This systematic review and meta-analysis examines data from studies published since 2010 to update estimates for the prevalence and incidence of dry eye and meibomian gland dysfunction in the US population.
Key Points
Question
What is the prevalence and incidence of dry eye and meibomian gland dysfunction (MGD) in the US population from studies published after January 1, 2010?
Findings
In this systematic review and meta-analysis, the pooled prevalence of dry eye was 8.1% (95% prediction interval [PI] 0%-98.9%) in 3 studies without high risk of bias and judged to be representative of the US population. Pooled prevalence of MGD was 21.2% (95% PI, 0%-100%) in 3 studies, and incidence of dry eye ranged from 3.5% to 7.8%.
Meaning
Current epidemiological studies provide uncertain estimates of the prevalence and incidence of dry eye and MGD in the United States.
Abstract
Importance
Dry eye is a common clinical manifestation, a leading cause of eye clinic visits, and a significant societal and personal economic burden in the United States. Meibomian gland dysfunction (MGD) is a major cause of evaporative dry eye.
Objective
To conduct a systematic review and meta-analysis to obtain updated estimates of the prevalence and incidence of dry eye and MGD in the United States.
Data Sources
Ovid MEDLINE and Embase.
Study Selection
A search conducted on August 16, 2021, identified studies published between January 1, 2010, and August 16, 2021, with no restrictions regarding participant age or language of publication. Case reports, case series, case-control studies, and interventional studies were excluded.
Data Extraction and Synthesis
The conduct of review followed a protocol registered on PROSPERO (CRD42021256934). PRISMA guidelines were followed for reporting. Joanna Briggs Institute and Newcastle Ottawa Scale tools were used to assess risk of bias. Data extraction was conducted by 1 reviewer and verified by another for accuracy. Prevalence of dry eye and MGD were combined in separate meta-analyses using random-effects models.
Main Outcomes and Measures
Prevalence and incidence of dry eye and MGD in the United States. Summary estimates from meta-analysis of dry eye and MGD prevalence with 95% CI and 95% prediction intervals (95% PI).
Results
Thirteen studies were included in the systematic review. Dry eye prevalence was reported by 10 studies, dry eye incidence by 2 studies, and MGD prevalence by 3 studies. Meta-analysis estimated a dry eye prevalence of 8.1% (95% CI, 4.9%-13.1%; 95% PI, 0%-98.9%; 3 studies; 9 808 758 participants) and MGD prevalence of 21.2% (95% CI, 7.2%-48.3%; 95% PI, 0%-100%; 3 studies; 19 648 participants). Dry eye incidence was 3.5% in a population 18 years and older and 7.8% in a population aged 68 years and older. No studies reported MGD incidence.
Conclusions and Relevance
This systematic review and meta-analysis demonstrated uncertainty about the prevalence and incidence of dry eye and MGD in the United States. Population-based epidemiological studies that use consistent and validated definitions of dry eye and MGD are needed for higher-certainty estimates of dry eye and MGD prevalence and incidence in the United States.
Introduction
The Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop II (DEWS-II) defines dry eye disease (DED) as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”1 Because there is no gold standard diagnostic test for DED,1 we use the term dry eye to describe various presentations of ocular discomfort and visual disturbance caused by tear film abnormalities.
Dry eye can be categorized as aqueous deficient, evaporative, or mixed mechanisms. Aqueous deficiency arises from reduced lacrimal gland secretion, and evaporative disease arises from abnormalities in the tear film lipid layer.2,3 Meibomian gland dysfunction (MGD) is a major cause of disruption of the tear film lipid layer and consequently of DED.4
Dry eye is a common clinical manifestation, a leading driver of eye clinic visits, and a considerable burden to patients and society. In 2011, the estimated direct economic cost to the US health care system for dry eye therapy was $3.8 billion per year and the estimated total societal cost was $55.4 billion per year.5 More recently, total US per capita expenditure for all ophthalmic medication classes has been shown to be highest for dry eye medications.6
Global burden of disease estimates for blindness and vision impairment do not include dry eye prevalence.7 The TFOS DEWS-II report estimated dry eye to be prevalent in 5% to 50% of populations worldwide, depending on disease definition and other contextual factors.8 The US prevalence of symptomatic dry eye was reported by the TFOS DEWS-II report to range from 6.8% in the Physicians’ Health Study to 21.6% in the Beaver Dam Eye Study.8,9,10 The prevalence of MGD has been reported to vary widely, from 3.5% to 69.3% depending on study design and setting, population characteristics, and definition of MGD.11 The objective of the current systematic review and meta-analysis is to provide updated estimates of the prevalence and incidence of dry eye and MGD in the United States.
Methods
We registered the review prospectively on PROSPERO (CRD42021256934) and reported it in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE)12 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.13 We followed the methods established in the published protocol.14
Eligibility Criteria
We included population-based, clinic-based, and secondary health care database studies that reported prevalence or incidence of dry eye or MGD in the United States. We included studies using both population-based and clinic-based samples. As there remains variability in the diagnostic criteria for dry eye, we did not place restrictions on the diagnostic criteria used to define dry eye in the included studies. We followed guidance from the TFOS DEWS-II report to categorize case definitions, including: (1) Women’s Health Study criteria (ie, self-reported physician diagnosis and/or self-reported constant or often symptoms),15 (2) dry eye symptoms when signs were not measured (eg, measured by the 5-item Dry Eye Questionnaire), (3) dry eye clinical signs when symptoms were not measured (eg, tear breakup time), (4) combination of dry eye signs and symptoms (distinct from Women’s Health Study criteria), and (5) MGD (eg, meiboscore).8 We also accepted dry eye and MGD definitions based on relevant Current Procedural Terminology and International Classification of Disease (ICD) codes.
We excluded case reports, case series, case-control studies, and interventional studies. We excluded studies with fewer than 73 total participants so their 95% CIs around estimates would not be wider than our anticipated minimum population prevalence.16
Search Strategies
In collaboration with a University of Colorado School of Medicine librarian, we searched Ovid MEDLINE and Embase on August 16, 2021, limiting the search to studies published on or after January 1, 2010, to provide current estimates of dry eye and MGD frequency. We included relevant controlled vocabulary terms (ie, medical subject headings in MEDLINE, Emtree terms in Embase) and text words (eTable 1 in the Supplement). We hand-searched the reference lists of included studies.
We searched the Cochrane Eyes and Vision US Satellite (CEV@US) database of systematic reviews on April 28, 2022, for systematic reviews tagged with condition: dry eye and review type: epidemiology – prevalence/incidence and reviewed available reference lists of relevant systematic reviews.17 We also searched the World Health Organization site on April 28, 2022, for the keywords dry eye and meibomian gland dysfunction. We retrieved the World Health Organization World Report on Vision and reviewed its reference lists.18 Further, we searched the reference list of the American Academy of Ophthalmology Dry Eye Syndrome Preferred Practice Pattern 2018 guidance.19
Study Selection
Two investigators independently screened each record for eligibility at both the title/abstract and full-text stages in Covidence.20 We contacted authors for further information and clarifications when required. If the authors did not respond within 4 weeks, we used the reported information to determine eligibility. We resolved discrepancies during the title/abstract and full-text screening stages by discussion or adjudication by a third investigator where needed.
Data Extraction and Risk-of-Bias Assessment
One investigator extracted all relevant study characteristics, methods, and results from each included study using the Systematic Review Data Repository Plus.21 An independent investigator verified all extracted data. We resolved discrepancies during data extraction by discussion or adjudication by a third investigator where needed. Data only available in graphical format were extracted by 2 investigators, independently, using an online graph digitizer.22 We analyzed the mean of the 2 independent investigators’ extracted values.
Two investigators independently assessed the risk of bias in each included study. For prevalence studies, we used the tool proposed by Hoy et al.23 For incidence studies, we used items from the Joanna Briggs Institute Checklist for Cohort Studies and the Newcastle Ottawa Scale for Cohort Studies.24,25 We resolved discrepancies during the risk-of-bias assessment by discussion or adjudication by a third investigator where needed.
Evaluation of Heterogeneity and Data Synthesis
We summarized data from each study in evidence tables. We investigated clinical heterogeneity by assessing diversity in population (eg, age, sex distribution) and disease characteristics (eg, DED, MGD). We assessed methodological heterogeneity by evaluating study designs and risk of bias. We assessed statistical heterogeneity by calculating the amount of heterogeneity (τ2) and the contribution of heterogeneity to the total variability across studies (I2).26
We meta-analyzed prevalence proportions using a generalized linear mixed model27 through the metaprop package in R version 4.1.2.28,29 We reported 95% CIs and 95% prediction intervals (PIs) for summary estimates. Prediction intervals predict the range in which the next sampled prevalence estimate will lie and are suggested to be more appropriate to evaluate and incorporate uncertainty in proportional meta-analyses of large prevalence studies compared with attempting to minimize the I2 statistic via sensitivity analyses.30 Our primary analysis excluded dry eye prevalence estimates derived from single-institution clinic-based studies, studies judged to be unrepresentative of the US population, and studies with high risk of bias.
Deviations From Protocol
We were unable to cross-walk prevalence from studies with diverse definitions because we did not find a methodologically robust study with a satisfactory reference standard to demonstrate the bias associated with each definition.14,31 We could not perform meta-regression on geo-environmental variables (eg, latitude, humidity) because of the lack of reported data.14,31
Results
Our search yielded 11 133 unique titles and abstracts. After screening 128 full-text reports, we included 13 studies (14 reports) (eFigure 1 in the Supplement)32,33,34,35,36,37,38,39,40,41,42,43,44,45 and provided reasons for excluding 14 studies that might have been expected to be included (eTable 2 in the Supplement).
Characteristics of Included Studies
The included studies covered 3 topics: dry eye prevalence (n = 10), dry eye incidence (n = 2), and MGD prevalence (n = 3) (Table 1).32,33,34,35,36,37,38,39,40,41,42,43,44,45 Data sources varied across the 13 studies (Table 1): health care system, multi-institutional, or nationwide studies using secondary data (n = 3)32,37,40; population-based epidemiological studies (n = 3)33,42,45; and single-institution clinic or hospital-based studies (n = 7).34,35,36,38,41,43,44 Definitions of dry eye varied across prevalence (Table 2) and incidence studies (Table 3): relevant ICD codes and/or data extracted from medical records (n = 6),32,38,39,40,41,43 symptom questionnaires without signs and/or self-reported diagnosis (n = 4),33,35,42,44 and signs alone (n = 1).45 Definitions of MGD also varied across studies (Table 2): relevant ICD codes and/or data extracted from medical records (n = 1)34 and signs alone (n = 2).35,36
Table 1. Study Characteristics.
| Design | Characteristics of study population | ||||||
|---|---|---|---|---|---|---|---|
| Source | Etiology (DED and/or MGD) | Source population (Census Bureau division) | Data source (national, regional, local) | Sampling scheme (response rate) | Female, % | Age, y | Race and ethnicity, % |
| Prevalence | |||||||
| Dana et al,32 2019 | DED | DOD military and civilian facilities (nationwide)a | Health care system, multi-institutional, or nationwide study using secondary data (national) | Full census or registry (NA) | 48.1 | 2-17 y: 20.8%; 18-39 y: 34.6%; 40-49 y: 11.2%; ≥50 y: 33.4% | NR |
| Paulsen et al,33 2014 | DED | Beaver Dam, Wisconsin, (East North Central) | Population-based epidemiological study (regional) | Unspecified (99.7%)b | 54.6 | Mean (range): 49 (21-84)c | NR |
| Davis et al,38 2015 | DED | University of North Carolina outpatient clinics (South Atlantic) | Single-institution clinic- or hospital-based study (local) | Full census or registry (NA) | NR | NR | NR |
| Lee et al, 201839 and 201740 | DED | Veteran Affairs health care system (nationwide) | Health care system, multi-institutional, or nationwide study using secondary data (national) | Full census or registry (NA) | 4.9 | Mean (SD): 65.0 (15.0) | White: 75.3; Black: 19.7 |
| Yalamanchili et al,34 2018 | MGD | University of North Carolina ophthalmology system (South Atlantic) | Single-institution clinic- or hospital-based study (local) | Full census or registry (NA) | NR | NR | NR |
| Russo and Bass,41 2021 | DED | Boston Health Care for the Homeless Program, Boston, Massachusetts (New England) | Single-institution clinic- or hospital-based study (local) | Consecutive enrollment (NR) | 25.9 | Mean (range) [IQR]: 52.7 (18-84) [40-60] | White: 40.8; Black: 33.3; Asian: 1.9; Hispanic and/or Latino: 13.9; American Indian or Alaska Native: 0.5; other: 3.3 |
| Farrand et al,42 2017 | DED | US census regions: Northeast, Midwest, South, West (nationwide) | Population-based epidemiological study (national) | Random (from panel of self-selected online volunteers) (NR) | 51.6 | 18-49 y: 52.8%; ≥50 y: 47.2% | White: 72.4; Black: 11.5; Asian: 5.0; Hispanic: 8.2; other 2.9 |
| Stull et al,43 2017 | DED | Temple University ophthalmology and optometry outpatient clinics, Philadelphia, Pennsylvania (Middle Atlantic) | Single-institution clinic- or hospital-based study (local) | Unspecified (86.9%) | 63.0 | Mean (SD) [range]: 58.0 (16.1) [18-93] | White: 22.8; African American: 60.5; Asian: 3.8; Hispanic/Latino: 11.0; other: 2.0 |
| Chang et al,44 2018 | DED | Bascom Palmer Eye Institute, Miami, Florida (South Atlantic) | Single-institution clinic- or hospital-based study (local) | Consecutive enrollment (95.5%) | 67.8 | Mean (SD) [range]: 46.3 (13) [19-77] | White: 75.5; Black: 18.0; Hispanic: 51.5 |
| Yeh and Lin,35 2017 | DED, MGD | University of California, Berkeley, Clinical Research Center (Pacific) | Single-institution clinic- or hospital-based study (local) | Unspecified (NR) | 70.3 | Mean (SD) [range]: 22.3 (4) [18-41] | Asian: 54.5; Non-Asian (White/Hispanic): 28.7; other: 16.8 |
| Manoj,45 2016 | DED | NR | Population-based epidemiological study | Unspecified (NR) | NR | NR | NR |
| Alghamdi et al,36 2016 | MGD | Miami Veteran Affairs Medical Center, Miami, Florida (South Atlantic) | Single-institution clinic- or hospital-based study (local) | Unspecified (NR) | 9.0 | Mean (SD) [range]: 63 (11) [27-89] | NR |
| Incidence | |||||||
| Chi et al,37 2012 | DED | NR | Health care system, multi-institutional, or nationwide study using secondary data (national) | 5% Sample of Medicare beneficiaries’ Part B claims to identify beneficiaries with DED diagnoses (NR) | NR | ≥68 | NR |
| Dana et al,32 2019 | DED | DOD military and civilian facilities (nationwide)a | Health care system, multi-institutional, or nationwide study using secondary data (national) | Full census or registry (NA) | NR | ≥18 | NR |
Abbreviations: DED, dry eye disease; DOD, US Department of Defense; MGD, meibomian gland dysfunction; NA, not applicable; NR, not reported.
US Department of Defense Military Health System data.
Adult offspring of the participants in the Epidemiology of Hearing Loss study were invited to participate.
Participants by age group: 21-34 years: 179 (5.5%); 35-44 years: 931 (28.4%); 45-54 years: 1227 (37.5%); 55-64 years: 710 (21.7%); 65-84 years: 228 (7.0%).
Table 2. Prevalence of Dry Eye and Meibomian Gland Dysfunction Results.
| Source | Etiology (DED and/or MGD) | Measurement method: components contributing to diagnostic standards | Diagnostic standards | Prevalence denominator, No. | Prevalence numerator, No. | Prevalence, % (95% CI) | Point or period of data collection | Characteristics used to stratify prevalence or report an association | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Dana et al,32 2019 | DED | Relevant ICD codesa; CPT codes for procedures and prescriptionsb; prescription fillsc | (1) Overall DED | (1) 9 732 272 | (1) 513 988 | (1) 5.3 (5.3-5.3)g | Period: 2003-2015 (8 y) | Age, sex, year of prevalence estimate (annual prevalence)h; NRi; NRj | M |
| (2) Unspecified DEDd | (2) 9 732 272 | (2) 207 727 | (2) 2.1 (2.1-2.1)g | ||||||
| (3) Specified DEDe | (3) 9 732 272 | (3) 24 809 | (3) 0.3 (0.3-0.3)g | ||||||
| (4) Sjögren-type DEDf | (4) 9 732 272 | (4) 17 403 | (4) 0.2 (0.2-0.2)g | ||||||
| Paulsen et al,33 2014 | DED | Symptomsk | Symptoms (questionnaire)k | 3275 | 475 | 14.5 (13.3-15.8)g | Period: 2005-2008 (3 y) | NRh; NRi; age, sex, medical comorbiditiesl, medication usej,m | L |
| Davis et al,38 2015 | DED | Relevant ICD codes | ICD-9 coden | 460 611 | 7207 | 1.6 (1.5-1.6)g | Period: 2008-2013 (5 y) | NRh; medical comorbiditiesi,o; NRj | H |
| Lee et al, 201839 and 201740 | DED | Relevant ICD codes | (1) ICD-9 codep | (1) 3 265 894 | (1) 854 480 | (1) 26.2 (26.1-26.2)g | Period: 2010-2014 (4 y) | NRh; age, sex, race and ethnicity, medical comorbidities, medication usei; NRj | M |
| (2) ICD-9 codeq | (2) 3 265 894 | (2) 843 194 | (2) 25.8 (25.8-25.9)g | ||||||
| (3) ICD-9 codesr | (3) 3 265 894 | (3) 9315 | (3) 0.3 (0.3-0.3)g | ||||||
| (4) ICD-9 codess | (4) 3 265 894 | (4) 28 407 | (4) 0.9 (0.9-0.9)g | ||||||
| (5) ICD-9 codest | (5) 3 265 894 | (5) 959 881 | (5) 29.4 (29.3-29.4) | ||||||
| (6) ICD-9 codesu | (6) 3 265 894 | (6) 186 299 | (6) 5.7 (5.7-5.7)g | ||||||
| Russo and Bass,41 2021 | DED | Relevant ICD codes; other | ICD-10 codes and other (diagnoses contained in the attending optometrist’s assessment and plan) | 424 | 121 | 28.5 (24.3-33.1)g | Period: 2016-2017 (1-2 y) | NRh; NRi; NRj | H |
| Farrand et al,42 2017 | DED | Self-reported diagnosisv; symptomsv | (1) Self-reported diagnosis with symptoms | (1) 73 211 | (1) 5051 | (1) 6.9 (6.7-7.1)g | Point: 2013 | Age, sex, race and ethnicity, US region, dry eye disease severityh; NRi; age, sex, race/ethnicity, US regionj | M |
| (2) Self-reported diagnosis with symptomsw | (2) 73 211 | (2) NR | (2) 6.8 (6.6-7.0) | ||||||
| (3) Symptoms without self-reported diagnosis | (3) 75 000 | (3) 1789 | (3) 2.4 (2.3-2.5)g | ||||||
| (4) Symptoms without self-reported diagnosisw | (4) 75 000 | (4) NR | (4) 2.5 (2.3-2.6) | ||||||
| Stull et al,43 2017 | DED | Other | Other (extracted from patient medical records) | 400 | 187 | 46.8 (41.8-51.8)g | Point: 2014/2015 | NRh; NRi; NRj | H |
| Chang et al,44 2018 | DED | Symptomsx | (1) DEQ-5 ≥6 | (1) 233 | (1) 94 | (1) 40.3 (34.0-47.0)g | Point: 2016 | Dry eye disease severityh; age, sex, race and ethnicity, medical comorbidities, medication usei; NRj | M |
| (2) DEQ-5 ≥12 | (2) 233 | (2) 24 | (2) 10.3 (6.7-14.9)g | ||||||
| (3) Chief concern dry eye symptoms | (3) 233 | (3) 60 | (3) 25.8 (20.3-31.9)g | ||||||
| Manoj,45 2016 | DED | Signs | (1) Tear hyperosmolarity | (1) 9947 | (1) 4605g | (1) 46.3 (45.3-47.3)g | NR | Sex, dry eye disease severityh; medical comorbiditiesi; NRj | H |
| (2) Tear osmolarity ≥316 mOsm/L | (2) 9947 | (2) 2885g | (2) 29.0 (28.1-29.9)g | ||||||
| Yeh and Lin,35 2017y | DED; MGD | Symptomsz; signsaa | (1) SPEED ≥9 | (1) 101 | (1) 19 | (1) 18.8 (11.7-27.8)g | Point: NR | Dry eye disease severityh; age, sex, race and ethnicity, medication usei; NRj | H |
| (2) SPEED ≥6 | (2) 101 | (2) 43 | (2) 42.6 (32.8-52.8)g | ||||||
| (3) Meiboscore = 3 | (3) 101 | (3) 12 | (3) 11.9 (6.2-19.8)g | ||||||
| Alghamdi et al,36 2016 | MGD | Signsbb,cc | (1) Eyelid vascularity score ≥2 | (1) 233 | (1) 39 | (1) 16.7 (12.2-22.2)g | Point: NR | Age, sex, race and ethnicity, medical comorbidities, medication useh; NRi; NRj | H |
| (2) Meibum quality score ≥2 | (2) 233 | (2) 129 | (2) 55.4 (48.7-61.9)g | ||||||
| Yalamanchili et al,34 2018 | MGD | Relevant ICD codes | (1) ICD and EMR codes for MGD and MGD spectrum disorders | (1) 19 314 | (1) 2002 | (1) 10.4 (9.9-10.8)g | Period: 2014-2017 (3 y) | Age, race and ethnicityh; NRi; NRj | H |
Abbreviations: CPT, Current Procedural Terminology; DED, dry eye disease; DEQ-5, 5-item dry eye questionnaire; EMR, electronic medical record; H, high; ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; L, low; M, moderate; MGD, meibomian gland dysfunction; NR, not reported; SPEED, Standardized Patient Evaluation of Eye Dryness.
Driving indicators: 370.33, keratoconjunctivitis sicca; 370.34, exposure keratoconjunctivitis; 372.53, conjunctival xerosis; 375.15, tear film insufficiency, unspecified; 710.20, sicca syndrome, Sjögren; nondriving indicators: 370.20, superficial keratoconjunctivitis; 370.21, punctate keratitis; 714.0, rheumatoid arthritis; 695.4, discoid lupus erythematosus; 710.0, systemic lupus erythematosus; 373.34, discoid lupus erythematosus of eyelid.
Driving indicators: 68 760, closure of the lacrimal punctum (by thermocauterization, ligation, or laser surgery); 68 761, punctal plugs; 09.91, obliteration of lacrimal punctum.
Driving indicator: restasis prescription fill for cyclosporine ophthalmic emulsion.
Using ICD-9 codes 375.15 and 370.33, at least 1 of each or 2 of the same.
Using ICD-9 codes nondriving indicators 370.21 and 370.20, at least 1 of 370.21 or 370.20 and 1 driving indicator (375.15 or 370.33).
Using ICD-9 code driving indicator 710.2, at least 2 of 710.2 OR 1 of 710.2 and 1 driving indicator (375.15 or 370.33).
Derived by binomial “exact” calculation.
Stratified by characteristic.
Characteristic included in a univariate analysis.
Characteristic included in a multivariate analysis.
Participants were asked “How often do you have dry eyes, a dry, gritty, or burning feeling?”; “How much does the dryness in your eyes bother you?”; “Is there a season of the year when the dryness in your eyes is the worst?”; and “Are you currently using eye drops at least once a day for dry eyes?” Participants who reported that symptoms were present sometimes or more often and that they were moderately bothersome or greater, or those who reported currently using eye drops at least once a day for dry eyes, were considered to be cases.
Arthritis, osteoporosis, allergies, thyroid disease, migraine headache, history of head injury (no loss of consciousness, loss of consciousness <5 minutes, loss of consciousness ≥5 minutes).
Antihistamines, acetaminophen, benzodiazepine, antidepressants, steroids, multivitamin, hormones (women).
ICD-9 code 375.15.
Diabetes (ICD-9 codes 250.00-250.93).
ICD-9 code indicative of tear film dysfunction (sicca syndrome [710.2] or keratoconjunctivitis sicca [370.33] or tear film insufficiency [375.15]).
ICD-9 code 375.15.
ICD-9 code 710.2.
ICD-9 code 370.33.
“Any dry eye” (tear film insufficiency [375.15] or visual discomfort [368.13] or pain in or around eye [379.91] or sicca syndrome [710.2] or keratoconjunctivitis sicca [370.33]).
ICD-9 code indicative of ocular pain (visual discomfort [368.13] or pain in or around eye [379.91]).
Participants were asked whether they had ever experienced dry eye. Possible responses were yes or no. Those who answered no to ever experiencing dry eye were classified as non-DED and were not asked any other DED-related questions. All participants who said yes were asked a series of dry eye–related questions. The first was whether their dry eye had ever been diagnosed by a physician. Possible responses were yes or no. Those who answered yes to experiencing dry eye but no to being diagnosed by a physician were classified as symptomatic–undiagnosed. Those who confirmed both experiencing dry eye and physician diagnosis were classified as diagnosed-DED and were given a list of symptoms (pain, light sensitivity, gritty sensation, feeling of a foreign body or sand in the eye, itching, redness, and blurring of vision) and asked to select all that applied.
Adjusted using the Horvitz-Thompson estimator and inverse probability weighting.
Using the DEQ-5.
Individual patient data are available.
Using the SPEED.
Using the Meiboscore.
Using the eyelid vascularity score (0-3).
Using the meibum quality score (0-4).
Table 3. Incidence of Dry Eye Results.
| Source | Etiology (DED and/or MGD) | Measurement method: components contributing to diagnostic standards | Diagnostic standards | Incidence denominator | Incidence numerator | Incidence, % (95% CI) | Cumulative incidence or incidence rate (period of data collection) | Cumulative incidence normalized to 5-y incidence | Characteristics used to stratify incidence or report an association | Annual incidence, % | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chi et al,37 2012 | DED | Relevant ICD codesa | ICD-9 codes | NR | NR | 23.3 (NR) | Cumulative incidence (1994-2008, 15 y) | 7.8% | Year of incidence estimate (annual incidence)b; NRc; NRd | 1994: 1.17; 1995: 1.21; 1996: 1.24; 1997: 1.33; 1998: 1.35; 1999: 1.35; 2000: 1.40; 2001: 1.49; 2002: 1.58; 2003: 1.59; 2004: 1.62; 2005: 1.63; 2006: 2.11; 2007: 1.81; 2008: 1.83e | H |
| Dana et al,32 2019 | DED | Relevant ICD codesf; CPT codes for procedures and prescriptionsg; prescription fillsh | (1) Overall DED | (1) NRi | (1) 58 126j | (1) NR | Cumulative incidence (2008-2012, 5 y) | 3.5% (All ages); 0.96% (18-39 y); 2.60% (40-49 y); 6.48% (≥50 y) | Age, sex, year of incidence estimate (annual incidence)b; NRc; NRd | All ages: 2008: 0.55; 2009: 0.61; 2010: 0.68; 2011: 0.74; 2012: 0.87 | M |
| (2) Unspecified DEDk | (2) NRi | (2) 32 341j | (2) NR | 18-39 y: 2008: 0.15; 2009: 0.17; 2010: 0.18; 2011: 0.20; 2012: 0.26 | |||||||
| (3) Specified DEDl | (3) NRi | (3) 2705j | (3) NR | 40-49 y: 2008: 0.42; 2009: 0.46; 2010: 0.51; 2011: 0.56; 2012: 0.65 | |||||||
| (4) Sjögren-type DEDm | (4) NRi | (4) 1716j | (4) NR | ≥50 y: 2008: 1.03; 2009: 1.15; 2010: 1.28; 2011: 1.40; 2012: 1.62 |
Abbreviations: CPT, Current Procedural Terminology; DED, dry eye disease; H, high; ICD-9, International Classification of Diseases, Ninth Revision; M, moderate; NR, not reported.
ICD-9 codes 375.15 or 370.20 or 370.21 or 370.33 or 370.34 or 372.53 or 710.2.
Stratified by characteristic.
Characteristic included in univariate analysis.
Characteristic included in multivariate analysis.
Extracted using an online graph digitizer.
Driving indicators: 370.33, keratoconjunctivitis sicca; 370.34, exposure keratoconjunctivitis; 372.53, conjunctival xerosis; 375.15, tear film insufficiency, unspecified; 710.20, sicca syndrome, Sjögren; nondriving indicators: 370.20, superficial keratoconjunctivitis; 370.21, punctate keratitis; 714.0, rheumatoid arthritis; 695.4, discoid lupus erythematosus; 710.0, systemic lupus erythematosus; 373.34, discoid lupus erythematosus of eyelid.
Driving indicators: 68 760, closure of the lacrimal punctum (by thermocauterization, ligation, or laser surgery); 68 761, punctal plugs; 09.91, obliteration of lacrimal punctum.
Driving indicator: restasis prescription fill for cyclosporine ophthalmic emulsion.
Reported as approximately 7.4 million.
2012 Annual incidence numerator.
Using ICD-9 codes 375.15 and 370.33, at least 1 of each or 2 of the same.
Using ICD-9 codes nondriving indicators 370.21 and 370.20, at least 1 of 370.21 or 370.20 and 1 driving indicator (375.15 or 370.33).
Using ICD-9 codes driving indicator 710.2, at least 2 of 710.2 or 1 of 710.2 and 1 driving indicator (375.15 or 370.33).
Risk-of-Bias Assessment
Summaries of risk-of-bias assessments for the dry eye and MGD prevalence studies are presented in eTable 3 in the Supplement. Of the 10 studies that reported prevalence of DED, we judged overall risk of bias to be high in 5 studies (50%),35,38,41,43,45 moderate in 4 studies (40%),32,40,42,44 and low in 1 study (10%).33 We judged all 3 studies that reported prevalence of MGD to be at overall high risk of bias.34,35,36 Of the 2 studies that reported incidence of dry eye, we judged overall risk of bias to be high in Chi et al37 and moderate in Dana et al.32
Prevalence of Dry Eye
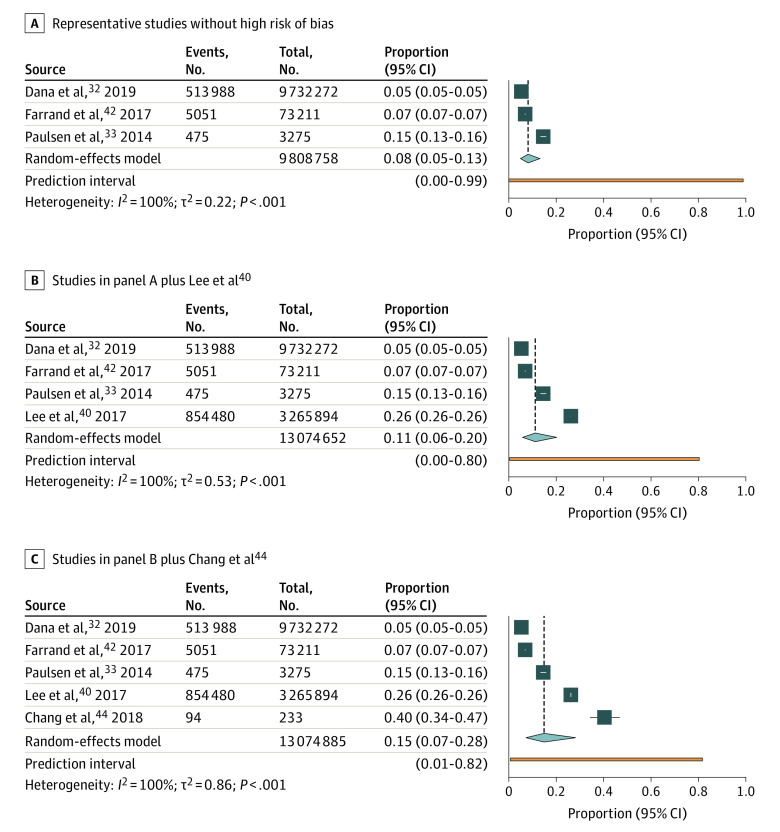
In our primary analysis, prevalence of dry eye ranged from 5.3% to 14.5%, with a summary estimate of 8.1% (95% CI, 4.9%-13.1%; τ2 = 0.22; I2 = 100%; 95% PI, 0%-98.9%; 3 studies, 9 808 758 participants) (Figure 1A). When we added the prevalence (26.2%) from Lee et al,40 the summary estimate increased to 11.1% (95% CI, 5.8%-20.2%; τ2 = 0.53; I2 = 100%; 95% PI, 0.4%-80.3%; 4 studies, 13 074 652 participants) (Figure 1B). When we added the prevalence (40.6%) from Chang et al,44 the summary estimate increased to 14.9% (95% CI, 7.2%-28.2%; τ2 = 0.86; I2 = 100%; 95% PI, 0.7%-81.6%; 5 studies, 13 074 885 participants) (Figure 1C). When all studies were included, prevalence ranged from 1.6% to 46.8%, with a summary estimate of 17.4% (95% CI, 8.9%-31.4%; τ2 = 1.57; I2 = 100%; 95% PI, 1.0%-81.4%; 10 studies, 13 546 368 participants) (eFigure 2 in the Supplement).
Figure 1. Meta-analysis of the Prevalence of Dry Eye in the US Population.
A, Prevalence reported in studies without high risk of bias and judged to be representative of the general population. B, Prevalence reported in studies without high risk of bias and judged to be representative of the general population plus Lee et al40 (a nationwide Veterans Affairs health system database study with a 95.1% male population). C, Prevalence reported in studies without high risk of bias and judged to be representative of the general population plus Lee et al40 (a nationwide Veterans Affairs health system database study with a 95.1% male population) and Chang et al44 (a single-institution clinic-based study that used consecutive enrollment from a comprehensive eye clinic in Miami, Florida).
Associations With Prevalence of Dry Eye
Summaries of the association with age, sex, race and ethnicity, medical comorbidities, medication use, and region of the United States are outlined in eTables 4-6 in the Supplement. Prevalence of dry eye appears to increase with age,32,33,42 female sex,32,33,39,40,42,44,45 diabetes and diabetic complications,38,39,40,44 and southern US regions.42 There is inconsistent evidence regarding the association between dry eye prevalence and race and ethnicity or medication use.39,40,42,44
Incidence of Dry Eye
Only 2 studies contributed to dry eye incidence estimates, and after normalization, the 5-year incidence was 3.5% in the population 18 years and older and 7.8% in the population 68 years and older (Table 3).32,37 The age-stratified and annual incidence, reported by Chi et al37 and Dana et al,32 are presented in Table 3. Dana et al32 reported the incidence in the population 50 years and older as 6.5%, which is comparable with 7.8% in the population 68 years and older reported by Chi et al.37 Because of the lack of well-defined incidence denominators and the heterogeneity between the 2 studies, we did not meta-analyze the incidence data.
Prevalence of MGD
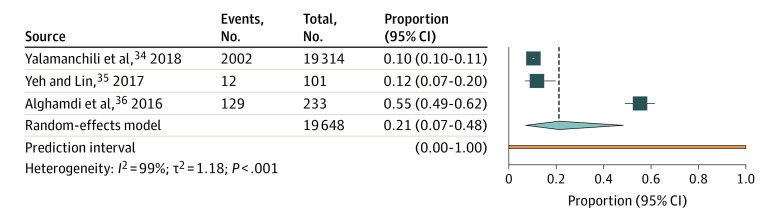
Prevalence of MGD ranged from 10.4% to 55.4% in the 3 included studies.34,35,36 The pooled estimate of MGD prevalence was 21.2% (95% CI, 7.2%-48.3%; τ2 = 1.18; I2 = 99%; 95% PI, 0%-100%; 3 studies, 19 648 participants) (Figure 2). There is inconsistent evidence whether prevalence of MGD increases with age. Yalamanchili et al34 reported that prevalence was 7.7% in the group aged 50 to 59 years and 13.7% in the group aged 80 to 89 years (eTable 4 in the Supplement)34; however, Yeh et al35 reported a nonsignificant association with age in a univariate regression model (eTable 5 in the Supplement). We found no study that reported incidence of MGD.
Figure 2. Meta-analysis of the Prevalence of Meibomian Gland Dysfunction in the US Population.
Discussion
In this systematic review and meta-analysis, we searched for studies that estimated the prevalence or incidence of DED or MGD in the US population after 2010. Our meta-analysis showed that the pooled prevalence of dry eye was 8.1% and the pooled prevalence of MGD was 21.2%. Incidence of dry eye was 3.5% (population aged ≥18 years) and 7.8% (population aged ≥68 years).32,37
The wide range of prevalence estimates for dry eye reflect the notable clinical and methodological heterogeneity across studies. Studies included in the meta-analysis had diverse population characteristics, variations in study designs and settings, and heterogenous definitions of dry eye, which increase our uncertainty in the summary prevalence estimate. Dana et al32 analyzed a nationwide database of US Department of Defense staff and their families. They defined dry eye with relevant diagnostic codes and reported prevalence of dry eye as 5.3%,32 whereas Paulsen et al33 and Farrand et al42 were both population-based epidemiological studies and defined dry eye with self-reported diagnosis and/or symptoms. Farrand et al42 (prevalence estimate of 6.9%) was a nationwide study that randomly sampled from a self-selected panel of online volunteers, and Paulsen et al33 (prevalence estimate of 14.5%) was a regional study sampled from offspring from the Beaver Dam Study, Wisconsin. Population age differences may, at least in part, explain the heterogeneity in the prevalence estimates between studies.33,42
The inclusion of unrepresentative studies and studies at high risk of bias in the meta-analysis appears to inflate the pooled prevalence of dry eye in the US population. Lee et al40 used a nationwide database and defined dry eye with relevant diagnostic codes; however, the diagnostic codes were not the same as those used by Dana et al.32 Furthermore, although Lee et al40 may be representative of the Veterans Affairs health care system population, given the predominantly male population and the unique exposures in the veteran population, the prevalence estimate of 26.2% is unlikely to adequately represent the US population.40 Chang et al44 used consecutive enrollment of patients from a single-institution comprehensive eye clinic in Miami, Florida, and defined dry eye as a score of 6 or higher on the 5-item Dry Eye Questionnaire. The prevalence of 40.3% is unlikely to adequately represent the US population because of the enriched eye clinic–derived population and the specifics of the environment and climate in Miami.
A set of working diagnostic criteria for DED is necessary to allow standardization and comparisons in dry eye epidemiological studies. Such efforts have been successfully implemented in the fields of glaucoma, age-related macular degeneration, and uveitis.46,47,48
Understanding of the pathophysiology of dry eye has evolved considerably over the past decade, and this is reflected in the updated definition of the disease provided by the TFOS DEWS-II report.1 However, although consensus on a battery of diagnostic tests has been published, these have not been implemented in any epidemiological studies in the United States.49
Several studies included in the current systematic review reported dry eye prevalence according to several diagnostic criteria.32,40,42 However, because these multiple criteria were not replicated across studies,32,40 this only compounded uncertainty regarding the underlying population prevalence.
Similar methodological challenges have been encountered by other systematic reviews of dry eye burden, and they have been handled differently across reviews. A systematic review of the burden of DED in Arab populations in the Middle East, which reported prevalence ranging from 6.8% in Egypt to 69.0% in Palestine, did not attempt to combine results across studies in a meta-analysis.50 However, a systematic review of the burden of DED in Africa reported that prevalence ranged from 6.6% to 78.4% and the pooled prevalence was 42.0%. A systematic review of burden of DED in China reported that the pooled prevalence of dry eye by symptoms and signs was 18.5% and by symptoms only was 38.9%.51,52
In other large population-based studies, the prevalence of dry eye has been reported as 22.0% in Canada using the 5-item Dry Eye Questionnaire53 and 9.1% in Germany using the Women’s Health Study questionnaire,54 and the 6-year incidence of dry eye has been reported as 6.1% in Singapore.55 However, dry eye prevalence estimates from global populations cannot be easily applied to their respective US-based ethnic groups (eg, estimates from China cannot be applied to Chinese American individuals) because of the reported effect of local and regional geo-environmental influences.56
In the US population, dry eye epidemiology studies published between 1997 and 2009 reported prevalence and incidence of dry eye and MGD.15,57,58,59 Prevalence of dry eye was 4.3% in the Physicians’ Health Study, 7.8% in the Women’s Health Study, 14.4% in the Beaver Dam Study, and 14.6% in the Salisbury Eye Study. Although we excluded these studies (because of our intent to obtain recent estimates), their reported estimates are consistent with our summary estimate of 8.1%. The Beaver Dam Study reported a 5-year cumulative incidence of 13.3% and a 10-year cumulative incidence of 21.6%.9,10 However, these estimates are higher than reported in the 2 incidence studies included in our review, which may be due to diversity in study designs and contexts.
A systematic review of the global burden of MGD reported that the pooled global prevalence was 35.8%, and before 2010, the prevalence in the United States ranged from 3.5% in a population-based study to 38.9% in a hospital-based study.60 In Japan, the Hirado-Takushima Study reported the prevalence of MGD as 32.9%, the prevalence of DED as 33.4%, and the coexistence with of MGD and DED in 12.4%.61 We were unable to report the prevalence of coexisting MGD and DED.
We report that prevalence of MGD ranged from 10.4% to 55.4%, with a summary prevalence of 21.2%, in studies published since 2010. Our MGD prevalence estimates were derived from hospital-based studies using MGD-specific ICD codes or objective signs of MGD to define cases. Differences in the clinical signs used to diagnose MGD may explain the heterogeneity, but the very wide confidence intervals make the prevalence estimates very uncertain. We did not identify any MGD incidence studies, which is a similar finding to that of the International Workshop on Meibomian Gland Dysfunction in 2011.11 Given the lack of population-based prevalence studies for MGD and the lack of incidence studies for MGD, contemporary longitudinal population-based studies are needed.
Limitations
There are limitations to our meta-analysis that should be acknowledged. As mentioned above, studies included in the meta-analysis had diverse population characteristics, variations in designs and settings, and heterogenous definitions of dry eye. Diagnostic codes and criteria differed across studies. Future epidemiological studies would benefit from the development of a standard set of diagnostic criteria for DED. In addition, a lack of MGD incidence studies and differences in the clinical signs used to diagnose MGD undermined the certainty of the prevalence estimates in this review. However, our pooled estimated of dry eye prevalence was consistent with those reported by previous studies conducted in the time frame prior to that for our review.
Conclusions
In this systematic review and meta-analysis, the prevalence of dry eye ranges from 5.3% to 14.5%, and the prevalence of MGD ranges from 10.4% to 55.4%. The 5-year incidence of dry eye was 3.5% in a population 18 years and older and 7.8% in a population 68 years and older. This study is the first to our knowledge to provide a pooled estimate of dry eye prevalence in the United States. However, the evidence provides uncertain estimates of dry eye burden and highlights the need for further studies that minimize risk of bias and use validated diagnostic criteria.
eFigure 1. PRISMA search flow diagram
eFigure 2. Forest plot of dry eye prevalence including all studies
eTable 1. MEDLINE and Embase search strategies
eTable 2. Excluded full texts
eTable 3. Risk of bias assessments for prevalence and incidence studies
eTable 4. Stratified associations with dry eye and meibomian gland dysfunction
eTable 5. Univariable model associations with dry eye and meibomian gland dysfunction
eTable 6. Multivariable model associations with dry eye and meibomian gland dysfunction
References
- 1.Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-283. doi: 10.1016/j.jtos.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 2.Pucker AD, Haworth KM. The presence and significance of polar meibum and tear lipids. Ocul Surf. 2015;13(1):26-42. doi: 10.1016/j.jtos.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 3.Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film: a review. Exp Eye Res. 2015;137:125-138. doi: 10.1016/j.exer.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 4.Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922-1929. doi: 10.1167/iovs.10-6997a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379-387. doi: 10.1097/ICO.0b013e3181f7f363 [DOI] [PubMed] [Google Scholar]
- 6.Chen EM, Kombo N, Teng CC, Mruthyunjaya P, Nwanyanwu K, Parikh R. Ophthalmic medication expenditures and out-of-pocket spending: an analysis of United States prescriptions from 2007 through 2016. Ophthalmology. 2020;127(10):1292-1302. doi: 10.1016/j.ophtha.2020.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourne R, Steinmetz JD, Flaxman S, et al. ; GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study . Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e130-e143. doi: 10.1016/S2214-109X(20)30425-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334-365. doi: 10.1016/j.jtos.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 9.Moss SE, Klein R, Klein BEK. Incidence of dry eye in an older population. Arch Ophthalmol. 2004;122(3):369-373. doi: 10.1001/archopht.122.3.369 [DOI] [PubMed] [Google Scholar]
- 10.Moss SE, Klein R, Klein BEK. Long-term incidence of dry eye in an older population. Optom Vis Sci. 2008;85(8):668-674. doi: 10.1097/OPX.0b013e318181a947 [DOI] [PubMed] [Google Scholar]
- 11.Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52(4):1994-2005. doi: 10.1167/iovs.10-6997e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372(71):n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCann P, Abraham AG, Gregory DG, et al. Prevalence and incidence of dry eye in the USA: a systematic review protocol. BMJ Open. 2021;11(11):e056203. doi: 10.1136/bmjopen-2021-056203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318-326. doi: 10.1016/S0002-9394(03)00218-6 [DOI] [PubMed] [Google Scholar]
- 16.Lemeshow S, Hosmer DW Jr, Klar J, Lwanga SK. Adequacy of Sample Size in Health Studies. World Health Organization; 1990. [Google Scholar]
- 17.Lê JT, Qureshi R, Rouse B, et al. Development and content of a database of systematic reviews for eyes and vision. Eye (Lond). 2022;36(4):883-885. doi: 10.1038/s41433-021-01514-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . World report on vision. Published October 8, 2019. https://www.who.int/publications/i/item/9789241516570
- 19.Akpek EK, Amescua G, Farid M, et al. ; American Academy of Ophthalmology Preferred Practice Pattern Cornea and External Disease Panel . Dry Eye Syndrome Preferred Practice Pattern®. Ophthalmology. 2019;126(1):286-P334. doi: 10.1016/j.ophtha.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 20.Veritas Health Innovation Melbourne Australia . Covidence systematic review software. Accessed September 23, 2022. https://www.covidence.org.
- 21.Li T, Vedula SS, Hadar N, Parkin C, Lau J, Dickersin K. Innovations in data collection, management, and archiving for systematic reviews. Ann Intern Med. 2015;162(4):287-294. doi: 10.7326/M14-1603 [DOI] [PubMed] [Google Scholar]
- 22.Ankit Rohatgi . WebPlotDigitizer. Published online 2021. https://automeris.io/WebPlotDigitizer/
- 23.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934-939. doi: 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 24.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Systematic reviews of prevalence and incidence. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. JBI; 2020, doi: 10.46658/JBIMES-20-06. [DOI] [Google Scholar]
- 25.Wells GA, Wells G, Shea B, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Published 2014. Accessed April 28, 2022. https://www.ohri.ca//programs/clinical_epidemiology/oxford.Asp
- 26.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stijnen T, Hamza TH, Özdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29(29):3046-3067. doi: 10.1002/sim.4040 [DOI] [PubMed] [Google Scholar]
- 28.R Core Team . R: a language and environment for statistical computing.
- 29.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153-160. doi: 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Migliavaca CB, Stein C, Colpani V, et al. ; Prevalence Estimates Reviews-Systematic Review Methodology Group (PERSyst) . Meta-analysis of prevalence: I2 statistic and how to deal with heterogeneity. Res Synth Methods. 2022;13(3):363-367. doi: 10.1002/jrsm.1547 [DOI] [PubMed] [Google Scholar]
- 31.Flaxman AD, Vos T, Murray CJ. An Integrative Metaregression Framework for Descriptive Epidemiology. University of Washington Press; 2015. [Google Scholar]
- 32.Dana R, Bradley JL, Guerin A, et al. Estimated prevalence and incidence of dry eye disease based on coding analysis of a large, all-age United States health care system. Am J Ophthalmol. 2019;202:47-54. doi: 10.1016/j.ajo.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 33.Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring Study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799-806. doi: 10.1016/j.ajo.2013.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yalamanchili SP, Donaldson J, Ward MF, Davis RM. Ethnic and racial differences in prevalence of meibomian gland dysfunction within the older population. J Am Geriatr Soc. 2018;66:S290. [Google Scholar]
- 35.Yeh TN, Lin MC. Risk factors for severe meibomian gland atrophy in a young adult population: a cross-sectional study. PLoS One. 2017;12(9):e0185603. doi: 10.1371/journal.pone.0185603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alghamdi YA, Mercado C, McClellan AL, Batawi H, Karp CL, Galor A. Epidemiology of meibomian gland dysfunction in an elderly population. Cornea. 2016;35(6):731-735. doi: 10.1097/ICO.0000000000000815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chi SL, Acquah KF, Richard MJ, Lee PP, Sloan FA. Longitudinal evidence on punctal plug use in an elderly population. Ophthalmic Plast Reconstr Surg. 2012;28(4):289-293. doi: 10.1097/IOP.0b013e31825ca599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis RM, Lefebvre CG, Van Der Vaart R, Weaver MA. Dry eye disease and diabetes mellitus. Diabetes. 2015;64:174. [Google Scholar]
- 39.Lee CJ, Felix ER, Levitt RC, et al. Traumatic brain injury, dry eye and comorbid pain diagnoses in US veterans. Br J Ophthalmol. 2018;102(5):667-673. doi: 10.1136/bjophthalmol-2017-310509 [DOI] [PubMed] [Google Scholar]
- 40.Lee CJ, Levitt RC, Felix ER, Sarantopoulos CD, Galor A. Evidence that dry eye is a comorbid pain condition in a U.S. veteran population. Pain Rep. 2017;2(6):e629-e629. doi: 10.1097/PR9.0000000000000629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo D, Bass O. Visual and ocular conditions among a homeless adult population of Boston. Optom Vis Sci. 2021;98(4):362-366. doi: 10.1097/OPX.0000000000001674 [DOI] [PubMed] [Google Scholar]
- 42.Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90-98. doi: 10.1016/j.ajo.2017.06.033 [DOI] [PubMed] [Google Scholar]
- 43.Stull C, Valdes-Rodriguez R, Shafer BM, et al. The prevalence and characteristics of chronic ocular itch: a cross-sectional survey. Itch (Phila). 2017;2(1):e4. doi: 10.1097/itx.0000000000000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang VS, Rose TP, Karp CL, Levitt RC, Sarantopoulos C, Galor A. Neuropathic-like ocular pain and nonocular comorbidities correlate with dry eye symptoms. Eye Contact Lens. 2018;44(suppl 2):S307-S313. doi: 10.1097/ICL.0000000000000463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manoj VS. Prevalence of hyperosmolarity and symptoms of DED in the US population. Invest Ophthalmol Vis Sci. 2016;57(12):2860. [Google Scholar]
- 46.Ferris FL III, Wilkinson CP, Bird A, et al. ; Beckman Initiative for Macular Research Classification Committee . Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-851. doi: 10.1016/j.ophtha.2012.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238-242. doi: 10.1136/bjo.86.2.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Gelder RN, Sen HN, Tufail A, Lee AY. Here comes the SUN (part 2): standardization of uveitis nomenclature for disease classification criteria. Am J Ophthalmol. 2021;228:A2-A6. doi: 10.1016/j.ajo.2021.05.006 [DOI] [PubMed] [Google Scholar]
- 49.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539-574. doi: 10.1016/j.jtos.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 50.Aljarousha M, Badarudin N, Azemin M, Aljeesh Y, Abuimara A. A systematic review on prevalence, risk factors, clinical diagnosis and medical management of dry eye disease in the Arab population. Afr Vision Eye Health. 2021;80(1):a591. doi: 10.4102/aveh.v80i1.591 [DOI] [Google Scholar]
- 51.Song P, Xia W, Wang M, et al. Variations of dry eye disease prevalence by age, sex and geographic characteristics in China: a systematic review and meta-analysis. J Glob Health. 2018;8(2):020503. doi: 10.7189/jogh.08.020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akowuah PK, Kobia-Acquah E. Prevalence of dry eye disease in Africa: a systematic review and meta-analysis. Optom Vis Sci. 2020;97(12):1089-1098. doi: 10.1097/OPX.0000000000001610 [DOI] [PubMed] [Google Scholar]
- 53.Caffery B, Srinivasan S, Reaume CJ, et al. Prevalence of dry eye disease in Ontario, Canada: a population-based survey. Ocul Surf. 2019;17(3):526-531. doi: 10.1016/j.jtos.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 54.Vehof J, Snieder H, Jansonius N, Hammond CJ. Prevalence and risk factors of dry eye in 79,866 participants of the population-based Lifelines cohort study in the Netherlands. Ocul Surf. 2021;19:83-93. doi: 10.1016/j.jtos.2020.04.005 [DOI] [PubMed] [Google Scholar]
- 55.Man REK, Veerappan AR, Tan S-P, et al. Incidence and risk factors of symptomatic dry eye disease in Asian Malays from the Singapore Malay Eye Study. Ocul Surf. 2017;15(4):742-748. doi: 10.1016/j.jtos.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 56.Galor A, Kumar N, Feuer W, Lee DJ. Environmental factors affect the risk of dry eye syndrome in a United States veteran population. Ophthalmology. 2014;121(4):972-973. doi: 10.1016/j.ophtha.2013.11.036 [DOI] [PubMed] [Google Scholar]
- 57.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127(6):763-768. doi: 10.1001/archophthalmol.2009.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schein OD, Muñoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124(6):723-728. doi: 10.1016/S0002-9394(14)71688-5 [DOI] [PubMed] [Google Scholar]
- 59.Moss SE, Klein R, Klein BEK. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264-1268. doi: 10.1001/archopht.118.9.1264 [DOI] [PubMed] [Google Scholar]
- 60.Hassanzadeh S, Varmaghani M, Zarei-Ghanavati S, Heravian Shandiz J, Azimi Khorasani A. Global prevalence of meibomian gland dysfunction: a systematic review and meta-analysis. Ocul Immunol Inflamm. 2021;29(1):66-75. doi: 10.1080/09273948.2020.1755441 [DOI] [PubMed] [Google Scholar]
- 61.Arita R, Mizoguchi T, Kawashima M, et al. Meibomian gland dysfunction and dry eye are similar but different based on a population-based study: the Hirado-Takushima Study in Japan. Am J Ophthalmol. 2019;207:410-418. doi: 10.1016/j.ajo.2019.02.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA search flow diagram
eFigure 2. Forest plot of dry eye prevalence including all studies
eTable 1. MEDLINE and Embase search strategies
eTable 2. Excluded full texts
eTable 3. Risk of bias assessments for prevalence and incidence studies
eTable 4. Stratified associations with dry eye and meibomian gland dysfunction
eTable 5. Univariable model associations with dry eye and meibomian gland dysfunction
eTable 6. Multivariable model associations with dry eye and meibomian gland dysfunction