Abstract
The study of antibody avidity changes during infection has improved the understanding of the pathologic processes involved in several infectious diseases. In some infections, like toxoplasmosis, this information is being used for diagnostic purposes. Results of the evolution of antibody avidity for different specific antigens in Trypanosome cruzi-infected rats are presented. A Western blotting technique, combined with avidity analysis to identify antigens that elicit high-avidity antibodies, is suggested. In this system, antibodies showed high avidity values only during the chronic phase of infection and only in relation to antibodies against 21-, 33-, 41-, 42-, 56-, 58-, 66-, and 72-kDa antigens. Finally, a 97-kDa T. cruzi antigen, which was recognized by high-avidity antibodies and occurred in noninfected rats, was identified. These results allow us to evaluate the different antigens in chagasic infection. Our results show that with the correct choice of antigen it is possible to detect differences in maturation of antibodies and to discriminate, in an experimental model, between recent (acute) and chronic infections.
Chagas' disease is caused by the hematic protozoan Trypanosoma cruzi. The infectious process in humans is characterized by an initial phase with large quantities of parasites in peripheral blood. The presence of the parasite in the bloodstream is partially controlled by the immune response. In most cases, the infection evolves toward a chronic phase characterized by high titers of immunoglobulin G (IgG) antibodies, by low and intermittent parasitemia, and very frequently by the absence of symptoms.
As in other parasitic and infectious diseases, the monitoring of antibody responses and antigens involved in different stages of the infection may give information for the comprehension of the mechanisms of immune system control over the parasite (1, 14, 17, 20, 24). In different infectious processes the maturation of antibodies, measured in terms of avidity, has been determined in order to find a correlation with the evolution of the infection (7, 8, 10, 18, 25).
An optimization process of the antibody binding properties has been described in long-term infection (19): somatic hypermutation of sequences encoding the variable region of light and heavy chains of immunoglobulins occurs, and B lymphocytes producing better quality antibodies, mainly in terms of avidity, are then selected. During this stage, some serum immunoglobulins evolve from low to high avidity. On the basis of this mechanism, methods have been developed to age a variety of infective diseases by using a simple method based on the selective unbinding of antibodies with chaotropic agents and the assessment of the evolution of their functional avidity (4, 9, 21). Another type of information that it is possible to obtain from the determination of the avidity index is the discrimination between autoantibodies and cross-reacting antibodies generated by an infectious process (17).
In this work, the evolution of the antibody response to different T. cruzi antigens in a well characterized Chagas' infection experimental model (6, 22) was studied. Antigens that elicit antibodies with different avidity patterns were identified.
MATERIALS AND METHODS
Experimental infection of rats.
Sera used in the present study were obtained from I strain rats experimentally infected on day 21 after birth. This strain is derived from a cross between Rattus novergicus and eIIM rats. Rats were infected with T. cruzi trypomastigotes (Tulahuen strain) by the subcutaneous route. Infective parasites were maintained by serial passage in CBi mice. Different groups of six rats each were sacrificed 7, 15, 30, and 60 days after inoculation. Sera were obtained from blood taken by cardiac puncture. Control sera were obtained the same way from noninfected rats.
Determination of blood parasitemia.
Bloodstream forms of T. cruzi were assessed under standardized conditions by direct microscopic observation of 5 μl of heparinized blood at 7, 15, 30, and 60 days. Data were expressed as the mean ± the standard deviation of parasites per 100 fields for each group.
Parasite antigens.
Epimastigotes of the Tulahuen strain were grown in liver infusion tryptose medium (3). Parasites were washed with phosphate-buffered saline (PBS), resuspended in distilled water with 1 mM phenylmethylsulfonyl fluoride (Sigma), and clarified by centrifugation at 10,000 × g at 4°C for 30 min after two freeze-thaw cycles. The supernatant, containing a complex mixture of T. cruzi antigens, was used in enzyme-lynked immunosorbent assays (ELISAs) and Western blotting (WB) assays as described below.
Determination of kinetics of anti-T. cruzi antibody levels in rat serum by ELISA.
Serum pools from the different groups of infected and noninfected animals were used to study the levels of total antibodies and IgG antibodies against T. cruzi antigens. Antigens described above were diluted to 10 μg/ml with pH 9.6 carbonate buffer; microtiter plates (Costar, Cambridge, Mass.) were coated with 0.1 ml of diluted antigen per well and incubated overnight at 4°C. Plates were washed with PBS, blocked with 0.2 ml of 5% nonfat milk (Molico-Nestle) in PBS for 1 h at 37°C, and washed three times with PBS–0.01% Tween 20 (Sigma, St. Louis, Mo.). Each pool, diluted 1:100, was then incubated for 1 h at 37°C in six parallel wells. After washing with PBS–0.01% Tween, half of the wells were incubated with anti-rat IgG– and IgM–peroxidase conjugates (Jackson Inc., West Grove, Pa.) and the remaining ones were incubated with anti-rat IgG–peroxidase conjugate (Jackson Inc.). Wells were incubated for 1 h at 37°C, washed three times with PBS–0.01% Tween, and developed with hydrogen peroxide-3,3′,5,5′-tetramethylbenzidine (RDI, Flanders, N.J.). The reaction was blocked after 15 min with 2 N H2SO4, and optical density at 450 nm (OD450) was read (MAXline microplate reader; Molecular Devices, Sunnyvale, Calif.).
Avidity ELISA.
Plates were coated as described above and incubated for 30 min with 6 M urea (ICN, Costa Mesa, Calif.) in PBS. Microplates were then washed with PBS and blocked with 0.2 ml of PBS–5% nonfat milk for 1 h at 37°C. Each serum sample was diluted 1:100 with 1% nonfat milk in PBS and incubated in six parallel wells for 60 min. After incubation, microplates were thoroughly washed with PBS–0.01% Tween 20. The three wells containing each serum sample were treated with PBS–6 M urea (ICN) for 30 min. The three remaining wells for each serum sample were incubated with PBS. After washing, samples were incubated with specific anti-rat IgG antibody–peroxidase conjugate (Jackson) for 60 min and then washed and developed with hydrogen peroxide-3,3′,5,5′-tetramethylbenzidine (RDI). The reaction was blocked after 15 min with 2 N H2SO4 and read at 450 nm as described earlier.
The avidity index (AI) was defined as mean OD450 of urea-treated wells/mean OD450 of urea-untreated wells × 100. AI was calculated in triplicate for the first, second, and third pairs of treated and untreated wells, and mean values and standard deviations were calculated.
Avidity WB.
Parasite extracts were separated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis as previously described (12) and then electroblotted to nitrocellulose membranes. Strips were washed in PBS for 5 min and then incubated with PBS–6 M urea for 30 min. The membranes were blocked for 1 h at room temperature with PBS–5% nonfat milk. Membranes were washed with PBS and incubated with primary antibody (1:50 rat serum dilution in PBS–1% nonfat milk, two strips each) at room temperature for 60 min. Membranes were washed three times for 5 min and then one strip was incubated for 30 min with PBS–6 M urea at room temperature for 30 min while the other one was incubated with PBS. All the strips were submitted to three washing cycles with PBS, and then membranes were incubated with anti-rat IgG antibody–peroxidase conjugate (Jackson Inc.). After a new washing step (three times), the strips were treated with 0.4% hydrogen peroxide 3,3′-diaminobenzidine tetrahydrochloride (Sigma Chemical Co.) in PBS.
Statistical analysis.
Statistical differences among groups for avidity indexes between 15, 30, and 60 days were calculated by using a one-way analysis of variance.
RESULTS
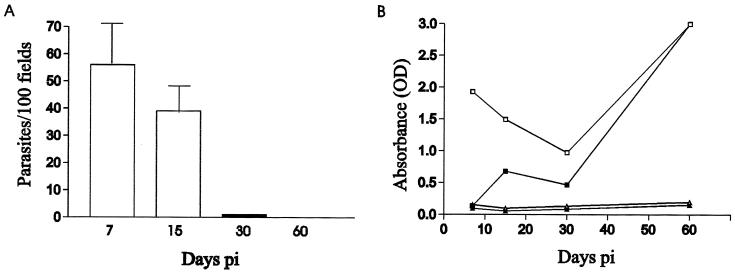
As mentioned earlier, a well-characterized experimental model using 21-day-old experimentally infected rats was chosen (6, 22). The acute phase of the infection is defined as the period in which rats present high parasitemia. When parasites can no longer be detected in the bloodstream, the chronic phase begins. The parasitemia was analyzed during infection (Fig. 1A). As described in previous reports, the peak was reached on day 7 postinfection (p.i.) and parasites could not be detected after day 30 (22).
FIG. 1.
Evolution of parasitemia and antibody levels in rat sera. (A) Clearance of blood-circulating parasites in T. cruzi-infected rats. The bars represent means ± standard deviations of parasites in 100 fields. (B) Kinetics after T. cruzi infection in control and infected rats on days 7, 15, 30, and 60 p.i. ▵, total antibody after inoculation in controls rats; ▴, IgG-specific antibody after inoculation in control rats; □, total antibody after inoculation in infected rats; ■, IgG-specific antibody after inoculation in infected rats. Points are averages of triplicate experiments. Standard deviations are less than 10%. All groups were assayed on the same ELISA plate. OD was obtained for each group with both conjugates in order to assess the serological response from the onset of the infection.
Serologic status during the acute and chronic phases of infection was determined by follow-up of specific gamma globulin and IgG isotypes by using ELISA (Fig. 1B). Levels of IgG isotype antibodies increased and showed a small peak on day 15 p.i. Specific gamma globulin levels decreased from day 7 to day 30 p.i. when the lowest levels of both IgG plus IgM and IgG isotype were observed. Then both values increased, mainly due to IgG isotype, reaching the maximum on day 60 p.i.
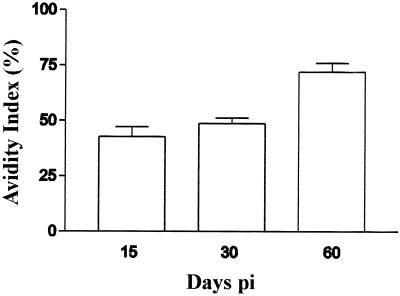
Avidity ELISAs using sera taken from infected animals were performed. The corresponding indices and evolution of antibody avidity against total antigens were determined (Fig. 2). Specific IgG isotype levels on day 7 p.i. were not detectable by ELISA, so in this case the avidity index could not be calculated. From 15 to 30 days p.i., no modifications in the avidity indices were shown by ELISA. However, the avidity index of antibodies from infected animals increased 23.3% ± 2.7% on day 60 p.i.
FIG. 2.
Evolution of the avidity index in sera pools from infected rats on days 15, 30, and 60 p.i. Differences were statistically significant at day 60 p.i. (P < 0.0002).
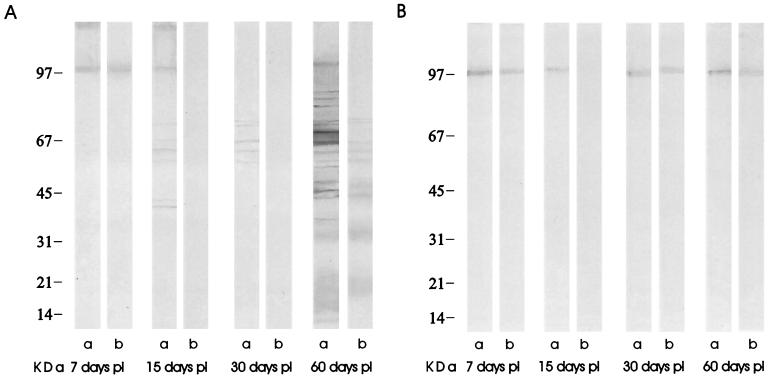
WB and avidity WB assays were performed in order to recognize the antigens involved in avidity maturation (Fig. 3). The avidity Western blot patterns are summarized in Table 1. High-avidity antibodies recognized at day 7 p.i. included a 97-kDa band in both infected and control rats. Low-avidity antibodies that eluted with the urea solution were found against seven major bands (41, 42, 56, 58, 66, 72, and 97 kDa) on day 15 p.i. The situation was quite similar on day 30 p.i.: low-avidity antibodies against five major bands (56, 58, 66, 70, and 72 kDa) were found and completely eluted with urea-PBS, but antigens of 41, 42, and 97 kDa were not recognized. On the other hand, a new band of low-avidity antibodies against a 71-kDa antigen appeared and evolved to high-avidity antibodies on day 60 p.i.
FIG. 3.
(A) WB of sera from different groups of infected rats; non-urea-eluted antibodies (a) and urea-eluted antibodies (b) were measured on days 7, 15, 30, and 60 p.i. (B) WB of control sera on days 7, 15, 30, and 60 p.i., with non-urea-eluted antibodies (a) and urea-eluted antibodies (b). Strips were incubated with peroxidase-conjugated anti-rat IgG. Apparent molecular mass is shown on the left.
TABLE 1.
Pattern of antigens recognized by antibodies at different times after infection in rats
| Antigen (kDa) | Detection of antibody
|
|||
|---|---|---|---|---|
| Day 7 | Day 15 | Day 30 | Day 60 | |
| 16 | + | |||
| 18 | + | |||
| 21 | +a | |||
| 24 | + | |||
| 33 | +a | |||
| 36 | + | |||
| 37 | + | |||
| 41 | + | +a | ||
| 42 | + | +a | ||
| 47 | + | |||
| 48 | + | |||
| 52 | + | |||
| 56 | + | + | +a | |
| 58 | + | + | +a | |
| 66 | + | + | +a | |
| 70 | + | + | ||
| 72 | + | + | +a | |
| 89 | + | |||
| 91 | + | |||
| 95 | + | |||
| 97 | +a | + | + | |
Antibody specific to T. cruzi antigen that was not eluted with 6 M urea.
A clear differentiation was observed between antibodies evolving to high avidity on day 60 p.i. (those that recognized bands of 21, 33, 41, 42, 56, 58, 66, and 72 kDa) and those not evolving to high avidity (the ones recognizing bands of 16, 18, 24, 36, 37, 47, 48, 52, 71, 89, 91, 95, and 97 kDa). A 97-kDa band with high avidity was observed in all newborns at 7 days p.i. in controls as well as in inoculated rats; this band disappears in inoculated rats at 15 days p.i. and is observed again with low avidity at 30 and 60 days p.i.
DISCUSSION
As previously reviewed, an avidity ELISA using complex antigens enables us to measure antibody avidity and determine primary infection in several diseases. Many diagnostic studies for other infections have been performed this way (8, 13, 20). The authors observed a high correlation between acute infection and low avidity indices calculated by using the avidity ELISA technique. It has been suggested that high avidity indices are chronic infection markers. However, low avidity indices cannot always be considered acute infection markers, since it was found that some individuals do not develop high-avidity antibodies during the chronic phase of toxoplasmosis (5, 20). Recently, an avidity determination by WB has been suggested for discriminating antigens related to high-avidity antibodies from those related to low-avidity antibodies in the acute phase of infection (15). In experimental rat infection by T. cruzi, both avidity ELISA and avidity WB showed that high-avidity antibodies appeared on day 60 p.i. Although avidity WB and avidity ELISA are techniques based on different principles, with differences in terms of sensitivity and the way in which antigens are presented to antibodies, results with the two techniques were qualitatively comparable. In fact, both methods showed a qualitative difference in terms of avidity on day 60 p.i., compared to avidity on days 15 and 30 p.i. Parasitemia and antibody curves indicated that, at this point, rat infection had reached the chronic stage.
Avidity WB proved to be a valuable technique to identify antigens bound to high- or low-avidity antibodies. It reveals the avidity evolution, which is modulated in different ways by different antigens. This suggests that antibody avidity evolution is induced by each antigen in an independent manner, as has already been reported for rubella (16), varicella-zoster virus (11), and human immunodeficiency virus (25) infections. These findings showed that different viral proteins had different maturation patterns during infection. It was also observed that avidity patterns appeared to be independent from antibody concentration, since the remaining signal after chaotropic elution was not related to the initial intensity of the bands. The most intense 70-kDa band observed on day 60 p.i. completely disappeared when treated with urea, while the light 21- and 33-kDa bands recognized by the same antibody pool did not elute when treated with urea. These results suggest, on one hand, that this technique is detecting antibody avidity and not antibody concentration. On the other hand, the results confirm previous findings, showing that there is no relationship between antibody concentration and avidity (24).
The quality of the antibody response was consequently heterogeneous, varying in time and with the antigen used. Antibodies against some specific antigens (bands of 41, 42, 56, 58, and 66 kDa) were found to increase their avidity. At the same time, specific antibodies for other antigens, in particular 70-kDa bands, did not increase their avidity. Avidity WB results were intriguing: high-avidity antibodies recognizing the 97-kDa band were universally present in offspring from both control and infected rats. It would be of interest to study the possible maternal origin of these high-avidity antibodies found at birth. The avidity of these antibodies decreased, showing a minimum at day 30 after birth, and then increased in noninfected control rats, perhaps due to the replacement of maternal antibodies. In infected rats, these antibodies did not recover their high avidity during the follow-up period of 60 days. The presence of this antibody population in this rat strain, which is capable of controlling the chagasic infection, suggests the possibility of a protective role for these antibodies.
Functional avidity of specific antibodies is a parameter that has been proposed to correlate with their protective activity. In this direction, previous work correlated antibody avidity with effector activity in terms of neutralizing capacity (2) or complement-mediated cytotoxicity (23). This work shows that there is a differential maturation of antibodies against different antigens associated with the immune response to T. cruzi.
Our results show that, in an experimental model and with a correct choice of antigen, it is possible to discriminate between recent (acute) and chronic infections. As a second step of this work, we are cloning these antigens and studying their application for determining avidities and their correlations with the clinical manifestations of the disease in human patients.
ACKNOWLEDGMENTS
This work was supported by Fundación de Investigaciones Biomédicas (grant number FIB 005/99). I.S.M. is a fellow of the Secretaria de Cultura de la Provincia de Santa Fe. M.G.R. is a fellow of the Universidad Nacional del Litoral. A.M.S. is a fellow of the Consejo Nacional de Investigaciones Científicas y Técnicas.
REFERENCES
- 1.Andersson A, Vetter V, Kreuzer L, Bauer G. Avidities of IgG directed against viral capsid antigen or early antigen: useful markers for significant Epstein-Barr serology. J Med Virol. 1994;43:238–244. doi: 10.1002/jmv.1890430308. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann M F, Kalinke U, Althage A, Freer G, Burkhart C, Roost H P, Aguet M, Hengartner H, Zinkernagel R M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2028. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 3.Camargo E P. Growth and differentiation in Trypanosoma cruzi. Origin of metacyclic trypanosomes in liquid media. Rev Inst Med Trop. 1964;6:93–100. [PubMed] [Google Scholar]
- 4.Camargo M E, da Silva S M, Leser P G, Granato C H. Avidez de anticorpos IgG especificos como marcadores de infecçao primária recente pelo Toxoplasma gondii. Rev Inst Med Trop. 1991;33:213–218. [PubMed] [Google Scholar]
- 5.Cozon G J, Ferrandiz J, Nebbi H, Wallon M, Peyron F. Estimation of the avidity of immunoglobulin G for routine diagnosis of chronic Toxoplasma gondii infection in pregnant women. Eur J Clin Microbiol Infect Dis. 1988;17:32–36. doi: 10.1007/BF01584360. [DOI] [PubMed] [Google Scholar]
- 6.Davila H, Revelli S, Moreno H, Valenti J, Musso O, Poli H, Morini J, Botasso O. Infection with Trypanosoma cruzi during pregnancy in rats and a decrease in chronic myocardial lesions in their infected offspring. Am J Trop Med Hyg. 1994;50:506–511. doi: 10.4269/ajtmh.1994.50.506. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira M U, Kimura E A S, Souza J M, Katzin A M. The isotype composition and avidity of naturally acquired anti-Plasmodium falciparum antibodies: differential patterns in clinically immune Africans and Amazonian patients. Am J Trop Med Hyg. 1996;53:315–323. doi: 10.4269/ajtmh.1996.55.315. [DOI] [PubMed] [Google Scholar]
- 8.Hedman K, Lampalainen M, Söderlund M, Hedman L. Avidity of IgG in serodiagnosis of infectious diseases. Rev Med Microbiol. 1993;4:123–129. [Google Scholar]
- 9.Hedman K, Lamppalainen M I, Seppälä I, Mäkela O. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989;159:736–740. doi: 10.1093/infdis/159.4.736. [DOI] [PubMed] [Google Scholar]
- 10.Holliman R E. Recent developments in the diagnosis of toxoplasmosis. Serodiagn Immunother Infect Dis. 1994;6:5–16. [Google Scholar]
- 11.Kangro H O, Manzoor S, Harper D R. Antibody avidity following varicella zoster virus infection. J Med Virol. 1991;33:100–105. doi: 10.1002/jmv.1890330207. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Lamppalainen M, Koskela P M, Koskiniemiemi M, Ammala P, Hiilesmaa V, Teramo K, Raivio K O, Remington J S, Hedman K. Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of immunoglobulin G. J Infect Dis. 1993;67:691–697. doi: 10.1093/infdis/167.3.691. [DOI] [PubMed] [Google Scholar]
- 14.Lutz E, Ward K N, Gray J J. Maturation of antibody avidity after primary human cytomegalovirus infection is delayed in immunosuppressed solid organ transplant patients. J Med Virol. 1994;44:317–322. doi: 10.1002/jmv.1890440402. [DOI] [PubMed] [Google Scholar]
- 15.Marcolino P T, Silva D A O, Lesser P G, Camargo M E, Mineo J R. Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by Western blotting. Clin Diagn Lab Immunol. 2000;7:384–389. doi: 10.1128/cdli.7.3.384-389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauracher C A, Mitchel L A, Tingle A J. Differential IgG avidity to rubella virus structural proteins. J Med Virol. 1992;36:202–208. doi: 10.1002/jmv.1890360310. [DOI] [PubMed] [Google Scholar]
- 17.Motran C C, Cerbán F M, Rivarola H, Vottero de Cima E. Characterization of autoantibodies generated in mice by immunization with the C-terminal region of Trypanosoma cruzi ribosomal P1 and P2 proteins. Clin Immunol. 1999;91:17–24. doi: 10.1006/clim.1998.4678. [DOI] [PubMed] [Google Scholar]
- 18.Newkirk M M, Fournier M J, Shiroky J. Rheumatoid factor avidity in patients with rheumatoid arthritis: identification of pathogenic RFs which correlate with disease parameters and with the gal(0) glycoform of IgG. J Clin Immunol. 1995;15:250–257. doi: 10.1007/BF01540882. [DOI] [PubMed] [Google Scholar]
- 19.Nossal G J V. The molecular and cellular basis of affinity maturation in the antibody response. Cell. 1992;68:1–2. doi: 10.1016/0092-8674(92)90198-l. [DOI] [PubMed] [Google Scholar]
- 20.Paul M. Immunoglobulin G avidity in diagnosis of toxoplasmic lymphadenopathy and ocular toxoplasmosis. Clin Diagn Lab Immunol. 1999;6:514–518. doi: 10.1128/cdli.6.4.514-518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pullen G R, Fitzgerald M G, Hosking C S. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986;86:83–87. doi: 10.1016/0022-1759(86)90268-1. [DOI] [PubMed] [Google Scholar]
- 22.Revelli S S, Amerio N, Moreno H S, Valenti J L, Balbarrey H, Morini J C. Enfermedad de Chagas crónica en la rata. Características serológicas electrocardiográficas e histopatológicas. Medicina (Buenos Aires) 1980;40:69–76. [PubMed] [Google Scholar]
- 23.Schlesinger Y, Granoff D M. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA. 1992;267:1489–1494. [PubMed] [Google Scholar]
- 24.Sennhauser F H, Macdonald R A, Roberton D M, Hosking C S. Comparison of concentration and avidity of specific antibodies to E. coli in breast milk and serum. Immunology. 1989;66:394–397. [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas H I, Wilson S, O'Toole C M, Lister C M, Saeed A M, Watkins R P, Morgan-Capner P. Differential maturation of avidity of IgG antibodies to gp41, p24 and p17 following infection with HIV-1. Clin Exp Immunol. 1996;103:185–191. doi: 10.1046/j.1365-2249.1996.951642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]