MaxAlc can help understand the biological and medical consequences of habitual drinking.
Limitations
The current study has limitations. The MVP is a predominately male US military veteran sample, and the results reflect this demographic. Additionally, MaxAlc is a trait rather than a state measure, an advantage for genetic studies, but retrospective reports such as MaxAlc may be subject to longitudinal changes and reporting bias.45 MaxAlc also has sample size restrictions for non–European-ancestry participants, as is currently true for many genetic studies of complex traits.
Conclusions
The present study is valuable in understanding relevant mechanisms involved as normative alcohol consumption approaches habitual problematic use, and ultimately, AUD.46 These findings suggest that MaxAlc is genetically different from other measures of alcohol consumption and that genetic studies of heavier drinking can be scientifically and medically informative in relation to broader substance use problems, the presence of psychiatric comorbidities, and identifying relationships with alcohol-related disease. These efforts provide promise for the continued advancement of our understanding of genetic influences across the spectrum of alcohol consumption levels and AUD and how these levels of alcohol use relate to other psychiatric and health conditions.
eAppendix. Supplemental Methods
eReferences.
eFigure 1. Screenshot of the MVP MaxAlc Survey Item Captured From Million Veteran Program Lifestyle Survey
eFigure 2. Distribution of MVP Participant’s MaxAlc Survey Item Responses
eFigure 3. Manhattan Plot of European Ancestry MaxAlc GWAS
eFigure 4. Manhattan Plot of African Ancestry MaxAlc GWAS
eFigure 5. Manhattan Plot of European Ancestry MaxAlc Gene-Based Results
eFigure 6. Tissue Enrichment in European Ancestry MaxAlc Analysis
eFigure 7. Manhattan Plot of African Ancestry MaxAlc Gene-Based Results
eFigure 8. Tissue Enrichment in African Ancestry MaxAlc Analysis
eFigure 9. Mendelian Randomization, Scatter Plot
eFigure 10. Mendelian Randomization, Forest Plot
eFigure 11. Mendelian Randomization, Leave-one-out Plot
eFigure 12. Mendelian Randomization, Funnel Plot
eFigure 13. Manhattan Plot of MaxAlc MTAG GWAS Results
eFigure 14. Manhattan Plot of PAU MTAG GWAS Results
eFigure 15. Circos Plots for Chromosomes Containing Genome-Wide Significant Loci for the MaxAlc GWAS
eFigure 16. Circos Plots for Chromosomes Containing Genome-Wide Significant Loci for the MaxAlc MTAG GWAS
eTable 1. MaxAlc MVP Participant Demographics Table
eTable 2. MaxAlc European-Ancestry Gene Based Results
eTable 3. MaxAlc European-Ancestry Gene Set Results
eTable 4. MaxAlc European-Ancestry Tissue Enrichment Results
eTable 5. MaxAlc African-Ancestry Gene Based Results
eTable 6. MaxAlc African-Ancestry Gene Set Results
eTable 7. MaxAlc African-Ancestry Tissue Enrichment Results
eTable 8. MaxAlc Alcohol Trait Genetic Correlation Results
eTable 9. MaxAlc Alcohol Psychiatric Trait Genetic Correlation Results
eTable 10. Genomic-SEM Exploratory Factor Analysis (EFA) Results
eTable 11. Genomic-SEM Confirmatory Factor Analysis (CFA) Results
eTable 12. Mendelian Randomization (MR) MaxAlc Liver Enzyme Genetic Correlation Results
eTable 13. Mendelian Randomization (MR) Results
eTable 14. Mendelian Randomization Robust Adjusted Profile Score (MR-RAPs) Results
eTable 15. MaxAlc Multi-trait Analysis of GWAS Results (with Problematic Alcohol Use)
eTable 16. Problematic Alcohol Use Multitrait Analysis of GWAS Results (with MaxAlc)
eTable 17. MaxAlc and Problematic Alcohol Use Multitrait Analysis of GWAS Results Comparison
Nonauthor Collaborators
References
- 1.Deak JD, Miller AP, Gizer IR. Genetics of alcohol use disorder: a review. Curr Opin Psychol. 2019;27:56-61. doi: 10.1016/j.copsyc.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelernter J, Polimanti R. Genetics of substance use disorders in the era of big data. Nat Rev Genet. 2021;22(11):712-729. doi: 10.1038/s41576-021-00377-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kranzler HR, Zhou H, Kember RL, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 2019;10(1):1499. doi: 10.1038/s41467-019-09480-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H, Sealock JM, Sanchez-Roige S, et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat Neurosci. 2020;23(7):809-818. doi: 10.1038/s41593-020-0643-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Roige S, Palmer AA, Fontanillas P, et al. ; 23andMe Research Team, the Substance Use Disorder Working Group of the Psychiatric Genomics Consortium . Genome-wide association study meta-analysis of the Alcohol Use Disorders Identification Test (AUDIT) in two population-based cohorts. Am J Psychiatry. 2019;176(2):107-118. doi: 10.1176/appi.ajp.2018.18040369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu M, Jiang Y, Wedow R, et al. ; 23andMe Research Team; HUNT All-In Psychiatry . Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237-244. doi: 10.1038/s41588-018-0307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallard TT, Savage JE, Johnson EC, et al. Item-level genome-wide association study of the alcohol use disorders identification test in three population-based cohorts. Am J Psychiatry. 2022;179(1):58-70. doi: 10.1176/appi.ajp.2020.20091390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelernter J, Sun N, Polimanti R, et al. ; Department of Veterans Affairs Cooperative Studies Program (No. 575B); Million Veteran Program . Genome-wide association study of maximum habitual alcohol intake in >140,000 U.S. European and African American veterans yields novel risk loci. Biol Psychiatry. 2019;86(5):365-376. doi: 10.1016/j.biopsych.2019.03.984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutledge PC, Park A, Sher KJ. 21st Birthday drinking: extremely extreme. J Consult Clin Psychol. 2008;76(3):511-516. doi: 10.1037/0022-006X.76.3.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edenberg HJ. How much do you drink on your heavy drinking day? Biol Psychiatry. 2019;86(5):330-332. doi: 10.1016/j.biopsych.2019.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaziano JM, Concato J, Brophy M, et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214-223. doi: 10.1016/j.jclinepi.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen XT, Whitbourne SB, Li Y, et al. ; VA Million Veteran Program . Data resource profile: self-reported data in the Million Veteran Program: survey development and insights from the first 850 736 participants. Int J Epidemiol. Published online June 24, 2022. doi: 10.1093/ije/dyac133 [DOI] [PubMed] [Google Scholar]
- 13.Little J, Higgins JPT, Ioannidis JPA, et al. ; Strengthening the Reporting of Genetic Association Studies . Strengthening the Reporting of Genetic Association Studies (STREGA): an extension of the STROBE statement. PLoS Med. 2009;6(2):e22. doi: 10.1371/journal.pmed.1000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190-2191. doi: 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auton A, Brooks LD, Durbin RM, et al. ; 1000 Genomes Project Consortium . A global reference for human genetic variation. Nature. 2015;526(7571):68-74. doi: 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8(1):1826. doi: 10.1038/s41467-017-01261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulik-Sullivan BK, Loh P-R, Finucane HK, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291-295. doi: 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88(6):791-804. doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 20.Luo Y, Li X, Wang X, et al. ; 23 and Me Research Team; SIGMA Type 2 Diabetes Consortium . Estimating heritability and its enrichment in tissue-specific gene sets in admixed populations. Hum Mol Genet. 2021;30(16):1521-1534. doi: 10.1093/hmg/ddab130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey DF, Gelernter J, Polimanti R, et al. ; Million Veteran Program . Reproducible genetic risk loci for anxiety: results from ∼200,000 participants in the Million Veteran Program. Am J Psychiatry. 2020;177(3):223-232. doi: 10.1176/appi.ajp.2019.19030256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey DF, Stein MB, Wendt FR, et al. ; 23andMe Research Team; Million Veteran Program . Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat Neurosci. 2021;24(7):954-963. doi: 10.1038/s41593-021-00860-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein MB, Levey DF, Cheng Z, et al. ; Department of Veterans Affairs Cooperative Studies Program (no. 575B); VA Million Veteran Program . Genome-wide association analyses of post-traumatic stress disorder and its symptom subdomains in the Million Veteran Program. Nat Genet. 2021;53(2):174-184. doi: 10.1038/s41588-020-00767-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardiñas AF, Holmans P, Pocklington AJ, et al. ; GERAD1 Consortium; CRESTAR Consortium . Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50(3):381-389. doi: 10.1038/s41588-018-0059-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullins N, Kang J, Campos AI, et al. ; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium; Bipolar Disorder Working Group of the Psychiatric Genomics Consortium; Eating Disorders Working Group of the Psychiatric Genomics Consortium; German Borderline Genomics Consortium; MVP Suicide Exemplar Workgroup; VA Million Veteran Program . Dissecting the shared genetic architecture of suicide attempt, psychiatric disorders, and known risk factors. Biol Psychiatry. 2022;91(3):313-327. doi: 10.1016/j.biopsych.2021.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grotzinger AD, Rhemtulla M, de Vlaming R, et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat Hum Behav. 2019;3(5):513-525. doi: 10.1038/s41562-019-0566-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pazoki R, Vujkovic M, Elliott J, et al. ; Lifelines Cohort Study; VA Million Veteran Program . Genetic analysis in European ancestry individuals identifies 517 loci associated with liver enzymes. Nat Commun. 2021;12(1):2579. doi: 10.1038/s41467-021-22338-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13(11):e1007081. doi: 10.1371/journal.pgen.1007081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693-698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Q, Wang J, Hemani G, Bowden J, Small D. Statistical inference in two-sample summary-data mendelian randomization using robust adjusted profile score. Ann Stat. 2020;48(3):1742-1769. doi: 10.1214/19-AOS1866 [DOI] [Google Scholar]
- 31.Turley P, Walters RK, Maghzian O, et al. ; 23andMe Research Team; Social Science Genetic Association Consortium . Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018;50(2):229-237. doi: 10.1038/s41588-017-0009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Institute on Alcohol Abuse and Alcoholism (NIAAA) . NIAAA council approves definition of binge drinking. NIAAA Newsletter, No. 3, Winter 2004. Accessed April 20, 2022. https://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf
- 33.Edenberg HJ, McClintick JN. Alcohol dehydrogenases, aldehyde dehydrogenases, and alcohol use disorders: a critical review. Alcohol Clin Exp Res. 2018;42(12):2281-2297. doi: 10.1111/acer.13904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomasson HR, Edenberg HJ, Crabb DW, et al. Alcohol and aldehyde dehydrogenase genotypes and alcoholism in Chinese men. Am J Hum Genet. 1991;48(4):677-681. [PMC free article] [PubMed] [Google Scholar]
- 35.Gelernter J, Kranzler HR, Sherva R, et al. Genome-wide association study of alcohol dependence: significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2014;19(1):41-49. doi: 10.1038/mp.2013.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong VW, Kuang A, Danning RD, et al. A genome-wide association study of bitter and sweet beverage consumption. Hum Mol Genet. 2019;28(14):2449-2457. doi: 10.1093/hmg/ddz061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanier SA, Hayes JE, Duffy VB. Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiol Behav. 2005;83(5):821-831. doi: 10.1016/j.physbeh.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 38.Ong J-S, Hwang L-D, Zhong VW, et al. Understanding the role of bitter taste perception in coffee, tea and alcohol consumption through mendelian randomization. Sci Rep. 2018;8(1):16414. doi: 10.1038/s41598-018-34713-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hennet T. The galactosyltransferase family. Cell Mol Life Sci. 2002;59(7):1081-1095. doi: 10.1007/s00018-002-8489-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waszkiewicz N, Szajda SD, Kępka A, Szulc A, Zwierz K. Glycoconjugates in the detection of alcohol abuse. Biochem Soc Trans. 2011;39(1):365-369. doi: 10.1042/BST0390365 [DOI] [PubMed] [Google Scholar]
- 41.Borges G, Bagge CL, Cherpitel CJ, Conner KR, Orozco R, Rossow I. A meta-analysis of acute use of alcohol and the risk of suicide attempt. Psychol Med. 2017;47(5):949-957. doi: 10.1017/S0033291716002841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards AC, Sundquist K, Sundquist J, Kendler KS, Larsson Lönn S. Genetic and environmental influences on the progression from alcohol use disorder to alcohol-related medical conditions. Alcohol Clin Exp Res. 2021;45(12):2528-2535. doi: 10.1111/acer.14731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boccuto L, Abenavoli L. Genetic and epigenetic profile of patients with alcoholic liver disease. Ann Hepatol. 2017;16(4):490-500. doi: 10.5604/01.3001.0010.0274 [DOI] [PubMed] [Google Scholar]
- 44.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38(4):263-355. doi: 10.1080/20014091084227 [DOI] [PubMed] [Google Scholar]
- 45.Xue A, Jiang L, Zhu Z, et al. Genome-wide analyses of behavioural traits are subject to bias by misreports and longitudinal changes. Nat Commun. 2021;12(1):20211. doi: 10.1038/s41467-020-20237-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kember RL, Vickers-Smith R, Zhou H, et al. Genetic underpinnings of the transition from alcohol consumption to alcohol use disorder: shared and unique genetic architectures in a cross-ancestry sample. medRxiv. Preprint posted online September 13, 2021. doi: 10.1101/2021.09.08.21263302 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Methods
eReferences.
eFigure 1. Screenshot of the MVP MaxAlc Survey Item Captured From Million Veteran Program Lifestyle Survey
eFigure 2. Distribution of MVP Participant’s MaxAlc Survey Item Responses
eFigure 3. Manhattan Plot of European Ancestry MaxAlc GWAS
eFigure 4. Manhattan Plot of African Ancestry MaxAlc GWAS
eFigure 5. Manhattan Plot of European Ancestry MaxAlc Gene-Based Results
eFigure 6. Tissue Enrichment in European Ancestry MaxAlc Analysis
eFigure 7. Manhattan Plot of African Ancestry MaxAlc Gene-Based Results
eFigure 8. Tissue Enrichment in African Ancestry MaxAlc Analysis
eFigure 9. Mendelian Randomization, Scatter Plot
eFigure 10. Mendelian Randomization, Forest Plot
eFigure 11. Mendelian Randomization, Leave-one-out Plot
eFigure 12. Mendelian Randomization, Funnel Plot
eFigure 13. Manhattan Plot of MaxAlc MTAG GWAS Results
eFigure 14. Manhattan Plot of PAU MTAG GWAS Results
eFigure 15. Circos Plots for Chromosomes Containing Genome-Wide Significant Loci for the MaxAlc GWAS
eFigure 16. Circos Plots for Chromosomes Containing Genome-Wide Significant Loci for the MaxAlc MTAG GWAS
eTable 1. MaxAlc MVP Participant Demographics Table
eTable 2. MaxAlc European-Ancestry Gene Based Results
eTable 3. MaxAlc European-Ancestry Gene Set Results
eTable 4. MaxAlc European-Ancestry Tissue Enrichment Results
eTable 5. MaxAlc African-Ancestry Gene Based Results
eTable 6. MaxAlc African-Ancestry Gene Set Results
eTable 7. MaxAlc African-Ancestry Tissue Enrichment Results
eTable 8. MaxAlc Alcohol Trait Genetic Correlation Results
eTable 9. MaxAlc Alcohol Psychiatric Trait Genetic Correlation Results
eTable 10. Genomic-SEM Exploratory Factor Analysis (EFA) Results
eTable 11. Genomic-SEM Confirmatory Factor Analysis (CFA) Results
eTable 12. Mendelian Randomization (MR) MaxAlc Liver Enzyme Genetic Correlation Results
eTable 13. Mendelian Randomization (MR) Results
eTable 14. Mendelian Randomization Robust Adjusted Profile Score (MR-RAPs) Results
eTable 15. MaxAlc Multi-trait Analysis of GWAS Results (with Problematic Alcohol Use)
eTable 16. Problematic Alcohol Use Multitrait Analysis of GWAS Results (with MaxAlc)
eTable 17. MaxAlc and Problematic Alcohol Use Multitrait Analysis of GWAS Results Comparison
Nonauthor Collaborators