Abstract
SARS-CoV-2 has caused a global pandemic and an unprecedented public health crisis, infecting more than 580 million people worldwide. Moreover, recent evidence has suggested the emergence of a new syndrome known as Long-COVID, a term used to describe a diverse set of physical and mental symptoms that persist after a diagnosed SARS-CoV-2 infection. Epidemiological data have identified myalgias, muscle and joint dysfunction, and bone fragility as common sequelae in patients with moderate and severe forms of this disease. Significant musculoskeletal dysfunction has also been detected in some healed patients, although knowledge about pathophysiological mechanisms of Long-COVID is still rather scarce. In this context, SARS-CoV-2 infection has been suggested to amplify the effects of aging on the musculoskeletal system by aggravating the osteosarcopenic state. Based on this evidence, our review focused on the muscle and bone tissue alterations induced by SARS-CoV-2 infection and Long-COVID, summarizing the current knowledge on the underlying biological mechanisms and highlighting the need for a multidisciplinary approach to predict the musculoskeletal targets and long-term consequences of COVID-19 disease.
Keywords: Aging, Long-COVID, musculoskeletal system, osteosarcopenia, SARS-CoV-2 infection
Introduction
The new coronavirus SARS-CoV-2, which suddenly emerged in December 2019 in the city of Wuhan, is still haunting the entire human race, affecting not only health, but also global socioeconomic systems. According to the most recent estimates provided by the World Health Organization (WHO), there have been approximately 580 million confirmed cases and around 6.5 million confirmed deaths worldwide since the pandemic began, highlighting how the SARS-CoV-2 outbreak is a true health emergency of international significance.1
The clinical manifestations of SARS-CoV-2 are not yet fully known, but symptoms vary from asymptomatic or mild to severe. Moreover, susceptibility to SARS-CoV-2 infection appears not to vary with age or gender. However, several evidence suggest that among all known patients, those who are older, male and have underlying comorbidities are more likely to have a more severe course of COVID-19, and a higher risk of developing complications.2
Undoubtedly, aging has been associated with worse outcomes, due to pathophysiological changes in the respiratory system and, especially, the upper airways, considered the entry site for SARS-CoV-2 infection.3 Coronavirus infectivity has been found to depend on its entry via the binding of its viral spike (S) protein to the receptor of angiotensin-converting enzyme 2 (ACE2) and the triggering of the S protein by the type II transmembrane serine protease (TMPRSS2). Interestingly, a decrease in ACE2 activity is known to increase the severity of lung lesions.4,5 Particularly, a strong association was found between reduced ACE2 levels in older patients and greater severity of disease, which in some cases progressed to acute respiratory distress syndrome (ARDS).6 In fact, according to data provided by the Istituto Superiore di Sanità (ISS) in Italy, ARDS was observed in approximately 97% of patients who died in hospital, who were predominantly elderly and with acute heart, liver and kidney function disorders.7–9
Interestingly, a possible gender difference in the susceptibility and progression of COVID-19 has been proposed to depend on the ACE2 localization on the X chromosome.10 In this regard, Fan et al.11 recently observed that gender significantly influences the aberrant methylation of the ACE2 promoter, possibly making men more susceptible to the risk of COVID-19 infection. In contrast, other evidence has found no significant differences in ACE2 expression between males and females, making this hypothesis less likely.12
The rapid identification of people at risk and the management of infected patients have been key to slowing the spread of COVID-19 worldwide, through a pathway based on early identification, immediate isolation, implementation of appropriate infection prevention and control measures, and provision of optimized supportive care.13,14 However, the emergence of many new variants of concern over time has increased the transmissibility or virulence of SARS-CoV-2, suggesting the need to develop efficient public health measures and vaccination programmes through a timely and science-based global response.15,16 Indeed, thanks to the relentless efforts of scientific research, a clear picture of the structure and genetic composition of SARS-CoV-2 is now known, and its host source, epidemiological characteristics and the histopathological changes that occur in lung tissue in response to infection.17–20 However, many questions remain unanswered, including the production of new strains and the mechanisms of infection spread to other target tissues, including the heart, intestine, kidney, bone, muscle, skin, liver, and some brain areas, with pathological changes very similar to those found in severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS) viral infections.21
In this context, the aim of our review was to summarize the current scientific knowledge on how SARS-CoV-2 infection could amplify the effects of aging, especially on the musculoskeletal system, and exacerbate the osteosarcopenic state, characterized by concomitant bone loss and muscle atrophy, suggesting the need for a multidisciplinary approach to help predict the musculoskeletal targets and long-term consequences of COVID-19 disease.
Literature search strategy
For this narrative review, 98 articles were selected from the MEDLINE bibliographic database, published between 1945 (start date) and 2022. Articles concerning SARS-Cov-2 infection and, specifically, the effects of Long-COVID on the musculoskeletal system were included. The search strategy was based on the use or combination of the following keywords: ‘SARS-CoV-2 infection’; ‘Long-COVID’; ‘musculoskeletal system’; ‘osteoporosis’; ‘sarcopenia’; ‘osteosarcopenia’; ‘aging’; ‘fragility fractures’; ‘prevention’. The search process was carried out on a worldwide basis, without excluding specific geographical areas or different ethnic groups. Language and species filters were applied to the list of results to eliminate non-English articles.
The musculoskeletal system as a potential target
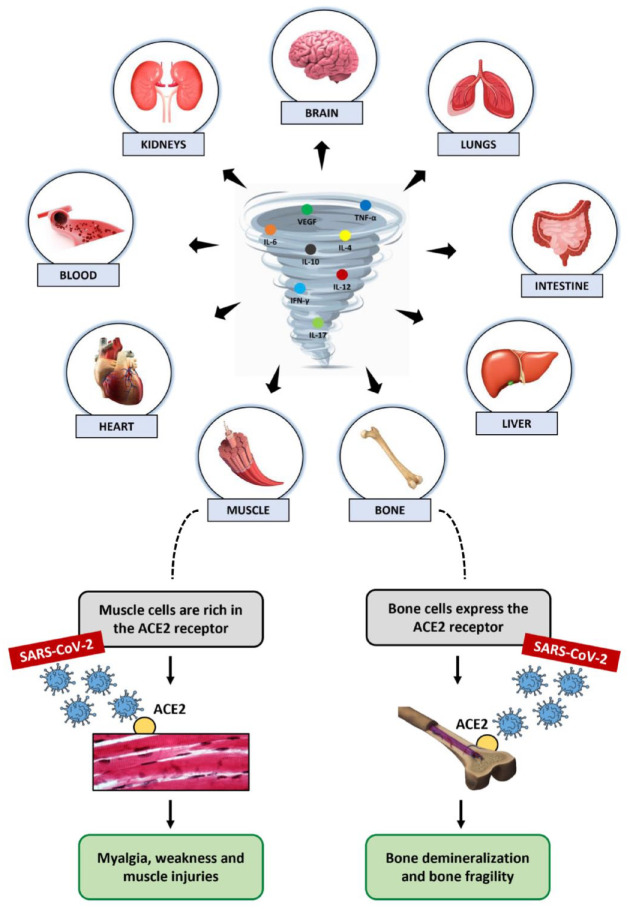
SARS-CoV-2 infection causes severe acute inflammation, triggering a storm of cytokines and inducing multi-organ damage that also involves the musculoskeletal system (Figure 1).22–24 According to Goshua et al.,25 such injury could be mediated by endothelial damage and a propensity for micro- and macro-thrombosis, through indirect mechanisms responsible for cytokine release and subsequent homeostatic disruption. Alternatively, Ferrandi and colleagues proposed that the inflammation of musculoskeletal tissue could depend on a direct interaction of skeletal muscle cells with SARS-CoV-2 via ACE2.26 Indeed, the ACE2 expression in skeletal muscle is well documented and its potential susceptibility to coronavirus is partially supported by clinical evidence pointing to pain, weakness and skeletal muscle injury as common symptoms of COVID-19 disease.27–29 In addition, several pro-inflammatory cytokines, such as interferon gamma (IFN-γ), interleukin 1 beta (IL-1β), interleukin 6 (IL-6), interleukin 17 (IL-17) and tumour necrosis factor alpha (TNF-α), whose levels were found to be elevated in COVID-19 patients, could directly induce proteolysis of muscle fibres and decrease protein synthesis, with a significant impact on skeletal muscle.30,31 Particularly, IL-1β and TNF-α are well known to inhibit satellite cell proliferation and differentiation, just as IL-6 is known to promote muscle fibroblast activity and lead to fibrosis, impairing muscle function and increasing susceptibility to injury.32–34
Figure 1.
SARS-CoV-2 infection is responsible for severe acute inflammation, triggering a storm of cytokines and inducing multi-organ damage. The musculoskeletal system is also one of the target tissues infected by the virus. The ACE2 expression in muscle and bone cells could promotes virus binding, causing myalgia, weakness, and injury in muscles, and demineralization and fragility in bones.
Among the possible damage caused by the cytokine storm, elevated c-reactive protein (CRP) concentration and subsequent mitochondrial damage have also been correlated with the onset of sarcopenia and frailty in COVID-19 patients.35 In fact, a role for systemic inflammation has also been proposed in the involvement of bone and joint tissue in infected patients, although the underlying mechanisms are still largely unknown.31 Specifically, the inflammatory cytokines detected in large quantities in COVID-19 patients with a severe disease course, such as IL-1β, IL-6, TNF-α, granulocyte colony-stimulating factor (G-CSF), interferon gamma-induced protein 10 (IP-10) and monocyte chemoattractant protein-1 (MCP-1), are known to be mediators normally involved in bone turnover, with a role in osteoclast formation and increased bone resorption capacity via the receptor activator of nuclear-factor κB (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) pathway.17 In this regard, Zheng et al.36 suggested that the evolution of inflammation from acute to chronic could promote the recruitment of osteoclasts by inflammatory cytokines, reducing osteoblastogenesis and triggering bone loss. In addition, IL-1β, IL-6, IL-17 and TNF-α could induce chondrolysis, causing arthralgia and osteoarthritis, and impair the normal biological activity of tenocytes, altering matrix remodelling and exacerbating tendon degenerative disorders.
Long-COVID: epidemiology, pathogenesis and clinical manifestations
People infected with SARS-CoV-2 commonly develop symptoms a few days after exposure, and most show complete recovery within a few weeks after infection. However, ongoing follow-up of patients healed of COVID-19 has shown that one or more symptoms persist in a substantial proportion of people, even weeks or months after recovery.37,38 In this regard, an Italian patient in May 2020 used the term ‘Long-COVID’ to describe the persistence of symptoms weeks or months after the initial SARS-CoV-2 infection. Since then, it has passed through various social media outlets to clinical experts and formal policymakers, acquiring a reasonable consistency. Thus, the phenomenon of Long-COVID, or post-COVID syndrome, has become an emerging problem and, according to the most recent estimates, could produce another public health crisis like the pandemic itself.39,40
The follow-up of patients healed of infection allowed the identification of some factors commonly associated with the Long-COVID development. Among these, age and gender seem to play a key role, as patients with persistent symptoms were generally men about 4 years older than those without symptoms.41 The presence of comorbidities and the number of symptoms characterizing the acute phase of the disease have also been suggested to increase the risk of Long-COVID.42
Several scientific evidences have shown a prevalence of Long-COVID after acute SARS-CoV-2 infection of between 10% and 30%.37 Specifically, Dennis et al.43 conducted a prospective observational cohort study to assess medium-term organ impairment in individuals with a mean age of 45 years and persistent symptoms after initial recovery from acute SARS-CoV-2 infection compared to healthy age-matched controls. Of a total of 201 patients, 42% had 10 or more Long-COVID symptoms 4 months after initial SARS-CoV-2 infection, including fatigue, muscle pain, breathlessness and headache, while 60% had severe Long-COVID symptoms associated with multi-organ impairment. In agreement, other studies reported persistence of symptoms in 32% of COVID-19 outpatients from Switzerland and 83% of COVID-19 inpatients from Italy.44 Daugherty and colleagues’ retrospective analysis of 193,113 patients also identified more than 50 clinical sequelae, or types of Long-COVID, during the 4 months following the acute illness. Interestingly, 14% of patients aged ⩽ 65 years developed a new clinical condition requiring medical care, suggesting that although older people with pre-existing conditions and hospitalized due to COVID-19 were at higher risk, younger people without pre-existing conditions or not hospitalized for the infection also had an increased risk of Long-COVID.45 Finally, although most evidence reports the development of chronic persistent symptoms mostly in adult individuals above 40 years of age, the Long-COVID phenomenon has surprisingly also been found in asymptomatic children. In this regard, Buonsenso et al.46 reported that out of a total of 129 children aged ⩽ 18 years with a diagnosis of COVID-19, of whom 33 were asymptomatic and 96 symptomatic, 2.3% developed multi-system inflammatory syndrome in children (MIS-C) and 1.6% developed myocarditis, 35.7% had one or two Long-COVID symptoms, 22.5% had three or more symptoms and 41.8% recovered completely.
The pathophysiological mechanisms of Long-COVID are still largely unknown, and several hypotheses have been put forward in this field. Among these, there is complete agreement that SARS-CoV-2 infects the organism after exploiting its tropism in several tissues. This results in immune system dysfunction, increased inflammatory processes and impairment of several other systems, leading to Long-COVID.47,48 Particularly, Khazaal and colleagues suggested that the virus persistence in the body after the acute phase is the main responsible, as the virus would appear not to be eliminated in Long-COVID patients, but rather could reactivate with harmful effects that persist over time.49 The multi-organ and multi-system impact of the virus is closely dependent on the ACE2 expression in many cell types, including neurons, astrocytes, oligodendrocytes and microglial cells.50 Such viral neurotropism could contribute to the Long-COVID pathophysiology, potentially explaining olfactory disorders or manifestations related to autonomic nervous system dysfunction.51 Second, Baig52 proposed that oxidative stress and inflammation are responsible for a weak immunological response and incomplete virus eradication. This hypothesis, confirmed by the identification of viral particles in various organs after acute infection, could explain the onset of deleterious disorders, including autoimmune manifestations, activation of the coagulation and fibrosis pathways and metabolic disorders.53,54 Finally, deconditioning and psychological problems, such as post-traumatic stress disorder (PTSD), may also contribute to the Long-COVID development.55
Long-term consequences of COVID-19: a shortcut to osteosarcopenia
Osteosarcopenia is a newly defined syndrome that describes the coexistence of sarcopenia and osteoporosis, two age-related musculoskeletal disorders increasingly recognized to be associated with significant morbidity, mortality and socioeconomic costs.56 The pathogenesis of osteosarcopenia is multifactorial, with several mechanical, biochemical, genetic and lifestyle factors all contributing to the regulation of the muscle-bone unit.57 The balance between these factors is crucial, and a disruption of it leads to innumerable bone and muscle risks and complications, causing deterioration of bone microarchitecture in association with the loss of muscle mass, strength and function.58–60 In this context, SARS-CoV-2 infection has been suggested to amplify the effects of aging on the musculoskeletal system by aggravating the osteosarcopenic state. This is true both for COVID-19 patients with a severe disease course, where prolonged intensive care unit induced loss of muscle mass and strength, weakness and persistent pain, and for all other patients, where reduced physical activity and walking due to restriction measures increased muscle atrophy and the risk of fragility fractures.61
Particularly, the COVID-19 pandemic has been suggested to have caused an increase in risk factors for the incidence and progression of sarcopenia, especially in the elderly, such as decreased physical activity and unhealthy eating habits caused by social isolation.62–65 The inflammatory reaction caused by COVID-19 has also been correlated with metabolic stress and muscle catabolism.66,67 However, as suggested by Wang and colleagues, the interaction between COVID-19 and sarcopenia could be bidirectional and trigger a vicious circle.68 In fact, the sarcopenic condition is known to be associated with chronic inflammation, malnutrition, metabolic and endocrine dysregulation, and various systemic dysfunctions, thus representing a key risk factor in the increased vulnerability of the elderly to COVID-19.69,70
The negative impact of sarcopenia on the COVID-19 treatment was partially supported by some indirect evidence showing that sarcopenic patients had impaired respiratory muscle strength and respiratory function, which was detrimental to the treatment of severe pneumonia and ARDS. 71 In addition, Okazaki and colleagues observed that sarcopenia is a risk factor for aspiration pneumonia in the elderly adult population due to dysfunction of the swallowing muscles, worsening the condition of bedridden patients with SARS-CoV-2 infection.72 Therefore, it is to be expected that sarcopenic patients have higher infection and mortality rates in association with greater disease severity.
Myalgias and generalized weakness were found in a quarter to a half of symptomatic COVID-19 patients.73,74 Although a correlation between the onset of muscle pain and disease severity has not been found, computed tomography (CT) and X-ray imaging investigations have shown that muscle pain on admission is associated with abnormal images of the lung and predicts a poor prognosis, especially in the elderly, suggesting myalgia as an important predictor for disease severity.75 Furthermore, several evidences suggested that myalgia, which occurs at the onset of SARS-CoV-2 infection, may not disappear in the acute phase of the disease, becoming a predominant feature of Long-COVID.76–80 In this regard, longitudinal studies conducted in Turkey, France and Italy showed that 6 months after discharge, approximately 60% of patients still suffered from at least one SARS-CoV-2-related symptom, of which the most common were fatigue, myalgia and joint pain with an average prevalence of 30%, 20% and 15%, respectively.81,82 Noteworthy, recent surveys have suggested that between 4.6% and 12.1% of patients still suffer from joint pain during the first year after infection, and that Long-COVID pain is associated with fatigue and is more prevalent in women.83,84
An emerging feature of COVID-19 is a clinically relevant osteo-metabolic phenotype characterized by acute diffuse hypocalcemia and chronic hypovitaminosis D, and a high prevalence of morphometric vertebral fractures.85 This phenotype has been associated with a more severe form of the disease and higher mortality, confirming a role for SARS-CoV-2 also on bone tissue and mineral metabolism.86 Specifically, hypocalcemia was found in 62.6–87.2% of COVID-19 patients, in association with increased markers of inflammation and thrombosis, a greater need for hospitalization and a higher mortality rate.87 Low calcium levels have been mainly related to malnutrition and hypovitaminosis D, which in turn is known to be associated with a significantly higher mortality risk in COVID-19 patients due to its immunomodulating action on the immune system.88 Therefore, immobilization due to hospitalization related to SARS-CoV-2 infection or isolation and quarantine, in combination with vitamin D deficiency and hypocalcemia, are all factors that could contribute to bone demineralization and the subsequent onset of osteoporosis.
Table 1 summarizes all the proposed mechanisms that correlate with worsening osteosarcopenic status in COVID-19 patients.
Table 1.
A summary table on possible mechanisms of osteosarcopenia in Long-COVID and possible prevention and treatment strategies.
| References | Possible mechanisms aggravating osteosarcopenic status in COVID-19 patients | Possible prevention and treatment strategies |
|---|---|---|
| Suzuki et al.,62
Yamada et al.,63 Ali and Kunugi,64 Visser et al.65 |
Increased risk factors for osteosarcopenia, such as reduced physical activity and unhealthy eating habits caused by social isolation | Adequate protein support along with aerobic and endurance exercise preserves skeletal muscle mass Adequate rehabilitation improves the respiratory, functional and cognitive condition of patients, improving quality of life and reducing hospitalization |
| Meftahi et al.,66 Bano et al.67 | The inflammatory reaction associated with metabolic stress and muscle catabolism | |
| Zhang et al.75 | Myalgia is an important predictor of COVID-19 disease severity, given the association found between muscle pain on admission and pulmonary abnormalities in the elderly | |
| di Filippo et al.85 | A clinically relevant osteo-metabolic phenotype appeared, characterized by acute diffuse hypocalcemia, chronic hypovitaminosis D and a high prevalence of morphometric vertebral fractures | |
| di Filippo et al.87 | Hypocalcemia has been associated with increased markers of inflammation and thrombosis, increased need for hospitalization and higher mortality rates | |
| Carpagnano et al.88 | Immobilization due to hospitalization for SARS-CoV-2 infection or isolation and quarantine, in combination with vitamin D deficiency, are all factors that could contribute to bone demineralization |
Long-COVID-related osteosarcopenia: possible prevention and treatment strategies
The clinical management of patients with osteosarcopenia is already complex and neglected under normal circumstances, as there is still no specific diagnostic and therapeutic protocol for the condition due to its recent definition. However, a new potential predictive model has recently been identified on the basis of the correlation between the variables T-score and Handgrip test, suggesting it as a new parameter for a faster and more targeted diagnosis of osteosarcopenia.89
Undoubtedly, prevention is fundamental and represents a goal that, if achieved, makes it possible not only to improve individuals’ quality of life but also to reduce the socioeconomic and health costs associated with such a widespread disease.90 The osteosarcopenia development can be prevented by acting on modifiable risk factors. Indeed, a healthy lifestyle and regular exercise are currently the first-line choices for the prevention and treatment of osteosarcopenia (Table 1).91 These recommendations are the same as those provided by the clinical guidelines for the management of COVID-19 or post-COVID syndrome patients. Indeed, since SARS-CoV-2 infection has been suggested to cause muscle wasting and bone demineralization, several evidences agree that interventions targeting musculoskeletal tissue could break the vicious circle and benefit the treatment of both COVID-19 disease and osteoporosis and sarcopenia (Figure 2).68 In this regard, aerobic and resistance exercises are known to improve muscle protein synthesis by sensitizing muscles to insulin- or amino acid-mediated anabolic actions.92,93 Furthermore, adequate protein support together with physical activity successfully preserves skeletal muscle mass.94,95 Finally, adequate rehabilitation has been recommended to prevent the development of Long-COVID already in intensive care units, as soon as sedation and clinical stability permit. Indeed, pulmonary rehabilitation is known to improve patients’ breathing, exercise capacity, muscle strength, quality of life and functional outcome. In association, early mobilization could improve respiratory, functional and cognitive conditions in these patients, even reducing hospital stay.96,97 Similarly, adequate physical rehabilitation is recommended for non-hospitalized Long-COVID patients to improve their ability to engage in activities of daily living.98
Figure 2.
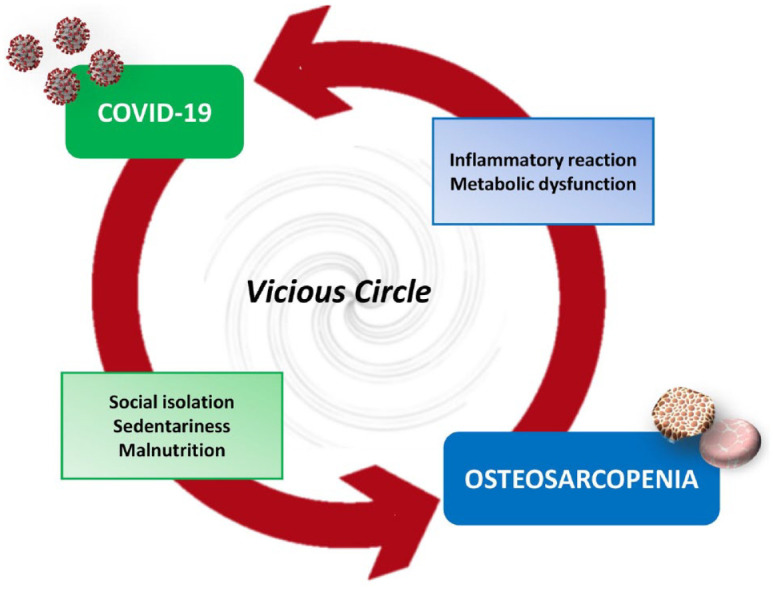
The interaction between COVID-19 and osteosarcopenia could be bidirectional and trigger a vicious circle. Indeed, factors associated with SARS-CoV-2 infection, such as social isolation, sedentariness and malnutrition, are known to promote osteosarcopenia. Conversely, events triggering osteosarcopenia, such as inflammatory reaction and metabolic dysfunction, are also factors associated with COVID-19 disease.
Conclusion
The continued growth of our experience with SARS-CoV-2 is closely associated with a better understanding of the short- and long-term complications caused by COVID-19 disease. Undoubtedly, the onset of musculoskeletal dysfunction as a sequelae of SARS-CoV-2 infection and the potential development of osteosarcopenia that follows may adversely affect the clinical course in older individuals, thus increasing the already high burden of the disease and underlining the need for appropriate patient assessment, including personalized rehabilitation and dietary approaches. Indeed, therapeutic options are currently limited, as the understanding of the biological mechanisms underlying Long-COVID is insufficient. However, most evidence agrees with the adoption of a multidisciplinary approach for long-term monitoring of ongoing symptoms, so that musculoskeletal targets can be predicted and potential complications can be identified. Further studies will be necessary for a deeper understanding of musculoskeletal changes and underlying determinants to alleviate clinical symptoms and improve quality of life in COVID-19 or Long-COVID patients.
Acknowledgments
The authors thank the Italian Study Group in Orthopaedics of Severe Osteoporosis (GISOOS) for supporting this work.
Footnotes
ORCID iDs: Virginia V. Visconti  https://orcid.org/0000-0002-4543-7770
https://orcid.org/0000-0002-4543-7770
Ida Cariati  https://orcid.org/0000-0002-2102-5034
https://orcid.org/0000-0002-2102-5034
Contributor Information
Umberto Tarantino, Department of Clinical Sciences and Translational Medicine, University of Rome ‘Tor Vergata’, Rome, Italy; Department of Orthopaedics and Traumatology, ‘Policlinico Tor Vergata’ Foundation, Rome, Italy.
Virginia V. Visconti, Department of Clinical Sciences and Translational Medicine, University of Rome ‘Tor Vergata’, Rome, Italy
Roberto Bonanni, Department of Clinical Sciences and Translational Medicine, University of Rome ‘Tor Vergata’, Rome, Italy.
Andrea Gatti, Department of Orthopaedics and Traumatology, ‘Policlinico Tor Vergata’ Foundation, Rome, Italy.
Martina Marcozzi, Department of Orthopaedics and Traumatology, ‘Policlinico Tor Vergata’ Foundation, Rome, Italy.
Davide Calabrò, Department of Orthopaedics and Traumatology, ‘Policlinico Tor Vergata’ Foundation, Rome, Italy.
Ida Cariati, Department of Clinical Sciences and Translational Medicine, University of Rome ‘Tor Vergata’, Via Montpellier 1, 00133 Rome, Italy.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Umberto Tarantino: Conceptualization; Writing – original draft; Writing – review & editing.
Virginia Veronica Visconti: Data curation; Investigation; Methodology.
Roberto Bonanni: Data curation; Investigation; Methodology.
Andrea Gatti: Investigation; Visualization.
Martina Marcozzi: Investigation; Visualization.
Davide Calabrò: Investigation; Visualization.
Ida Cariati: Conceptualization; Data curation; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. Long B, Carius BM, Chavez S, et al. Clinical update on COVID-19 for the emergency clinician: presentation and evaluation. Am J Emerg Med 2022; 54: 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Libertini G, Corbi G, Cellurale M, et al. Age-related dysfunctions: evidence and relationship with some risk factors and protective drugs. Biochemistry 2019; 84: 1442–1450. [DOI] [PubMed] [Google Scholar]
- 4. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lan J, Ge J, Yu J, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020; 581: 215–220. [DOI] [PubMed] [Google Scholar]
- 6. AlGhatrif M, Cingolani O, Lakatta EG. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: insights from cardiovascular aging science. JAMA Cardiol 2020; 5: 747–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lippi G, Mattiuzzi C, Sanchis-Gomar F, et al. Clinical and demographic characteristics of patients dying from COVID-19 in Italy vs China. J Med Virol 2020; 92: 1759–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J 2020; 55: 2000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu K, Chen Y, Lin R, et al. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect 2020; 80: e14–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jin JM, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health 2020; 8: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan R, Mao SQ, Gu TL, et al. Preliminary analysis of the association between methylation of the ACE2 promoter and essential hypertension. Mol Med Rep 2017; 15: 3905–3911. [DOI] [PubMed] [Google Scholar]
- 12. Mjaess G, Karam A, Aoun F, et al. COVID-19 and the male susceptibility: the role of ACE2, TMPRSS2 and the androgen receptor. Prog Urol 2020; 30: 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu J, Liu S. The management of coronavirus disease 2019 (COVID-19). J Med Virol 2020; 92: 1484–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alavi-Moghaddam M. A novel coronavirus outbreak from Wuhan city in china, rapid need for emergency departments preparedness and response; a letter to editor. Arch Acad Emerg Med 2020; 8: e12. [PMC free article] [PubMed] [Google Scholar]
- 15. Krause PR, Fleming TR, Longini IM, et al. SARS-CoV-2 variants and vaccines. N Engl J Med 2021; 385: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boehm E, Kronig I, Neher RA, et al. Novel SARS-CoV-2 variants: the pandemics within the pandemic. Clin Microbiol Infect 2021; 27: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lam TT, Jia N, Zhang YW, et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020; 583: 282–285. [DOI] [PubMed] [Google Scholar]
- 19. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020; 63: 457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8: 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tabary M, Khanmohammadi S, Araghi F, et al. Pathologic features of COVID-19: a concise review. Pathol Res Pract 2020; 216: 153097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324: 782–793. [DOI] [PubMed] [Google Scholar]
- 23. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020; 75: 1564–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol 2021; 93: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 2020; 7: e575–e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrandi PJ, Alway SE, Mohamed JS. The interaction between SARS-CoV-2 and ACE2 may have consequences for skeletal muscle viral susceptibility and myopathies. J Appl Physiol 2020; 129: 864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Motta-Santos D, Dos Santos RA, Oliveira M, et al. Effects of ACE2 deficiency on physical performance and physiological adaptations of cardiac and skeletal muscle to exercise. Hypertens Res 2016; 39: 506–512. [DOI] [PubMed] [Google Scholar]
- 28. Riquelme C, Acuña MJ, Torrejón J, et al. ACE2 is augmented in dystrophic skeletal muscle and plays a role in decreasing associated fibrosis. PLoS ONE 2014; 9: e93449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Y-C, Bai W-Z, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 2020; 92: 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forcina L, Miano C, Scicchitano BM, et al. Increased circulating levels of interleukin-6 affect the redox balance in skeletal muscle. Oxid Med Cell Longev 2019; 2019: 3018584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Disser NP, De Micheli AJ, Schonk MM, et al. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am 2020; 102: 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Broussard SR, McCusker RH, Novakofski JE, et al. IL-1beta impairs insulin-like growth factor i-induced differentiation and downstream activation signals of the insulin-like growth factor i receptor in myoblasts. J Immunol 2004; 172: 7713–7720. [DOI] [PubMed] [Google Scholar]
- 33. Otis JS, Niccoli S, Hawdon N, et al. Pro-inflammatory mediation of myoblast proliferation. PLoS ONE 2014; 9: e92363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madaro L, Passafaro M, Sala D, et al. Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat Cell Biol 2018; 20: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piotrowicz K, Ga˛sowski J. Risk factors for frailty and cardiovascular diseases: are they the same. Adv Exp Med Biol 2020; 1216: 39–50. [DOI] [PubMed] [Google Scholar]
- 36. Zheng K, Zhang WC, Xu YZ, et al. COVID-19 and the bone: underestimated to consider. Eur Rev Med Pharmacol Sci 2020; 24: 10316–10318. [DOI] [PubMed] [Google Scholar]
- 37. Sisó-Almirall A, Brito-Zerón P, Conangla Ferrín L, et al. Long covid-19: proposed primary care clinical guidelines for diagnosis and disease management. Int J Environ Res Public Health 2021; 18: 4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep 2021; 11: 16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garg M, Maralakunte M, Garg S, et al. The conundrum of ‘Long-COVID-19’: a narrative review. Int J Gen Med 2021; 14: 2491–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parums DV. Editorial: long COVID, or post-COVID syndrome, and the global impact on health care. Med Sci Monit 2021; 27: e933446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nabavi N. Long covid: how to define it and how to manage it. BMJ 2020; 370: m3489. [DOI] [PubMed] [Google Scholar]
- 42. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021; 27: 626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 2021; 11: e048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324: 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Daugherty SE, Guo Y, Heath K, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2021; 373: n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buonsenso D, Munblit D, De Rose C, et al. Preliminary evidence on long COVID in children. Acta Paediatr 2021; 110: 2208–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Silva Andrade B, Siqueira S, de Assis Soares WR, et al. Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses 2021; 13: 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khazaal S, Harb J, Rima M, et al. The pathophysiology of long COVID throughout the renin-angiotensin system. Molecules 2022; 27: 2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lancet T. Facing up to long COVID. Lancet 2020; 396: 1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Castanares-Zapatero D, Chalon P, Kohn L, et al. Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med 2022; 54: 1473–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baig AM. Deleterious outcomes in long-hauler COVID-19: the effects of SARS-CoV-2 on the CNS in chronic COVID syndrome. ACS Chem Neurosci 2020; 11: 4017–4020. [DOI] [PubMed] [Google Scholar]
- 53. de Melo GD, Lazarini F, Levallois S, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med 2021; 13: eabf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021; 591: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Forte G, Favieri F, Tambelli R, et al. COVID-19 pandemic in the Italian population: validation of a post-traumatic stress disorder questionnaire and prevalence of PTSD symptomatology. Int J Environ Res Public Health 2020; 17: 4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zanker J, Duque G. Osteosarcopenia: the path beyond controversy. Curr Osteoporos Rep 2020; 18: 81–84. [DOI] [PubMed] [Google Scholar]
- 57. Cariati I, Scimeca M, Bonanni R, et al. Role of myostatin in muscle degeneration by random positioning machine exposure: an in vitro study for the treatment of sarcopenia. Front Physiol 2022; 13: 782000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol 2021; 6: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bonanni R, Cariati I, Tancredi V, et al. Chronic pain in musculoskeletal diseases: do you know your enemy? J Clin Med 2022; 11: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cariati I, Bonanni R, Scimeca M, et al. Exposure to random positioning machine alters the mineralization process and PTX3 expression in the SAOS-2 cell line. Life 2022; 12: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tarantino U, Cariati I, Tancredi V, et al. State of fragility fractures management during the covid-19 pandemic. Int J Environ Res Public Health 2020; 17: 7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Suzuki Y, Maeda N, Hirado D, et al. Physical activity changes and its risk factors among community-dwelling Japanese older adults during the COVID-19 epidemic: associations with subjective well-being and health-related quality of life. Int J Environ Res Public Health 2020; 17: 6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yamada M, Kimura Y, Ishiyama D, et al. Effect of the COVID-19 epidemic on physical activity in community-dwelling older adults in Japan: a cross-sectional online survey. J Nutr Health Aging 2020; 24: 948–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ali AM, Kunugi H. COVID-19: a pandemic that threatens physical and mental health by promoting physical inactivity. Sports Med Health Sci 2020; 2: 221–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Visser M, Schaap LA, Wijnhoven HAH. Self-reported impact of the COVID-19 pandemic on nutrition and physical activity behaviour in dutch older adults living independently. Nutrients 2020; 12: 3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meftahi GH, Jangravi Z, Sahraei H, et al. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: the contribution of ‘inflame-aging’. Inflamm Res 2020; 69: 825–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bano G, Trevisan C, Carraro S, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas 2017; 96: 10–15. [DOI] [PubMed] [Google Scholar]
- 68. Wang PY, Li Y, Wang Q. Sarcopenia: an underlying treatment target during the COVID-19 pandemic. Nutrition 2021; 84: 111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ali AM, Kunugi H. Approaches to nutritional screening in patients with coronavirus disease 2019 (COVID-19). Int J Environ Res Public Health 2021; 18: 2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tarantino U, Cariati I, Marini M, et al. Effects of simulated microgravity on muscle stem cells activity. Cell Physiol Biochem 2020; 54: 736–747. [DOI] [PubMed] [Google Scholar]
- 71. Ohara DG, Pegorari MS, Oliveira Dos Santos NL, et al. Cross-sectional study on the association between pulmonary function and sarcopenia in Brazilian community-dwelling elderly from the Amazon region. J Nutr Health Aging 2020; 24: 181–187. [DOI] [PubMed] [Google Scholar]
- 72. Okazaki T, Ebihara S, Mori T, et al. Association between sarcopenia and pneumonia in older people. Geriatr Gerontol Int 2020; 20: 7–13. [DOI] [PubMed] [Google Scholar]
- 73. Paliwal VK, Garg RK, Gupta A, et al. Neuromuscular presentations in patients with COVID-19. Neurol Sci 2020; 41: 3039–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol 2020; 92: 1902–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang X, Cai H, Hu J, et al. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int J Infect Dis 2020; 94: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vehar S, Boushra M, Ntiamoah P, et al. Post-acute sequelae of SARS-CoV-2 infection: caring for the ‘long-haulers’. Cleve Clin J Med 2021; 88: 267–272. [DOI] [PubMed] [Google Scholar]
- 77. Aiyegbusi OL, Hughes SE, Turner G, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med 2021; 114: 428–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tosato M, Carfì A, Martis I, et al. Prevalence and predictors of persistence of COVID-19 symptoms in older adults: a single-center study. J Am Med Dir Assoc 2021; 22: 1840–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tuzun S, Keles A, Okutan D, et al. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. Eur J Phys Rehabil Med 2021; 57: 653–662. [DOI] [PubMed] [Google Scholar]
- 80. Fernández-de-Las-Peñas C, Rodríguez-Jiménez J, Fuensalida-Novo S, et al. Myalgia as a symptom at hospital admission by severe acute respiratory syndrome coronavirus 2 infection is associated with persistent musculoskeletal pain as long-term post-COVID sequelae: a case-control study. Pain 2021; 162: 2832–2840. [DOI] [PubMed] [Google Scholar]
- 81. Ghosn J, Piroth L, Epaulard O, et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect 2021; 27: 1041e1–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Karaarslan F, Güneri FD, Kardeş S. Long COVID: rheumatologic/musculoskeletal symptoms in hospitalized COVID-19 survivors at 3 and 6 months. Clin Rheumatol 2022; 41: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fernández-de-Las-Peñas C, Navarro-Santana M, Plaza-Manzano G, et al. Time course prevalence of post-COVID pain symptoms of musculoskeletal origin in patients who had survived to severe acute respiratory syndrome coronavirus 2 infection: a systematic review and meta-analysis. Pain 2021; 163: 1220–1231. [DOI] [PubMed] [Google Scholar]
- 84. Sykes DL, Holdsworth L, Jawad N, et al. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it. Lung 2021; 199: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. di Filippo L, Frara S, Giustina A. The emerging osteo-metabolic phenotype of COVID-19: clinical and pathophysiological aspects. Nat Rev Endocrinol 2021; 17: 445–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lauwers M, Au M, Yuan S, et al. COVID-19 in joint ageing and osteoarthritis: current status and perspectives. Int J Mol Sci 2022; 23: 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. di Filippo L, Doga M, Frara S, et al. Hypocalcemia in COVID-19: prevalence, clinical significance and therapeutic implications. Rev Endocr Metab Disord 2022; 23: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Carpagnano GE, Di Lecce V, Quaranta VN, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest 2021; 44: 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Tarantino U, Greggi C, Visconti VV, et al. T-score and handgrip strength association for the diagnosis of osteosarcopenia: a systematic review and meta-analysis. J Clin Med 2021; 10: 2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bonanni R, Cariati I, Tarantino U, et al. Physical exercise and health: a focus on its protective role in neurodegenerative diseases. J Funct Morphol Kinesiol 2022; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Inoue T, Maeda K, Nagano A, et al. Related factors and clinical outcomes of osteosarcopenia: a narrative review. Nutrients 2021; 13: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pallone G, Palmieri M, Cariati I, et al. Different continuous training modalities result in distinctive effects on muscle structure, plasticity and function. Biomed Rep 2020; 12: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cariati I, Bonanni R, Annino G, et al. Dose-response effect of vibratory stimulus on synaptic and muscle plasticity in a middle-aged murine model. Front Physiol 2021; 12: 678449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hashimoto Y, Kaji A, Sakai R, et al. Effect of exercise habit on skeletal muscle mass varies with protein intake in elderly patients with type 2 diabetes: a retrospective cohort study. Nutrients 2020; 12: 3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kirwan R, McCullough D, Butler T, et al. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. Geroscience 2020; 42: 1547–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Candan SA, Elibol N, Abdullahi A. Consideration of prevention and management of long-term consequences of post-acute respiratory distress syndrome in patients with COVID-19. Physiother Theory Pract 2020; 36: 663–668. [DOI] [PubMed] [Google Scholar]
- 97. Grigoletto I, Cavalheri V, Lima FF, et al. Recovery after COVID-19: the potential role of pulmonary rehabilitation. Braz J Phys Ther 2020; 24: 463–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after covid-19: qualitative study of 114 ‘long covid’ patients and draft quality principles for services. BMC Health Serv Res 2020; 20: 1144. [DOI] [PMC free article] [PubMed] [Google Scholar]