This phase 1 dose-escalation trial assesses the safety and tolerability of a combination of olaparib and radiotherapy in patients with triple-negative breast cancer with residual disease after neoadjuvant chemotherapy.
Key Points
Question
What is the safety profile of poly(adenosine diphosphate ribose) polymerase (PARP) inhibition with olaparib used as a radiosensitizer in combination with radiotherapy for early-stage, high-risk triple-negative breast cancer (TNBC)?
Findings
In this phase 1 dose-escalation trial of radiotherapy with concurrent PARP inhibition in 24 patients with TNBC with residual disease after neoadjuvant chemotherapy, olaparib was increased to a dose of 200 mg twice daily without dose-limiting toxic effects. The combination treatment was well tolerated, and no grade 3 or greater adverse events were observed.
Meaning
In this trial, olaparib used as a radiosensitizer in combination with radiotherapy for early-stage, high-risk TNBC was safe and well tolerated; this combination treatment should continue to be evaluated in larger trials.
Abstract
Importance
Triple-negative breast cancer (TNBC) cells are sensitive to poly(adenosine diphosphate ribose) polymerase (PARP) inhibitors used as radiosensitizers. Whether combining PARP inhibitors with radiotherapy in patients with TNBC would enhance the biological effectiveness of the irradiation and improve locoregional control is unclear.
Objective
To assess the safety and tolerability of PARP inhibition with olaparib used concurrently with radiotherapy in patients with TNBC with residual disease after neoadjuvant chemotherapy.
Design, Setting, and Participants
This phase 1 prospective dose-escalation trial (Olaparib and Radiation Therapy for TNBC [RadioPARP] trial) using a time-to-event continual reassessment method was performed from September 2017 to November 2019, with follow-up until November 2021. Participants had an incomplete pathologic response after neoadjuvant chemotherapy or unresectable TNBC despite previous neoadjuvant chemotherapy, an Eastern Cooperative Oncology Group Performance Status score of 0 or 1, and adequate organ functions.
Interventions
Olaparib was administered orally in the form of tablets and given at increasing doses (50 mg, 100 mg, 150 mg, or 200 mg twice daily). Olaparib therapy was started 1 week before radiotherapy and was continued concomitantly with radiotherapy. After breast-conserving surgery, a total dose of 50.4 Gy was delivered to the whole breast, with a 63-Gy simultaneously integrated boost to the tumor bed for patients younger than 60 years. After radical mastectomy or for unresectable tumors despite neoadjuvant chemotherapy, a total dose of 50.0 Gy was delivered to the chest wall (after mastectomy) or to the whole breast (for unresectable tumors). Regional lymph node stations could be treated with a total dose of 50.0 Gy to 50.4 Gy in cases of node-positive disease.
Main Outcomes and Measures
Main outcomes were the safety and tolerability of PARP inhibition with radiotherapy for early-stage, high-risk TNBC. Secondary outcomes included overall survival (OS) and event-free survival (EFS).
Results
Among the 24 patients included in the trial (100% female; median age, 46 years [range, 25-74 years]), no dose-limiting toxic effects were observed, and olaparib was escalated to 200 mg twice daily without reaching the maximum tolerated dose. No late treatment-related grade 3 or greater toxic effect was observed, and the maximum observed treatment-related toxic effects at the 2-year follow-up were grade 2 breast pain, fibrosis, and deformity in 1 patient (4.2%). Three-year OS and EFS were 83% (95% CI, 70%-100%) and 65% (95% CI, 48%-88%), respectively. Homologous recombination status was not associated with OS or EFS.
Conclusions and Relevance
The findings of this phase 1 dose-escalation trial suggest that PARP inhibition with olaparib concurrently with radiotherapy for early-stage, high-risk TNBC is well tolerated and should continue to be evaluated in further clinical trials.
Trial Registration
ClinicalTrials.gov Identifier: NCT03109080
Introduction
Triple-negative breast cancer (TNBC) cells frequently harbor defects in the homologous recombination pathway,1 which plays a prime role in DNA double-strand breaks repair. In a context of homologous recombination deficiency, use of poly(adenosine diphosphate ribose) polymerase (PARP) inhibitors indirectly increases the frequency of unrepaired double-strand break by affecting the base excision repair pathway after DNA-damaging stresses,2 such as chemotherapy or radiotherapy. Preclinical studies have confirmed the radiosensitizing properties of PARP inhibitors in breast cancer murine models.3
Approximately 15% of breast cancers are classified as TNBC, which is associated with a poor prognosis.4 In particular, the presence of residual active disease after neoadjuvant chemotherapy represents a pejorative situation with limited disease-free survival and a 5-year locoregional relapse-free survival estimated at approximately 90%.5,6 In this context, increasing the biological effectiveness of radiotherapy with radiosensitizers, such as PARP inhibitors, could theoretically achieve greater tumor control, resulting in improved clinical outcomes.
Two randomized clinical trials7,8 demonstrated that olaparib monotherapy, an oral PARP inhibitor, improved disease-free survival and progression-free survival in patients whose tumors were ERBB2 (formerly HER2 or HER2/neu) negative with a germline BRCA1 or BRCA2 (BRCA1/2) variant in adjuvant7 and metastatic8 settings while being clinically well tolerated. However, the safety of olaparib specifically used as a radiosensitizer in combination with radiotherapy in patients with TNBC has not been investigated to our knowledge. The Olaparib and Radiation Therapy for TNBC (RadioPARP) phase 1 dose-escalation trial aimed to assess the safety profile of olaparib used as a radiosensitizer in combination with radiotherapy in patients with early-stage, high-risk TNBC, defined by residual disease after neoadjuvant chemotherapy.
Methods
Study Design and Population
The RadioPARP trial was an open-label, single-arm, phase 1 dose-escalation trial that aimed to assess the safety profile of olaparib used as a radiosensitizer in combination with radiotherapy in patients with TNBC with residual disease after neoadjuvant chemotherapy (trial protocol is provided in the Supplement). The trial was conducted from September 2017 to November 2019, with follow-up until November 2021. Inclusion criteria were resected TNBC with an incomplete pathologic response after neoadjuvant chemotherapy or unresectable TNBC despite prior neoadjuvant chemotherapy. In addition, an Eastern Cooperative Oncology Group Performance Status score of 0 or 1 and adequate organ functions were required. Exclusion criteria were history of radiotherapy or treatment with PARP inhibitors, concurrent antineoplastic treatment, residual Common Terminology Criteria for Adverse Events (version 4.03)9 grade 3 or 4 toxic effects resulting from neoadjuvant chemotherapy, pregnancy, or substantial comorbidity. The trial was approved by the Institut Curie institutional review board and was conducted in accordance with the Declaration of Helsinki10 and with the national regulatory requirements of France; written informed consent was obtained from all patients.
Procedures
Olaparib was administered orally in the form of tablets and given at increasing doses (50 mg, 100 mg, 150 mg, or 200 mg twice daily); the maximum dose of 200 mg twice daily was chosen to limit risk of hematologic toxic effects while maintaining antitumor efficacy. For safety, a dose of 25 mg twice daily (dose −1) was included in case the first dose level of 50 mg twice daily was found to have an unacceptable clinical toxicity. Olaparib therapy was started 1 week before radiotherapy and was continued concomitantly with adjuvant radiotherapy. After breast-conserving surgery, a total dose of 50.4 Gy was delivered in 28 fractions of 1.8 Gy to the whole breast, with a 63-Gy simultaneously integrated boost to the tumor bed for patients younger than 60 years delivered using a volumetric-modulated arc therapy technique. After radical mastectomy or for unresectable tumors despite neoadjuvant chemotherapy, a total dose of 50.0 Gy was delivered in 25 fractions of 2.0 Gy to the chest wall (after mastectomy) or to the whole breast (for unresectable tumors). In addition, regional lymph node stations (comprising the axillary, subclavicular, and supraclavicular nodes and the internal mammary chains) could be treated with a total dose of 50.0 Gy to 50.4 Gy in cases of node-positive disease. Clinical target volumes were delineated in accordance with the European Society for Radiotherapy and Oncology (ESTRO) guidelines11 to ensure reproducibility and homogeneity. Planned target volumes were obtained by adding an isotropic 0.5-cm margin to the clinical target volumes. Starting in April 2019, patients with an incomplete pathologic response after neoadjuvant chemotherapy additionally received adjuvant oral capecitabine,12 which was started 4 weeks after the end of radiotherapy to limit interferences concerning evaluation of the toxicity of the combination treatment.
Patients were evaluated weekly during the irradiation, at 2 weeks and at 6 weeks after radiotherapy completion, and every 6 months thereafter. Each visit consisted of a clinical examination and an interval history anamnesis to look for potential adverse events (AEs), classified according to the Common Terminology Criteria for Adverse Events, version 4.03.9 Blood sample analyses were performed on a weekly basis during radiotherapy and at the 2- and 6-week visits. Breast imaging with mammography and ultrasonography was conducted annually thereafter. Supplementary laboratory tests or imaging examinations could be required at the discretion of the physician.
End Points and Statistical Analysis
The primary end point was to assess the maximum tolerated dose (MTD) of olaparib given in combination with radiotherapy. Dose allocation was centrally defined; a time-to-event continual reassessment method, which is a dose-escalation method relying on continuous dose-toxicity modeling, was used to progressively increase the olaparib dose through the 4 predefined dose levels.13 Intrapatient dose escalation and dose skipping were not authorized. At least 3 fully observed patients were required at a given dose before dose escalation. The MTD was defined as the olaparib dose associated with the modeled dose-limiting toxicity (DLT) probability closest to 25% during radiotherapy and the 6 following weeks without exceeding it. The DLT was defined as grade 3 dermatitis occurring before 40 Gy or any grade 4 dermatitis; any other nonhematologic grade 3 or greater toxic effect; a hematologic toxic effect corresponding to neutropenia (grade 4 if lasting ≥1 week or grade 3 with fever), thrombocytopenia (grade 3 if bleeding or grade ≥4), or anemia (grade ≥3); or any toxic effect requiring a radiotherapy delay of 7 or greater fractions or resulting in 4 or fewer weeks of olaparib administration. Given the number of prespecified dose levels in the trial, we planned to include a maximum of 30 patients if no stopping rule was reached earlier.
Secondary end points included overall survival (OS), event-free survival (EFS), and tolerance profile of the combination treatment. Overall survival was defined from the time of enrollment to death from any cause. Event-free survival was defined from the time of enrollment to local or metastatic relapse or death from any cause. Overall survival and EFS were analyzed by the Kaplan-Meier method. Exploratory subgroup analyses were performed according to the homologous recombination status, evaluated based on the number of large-scale genomic alterations (LGAs) obtained from whole-genome sequencing (WGS) at low coverage (shallow WGS).14 Homologous recombination deficiency is characterized by an increased number of large-scale intrachromosomal copy number alterations; LGAs are defined as intrachromosomal arm copy number alteration breaks with adjacent segments greater than 10 Mb. Homologous recombination deficiency was defined based on the number of whole-genome LGAs, with a minimum threshold of 20; non–homologous recombination deficiency status was defined by fewer than 15 LGAs and borderline status by 15 to 19 LGAs.14 Statistical analyses were performed using R, version 4.1.2 (R Project for Statistical Computing). Two-sided P = .05 was considered significant.
Results
Patients
Between September 2017 and November 2019, 27 patients with TNBC were screened for inclusion, and 24 patients (100% female; median age, 46 years [range, 25-74 years]) were enrolled in the trial (Table 1).15 Twenty-one patients (87.5%) had an incomplete pathologic response on postoperative histopathologic examination after neoadjuvant chemotherapy, and 3 patients (12.5%) had unresectable tumors despite having received neoadjuvant chemotherapy. Of the 21 patients who had undergone surgery before radiotherapy, 10 (47.6%) were treated with mastectomy and 11 (52.4%) with breast-conserving surgery. The 3 patients with unresectable tumors after neoadjuvant chemotherapy subsequently underwent radiotherapy followed by mastectomy; none of them had a complete pathologic response. Regional lymph node irradiation was conducted for 17 patients (70.8%), and 11 patients (45.8%) received adjuvant capecitabine. Genetic analyses were conducted for 22 patients (91.7%); of these, 14 (63.6%) had homologous recombination–deficient tumors and 7 (31.8%) had homologous recombination–proficient tumors evaluated by shallow WGS. Homologous recombination status was undetermined (borderline) for 1 patient (4.5%). The median number of whole-genome LGAs was 18 (range, 1-44). Among the 14 patients with homologous recombination–deficient tumors, deleterious tumor variants in BRCA1 were identified in 4 patients (28.6%), variants in BRCA2 in 3 (21.4%), and variants in RAD51C in 1 (7.1%). No patient with homologous recombination–proficient tumors had a BRCA1/2 variant. All patients received full-course combination treatment, and the median follow-up for all patients was 34 months (95% CI, 31-37 months).
Table 1. Patient Characteristics at Baseline.
| Characteristic | Patients (N = 24)a |
|---|---|
| Sex | |
| Female | 24 (100) |
| Male | 0 |
| Age, median (range), y | 46 (25-74) |
| ECOG performance status | |
| 0 | 17 (70.8) |
| 1 | 7 (29.2) |
| Clinical situation after neoadjuvant chemotherapy | |
| Incomplete pathologic response after surgery | 21 (87.5) |
| Inflammatory and unresectable | 2 (8.3) |
| Locoregional and unresectable | 1 (4.2) |
| T categoryb | |
| T1 | 4 (16.7) |
| T2 | 14 (58.3) |
| T3 | 4 (16.7) |
| T4 | 2 (8.3) |
| N categoryb | |
| N0 | 13 (54.2) |
| N1 | 10 (41.7) |
| N2 | 1 (4.2) |
| M categoryb | |
| M0 | 24 (100) |
| M1 | 0 |
| Residual disease burdenc | |
| I | 2 (9.5) |
| II | 15 (71.4) |
| III | 4 (19.0) |
| Ki-67, median (range), % | 60 (20-80) |
| Elston-Ellis graded | |
| 2 | 5 (20.8) |
| 3 | 18 (75.0) |
| Not available | 1 (4.2) |
| Homologous recombination deficiency | |
| Yes | 14 (58.3) |
| No | 7 (29.2) |
| Undetermined | 1 (4.2) |
| Not tested | 2 (8.3) |
| BRCA1/2 variant carrier | |
| Yes | 7 (29.2) |
| No | 15 (62.5) |
| Not tested | 2 (8.3) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Data are reported as number (percentage) of patients unless otherwise indicated.
According to American Joint Committee on Cancer criteria. N category percentages do not sum to 100% because of rounding.
The residual disease burden (residual cancer burden [RCB]) score can only be calculated after surgery.15 Thus, the RCB score was only available for 21 patients who were included in the trial after undergoing surgery. Percentages do not sum to 100% because of rounding.
Grades range from 1 to 3, with higher grades indicating poorly differentiated breast cancer and fast-growing cancer.
Safety
No DLT was observed at any olaparib dose level, and the MTD was not reached, allowing safe dose escalation of olaparib to 200 mg twice daily, the highest planned dose. No death occurred within 6 weeks after completion of treatment. Four patients (16.7%) received olaparib, 50 mg twice daily; 8 (33.3%) received 100 mg twice daily; 7 (29.2%) received 150 mg twice daily; and 5 (20.8%) received 200 mg twice daily. Acute non-DLT grade 3 and 4 AEs were restricted to grade 3 lymphocele in 1 patient (4.2%), grade 3 breast pain in 1 (4.2%), grade 3 radiodermatitis in 2 (8.3%), grade 3 lymphopenia in 8 (33.3%), and grade 4 lymphopenia in 3 (12.5%). Overall, 11 patients (45.8%) had grade 3 or 4 lymphopenia. Late AEs are shown in Table 2.9
Table 2. Treatment-Related Adverse Events Reported During Follow-up.
| Toxic effect | Toxic effects by olaparib dose and toxic effect grade, No.a | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg Twice daily | 100 mg Twice daily | 150 mg Twice daily | 200 mg Twice daily | |||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 1-y Follow-up (n = 23) | ||||||||||||||||
| Pain | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Fibrosis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Deformity | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Skin hyperpigmentation | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Telangiectasia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lymphedema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2-y Follow-up (n = 20) | ||||||||||||||||
| Pain | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fibrosis | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Deformity | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Skin hyperpigmentation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Telangiectasia | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Lymphedema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Toxic effect grades are based on Common Terminology Criteria for Adverse Events, version 4.03.9
Late grade 3 or greater AEs were limited to grade 4 thrombopenia in 1 patient 1 year after trial inclusion while the patient was receiving antineoplastic cytotoxic agents for metastatic progression; this event was considered unrelated to the combination treatment. No late treatment-related grade 3 or greater AE was recorded. At the 1-year follow-up visit, 23 patients (95.8%) were alive and assessed for toxic effects. Maximum observed toxic effects corresponded to grade 2 breast pain, fibrosis, and deformity in 1 patient who received olaparib, 100 mg twice daily. At the 2-year follow-up, 20 patients (83.3%) were assessed for toxic effects. The maximum observed toxic effects corresponded to grade 2 breast pain, fibrosis, and deformity in the same patient receiving olaparib, 100 mg twice daily. No treatment-related systemic grade 2 or greater AEs were observed. No patient developed a second cancer.
EFS and OS
Eight patients (33.3%) experienced disease control failure (at 2, 8, 11, 12, 14, 16, 23, and 28 months), and 5 patients (20.8%) died of their disease (at 5, 13, 15, 19, and 37 months). Of the 8 patients who experienced disease control failure, the first site of disease progression was locoregional for 2 (25%) and distant for 6 (75%). Median EFS and OS were not reached (Figure). Event-free survival rates were 88% (95% CI, 75%-100%) at 1 year, 71% (95% CI, 55%-92%) at 2 years, and 65% (95% CI, 48%-88%) at 3 years. Overall survival rates were 96% (95% CI, 88%-100%) at 1 year, 83% (95% CI, 70%-100%) at 2 years, and 83% (95% CI, 70%-100%) at 3 years. On univariate analysis, positive nodal status was associated with worse EFS (hazard ratio, 11.4; 95% CI, 1.39-93.9; P = .02) but this was not the case for the other clinical or tumor characteristics, including homologous recombination status (hazard ratio, 0.49; 95% CI, 0.11-2.19; P = .34). Overall survival was not significantly associated with any clinicobiological features.
Figure. Event-Free Survival and Overall Survival Among the 24 Patients Included in the Trial.
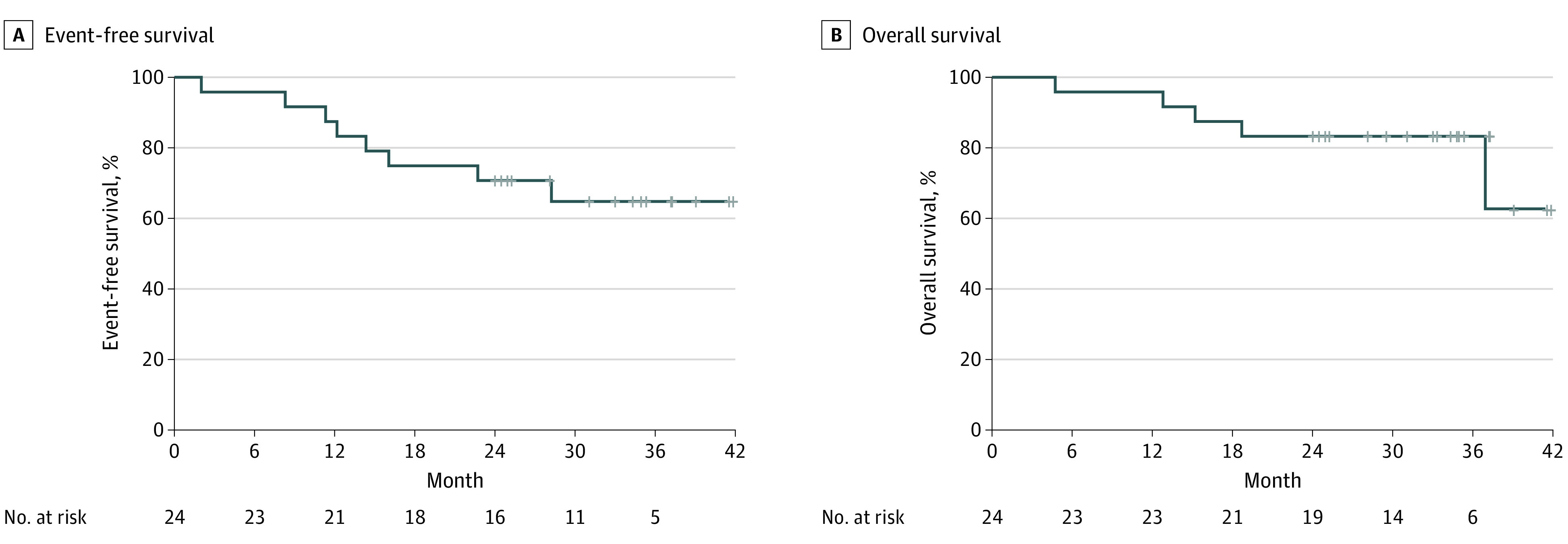
Event-free survival was defined from the time of enrollment to local or metastatic relapse or death from any cause. Overall survival was defined from the time of enrollment to death from any cause.
Discussion
In this phase 1 dose-escalation trial evaluating olaparib used as a radiosensitizer in combination with radiotherapy for TNBC with residual disease after neoadjuvant chemotherapy, no DLT was observed during the irradiation and the 6 following weeks, and the MTD of olaparib in this combination was not reached. In addition, with a median follow-up of 34 months, only 1 patient developed late treatment-related grade 2 AEs, and no late grade 3 or greater treatment-related AEs were observed, demonstrating the safety of this combination treatment for this indication. The late toxicity profile was similar to that commonly observed with standard radiotherapy protocols.16 These results suggest that use of olaparib as a radiosensitizer during breast radiotherapy may not be associated with an increased risk of late complications. Therefore, we recommend a radiosensitizing dose of 200 mg twice daily when combining olaparib with breast radiotherapy for future trials assessing the antitumor efficacy of this combination.
Veliparib, another oral PARP inhibitor, was evaluated in combination with locoregional postmastectomy radiotherapy for breast cancer management in a phase 1 trial that included 30 patients, with 16 treated for TNBC.17 Even though the definition of DLT was different from that in our study, 5 DLTs were reported and the MTD was defined at 100 mg twice daily. Of note, the late safety profile of veliparib in that trial was a subject of concern since the proportion of grade 3 or greater AEs reached 46.7% after 3 years, contrasting with the good tolerance profile observed with olaparib in the present study. The notable difference of the tolerance profile between both studies might be explained by the fact that fewer than half of the patients in our study underwent postmastectomy radiotherapy, which is associated with more frequent skin toxic effects than postlumpectomy radiotherapy.18 Another explanation for the difference may be the prescribed radiation doses since no scar boost was performed for postmastectomy radiotherapy in our study. In addition, when indicated, tumor bed boosts were delivered using a volumetric-modulated arc therapy technique, which may be associated with lower incidence of skin toxic effects compared with other techniques.16 Furthermore, we cannot rule out that some AEs might have been missed due to the follow-up intervals. Of note, although veliparib and olaparib have similar molecular mechanisms, preclinical studies have described notable differences in drug pharmacokinetics,19,20 which might have led to the difference in tolerance profiles. Given the lack of increased skin or lung toxic effects, it could also be hypothesized that olaparib might not have had a clinical radiosensitizing effect; in particular, increased radiation pneumonitis is often an early sign of radiosensitization.21 However, multiple preclinical studies have demonstrated the radiosensitizing effect of olaparib on breast cancer cell lines.3,22
The risk of acute hematologic toxic effects when combining PARP inhibitors with radiotherapy should be closely monitored. We reported a high frequency of lymphopenia, with a 45.8% rate of acute grade 3 or greater lymphopenia, which spontaneously resolved and may have resulted from a synergistic effect of olaparib with radiotherapy. In a phase 1 trial combining veliparib with radiotherapy and gemcitabine for locally advanced pancreatic cancer, a 96% rate of grade 3 or greater lymphopenia was observed.23 These concordant observations may justify repeated blood sample monitoring to help prevent potential opportunistic infections.
The OlympiA trial7 demonstrated that, among patients with high-risk nonmetastatic ERBB2-negative breast cancer and germline BRCA pathogenic variants, adjuvant olaparib after local treatment completion was associated with a significantly longer disease-free survival. While the study population was different from that in the present study, which was not restricted to patients with a BRCA variant, our results suggest that olaparib could be safely started earlier in combination with radiotherapy, which would allow continuation of systemic treatment during irradiation. In addition, 7 patients with homologous recombination deficiency in our trial did not have deleterious BRCA variants, suggesting that olaparib might not be solely limited to patients with a BRCA variant. Genetic characterization of homologous recombination status may become increasingly available for PARP-inhibitor trials. The efficacy of immunotherapy for resectable TNBC management24 raises the possibility of combining olaparib with checkpoint inhibitors in such situations, and a clinical trial is currently evaluating this combination treatment concomitantly with radiotherapy.25
Limitations
The limitations of the present study are inherent to dose-escalation trials, which include a limited number of patients and lack of a control group. Radiotherapy was delivered according to normofractionated regimens, and whether the same tolerance profile would be observed with hypofractionated regimens is an open question. As a phase 1 dose-escalation trial, there was no control group; thus, conclusions regarding the efficacy of the combination treatment are impossible. Two patients experienced a locoregional relapse, but this observation might have been related to selection bias since the study population corresponded to patients with high risk of poor tumor control. The absence of a significant survival difference between patients with homologous recombination proficiency and those with homologous recombination deficiency might be explained by the limited size of the trial, which was not powered to this end, or by a similar radiosensitizing effect of olaparib for patients with homologous recombination proficiency and those with homologous recombination deficiency. Whether or not the radiosensitizing effect of olaparib during radiotherapy may have a clinical impact is yet to be determined by dedicated randomized clinical trials. Chemotherapy-resistant TNBCs, which are defined by disease progression during neoadjuvant chemotherapy, represent a rare but challenging situation26 in which the radiosensitizing effect of olaparib could be evaluated in the neoadjuvant setting by the rate of pathologic complete response, which will be the objective of a dedicated phase 2 study (Y.K., written communication, December 2021).
Conclusions
In this phase 1 dose-escalation trial, olaparib used as a radiosensitizer in combination with breast radiotherapy in patients with TNBC was well tolerated. The MTD was not reached, and no late grade 3 or greater treatment-related toxic effect was reported. Our findings suggest that, for future trials evaluating the antitumor efficacy of this combination treatment, an olaparib dose of 200 mg twice daily should be considered.
Trial Protocol
References
- 1.Chopra N, Tovey H, Pearson A, et al. Homologous recombination DNA repair deficiency and PARP inhibition activity in primary triple negative breast cancer. Nat Commun. 2020;11(1):2662. doi: 10.1038/s41467-020-16142-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Underhill C, Toulmonde M, Bonnefoi H. A review of PARP inhibitors: from bench to bedside. Ann Oncol. 2011;22(2):268-279. doi: 10.1093/annonc/mdq322 [DOI] [PubMed] [Google Scholar]
- 3.Michmerhuizen AR, Pesch AM, Moubadder L, et al. PARP1 inhibition radiosensitizes models of inflammatory breast cancer to ionizing radiation. Mol Cancer Ther. 2019;18(11):2063-2073. doi: 10.1158/1535-7163.MCT-19-0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118(22):5463-5472. doi: 10.1002/cncr.27581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. doi: 10.1016/S0140-6736(13)62422-8 [DOI] [PubMed] [Google Scholar]
- 6.Swisher SK, Vila J, Tucker SL, et al. Locoregional control according to breast cancer subtype and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast-conserving therapy. Ann Surg Oncol. 2016;23(3):749-756. doi: 10.1245/s10434-015-4921-5 [DOI] [PubMed] [Google Scholar]
- 7.Tutt ANJ, Garber JE, Kaufman B, et al. ; OlympiA Clinical Trial Steering Committee and Investigators . Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394-2405. doi: 10.1056/NEJMoa2105215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523-533. doi: 10.1056/NEJMoa1706450 [DOI] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.03. June 14, 2010. Accessed September 1, 2017. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 10.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 11.Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114(1):3-10. doi: 10.1016/j.radonc.2014.11.030 [DOI] [PubMed] [Google Scholar]
- 12.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi: 10.1056/NEJMoa1612645 [DOI] [PubMed] [Google Scholar]
- 13.Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56(4):1177-1182. doi: 10.1111/j.0006-341X.2000.01177.x [DOI] [PubMed] [Google Scholar]
- 14.Eeckhoutte A, Houy A, Manié E, et al. ShallowHRD: detection of homologous recombination deficiency from shallow whole genome sequencing. Bioinformatics. 2020;36(12):3888-3889. doi: 10.1093/bioinformatics/btaa261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahleh Z, Sivasubramaniam D, Dhaliwal S, Sundarajan V, Komrokji R. Residual cancer burden in locally advanced breast cancer: a superior tool. Curr Oncol. 2008;15(6):271-278. doi: 10.3747/co.v15i6.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allali S, Carton M, Sarrade T, et al. CANTO-RT: skin toxicities evaluation of a multicentre large prospective cohort of irradiated patients for early-stage breast cancer. Int J Cancer. 2022;151(7):1098-1108. doi: 10.1002/ijc.34057 [DOI] [PubMed] [Google Scholar]
- 17.Jagsi R, Griffith KA, Bellon JR, et al. ; Translational Breast Cancer Research Consortium . Concurrent veliparib with chest wall and nodal radiotherapy in patients with inflammatory or locoregionally recurrent breast cancer: the TBCRC 024 phase I multicenter study. J Clin Oncol. 2018;36(13):1317-1322. doi: 10.1200/JCO.2017.77.2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parekh A, Dholakia AD, Zabranksy DJ, et al. Predictors of radiation-induced acute skin toxicity in breast cancer at a single institution: role of fractionation and treatment volume. Adv Radiat Oncol. 2017;3(1):8-15. doi: 10.1016/j.adro.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72(21):5588-5599. doi: 10.1158/0008-5472.CAN-12-2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.To C, Kim EH, Royce DB, et al. The PARP inhibitors, veliparib and olaparib, are effective chemopreventive agents for delaying mammary tumor development in BRCA1-deficient mice. Cancer Prev Res (Phila). 2014;7(7):698-707. doi: 10.1158/1940-6207.CAPR-14-0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan J, Cohen V, Agulnik J, et al. Unexpected high lung toxicity from radiation pneumonitis in a phase I/II trial of concurrent erlotinib with limited field radiation for intermediate prognosis patients with stage III or inoperable stage IIB non–small-cell lung cancer(NSCLC). Int J Radiat Oncol Biol Phys. 2009;75(3)(suppl):S110. doi: 10.1016/j.ijrobp.2009.07.267 [DOI] [Google Scholar]
- 22.Jang NY, Kim DH, Cho BJ, et al. Radiosensitization with combined use of olaparib and PI-103 in triple-negative breast cancer. BMC Cancer. 2015;15:89. doi: 10.1186/s12885-015-1090-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuli R, Shiao SL, Nissen N, et al. A phase 1 study of veliparib, a PARP-1/2 inhibitor, with gemcitabine and radiotherapy in locally advanced pancreatic cancer. EBioMedicine. 2019;40:375-381. doi: 10.1016/j.ebiom.2018.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmid P, Cortes J, Pusztai L, et al. ; KEYNOTE-522 Investigators . Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810-821. doi: 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 25.A study of radiation therapy with pembrolizumab and olaparib in women who have triple-negative breast cancer. ClinicalTrials.gov identifier: NCT04683679. Updated September 21, 2021. Accessed August 4, 2022. https://clinicaltrials.gov/ct2/show/NCT04683679
- 26.Loap P, Nicaise B, Laki F, et al. A therapeutic challenge: chemo-refractory non-metastatic inflammatory breast cancers. Clin Oncol (R Coll Radiol). 2022;34(3):e140. doi: 10.1016/j.clon.2022.01.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol


