Abstract
Background
Increasing proportions of smokers in Japan smoke <10 cigarettes per day (CPD). Yet, the health risks of low-intensity smoking in Asia are poorly understood.
Methods
We performed a pooled analysis of 410 294 adults from nine population-based prospective cohort studies participating in the Japan Cohort Consortium. Cigarette-use data were collected at each study baseline in 1983–1994. Study-specific hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause and cause-specific mortality were calculated using multivariable-adjusted Cox regression by CPD among current smokers and by age at cessation among former smokers, with never smokers as the referent group. Pooled HRs and CIs were computed using a random-effect model.
Results
The smoking prevalence was 54.5% in men and 7.4% in women. About 15.5% of male and 50.4% of female current smokers smoked 1–10 CPD (low-intensity). Both male and female low-intensity smokers had higher all-cause mortality risks than never smokers. Risks were further higher with increasing CPD in a dose–response manner. HRs (95% CIs) were 1.27 (0.97–1.66), 1.45 (1.33–1.59) and 1.49 (1.38–1.62) for 1–2, 3–5 and 6–10 CPD, respectively, in men; 1.28 (1.01–1.62), 1.49 (1.34–1.66) and 1.68 (1.55–1.81) for 1–2, 3–5 and 6–10 CPD, respectively, in women. Similar associations were observed for smoking-related causes of death. Among former low-intensity smokers, younger age at cessation was associated with lower mortality risk.
Conclusions
Smoking very low amounts was associated with increased mortality risks in Japan. All smokers should quit, even if they smoke very few CPD.
Keywords: Low-intensity smoking, cigarette, smoking, mortality, smoking-related death, cessation, Japan
Key Messages.
Increasing proportions of smokers in Japan smoke <10 cigarettes per day (CPD; low-intensity smoking), but the health risks of low-intensity smoking in Asia are poorly understood.
Low-intensity smokers, even at 1–2 CPD, had higher mortality risks for all causes and for a wide range of smoking-related causes of death than never smokers, with risks further increasing with higher CPD both in men and women.
Mortality risks were lower with younger age at cessation among male and female former smokers, including among those who used to smoke ≤10 CPD.
Even smoking very low amounts was associated with increased mortality risks in Japan. Thus, all smokers should quit, even if they smoke very few CPD.
Introduction
Tobacco use is the leading cause of premature death worldwide. Each year, more than 7 million people worldwide die as a result of tobacco use.1 Japan is among the 10 largest smoking populations in the world, along with other Asian countries such as China, India and Indonesia.2
Tobacco smoking has historically been uncommon among women in Asia. However, in more developed countries such as Japan, a substantial number of women smoke cigarettes. Smoking prevalence in Japanese women has declined slightly in the last two decades, from 10.9% in 1998 to 8.1% in 2018,3 but remains higher than in China (2.2%), India (2.8%) and other Asian countries.2,4 Traditionally, Japanese female smokers do not smoke heavily, yet the proportion of female smokers who smoke ≤10 cigarettes per day (CPD) (low-intensity) increased from 43.4% in 2003 to 53.4% in 2017.4
More men smoke cigarettes than women in Japan, but the prevalence among men has declined for the last two decades, from 50.8% in 1998 to 29.0% in 2018, as a result of tobacco-control efforts.3 Over time, Japanese male smokers are also smoking fewer CPD. The proportion of male smokers who smoked 1–10 CPD increased from 15.5% in 2003 to 34.0% in 2017.4
There is a common perception that low-intensity smoking poses little to no harm.5 Yet, a growing body of literature suggests that low-intensity smokers have an increased risk of death.6–13 Nevertheless, little is known about the health risks of low-intensity smoking in Asia. To date, no studies have focused on the health impact of low-intensity smoking in the Japanese population.
Herein, we pooled nine population-based prospective cohort studies participating in the Japan Cohort Consortium (JCC) to assess all-cause and cause-specific mortality risks among low-intensity smokers. A large number of participants in the pooled analysis enabled us to assess the mortality risks by detailed CPD among low-intensity smokers.
Methods
Study population
In the current pooled analysis, we included population-based prospective cohort studies participating in the JCC that (i) assessed cigarette smoking at study baseline in the mid-1980s to mid-1990s using a validated questionnaire; (ii) assessed CPD at the very low level (1–2 CPD); and (iii) had cause-of-death information during follow-up. Nine studies that met the criteria included (i) Japan Public Health Center-based Prospective Study, Cohort I (JPHC-I); (ii) Japan Public Health Center-based Prospective Study, Cohort II (JPHC-II); (iii) Japan Collaborative Cohort Study (JACC); (iv) Miyagi Cohort Study (MIYAGI); (v) Three-Prefecture Miyagi (3-Pref MIYAGI); (vi) Three-Prefecture Aichi (3-Pref AICHI); (vii) Ohsaki National Health Insurance Cohort Study (OHSAKI); (viii) Life Span Study (LSS); and (ix) Three-Prefecture Osaka (3-Pref OSAKA). Characteristics of the participating studies are described in Table 1. We excluded participants who (i) were >95 years old at baseline; (ii) did not have information on sex, age or region (for JPHC-I, JPHC-II, JACC and LSS); or (iii) did not report cigarette-smoking status (never, former or current) and/or current CPD. After the exclusions, the current pooled analysis included 410 294 adults.
Table 1.
Characteristics of the cohort studies in the pooled analysis
| Study | Age at baseline (years) | Baseline survey | Last follow-up time | Follow-up yearsa | Size of the analytic cohort |
Number of deaths |
Current smokers |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at starting smoking regularly (years)b |
Number of cigarettes per dayb |
|||||||||||
| Men | Women | Men | Women | Men | Women | Men | Women | |||||
| JPHC-I | 40–59 | 1990 | 2016 | 1 148 933 (26.5) | 23 258 | 26 485 | 6438 | 3552 | 20 (19–21) | 25 (20–35) | 20 (15–30) | 12 (8–20) |
| JPHC-II | 40–69 | 1993–1994 | 2016 | 1 259 354 (22.9) | 29 453 | 32 995 | 9914 | 6511 | 20 (19–21) | 25 (20–34) | 20 (20–30) | 15 (10–20) |
| JACC | 40–79 | 1988–1990 | 2009 | 1 781 319 (18.9) | 44 139 | 64 190 | 15 401 | 12 009 | 20 (20–22) | 30 (23–40) | 20 (15–25) | 10 (10–20) |
| MIYAGI | 40–64 | 1990 | 2017, 2014 (cause-specific) | 878 010 (27.6) | 19 750 | 17 796 | 7076 | 3517 | 20 (20–24) | 30 (23–39) | 20 (18–30) | 10 (8–20) |
| 3-Pref MIYAGI | ≥40 | 1984 | 1998 | 250 578 (15.0) | 10 150 | 11 737 | 2353 | 1900 | 20 (20–22) | 30 (23–40) | 20 (15–25) | 20 (10–20) |
| 3-Pref AICHI | 40–103 | 1985 | 2000 | 386 035 (15.2) | 15 746 | 17 783 | 3241 | 2639 | 20 (19–21) | 30 (23–37) | 20 (15–30) | 12 (10–20) |
| OHSAKI | 40–79 | 1994 | 2008 | 435 207 (13.2) | 20 403 | 19 875 | 4439 | 2357 | 20 (20–23) | 30 (23–40) | 20 (15–25) | 10 (10–20) |
| LSS | 46–104 | 1991 | 2003 | 258 482 (12.0) | 9148 | 14 789 | 1979 | 3091 | 20 (19–20) | 27 (22–35) | 20 (15–30) | 12 (10–20) |
| 3-Pref OSAKA | 40–99 | 1983–1985 | 2000 | 398 285 (15.0) | 15 881 | 16 716 | 4071 | 2997 | 20 (19–21) | 30 (23–38) | 20 (15–30) | 10 (7–20) |
| Total | 187 928 | 222 366 | 54 912 | 38 573 | ||||||||
JPHC-I, Japan Public Health Center-based Prospective Study, Cohort I; JPHC-II, Japan Public Health Center-based Prospective Study, Cohort II; JACC, Japan Collaborative Cohort Study; MIYAGI, Miyagi Cohort Study; 3-Pref MIYAGI, Three-Prefecture Miyagi; 3-Pref AICHI: Three-Prefecture Aichi; OHSAKI, The Ohsaki Cohort Study; LSS, Life Span Study; 3-Pref OSAKA, Three-Prefecture Osaka.
Total (median).
Median (interquartile range).
Cigarette smoking
Cigarette smoking was assessed in the self-administered questionnaire at the baseline of each study. Although there were slight differences in wording, smoking was assessed with generable consistency across included studies (Supplementary Table S1, available as Supplementary data at IJE online). The participants were grouped into never, former and current smokers. We further categorized current smokers at the study baseline by reported CPD: 1–2, 3–5, 6–10, 11–20, 21–30, 31–40, >40 CPD. Former smokers were categorized by age at smoking cessation (<30, 30–39, 40–49, 50–59, ≥60 years) and years since cessation at study baseline (≤2, 3–10, 11–20, >20 years). For certain causes of death for which the number of deaths in one category was less than five, we collapsed categories to create a wider range. Information on age at starting smoking regularly was also collected.
Mortality ascertainment
Study participants were followed for deaths through the end of follow-up and 93 485 deaths were identified. Death and cause of death were confirmed by examination of resident registration and death certificates, which are mandatory by law, collected by local governments and cover the entire Japanese population with nearly 100% completeness. Study participants were matched by name, date of birth, address and date of death, although the procedures slightly differ by study.14–19 Smoking-related causes of death were identified based on International Classification of Diseases and Health Related problems, Ninth Revision (ICD-9) and Tenth Revision (ICD-10) codes. Cause-specific mortality outcomes included all cancer, smoking-related cancer according to the International Agency for Research on Cancer and US Surgeon General Report,20–22 lung cancer, circulatory disease, ischaemic heart disease, cerebrovascular disease, subarachnoid haemorrhage and chronic lower respiratory disease. ICD codes for mortality outcomes are shown in Supplementary Table S2 (available as Supplementary data at IJE online).
Statistical analysis
Follow-up time was computed as person-years from baseline to date of death, migration from the study area or the end of follow-up, whichever occurred first. Study-specific hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause and cause-specific mortality risks were estimated using Cox proportional-hazards regression with never smokers as the referent group. Covariates in the full model were determined based on literature review, including age at baseline, alcohol intake (men: never, former, <1 time/week, regularly <23, 23 to <46, ≥46 g/d; women: never, former, <1 time/week, regularly <23, ≥23 g/d) and region (for JPHC-I, JPHC-II, JACC and LSS). Additional analyses included adjustment for second-hand smoking (current at home: almost never, hardly ever; current at work: yes, no; and childhood: yes, no, unknown) or age at smoking initiation (never, <19, 19–20, ≥21 years for men and <25, 25–29, 30–34, ≥35 years for women). The LSS did not collect second-hand-smoking data. Adjusting for physical activity or body weight did not change the results considerably. Among former smokers, we examined all-cause mortality by age at cessation and years since cessation at study baseline in men and women combined overall and in analyses restricted to former low-intensity smokers (1–10 CPD). We conducted three sensitivity analyses for all-cause mortality (i) excluding those who died within 2 years of follow-up, (ii) limiting follow-up to 10 years after baseline and (iii) excluding deaths occurring within 5 years after baseline among former smokers. Pooled HRs and CIs were calculated using a random-effects model. To assess heterogeneity, we used I2 statistics and Cochran’s Q chi-square test. Pooled analyses were conducted using STATA/SE 16.
Results
Table 1 describes nine cohort studies included in the pooled analysis. Of 410 294 participants aged 40–95 years, there were 187 928 (45.8%) men and 222 366 (54.2%) women. About 29.0% (n = 118 989) of the participants were current smokers, 15.4% (n = 63 353) were former smokers and 55.6% (n = 227 952) were never smokers. The distribution of smoking status differed considerably in men and women. In men, 54.5% were current smokers, 24.9% were former smokers and 20.6% were never smokers. In women, 7.4% were current smokers, 7.4% were former smokers and 85.2% were never smokers. About 20.3% (15.5% for men and 50.4% for women) of current smokers were low-intensity smokers (0.7% 1–2 CPD, 4.0% 3–5 CPD, 15.6% 6–10 CPD). Smoking patterns among current smokers were similar across the studies. In participating studies, the median age of starting smoking regularly was 20 years in men and ranged from 25 to 30 years in women. The median CPD in each cohort was 20 in men and 10–20 in women. Low-intensity smokers tended to start at older ages than heavier smokers (Supplementary Table S3, available as Supplementary data at IJE online). Among this group, the median age at starting smoking regularly ranged from 20 to 21 years in men and 28 to 35 years in women.
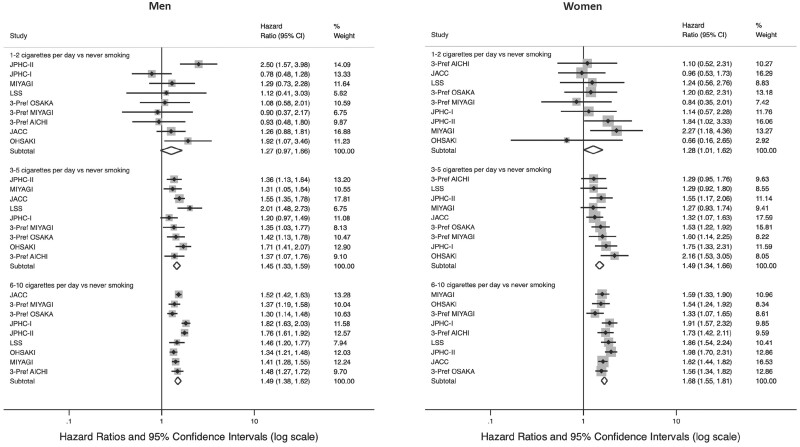
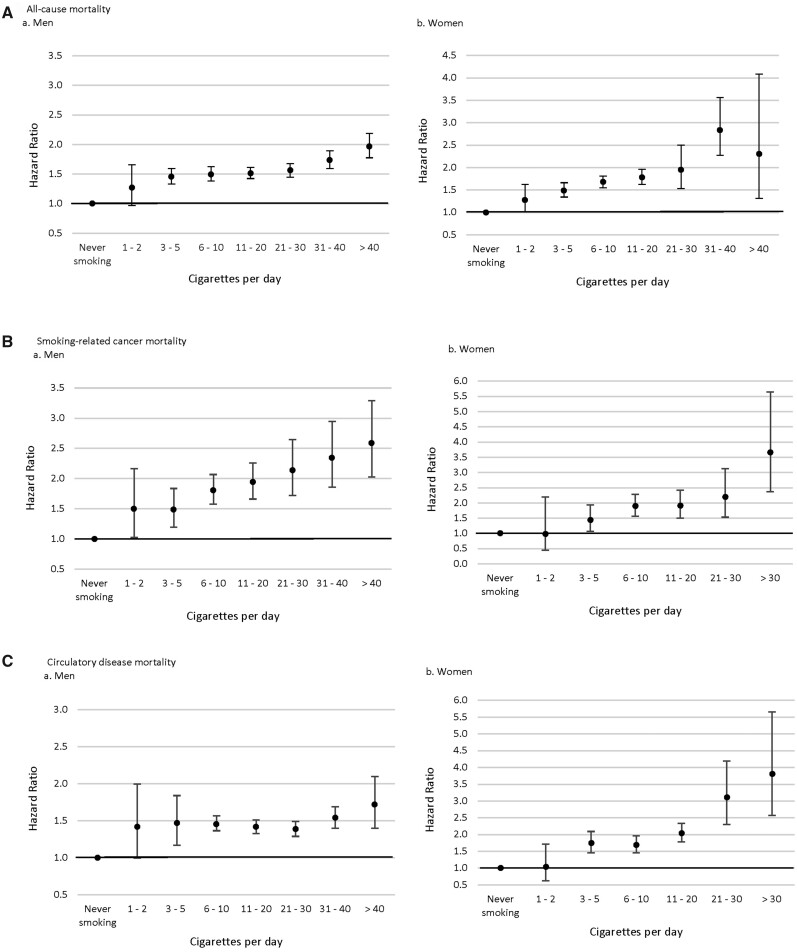
Higher all-cause-mortality risks were observed for both male and female low-intensity smokers compared with never smokers (Table 2 and Figures 1A and 2A). HRs (95% CIs) from the full model were 1.27 (0.97–1.66) for 1–2 CPD, 1.45 (1.33–1.59) for 3–5 CPD and 1.49 (1.38–1.62) for 6–10 CPD in men. Similarly, HRs and 95% CIs in women were 1.28 (1.00–1.62) for 1–2 CPD, 1.49 (1.34–1.66) for 3–5 CPD and 1.68 (1.55–1.81) for 6–10 CPD. Even higher risks were observed among those smoking >10 CPD. Additionally adjusting for second-hand smoking or age at smoking initiation did not change associations considerably. In sensitivity analyses, associations remained similar after limiting follow-up to 10 years or excluding deaths occurring within 2 years of the baseline.
Table 2.
All-cause mortality risk by number of cigarettes smoked per day in the pooled analysis of nine cohort studies in the Japan Cohort Consortium
| Never | Cigarettes smoked per day |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1–2 | 3–5 | 6–10 | 11–20 | 21–30 | 31–40 | >40 | ||
| Men | ||||||||
| Total n | 38 616 | 485 | 2641 | 12 709 | 53 894 | 19 845 | 10 035 | 2871 |
| Death, n | 8879 | 116 | 909 | 4805 | 16 948 | 4945 | 2446 | 660 |
| Full modela | ||||||||
| HR (95% CI) | 1.00 | 1.27 (0.97–1.66) | 1.45 (1.33–1.59) | 1.49 (1.38–1.62) | 1.51 (1.42–1.61) | 1.57 (1.45–1.68) | 1.73 (1.59–1.89) | 1.96 (1.77–2.18) |
| I2b | 48.33 | 37.31 | 78.84 | 83.80 | 74.31 | 67.01 | 32.71 | |
| Pc | 0.05 | 0.12 | <0.001 | <0.001 | <0.001 | 0.002 | 0.16 | |
| Additional adjustment for second-hand smokingd | ||||||||
| HR (95% CI) | 1.00 | 1.30 (0.96–1.74) | 1.42 (1.31–1.54) | 1.49 (1.36–1.64) | 1.50 (1.40–1.61) | 1.56 (1.43–1.70) | 1.72 (1.56–1.90) | 1.96 (1.74–2.19) |
| I2 | 56.52 | 18.60 | 84.28 | 86.04 | 80.28 | 74.26 | 42.36 | |
| P | 0.02 | 0.28 | <0.001 | <0.001 | <0.001 | <0.001 | 0.10 | |
| Additional adjustment for age at smoking initiatione | ||||||||
| HR (95% CI) | 1.00 | 1.30 (0.92–1.82) | 1.46 (1.24–1.72) | 1.44 (1.22–1.70) | 1.45 (1.24–1.71) | 1.48 (1.27–1.73) | 1.61 (1.35–1.92) | 1.78 (1.42–2.25) |
| I2 | 59.66 | 71.13 | 89.74 | 92.51 | 86.52 | 85.24 | 78.04 | |
| P | 0.01 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Follow-up up to 10 yearsa | ||||||||
| Death, n | 3589 | 63 | 502 | 2365 | 6867 | 1682 | 856 | 226 |
| HR (95% CI) | 1.00 | 1.53 (1.19–1.96) | 1.61 (1.47–1.77) | 1.50 (1.36–1.65) | 1.43 (1.35–1.51) | 1.37 (1.30–1.46) | 1.57 (1.42–1.74) | 1.60 (1.27–2.01) |
| I2 | 3.08 | 0 | 68.96 | 48.33 | 3.56 | 41.19 | 62.38 | |
| P | 0.41 | 0.68 | 0.001 | 0.04 | 0.41 | 0.08 | 0.004 | |
| Excluding deaths within 2 years of baselinea | ||||||||
| Total n | 38 032 | 480 | 2546 | 12 339 | 53 031 | 19 645 | 9938 | 2847 |
| Death, n | 8295 | 111 | 814 | 4435 | 16 085 | 4745 | 2349 | 636 |
| HR (95% CI) | 1.00 | 1.32 (1.00–1.74) | 1.44 (1.30–1.60) | 1.51 (1.40–1.62) | 1.54 (1.45–1.63) | 1.60 (1.49–1.71) | 1.77 (1.63–1.92) | 2.02 (1.82–2.24) |
| I2 | 49.93 | 49.32 | 74.58 | 80.05 | 71.27 | 62.98 | 28.74 | |
| P | 0.04 | 0.046 | <0.001 | <0.001 | 0.001 | 0.006 | 0.19 | |
| Women | ||||||||
| Total n | 189 336 | 405 | 2116 | 5796 | 6825 | 886 | 384 | 97 |
| Death, n | 31 387 | 68 | 460 | 1319 | 1318 | 152 | 80 | 12 |
| Full modela | ||||||||
| HR (95% CI) | 1.00 | 1.28 (1.01–1.62) | 1.49 (1.34–1.66) | 1.68 (1.55–1.81) | 1.78 (1.62–1.96) | 1.95 (1.53–2.50) | 2.84 (2.27–3.56) | 2.31 (1.31–4.08) |
| I2 | 0 | 18.63 | 42.89 | 62.19 | 48.69 | 0 | 0 | |
| P | 0.50 | 0.28 | 0.08 | 0.007 | 0.049 | 0.84 | 0.64 | |
| Additional adjustment for second-hand smokingc | ||||||||
| HR (95% CI) | 1.00 | 1.28 (0.97–1.68) | 1.57 (1.39–1.78) | 1.65 (1.52–1.80) | 1.80 (1.61–2.01) | 1.96 (1.50–2.57) | 2.74 (2.17–3.47) | 2.35 (1.33–4.16) |
| I2 | 15.06 | 18.96 | 46.22 | 68.41 | 54.24 | 0 | 0 | |
| P | 0.31 | 0.29 | 0.07 | 0.002 | 0.03 | 0.71 | 0.60 | |
| Additional adjustment for age at smoking initiationd | ||||||||
| HR (95% CI) | 1.00 | 1.23 (0.91–1.65) | 1.49 (1.23–1.80) | 1.52 (1.21–1.91) | 1.54 (1.16–2.02) | 1.48 (1.02–2.16) | 2.33 (1.75–3.10) | 2.00 (1.07–3.73) |
| I2 | 0 | 37.41 | 69.65 | 78.67 | 56.73 | 0 | 0 | |
| P | 0.70 | 0.12 | 0.001 | <0.001 | 0.018 | 0.78 | 0.79 | |
| Follow-up up to 10 yearsa | ||||||||
| Death, n | 12 764 | 37 | 264 | 658 | 570 | 59 | 32 | 3 |
| HR (95% CI) | 1.00 | 1.41 (1.04–1.91) | 1.71 (1.46–2.00) | 1.66 (1.48–1.85) | 1.60 (1.41–1.82) | 1.71 (1.32–2.21) | 2.57 (1.84–3.60) | 1.65 (0.53–5.11) |
| I2 | 0 | 37.71 | 39.93 | 49.45 | 0 | 0 | 0 | |
| P | 0.51 | 0.11 | 0.09 | 0.04 | 0.80 | 0.89 | 0.82 | |
| Excluding deaths within 2 years of baselinea | ||||||||
| Total n | 186 620 | 394 | 2072 | 5701 | 6758 | 881 | 380 | 97 |
| Death, n | 29 471 | 57 | 416 | 1224 | 1251 | 147 | 76 | 12 |
| HR (95% CI) | 1.00 | 1.20 (0.93–1.55) | 1.49 (1.34–1.66) | 1.69 (1.56–1.83) | 1.84 (1.69–2.01) | 2.01 (1.58–2.57) | 2.89 (2.30–3.63) | 2.45 (1.39–4.32) |
| I2 | 0 | 16.26 | 42.69 | 52.47 | 46.20 | 0 | 0 | |
| P | 0.52 | 0.30 | 0.08 | 0.04 | 0.06 | 0.93 | 0.64 | |
Full model was adjusted for age, alcohol intake (men: never, former, <1 time/week, regularly <23, 23 to <46, ≥46 g/d; women: never, former, <1 time/week, regularly <23, ≥23 g/d) and region (for the Japan Public Health Center-based Prospective Study, Cohort I, the Japan Public Health Center-based Prospective Study, Cohort II, the Japan Collaborative Cohort Study and the Life Span Study).
I 2 statistics describe the percentage of variation across studies due to heterogeneity rather than chance.
Cochrane’s Q statistics for a homogeneity test.
Adjusted for covariates in full model and second-hand smoking: current at home (almost never, hardly ever), current at work (yes, no) and childhood (yes, no, unknown); the Life Span Study was not included because second-hand smoking data were not available.
Adjusted for covariates in full model and age at starting smoking regularly: never, <19, 19–20, ≥21 years for men; never, <25, 25–29, 30–34, ≥35 years for women.
Figure 1.
All-cause mortality risk by number of cigarettes smoked per day among low-intensity smokers (≤10 cigarettes per day) in the pooled analysis in the Japan Cohort Consortiuma
aThe Cox proportional-hazards regression models were adjusted for age, alcohol intake (men: never, former, <1 time/week, regularly <23, 23 to <46, ≥46 g/d; women: never, former, <1 time/week, regularly <23, ≥23 g/d) and region (for the Japan Public Health Center-based Prospective Study Cohort I, the Japan Public Health Center-based Prospective Study Cohort II, the Japan Collaborative Cohort Study and the Life Span Study).
Higher risks relative to never smokers were observed among low-intensity smokers for smoking-related causes of death, although CIs were large for some outcomes reflecting small numbers (Table 3, Figures 1B–F and 2B–D, and Supplementary Table S4, available as Supplementary data at IJE online). In men, mortality risks for 1–2 CPD were higher than never smokers for smoking-related cancer (HR = 1.50, 95% CI = 1.03–2.17), lung cancer (HR = 4.05, 95% CI = 1.63–10.07), circulatory disease (HR = 1.42, 95% CI = 1.00–2.00) and subarachnoid haemorrhage (HR = 4.12, 95% CI = 1.69–9.99). For 3–5 CPD, increased risks were observed for all cancer (HR = 1.30, 95% CI = 1.12–1.51), smoking-related cancer (HR = 1.49, 95% CI = 1.20–1.84), lung cancer (HR = 2.04, 95% CI = 1.47–2.82) and circulatory disease (HR = 1.47, 95% CI = 1.17–1.84). Higher risks were observed among men who reported smoking >5 CPD, but the risk increased the most at the lowest level of CPD for certain causes of death, such as circulatory disease, ischaemic heart disease and respiratory disease. Similar results were found in women. Higher risks relative to never smokers were observed in the combined 1–5 CPD category for lung cancer (HR = 3.45, 95% CI = 2.19–5.45), ischaemic heart disease (HR = 2.24, 95% CI = 1.63–3.08), cerebrovascular disease (HR = 1.65, 95% CI = 1.31–2.10), subarachnoid haemorrhage (HR = 3.11, 95% CI = 1.93–5.00) and respiratory disease (HR = 5.29, 95% CI = 2.33–12.04) with higher risks among women who reported smoking more.
Table 3.
Cause-specific mortality risks by number of cigarettes smoked per day in the pooled analysis of nine cohort studies in the Japan Cohort Consortium
| Never | Cigarettes smoked per day |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1–2 | 3–5 | 6–10 | 11–20 | 21–30 | 31–40 | >40 | ||
| Men | ||||||||
| Total n | 38 616 | 485 | 2641 | 12 709 | 53 894 | 19 845 | 10 035 | 2871 |
| All cancer | ||||||||
| Death, n | 2630 | 35 | 237 | 1584 | 6559 | 2206 | 1097 | 300 |
| HR (95% CI)a | 1.00 | 1.29 (0.92–1.80) | 1.30 (1.12–1.51) | 1.65 (1.53–1.78) | 1.84 (1.69–1.99) | 2.09 (1.90–2.30) | 2.31 (2.08–2.58) | 2.65 (2.30–3.06) |
| I2b | 0 | 18.16 | 31.42 | 69.31 | 61.28 | 49.51 | 20.57 | |
| Pc | 0.63 | 0.28 | 0.17 | 0.001 | 0.008 | 0.045 | 0.26 | |
| Smoking-related cancer | ||||||||
| Death, n | 1846 | 29 | 185 | 1199 | 4912 | 1625 | 845 | 218 |
| HR (95% CI) | 1.00 | 1.50 (1.03–2.17) | 1.49 (1.20–1.84) | 1.81 (1.58–2.07) | 1.94 (1.66–2.26) | 2.14 (1.72–2.65) | 2.35 (1.86–2.95) | 2.59 (2.03–3.29) |
| I2 | 0 | 44.38 | 67.54 | 87.81 | 89.17 | 84.72 | 57.33 | |
| P | 0.82 | 0.07 | 0.002 | <0.001 | <0.001 | <0.001 | 0.02 | |
| Lung cancer | ||||||||
| Death, n | 277 | 5 | 41 | 359 | 1877 | 784 | 409 | 109 |
| HR (95% CI) | 1.00 | 4.05 (1.63–10.07) | 2.04 (1.47–2.82) | 3.16 (2.44–4.09) | 4.54 (3.44–6.00) | 6.87 (5.21–9.05) | 8.21 (6.19–10.90) | 10.31 (7.70–13.81) |
| I2 | 0 | 0 | 63.09 | 82.46 | 75.06 | 67.66 | 32.06 | |
| P | 0.40 | 0.61 | 0.006 | <0.001 | <0.001 | 0.002 | 0.16 | |
| Circulatory disease | ||||||||
| Death, n | 2493 | 33 | 268 | 1261 | 4167 | 1087 | 514 | 136 |
| HR (95% CI) | 1.00 | 1.42 (1.00–2.00) | 1.47 (1.17–1.84) | 1.45 (1.36–1.56) | 1.42 (1.33–1.51) | 1.39 (1.29–1.49) | 1.54 (1.40–1.69) | 1.72 (1.40–2.10) |
| I2 | 0 | 63.99 | 0 | 32.32 | 0 | 0 | 16.67 | |
| P | 0.70 | 0.005 | 0.77 | 0.16 | 0.64 | 0.91 | 0.29 | |
| Ischaemic heart disease | ||||||||
| Death, n | 522 | 6 | 55 | 304 | 1102 | 336 | 172 | 53 |
| HR (95% CI) | 1.00 | 1.95 (0.87–4.38) | 1.52 (0.92–2.51) | 1.61 (1.40–1.85) | 1.70 (1.54–1.87) | 1.87 (1.63–2.14) | 2.14 (1.80–2.55) | 2.78 (2.09–3.69) |
| I2 | 0 | 59.90 | 0 | 0 | 0 | 0 | 0 | |
| P | 0.10 | 0.01 | 0.85 | 0.64 | 0.68 | 0.90 | 0.90 | |
| Cerebrovascular disease | ||||||||
| Death, n | 1200 | 145 | 588 | 1741 | 426 | 183 | 49 | |
| HR (95% CI) | 1.00 | 1.50 (1.26–1.78) | 1.33 (1.21–1.47) | 1.22 (1.13–1.30) | 1.12 (1.00–1.25) | 1.15 (0.94–1.41) | 1.44 (1.07–1.92) | |
| I2 | 0 | 0 | 0 | 0 | 30.72 | 0 | ||
| P | 0.84 | 0.71 | 0.95 | 0.55 | 0.17 | 0.44 | ||
| Subarachnoid haemorrhage | ||||||||
| Death, n | 177 | 5 | 13 | 96 | 423 | 126 | 58 | 13 |
| HR (95% CI) | 1.00 | 4.12 (1.69–9.99) | 1.54 (0.87–2.72) | 1.60 (1.26–2.02) | 1.89 (1.39–2.56) | 1.67 (1.09–2.57) | 1.97 (1.19–3.26) | 2.31 (0.83–6.47) |
| I2 | 0 | 0 | 0 | 43.34 | 44.86 | 41.10 | 57.32 | |
| P | 0.89 | 0.48 | 0.61 | 0.08 | 0.07 | 0.12 | 0.07 | |
| Respiratory disease | ||||||||
| Death, n | 101 | 20 | 114 | 410 | 104 | 47 | 13 | |
| HR (95% CI) | 1.00 | 2.69 (1.66–4.37) | 2.41 (1.56–3.72) | 2.66 (1.87–3.79) | 3.69 (2.78–4.90) | 4.06 (2.50–6.61) | 7.15 (3.90–13.12) | |
| I2 | 0 | 59.56 | 64.74 | 3.69 | 35.91 | 0 | ||
| P | 0.72 | 0.01 | 0.004 | 0.40 | 0.14 | 0.50 | ||
| Women | ||||||||
| Total n | 189 336 | 405 | 2116 | 5796 | 6825 | 886 | 481 | |
| All cancer | ||||||||
| Death, n | 9081 | 17 | 111 | 410 | 422 | 52 | 34 | |
| HR (95% CI) | 1.00 | 1.38 (0.86–2.23) | 1.33 (1.07–1.67) | 1.76 (1.57–1.98) | 1.74 (1.57–1.93) | 1.87 (1.26–2.78) | 3.38 (2.40–4.77) | |
| I2 | 0 | 23.51 | 18.99 | 0 | 41.56 | 0 | ||
| P | 0.59 | 0.23 | 0.27 | 0.54 | 0.09 | 0.72 | ||
| Smoking-related cancer | ||||||||
| Death, n | 5717 | 6 | 75 | 273 | 300 | 33 | 22 | |
| HR (95% CI) | 1.00 | 0.98 (0.44–2.19) | 1.43 (1.06–1.93) | 1.89 (1.56–2.28) | 1.90 (1.50–2.42) | 2.19 (1.53–3.12) | 3.66 (2.37–5.64) | |
| I2 | 0 | 30.97 | 53.77 | 62.09 | 0 | 0 | ||
| P | 0.98 | 0.17 | 0.03 | 0.01 | 0.75 | 0.72 | ||
| Lung cancer | ||||||||
| Death, n | 1023 | 33 | 99 | 119 | 18 | 17 | ||
| HR (95% CI) | 1.00 | 3.45 (2.19–5.45) | 4.04 (2.84–5.75) | 4.79 (3.91–5.86) | 9.35 (5.80–15.08) | 19.59 (11.75–32.66) | ||
| I2 | 22.35 | 58.34 | 0 | 0 | 1.14 | |||
| P | 0.24 | 0.01 | 0.78 | 0.59 | 0.42 | |||
| Circulatory disease | ||||||||
| Death, n | 9533 | 15 | 157 | 393 | 402 | 50 | 26 | |
| HR (95% CI) | 1.00 | 1.03 (0.62–1.71) | 1.74 (1.45–2.09) | 1.69 (1.45–1.96) | 2.04 (1.78–2.33) | 3.11 (2.30–4.19) | 3.81 (2.57–5.66) | |
| I2 | 0 | 22.92 | 48.48 | 35.60 | 4.62 | 0 | ||
| P | 0.81 | 0.24 | 0.05 | 0.13 | 0.39 | 0.57 | ||
| Ischaemic heart disease | ||||||||
| Death, n | 1801 | 42 | 109 | 110 | 17 | 7 | ||
| HR (95% CI) | 1.00 | 2.24 (1.63–3.08) | 2.40 (1.97–2.93) | 2.77 (2.22–3.46) | 6.42 (3.70–11.13) | 10.64 (5.00–22.66) | ||
| I2 | 0 | 0 | 13.65 | 13.30 | 0 | |||
| P | 0.73 | 0.61 | 0.32 | 0.33 | 0.57 | |||
| Cerebrovascular disease | ||||||||
| Death, n | 4343 | 76 | 164 | 184 | 18 | 11 | ||
| HR (95% CI) | 1.00 | 1.65 (1.31–2.10) | 1.49 (1.22–1.83) | 1.98 (1.67–2.35) | 2.60 (1.61–4.20) | 3.63 (1.95–6.78) | ||
| I2 | 4.37 | 33.73 | 17.79 | 0 | 0 | |||
| P | 0.40 | 0.15 | 0.28 | 0.87 | 0.80 | |||
| Subarachnoid haemorrhage | ||||||||
| Death, n | 966 | 22 | 44 | 61 | 8 | |||
| HR (95% CI) | 1.00 | 3.11 (1.93–5.00) | 2.01 (1.30–3.12) | 2.52 (1.91–3.33) | 5.88 (2.91–11.89) | |||
| I2 | 15.77 | 39.09 | 0 | 0 | ||||
| P | 0.30 | 0.11 | 0.92 | 0.98 | ||||
| Respiratory disease | ||||||||
| Death, n | 282 | 6 | 26 | 28 | ||||
| HR (95% CI) | 1.00 | 5.29 (2.33–12.04) | 3.91 (2.56–5.97) | 6.83 (4.19–11.12) | ||||
| I2 | 0 | 0 | 22.52 | |||||
| P | 0.56 | 0.88 | 0.24 | |||||
Adjusted for age, alcohol intake (men: never, former, <1 time/week, regularly <23, 23 to <46, ≥46 g/d; women: never, former, <1 time/week, regularly <23, ≥23 g/d) and region (for the Japan Public Health Center-based Prospective Study, Cohort I, the Japan Public Health Center-based Prospective Study, Cohort II, the Japan Collaborative Cohort Study and the Life Span Study).
I 2 statistics describe the percentage of variation across studies due to heterogeneity rather than chance.
Cochrane’s Q statistics for a homogeneity test.
Figure 2.
All-cause and cause-specific mortality risk by number of cigarettes smoked per day among current smokersa (A) All-cause mortality, (B) Smoking-related cancer mortality, and (C) Circulatory disease mortality aHazard ratios and 95% confidence intervals were adjusted for age, alcohol intake (men: never, former, <1 time/week, regularly <23, 23 to <46, ≥46 g/d; women: never, former, <1 time/week, regularly <23, ≥23 g/d) and region (for the Japan Public Health Center-based Prospective Study, Cohort I, the Japan Public Health Center-based Prospective Study, Cohort II, the Japan Collaborative Cohort Study and the Life Span Study).
Lower mortality risks were generally observed among former smokers who had quit at younger ages (Table 4). Relative to never smokers, the HR (95% CI) for all-cause mortality for participants who quit at <30, 30–39, 40–49, 50–59 and ≥60 years old was 1.04 (0.91–1.20), 0.96 (0.89–1.05), 1.08 (1.01–1.15), 1.29 (1.19–1.40) and 1.37 (1.32–1.42), respectively, and 1.62 (95% CI = 1.56–1.69) for current smokers. A comparable association was observed among former low-intensity smokers. HRs were also generally lower with more years of cessation at study baseline, including among low-intensity smokers (Supplementary Table S5, available as Supplementary data at IJE online) and when we excluded deaths occurring within 5 years of study baseline (Supplementary Table S6, available as Supplementary data at IJE online).
Table 4.
All-cause mortality by age at cessation among all former smokers and former low-intensity smokers [≤10 cigarettes per day (CPD)] in the pooled analysis of nine cohort studies in the Japan Cohort Consortium
| Never smokers | Former smokers: age at cessation (years) |
Current smokersa | |||||
|---|---|---|---|---|---|---|---|
| <30 | 30–39 | 40–49 | 50–59 | ≥60 | |||
| All former smokers | |||||||
| Total, n | 227 952 | 3947 | 10 112 | 14 352 | 12 525 | 8656 | 119 500 |
| Death, n | 40 266 | 503 | 1367 | 2952 | 4616 | 4964 | 34 429 |
| HR (95% CI)b | 1.00 (ref.) | 1.04 (0.91–1.20) | 0.96 (0.89–1.05) | 1.08 (1.01–1.15) | 1.29 (1.19–1.40) | 1.37 (1.32–1.42) | 1.62 (1.56–1.69) |
| I2c | 41.30 | 33.82 | 41.17 | 77.87 | 0 | 69.94 | |
| Pd | 0.02 | 0.08 | 0.04 | <0.001 | 0.46 | < 0.001 | |
| Former low-intensity smokers of ≤10 CPD | |||||||
| Total, n | 227 952 | 1804 | 2564 | 3134 | 2702 | 2238 | 24 209 |
| Death, n | 40 266 | 215 | 409 | 629 | 938 | 1275 | 7701 |
| HR (95% CI) | 1.00 (ref) | 0.93 (0.77–1.13) | 1.05 (0.90–1.22) | 1.04 (0.93–1.16) | 1.22 (1.14–1.31) | 1.37 (1.28–1.47) | 1.58 (1.52–1.63) |
| I2 | 31.75 | 46.04 | 37.84 | 0 | 18.95 | 26.77 | |
| P | 0.08 | 0.02 | 0.05 | 0.56 | 0.4 | 0.14 | |
Current low-intensity smokers who smoked ≤10 CPD for the analysis of former low-intensity smokers.
Adjusted for age, alcohol intake (men: never, former, <1 time/week, regularly <23, 23 to <46, ≥46 g/d; women: never, former, <1 time/week, regularly <23, ≥23 g/d) and region (for the Japan Public Health Center-based Prospective Study, Cohort I, the Japan Public Health Center-based Prospective Study, Cohort II, the Japan Collaborative Cohort Study and the Life Span Study).
I 2 statistics describe the percentage of variation across studies due to heterogeneity rather than chance.
Cochrane’s Q statistics for a homogeneity test.
Discussion
In this pooled analysis of >410 000 adults from nine large prospective cohort studies in Japan, smokers who smoked ≤10 CPD had higher mortality risks for all causes and smoking-related causes of death than never smokers. Risks were higher at 1–2 CPD and increased with additional CPD. Former smokers, especially those who quit at younger ages, had lower mortality risks than current smokers, including those who had smoked ≤10 CPD.
Low-intensity smoking has sometimes been considered to be harmless, yet accumulating evidence shows increased disease and mortality risks.6–13,23–26 In the large US NIH-AARP cohort, lifetime smokers who consistently smoked <1 CPD had 1.64 (95% CI = 1.07–2.51) times and consistent smokers of 1–10 CPD had 1.87 (95% CI = 1.64–2.13) times the mortality risk of never smokers.10 In the US National Health Interview Survey, daily smokers who smoked ≤2 CPD had 1.42 (95% CI = 0.95,2.10) times and those who smoked 3–10 CPD had 2.07 (95% CI = 1.86,2.30) times the mortality risks of never smokers.12 The doubled mortality risk relative to never smokers was also observed for daily smokers who smoked ≤10 CPD in the US National Longitudinal Mortality Study.13
In Asia, to the best of our knowledge, there has been only one study assessing the mortality risks of low-intensity smoking. A recent pooled analysis of 16 prospective cohort studies in the Asia Cohort Consortium, including 6 studies in Japan, showed that the all-cause mortality risk was 1.27 (95% CI = 1.17–1.37) times and 1.40 (95% CI = 1.30–1.51) times the risk for <5 and 5–9 CPD, respectively, of never smoking. Increased mortality risks were observed for smoking-related causes of death.27 This previous analysis included studies from multiple countries in Asia, including India, Bangladesh, China, Taiwan and Singapore, where cigarette-smoking patterns (e.g. co-use of smokeless tobacco) are considerably different from those in Japan. Moreover, this previous study did not examine associations in men and women separately, despite different smoking prevalence and smoking patterns by sex in Asia.
The smoking prevalence in Japanese women has been historically low, as typically observed in many Asian countries, and it has decreased slightly for the past two decades (10.9% in 1998 and 8.1% in 2018).3 A majority of female smokers traditionally smoke ≤20 CPD and the proportion of female smokers who smoke ≤10 CPD has increased (43.4% in 2003 to 53.4% in 2017). In the current study, the smoking prevalence in women was 7.4% and about a half of current smokers reported smoking ≤10 CPD. Moreover, these female low-intensity smokers started smoking regularly at relatively older ages (median range: 28–35 years). Nevertheless, we found that female low-intensity smokers had higher mortality risks than never smokers. Although remaining higher than in the US and other developed countries, the prevalence of cigarette smoking among Japanese men has declined (50.8% in 1998, 36.8% in 2008 and 29.0% in 2018).3 Furthermore, an increasing proportion of male smokers smoke ≤10 CPD (15.5% in 2003 to 34.0% in 2017).4
The associations for low-intensity smoking found in the current study were somewhat weaker than those reported in US studies, as observed previously for lung-cancer risk in Japanese men.28 It might be because smokers in Japan generally start smoking at older ages.28 In the current study, male smokers typically started smoking at 20 years old, whereas US adults generally start smoking at 17 years old.13 Female smokers started smoking even later, especially among low-intensity smokers (28–35 years old). Observing the increasing proportion of low-intensity smokers and decreasing age at initiation of smoking in both sexes in Japan,4,28 future studies will be informative.
Decreases in smoking prevalence and in CPD have occurred concurrently in the past decades in Japan.3,4 Cessation has been shown to decrease mortality and disease risks in previous studies among heavier smokers.29 A few studies in the USA have shown that low-intensity smokers may also receive benefits from cessation.10,12,13 In the NIH-AARP cohort study, former smokers who had smoked ≤10 CPD but quit at ≥50 years old had similar mortality risks to those of current smokers, but the risk among those who quit in their 20s was similar to those of never smokers.10 A previous pooled analysis of cessation and incident cancer risks in the JCC showed that years since cessation were inversely associated with the risk of all cancer and smoking-related cancer.30 In the current study, mortality risks were lower with longer time since cessation at study baseline among former smokers who used to smoke ≤10 CPD, with the risk no longer increased 20 years after cessation relative to never smoking.
Our study had a number of strengths. Our study was large and included nine large prospective cohorts. Detailed assessment of cigarette smoking in each cohort enabled us to assess mortality risks in detailed CPD categories. By including the major large-scale population-based cohorts from multiple regions in Japan with overlapping birth cohorts, our findings are likely reflective of the general Japanese population. All Japanese citizens are registered in the residential and death registrations, which enabled nearly complete follow-up.
There are several limitations as well. Despite a large sample size, we lacked the statistical power for rare causes of death due to the small number of deaths. For those analyses, we collapsed the categories to create a wider range. Also, we were unable to examine non-daily smoking separately from daily smoking, as eight of nine included cohorts lacked this information on all of their participants. Cigarette smoking was assessed a single time at study baseline and thus it is possible that the participants’ smoking status and/or patterns changed during the follow-up. The JPHC-I and the JPHC-II, the two largest cohorts in this pooled analysis, reassessed smoking 5 years after the baseline. Among current smokers at both assessments, reported CPD were strongly correlated (Spearman’s correlation coefficient: 0.93). We also conducted multiple sensitivity analyses, limiting follow-up to 10 years or excluding deaths occurring within 2 years after baseline among current smokers, and excluding deaths occurring within 5 years of study baseline for former smokers. Such analyses had only a modest impact on our results. Nevertheless, there have been large declines in smoking in Japan over the past few decades. Although some recent quitters may have restarted smoking during follow-up, it also seems likely that a larger number of current smokers at baseline reduced their CPD or quit smoking during follow-up. Thus, our risk estimates for current smoking are likely underestimated.
In addition to studying cigarettes, future studies should also examine the health effects of heated tobacco products (HTPs). In Japan, the prevalence of HTPs has been increasing since the introduction of the most popular HTP in Japan, iQOS, in 2014. Although the health effects of HTPs remain unclear, surveys indicate that a majority of HTP users in Japan also use cigarettes.31 As HTPs appeared on the market many years after the enrolment of our studies, we were unable to assess mortality risks of users of HTPs who continued to smoke cigarettes at a low intensity. However, it seems plausible that such dual users are likely to have mortality risks that are at least as high as those observed among exclusive low-intensity cigarette smokers in the current study.
In summary, in this pooled analysis of nine cohort studies in Japan, low-intensity cigarette smokers had higher all-cause mortality risks relative to never smokers, even at the level of 1–2 CPD. Higher mortality risks were also observed for a wide range of smoking-related causes of death. Former smokers who quit at younger ages had lower mortality risks than those who quit at older ages. Our study provides evidence that both male and female cigarette smokers have higher mortality risks than never smokers, even if they only smoke a few CPD in Japan. All smokers should quit smoking, even if they smoke just a few CPD.
Supplementary data
Supplementary data are available at IJE online.
Ethics approval
All participating studies were approved by their respective institutional review boards. In JACC, written informed consent was obtained from all participants. In other cohorts (JPHC-I, JPHC-II, MIYAGI, 3-pref MIYAGI, 3-pref AICHI, OHSAKI, LSS and 3-pref OSAKA), the return of a completed questionnaire was considered as consent for study participation.
Funding
This work was supported by the Japan National Cancer Center Research and Development Fund [30-A-15] and the US National Cancer Institute, Intramural Research Program. The Radiation Effects Research Foundation (RERF) is funded by Japan and US governments [RERF Research Protocol A2-15].
Supplementary Material
Contributor Information
Maki Inoue-Choi, Metabolic Epidemiology Branch, Division of Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Neal D Freedman, Metabolic Epidemiology Branch, Division of Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Eiko Saito, Center for Cancer Control & Information Services, National Cancer Center, Tokyo, Japan.
Shiori Tanaka, Center for Public Health Sciences, National Cancer Center, Tokyo, Japan.
Mayo Hirabayashi, Center for Public Health Sciences, National Cancer Center, Tokyo, Japan.
Norie Sawada, Center for Public Health Sciences, National Cancer Center, Tokyo, Japan.
Shoichiro Tsugane, Center for Public Health Sciences, National Cancer Center, Tokyo, Japan.
Yoshiaki Usui, Division of Cancer Information and Control, Department of Preventive Medicine, Aichi Cancer Center Research Institute, Nagoya, Japan.
Hidemi Ito, Division of Cancer Information and Control, Department of Preventive Medicine, Aichi Cancer Center Research Institute, Nagoya, Japan.
Chaochen Wang, Department of Public Health, Aichi Medical University School of Medicine, Aichi, Japan.
Akiko Tamakoshi, Department of Public Health, Hokkaido University Graduate School of Medicine, Sapporo, Japan.
Taro Takeuchi, Department of Social Medicine, Osaka University Graduate School of Medicine, Osaka, Japan.
Yuri Kitamura, Department of Social Medicine, Osaka University Graduate School of Medicine, Osaka, Japan.
Mai Utada, Department of Epidemiology, Radiation Effects Research Foundation, Hiroshima, Japan.
Kotaro Ozasa, Department of Epidemiology, Radiation Effects Research Foundation, Hiroshima, Japan.
Yumi Sugawara, Division of Epidemiology, Department of Public Health and Forensic Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan.
Ichiro Tsuji, Division of Epidemiology, Department of Public Health and Forensic Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan.
Keiko Wada, Department of Epidemiology and Preventive Medicine, Gifu University Graduate School of Medicine, Gifu, Japan.
Chisato Nagata, Department of Epidemiology and Preventive Medicine, Gifu University Graduate School of Medicine, Gifu, Japan.
Taichi Shimazu, Center for Public Health Sciences, National Cancer Center, Tokyo, Japan.
Tetsuya Mizoue, Department of Epidemiology and Prevention, Center for Clinical Sciences, National Center for Global Health and Medicine, Tokyo, Japan.
Keitaro Matsuo, Division of Cancer Epidemiology and Prevention, Aichi Cancer Center Research Institute, Nagoya, Japan.
Mariko Naito, Department of Oral Epidemiology, Hiroshima University Graduate School of Biomedical and Health Sciences, Hiroshima, Japan and .
Keitaro Tanaka, Department of Preventive Medicine, Faculty of Medicine, Saga University, Saga, Japan.
Kota Katanoda, Center for Cancer Control & Information Services, National Cancer Center, Tokyo, Japan.
Manami Inoue, Center for Public Health Sciences, National Cancer Center, Tokyo, Japan.
Data Availability
The data underlying this manuscript cannot be shared publicly due to the privacy of study participants. A collaboration with each participating cohort study is required to access the data.
Author contributions
M.I.-C. designed the study, analysed the data and takes responsibility for the accuracy of the data analysis, drafted the manuscript, reviewed and edited the manuscript, and contributed to discussion; N.D.F. designed and supervised the study, reviewed and edited the manuscript, and contributed to discussion; M.I. supervised the study, reviewed and edited the manuscript, and contributed to discussion; S.T., M.H., Y.U., C.W. and T.T. analysed the data, reviewed and edited the manuscript, and contributed to discussion; N.S., S.T., H.I., A.T., Y.K., M.U., K.O., Y.S., I.T., K.W. and C.N. had full access to respective study data and take responsibility for the integrity of the data and the accuracy of the data analysis, reviewed and edited the manuscript, and contributed to discussion; E.S., T.S., T.M., K.M., M.N., K.T. and K.K. reviewed and edited the manuscript and contributed to discussion.
Conflict of interest
Dr Kota Katanoda received a JMWH Bayer Grant from 1 September 2017 to 31 August 2019 via the Japan Society for Menopause and Women's Health. Total funding: 1 million JPY.
References
- 1. WHO Report on the Global Tobacco Epidemic 2017—Monitoring Tobacco Use and Prevention Policies. Geneva: World Health Organization, 2017. [Google Scholar]
- 2.GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017;389:1885–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministry of Health, Labor and Welfare Tobacco or Health Tokyo, Japan: Ministry of Health, Labor and Welfare. http://www.health-net.or.jp/tobacco/menu02.html (15 January 2020, date last accessed).
- 4.National Cancer Center Cancer Information Service. Cancer statistics—smoking rate Tokyo, Japan: National Cancer Center, Center for Cancer Control and Information Services. https://ganjoho.jp/reg_stat/statistics/stat/smoking.html (24 August 2020, date last accessed).
- 5. Amrock SM, Weitzman M.. Adolescents' perceptions of light and intermittent smoking in the United States. Pediatrics 2015;135:246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosengren A, Wilhelmsen L, Wedel H.. Coronary heart disease, cancer and mortality in male middle-aged light smokers. J Intern Med 1992;231:357–62. [DOI] [PubMed] [Google Scholar]
- 7. Schane RE, Ling PM, Glantz SA.. Health effects of light and intermittent smoking: a review. Circulation 2010;121:1518–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prescott E, Scharling H, Osler M, Schnohr P.. Importance of light smoking and inhalation habits on risk of myocardial infarction and all cause mortality: a 22 year follow up of 12 149 men and women in the Copenhagen City Heart Study. J Epidemiol Community Health 2002;56:702–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bjartveit K, Tverdal A.. Health consequences of smoking 1-4 cigarettes per day. Tob Control 2005;14:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inoue-Choi M, Liao LM, Reyes-Guzman C, Hartge P, Caporaso N, Freedman ND.. Association of long-term, low-intensity smoking with all-cause and cause-specific mortality in the National Institutes of Health-AARP Diet and Health Study. JAMA Intern Med 2017;177:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christensen CH, Rostron B, Cosgrove C. et al. Association of cigarette, cigar, and pipe use with mortality risk in the US population. JAMA Intern Med 2018;178:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inoue-Choi M, McNeel TS, Hartge P, Caporaso N, Graubard BI, Freedman ND.. Non-daily cigarette smokers: mortality risks in the United States. Am J Prev Med 2019;56:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inoue-Choi M, Christensen CH, Rostron BL. et al. Dose-response association of low-intensity and nondaily smoking with mortality in the United States. JAMA Netw Open 2020;3:e206436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watanabe S, Tsugane S, Sobue T, Konishi M, Baba S.. Study design and organization of the JPHC study. Japan Public Health Center-based prospective study on cancer and cardiovascular diseases. J Epidemiol 2001;11:S3–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tamakoshi A, Ozasa K, Fujino Y. et al. ; JACC Study Group. Cohort profile of the Japan Collaborative Cohort Study at final follow-up. J Epidemiol 2013;23:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sado J, Kitamura T, Kitamura Y. et al. Coffee consumption and all-cause and cardiovascular mortality: three-prefecture cohort in Japan. Circ J 2019;83:757–66. [DOI] [PubMed] [Google Scholar]
- 17. Fukao A, Tsubono Y, Komatsu S. et al. A cohort study on the relation of lifestyle, personality and biologic markers to cancer in Miyagi, Japan: study design, response rate and profiles of the cohort subjects. J Epidemiol 1995;5:153–57. [Google Scholar]
- 18. Tsuji I, Nishino Y, Ohkubo T. et al. A prospective cohort study on National Health Insurance beneficiaries in Ohsaki, Miyagi Prefecture, Japan: study design, profiles of the subjects and medical cost during the first year. J Epidemiol 1998;8:258–63. [DOI] [PubMed] [Google Scholar]
- 19. Ozasa K, Shimizu Y, Suyama A. et al. Studies of the mortality of atomic bomb survivors, Report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res 2012;177:229–43. [DOI] [PubMed] [Google Scholar]
- 20.IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 100E. Personal Habits and Indoor Combustions. Lyon, France: International Agency for Research on Cancer, 2012. [PMC free article] [PubMed] [Google Scholar]
- 21. Secretan B, Straif K, Baan R. et al. A review of human carcinogens--Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009;10:1033–34. [DOI] [PubMed] [Google Scholar]
- 22.The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm (7 October 2021, date last accessed).
- 23. Hackshaw A, Morris JK, Boniface S, Tang JL, Milenkovic D.. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018;360:j5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bjerregaard BK, Raaschou-Nielsen O, Sorensen M. et al. The effect of occasional smoking on smoking-related cancers: in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes Control 2006;17:1305–09. [DOI] [PubMed] [Google Scholar]
- 25. Inoue-Choi M, Hartge P, Liao LM, Caporaso N, Freedman ND.. Association between long-term low-intensity cigarette smoking and incidence of smoking-related cancer in the National Institutes of Health-AARP cohort. Int J Cancer 2018;142:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomson B, Tapia-Conyer R, Lacey B. et al. Low-intensity daily smoking and cause-specific mortality in Mexico: prospective study of 150 000 adults. Int J Epidemiol 2021;50:955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang JJ, Yu D, Shu XO. et al. Quantifying the association of low-intensity and late initiation of tobacco smoking with total and cause-specific mortality in Asia. Tob Control 2021;30:328–35. [DOI] [PubMed] [Google Scholar]
- 28. Funatogawa I, Funatogawa T, Yano E.. Trends in smoking and lung cancer mortality in Japan, by birth cohort, 1949-2010. Bull World Health Organ 2013;91:332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smoking Cessation. A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Public Health Service, Office of the Surgeon General, 2020. [PubMed] [Google Scholar]
- 30. Saito E, Inoue M, Tsugane S. et al. ; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Smoking cessation and subsequent risk of cancer: a pooled analysis of eight population-based cohort studies in Japan. Cancer Epidemiol 2017;51:98–108. [DOI] [PubMed] [Google Scholar]
- 31. Sugiyama T, Tabuchi T.. Use of multiple tobacco and tobacco-like products including heated tobacco and e-cigarettes in Japan: a cross-sectional assessment of the 2017 JASTIS study. Int J Environ Res Public Health 2020;17:2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this manuscript cannot be shared publicly due to the privacy of study participants. A collaboration with each participating cohort study is required to access the data.