Abstract
Splenic-macrophage Fcγ receptors (FcγRs) participate in the pathophysiologies of immune-complex diseases and in host defense against infection. Modulation of macrophage FcγR expression is an immuno-therapeutic target. Glucocorticoids, sex steroids, and dopaminergic drugs modulate macrophage FcγR expression. Previous data indicate that estradiol increases macrophage FcγR expression. Nevertheless, the effects of clinically used estrogens upon macrophage FcγR expression are unknown. We assessed the effects of treatment with commonly used estrogens on the expression of macrophage FcγRs using a guinea pig experimental model. Six estrogens have been studied: ethynylestradiol (Et), mestranol (M), chlortianisene (Ct), promestriene, 17-epiestriol, and 17β-estradiol. Following in vivo treatment of guinea pigs, we determined the clearance of immunoglobulin G (IgG)-sensitized erythrocytes in vivo, the binding of IgG-sensitized erythrocytes by isolated splenic macrophages, and splenic-macrophage FcγR cell surface expression. Estrogens enhance the clearance of IgG-sensitized erythrocytes by increasing splenic-macrophage FcγR expression. Et, M, and Ct were more effective than the other estrogens. Flow cytometry and fluorescence microscopy with monoclonal antibodies demonstrated that estrogens increase the cell surface expression of FcγR1 and -2 more than that of FcγR2. These data indicate that treatment with commonly used estrogens enhances the clearance of IgG-sensitized cells by improving splenic-macrophage FcγR expression.
Splenic-macrophage Fcγ receptors (FcγRs) play a important role in the clearance of immune complexes (2, 3, 5, 12, 17, 18) and in host defense against infection (9, 16). Therefore, upregulation of macrophage FcγR expression is a potential therapeutic approach to those immune disorders.
Sex hormones may affect the clinical manifestations of autoimmune disorders (10, 13). In vitro data indicate that sex hormones have regulatory effects on lymphocyte and macrophage functions (6, 11, 19, 24, 25). Although the precise mechanisms by which these steroid hormones affect the immune system are not fully understood, our studies indicate that one effect is on macrophage FcγR expression (1, 7, 19, 20). Previous data indicate that estradiol increases macrophage FcγR expression (6). Nevertheless, the effects of synthetic estrogens commonly employed in the treatment of human conditions upon macrophage FcγR are presently unknown.
We have studied the effects of the treatment with estrogens approved for clinical use upon splenic-macrophage FcγR expression using a well-characterized experimental model, the guinea pig (7, 8, 15).
Treatment with estrogens of common clinical use improves the clearance of immunoglobulin G (IgG)-sensitized cells by enhancing the expression of both guinea pig splenic-macrophage FcγRs, FcγR2 and FcγR1-FcγR2 (6, 11, 19). Therefore, estrogens are candidate drugs for the treatment of disorders, like immune-complex diseases, whose sufferers benefit from an enhanced expression of the macrophage FcγR.
MATERIALS AND METHODS
All experiments were performed with 500- to 600-g male Duncan-Hartley guinea pigs obtained from Criffa, Barcelona, Spain. Guinea pigs were injected with equal volumes of a homogeneous suspension of estrogens in steroid suspension vehicle (SSV) (8, 15). Sham controls received 1 ml of SSV not containing estrogen. All animals were injected subcutaneously in the dorsal neck fat pad every afternoon for seven consecutive days and studied on the day after the last injection. The following estrogens were obtained from Steraloids, Inc. (Wilton, N.H.): ethynilestradiol (Et), mestranol (M), 17-epiestriol (Ep), and 17β-estradiol (E). Chlortianisene (Ct) and promestriene (Pm) were obtained from the pharmacy of our hospital. Doses of estrogens were selected on the basis of those previously used in the treatment of human conditions: 0.005 to 1 mg/kg of body weight for Et, 0.5 to 10 mg/kg for M, 0.5 to 10 mg/kg for Ct, 0.1 to 5 mg/kg for Pm, 2.5 to 10 mg/kg for Ep, and 2.5 to 10 mg/kg for E. Rabbit IgG anti-guinea pig red blood cell (RBC) antibodies were prepared as previously described, purified by Sephacryl S-300 gel filtration and quaternary aminoethyl ion-exchange chromatography (Pharmacia, Piscataway, N.J.), and free of IgM as determined by Ouchterlony analysis and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (7, 8, 15).
Clearance of IgG-coated erythrocytes.
Blood was drawn from anesthetized guinea pigs by cardiac puncture. Washed erythrocytes were radiolabeled with [51Cr]sodium chromate (Amersham, Madrid, Spain) and sensitized with an equal volume of IgG antibody, so as to be coated with approximately 3,500 IgG molecules per erythrocyte as described previously (8, 15). Treated animals were injected intravenously with 1.7 × 108 51Cr-labeled cells. Samples of blood were obtained 1 to 120 min after injection, and cell-associated radioactivity was measured in a gamma counter (Gamma 8000; Beckman Instruments, Inc., Fullerton, Calif.). Experiments were also performed with heat-altered erythrocytes to investigate splenic trapping mediated by nonimmune clearance, not only in sham controls but also in animals treated with high-dose estrogen (8, 15). Clearance curves were plotted by expressing the number of blood counts per minute at each time point as a percentage of the number of counts per minute at 5 min. Levels of clearance at 60, 90, and 120 min were analyzed to calculate a P value for the difference between control and experimental clearance curves using Student's t test. Clearance at each time point represents the mean (± standard error of the mean [SEM]) of results for at least six animals treated with a determined dose of estrogen, studied during 3 or more experimental days. Variations in levels of clearance among animals treated with various doses of estrogen was less than 10%. (In addition, for each day's clearance study, the percent inhibition of clearance (mean ± SEM) above the level of inhibition of the control was calculated at 90 and 120 min according to the formula 100 × [1 − (cpmc − cpmx)/(cpmc − cpmea)], where cpmc refers to counts per minute for the untreated control animal injected with unsensitized cells, cpmx refers to the experimental animal treated with steroid and injected with IgG-coated erythrocytes, and cpmea refers to control animals treated with SSV only (no estrogen) and injected with the control IgG-sensitized erythrocytes. A negative value for percent inhibition indicates enhancement of clearance. This formula compares results for treated animals with those for the control animals studied on the same experimental day and expresses the data as percentages of alteration of clearance, where 100% inhibition of clearance by estrogens corresponds to the situation in which the clearance of IgG-sensitized erythrocytes (cpmx) is identical to that of unsensitized erythrocytes (cpmc) (7, 8, 15).
Binding of IgG-coated erythrocytes by splenic macrophages in vitro.
Guinea pigs were sacrificed, splenectomy was performed immediately, and the spleens were placed in RPMI 1640 plus 10% heat-inactivated fetal calf serum plus glutamine (complete RPMI). Splenic macrophages were isolated by tissue grinding and sieving, discontinous Percoll gradient centrifugation, and plastic adherence as previously described (7, 8, 15). More than 95% of the resultant cells were viable mononuclear cells as determined by their ability to exclude trypan blue, and >90% of cells ingested latex beads and were stained with nonspecific sterase. Monolayers of adherent cells were prepared as previously described by incubating 106 cells on a glass coverslip in a 35-mm-diameter plastic petri dish at 37°C for 45 min under 5% CO2 (1, 2, 7, 8, 13, 20). More than 95% of the cells were adherent to glass. For experiments studying FcγR activity in vitro, guinea pig erythrocytes were coated with 800 molecules of IgG per erythrocyte as described above and 1 ml of erythrocytes (5 × 107 cells/ml) was incubated with the macrophage monolayers at 37°C under 5% CO2 for 20 min. The monolayers were washed, air dried, and stained with Wright-Giemsa, and 200 consecutive macrophages were inspected under an oil immersion lens for the number of erythrocytes bound per cell. The number of macrophages which bound ≥3 IgG-sensitized erythrocytes was then determined (7, 8, 15, 20).
Flow cytometry.
Monoclonal antibodies (MAbs) with specificities for guinea pig macrophage FcγR1-FcγR2 (VIA2 IgG1) and FcγR2 (VIIA1 IgG1) (22) were used in indirect immunofluorescence binding experiments to assess FcγR protein surface expression. These MAbs were the generous gift of I. Yamashita and T. Nakamura, Sapporo, Japan. Cells (5 × 105) were incubated with saturating concentrations of each MAb for 60 min at 4°C and washed twice with phosphate-buffered saline containing 0.5% bovine serum albumin and 0.02% sodium azide. To measure bound antibody, a fluorescein isothiocyanate-labeled goat anti-mouse antibody (Tago, Inc., Burlingame, Calif.) was added for 30 min at 4°C. The cells were again washed twice and fixed with 4% paraformaldehyde. Cell-associated fluorescence was measured using a FACSTAR cytometer with Consort-32 software (Becton Dickinson & Co., Mountain View, Calif.). For all samples, 10,000 events were recorded on a logarithmic fluorescence scale and the mean fluorescence intensity (MFI) for each sample was determined using Consort-32 software. In order to correct for autofluorescence, the MFI of a nonreactive murine IgG1 antibody (M3) was subtracted from the MFI of the anti-FcγR1-FcγR2- and anti-FcγR2-stained cells. Percent change in fluorescence intensity was calculated by the formula [(MFI of anti-FcγR-treated cells − MFI of M3-treated cells)/(MFI of cells not treated with anti-FcγR − MFI of cells not treated with M3)]−1 × 100.
To demonstrate the specificity of the estrogen effect on FcγR expression, we included an additional control with an irrelevant guinea pig pan-macrophage surface antigen, GPB (Seralab Ltd., Sussex, England). Treatment with estrogens did not significantly alter the cell surface expression of this pan-macrophage antigen and enhanced the expression of FcγR1-FcγR2 and FcγR2.
Effect of in vivo estrogens on membrane mobility of FcγR1-FcγR2 and FcγR2.
Immunofluorescence capping experiments were performed in order to examine any possible effects of in vivo-administered estrogens on the membrane mobilities of FcγR1-FcγR2 and FcγR2. To this end in vitro capping experiments were performed comparing splenic macrophages isolated from treated animals to those from sham controls. Et (0.01, 0.1, and 1 mg/kg), M (1, 5, and 10 mg/kg), and Ct (1, 5, and 10 mg/kg) were the chosen estrogens. Splenic macrophages (5 × 105) from guinea pigs treated with different doses of the most effective estrogens for 7 days or from sham-treated animals were incubated with saturating concentrations of MAbs for 30 min at 0°C on ice. After two washes at 0°C in phosphate-buffered saline–0.5% bovine serum albumin without sodium azide, fluorescein isothiocyanate-labeled goat anti-mouse antibody was added as in the flow cytometry experiments. Cells were incubated at either 0 or 37°C for 20 min, washed, fixed in paraformaldehyde, and spun onto microscope slides in a centrifuge. Several hundred cells per slide were examined under epifluorescence with a fluorescent microscope (Carl Zeiss, Oberkochen, Germany) (data not shown).
Statistics.
To determine whether the difference between two means was significant, the unpaired or paired t test was used.
RESULTS
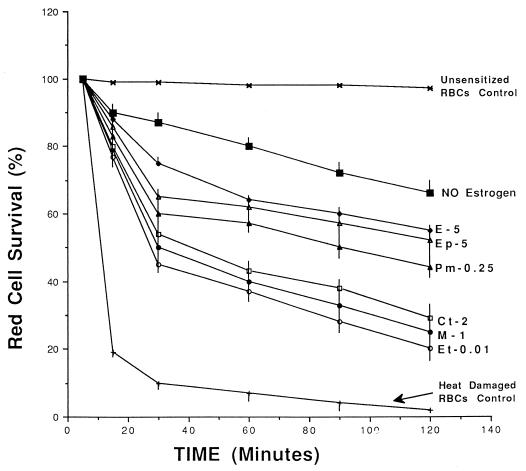
Six estrogens, Et, M, Ct, Pm, Ep, and E, were studied. We examined the clearance of IgG-sensitized RBCs in animals treated with estrogens for 7 days to assess their in vivo effects on the expression of splenic-macrophage FcγRs. Treatment with any of the estrogens significantly enhanced the clearance of IgG-sensitized erythrocytes in more than 90% of the animals at 120 min compared with that of simultaneously tested sham controls (Fig. 1). Et, M, and Ct increased the clearance of IgG-sensitized RBCs more efficiently than Pm, Ep, or E. The enhancement of clearance was dose related (data not shown). No significant inhibition of clearance was observed at doses of estrogens below those indicated in Fig. 1.
FIG. 1.
In vivo clearance of IgG-sensitized RBCs in guinea pigs treated with minimal effective doses of estrogens. Numbers represent the doses of estrogens used (in milligrams per kilogram per day). Red cell survival is the percentage of 51Cr-labeled IgG-senstized RBCs (± SEM) remaining in the circulation at each indicated time point. Clearance of IgG-sensitized RBCs at 120 min was significantly enhanced by Et at 0.01 mg/kg (P < 0.001), M at 1 mg/kg (P < 0.001), Ct at 1 mg/kg (P < 0.001), Pm at 0.25 mg/kg (P < 0.01), Ep at 5 mg/kg (P < 0.01), and E at 5 mg/kg (P < 0.01). Survival of heat-damaged RBCs was not significantly different in estrogen-treated animals from that in sham controls (no estrogen).
The effect of estrogens on the function of the splenic-macrophage FcγRs was assessed in vitro after splenic-macrophage isolation. Treatment with estrogens has no consistent effect on the yield or viability of mononuclear cells isolated from the spleen. FcγR activity was determined by the in vitro binding of IgG-sensitized erythrocytes (Table 1). Following treatment with any of the estrogens, the percentage of isolated splenic macrophages binding ≥3 IgG-sensitized erythrocytes was significantly higher than that of macrophages isolated from sham animals. Et was the most active estrogen, enhancing the recognition of IgG-sensitized RBCs by isolated splenic macrophages (P < 0.05). The lowest doses of estrogens that inhibited the binding of IgG-sensitized erythrocytes by splenic macrophages are indicated in Table 1.
TABLE 1.
In vitro binding of IgG-sensitized RBCs by isolated splenic macrophages and enhancement by treatment with estrogens
| Estrogen dose (mg/kg) | % of splenic macrophages binding ≥3 IgG-sensitized RBCs ± SEMa |
|---|---|
| None | 47 ± 3 |
| Et | |
| 0.005 | 56 ± 2* |
| 0.010 | 62 ± 3 |
| 0.100 | 76 ± 4 |
| 1.000 | 89 ± 3 |
| M | |
| 0.5 | 58 ± 3* |
| 1 | 64 ± 2 |
| 5 | 70 ± 3 |
| 10 | 83 ± 3 |
| Ct | |
| 0.5 | 55 ± 2* |
| 1 | 60 ± 2 |
| 5 | 66 ± 2 |
| 10 | 73 ± 3 |
| Pm | |
| 0.10 | 54 ± 2* |
| 0.25 | 58 ± 2 |
| 1 | 61 ± 2 |
| 5 | 66 ± 3 |
| Ep | |
| 2.5 | 59 ± 3* |
| 5 | 63 ± 3 |
| 10 | 70 ± 3 |
| E | |
| 2.5 | 57 ± 2* |
| 5 | 61 ± 3 |
| 10 | 68 ± 3 |
Splenic macrophages were isolated from animals treated for 7 days with the indicated estrogens at the indicated doses (in milligrams per kilogram per day). The percentages (± SEM) of splenic macrophages binding ≥3 IgG-sensitized RBCs over the number bound by macrophages in sham controls are indicated as an index of splenic-macrophage FcγR function. *, P < 0.01. P < 0.001 in all other cases.
We further studied the effect of the treatment with estrogens on splenic macrophage FcγR cell surface expression. Guinea pig macrophages express two classes of FcγRs: FcγR1-FcγR2 and FcγR2 (22). We examined the effect of estrogens on the expression of both FcγR1-FcγR2 and FcγR2 by isolated splenic macrophages, using flow cytometry with specific MAbs for these receptors (Table 2). Treatment with estrogens significantly increased the expression of both guinea pig macrophage FcγRs, FcγR1-FcγR2 and FcγR2. As shown in Table 2, the estrogen-mediated inhibition of macrophage FcγR expression was dose dependent. Minimal effective doses are indicated. Et, M, and Ct were more effective than Pm, Ep, or E, and all of them had a greater effect on FcγR1-FcγR2 than on FcγR2.
TABLE 2.
Enhancement of splenic-macrophage FcγR MFI by treatment with estrogens
| Estrogen dose (mg/kg) | % Enhancement (± SEM) of MFI ofa
|
|
|---|---|---|
| FcγR1-FcγR2 | FcγR2 | |
| Et | ||
| 0.002 | 17 ± 1* | 11 ± 1* |
| 0.005 | 25 ± 1 | 20 ± 1 |
| 0.010 | 38 ± 2 | 26 ± 1 |
| 0.100 | 46 ± 2 | 37 ± 2 |
| 1.000 | 68 ± 2 | 44 ± 2 |
| M | ||
| 0.25 | 15 ± 1* | 10 ± 1* |
| 0.50 | 30 ± 2 | 25 ± 1 |
| 1.00 | 41 ± 2 | 33 ± 2 |
| 5.00 | 59 ± 2 | 40 ± 2 |
| Ct | ||
| 0.25 | 16 ± 1* | 12 ± 1* |
| 0.50 | 25 ± 2 | 20 ± 2 |
| 1.00 | 42 ± 2 | 29 ± 2 |
| 5.00 | 56 ± 2 | 38 ± 3 |
| Pm | ||
| 0.05 | 16 ± 1* | 11 ± 1* |
| 0.10 | 24 ± 2 | 19 ± 1 |
| 0.50 | 39 ± 2 | 28 ± 2 |
| 1.00 | 50 ± 2 | 35 ± 2 |
| Ep | ||
| 1.00 | 15 ± 1* | 10 ± 1* |
| 2.50 | 30 ± 2 | 22 ± 2 |
| 5.00 | 47 ± 2 | 31 ± 2 |
| E | ||
| 1.00 | 16 ± 1* | 11 ± 1* |
| 2.50 | 32 ± 2 | 22 ± 2 |
| 5.00 | 46 ± 2 | 32 ± 2 |
Animals were treated for 7 days with the indicated estrogens at different doses (in milligrams per kilogram per day). The percentages of enhancement of the MFIs (± SEM) for both guinea pig macrophage FcγRs, FcγR1-FcγR2 and FcγR2, over the MFI exhibited by sham controls are indicated. *, P < 0.01; P < 0.001 in all other cases.
Immunofluorescence capping experiments were performed to examine the effects of in vivo-administered estrogens on the membrane mobilities of FcγR1-FcγR2 and FcγR2. We consider whether the highly lipophilic estrogen molecules might alter the mobilities of surface membrane receptors, thus contributing to the stimulatory effects observed on FcγR1-FcγR2 and FcγR2 expression. Cells incubated at 0°C to prevent membrane movement showed a uniform diffuse ring pattern when they were stained for either FcγR1-FcγR2 or FcγR2. When incubated at 37°C, the majority of cells displayed aggregates or patches of membrane fluorescence, with some cells showing an intense polar distribution of staining for both FcγRs, similar to that reported for the ligand-induced capping of lymphocyte surface Ig (21). No significant differences were observed between results for sham- and estrogen-treated animals for either FcγR1-FcγR2 or FcγR2 staining intensity or distribution (data not shown). Estrogens do not appear to have a major effect on the membrane mobilities of these receptors.
Our data indicate that treatment with estrogens approved for clinical use enhances the clearance of IgG-sensitized cells by improving the cell surface expression of splenic-macrophage FcγRs.
DISCUSSION
Macrophage FcγRs play an important role in the regulation of the immune response, in host defense against infection, and in the pathophysiologies of immune disorders (4, 9, 16). Thus, the modulation of macrophage FcγR expression is a therapeutic target for immune-mediated diseases (4).
Neuroendocrine mechanisms are relevant to the pathophysiologies of immune-mediated disorders (14). We have been interested in the modulation of FcRs for IgG by neuroendocrine actions as a new form of FcγR-directed therapy. Our guinea pig animal model has been useful in understanding the pathophysiologies of immune cytopenias (17, 18), as well as the effects of glucocorticoids, sex hormones, and dopaminergic drugs on macrophage FcγR expression (6–8, 15, 19).
We have previously observed that estradiol increases the clearance of IgG-sensitized cells by enhancing splenic-macrophage FcγR expression (6, 19). The effect of clinically used estrogens, other than estradiol, on the clearance of IgG-containing immune complexes has not previously been assessed. Therefore, we studied the effect of treatment with estrogens on splenic-macrophage FcγR expression. Estrogens increased the clearance of IgG-sensitized cells by enhancing the expression of splenic-macrophage FcγRs. Et, M, and Ct were more effective than Pm, Ep, and E.
Two FcγR types, FcγR2 and FcγR1-FcγR2 have been identified in guinea pig macrophages (22). Our data suggest that both receptors are expressed on essentially all splenic macrophages and participate in the binding of IgG-sensitized erythrocytes (7, 8, 15). Immunofluorescence capping experiments were performed to examine any possible effects of in vivo-administered estrogens on the membrane mobilities of FcγR1-FcγR2 and FcγR2 (21). Estrogens do not appear to have a major effect on the membrane mobilities of these receptors, suggesting that their stimulatory effects are likely at the level of surface receptor expression.
The precise homology between guinea pig and human macrophage FcγRs has not been established. Nevertheless, experimental studies using the guinea pig model have contributed to our understanding of the pathophysiologies and therapeutic mechanisms involving macrophage FcγRs in human immunity-mediated disorders (1, 7, 8, 15, 17–20). There is substantial similarity between humans and guinea pigs in their responses to steroids. Both species are steroid resistant and are similar in their steroid metabolisms (23). We have previously measured the circulating levels of steroid hormones in guinea pigs and observed that they correlate with the administered in vivo dose and with the steroid levels observed during changes in the hormonal state in humans (7, 8, 15, 19). As we have observed with other steroids, like glucocorticoids, androgens, and progesterones (7, 8, 15), guinea pig macrophage receptor FcγR1-FcγR2 in our experiments appears to be more responsive to such estrogen-induced modulatory signals than the other macrophage FcγR, FcγR2.
Our results indicate that treatment with the commonly employed estrogens Et, M, Ct, Ep, and E enhances ithe clearance of IgG-sensitized cells by increasing the expression of splenic-macrophage FcγRs. Guinea pig macrophage FcγR1-FcγR2 expression is more responsive to enhancement than is the other macrophage FcγR, FcγR2.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministerio de Educación y Ciencia (PM92-0259 and RE90-28515062) and the Consejeria de Educación, Junta Andalucia (Grupo 3224).
REFERENCES
- 1.Atkinson J P, Schreiber A D, Frank M M. Effects of corticosteroids and splenectomy on the immune clearance and destruction of erythrocytes. J Clin Investig. 1973;52:1509–1517. doi: 10.1172/JCI107325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchette V, Imbach P, Andrew M, et al. Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet. 1994;344:703–707. doi: 10.1016/s0140-6736(94)92205-5. [DOI] [PubMed] [Google Scholar]
- 3.Bussel J B, Kimberly R P, Inman R D, et al. Intravenous gammaglobulin treatment of chronic idiopathic thrombocytopenic purpura. Blood. 1983;62:480–486. [PubMed] [Google Scholar]
- 4.Deo Y M, Graziano R F, Repp R, van de Winkel J G J. Clinical significance of IgG Fc receptors and Fcγ-directed immunotherapies. Immunol Today. 1997;18:127–135. doi: 10.1016/s0167-5699(97)01007-4. [DOI] [PubMed] [Google Scholar]
- 5.Fehr J, Hoffman V, Kappeler U. Transient reversal of thrombocytopenia in idiopathic thrombocytopenic purpura by high dose intravenous gammaglobulin. N Engl J Med. 1982;306:1254–1258. doi: 10.1056/NEJM198205273062102. [DOI] [PubMed] [Google Scholar]
- 6.Friedman D, Nettl F, Schreiber A D. Effect of estradiol and steroid analogues on the clearance of immunoglobulin G coated erythrocytes. J Clin Investig. 1985;75:162–167. doi: 10.1172/JCI111669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez F, Ruiz P, Briceño F, Lopez R, Michan A. Treatment with progesterone analogues decreases macrophage Fcγ receptor expression. Clin Immunol Immunopathol. 1998;89:231–239. doi: 10.1006/clin.1998.4602. [DOI] [PubMed] [Google Scholar]
- 8.Gomez F, Ruiz P, Briceño F, Rivera C, Lopez R. Macrophage Fcγ receptor expression is altered by treatment with dopaminergic drugs. Clin Immunol. 1999;90:375–387. doi: 10.1006/clim.1998.4665. [DOI] [PubMed] [Google Scholar]
- 9.Gomez F, Ruiz P, Schreiber A D. Impairment of Fcγ-receptor predisposes to severe infection in alcoholic cirrhosis of the liver. N Engl J Med. 1994;331:1122–1128. doi: 10.1056/NEJM199410273311704. [DOI] [PubMed] [Google Scholar]
- 10.Goodhue P A, Evans T S. Idiopathic thrombocytopenic purpura and pregnancy. Obstet Gynecol Surg. 1962;18:671. doi: 10.1097/00006254-196310000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Holdstock G, Chastenay B F, Krawitt E L. Effects of testosterone, estradiol and progesterone on immune regulation. Clin Exp Immunol. 1982;47:162–168. [PMC free article] [PubMed] [Google Scholar]
- 12.Kimberly R P, Salmon J E, Bussel J B, et al. Modulation of mononuclear phagocyte function by intravenous gammaglobulin. J Immunol. 1984;132:745–750. [PubMed] [Google Scholar]
- 13.Masi A T. Neuroendocrine immune mechanisms in rheumatic diseases: an overview and future implications. Rheum Dis Clin North Am. 2000;26:1003–1007. doi: 10.1016/s0889-857x(05)70181-2. [DOI] [PubMed] [Google Scholar]
- 14.Masi A T, Bijlsma J W J, Chikanza I C, Cutolo M, editors. Neuroendocrine mechanisms in rheumatic diseases. Rheum Dis Clin North Am. 2000;26:693–1018. [Google Scholar]
- 15.Ruiz P, Gomez F, King M, Lopez R, Darby C, Schreiber A D. In vivo glucocorticoid modulation of guinea pig splenic macrophage Fcγ receptors. J Clin Investig. 1991;88:149–157. doi: 10.1172/JCI115271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz P, Gomez F, Schreiber A D. Impaired macrophage Fcγ receptor function in chronic renal failure. N Engl J Med. 1990;322:717–722. doi: 10.1056/NEJM199003153221102. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber A D, Frank M M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. I. In vivo effects of IgG and IgM complement-fixing sites. J Clin Investig. 1972;81:575–579. doi: 10.1172/JCI106846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber A D, Frank M M. Role of antibody and complement in the immune clearance and destruction of erythrocytes. II. Molecular nature of IgG and IgM complement-fixing sites and effects of their interaction with serum. J Clin Investig. 1972;51:583–587. doi: 10.1172/JCI106847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schreiber A D, Nettl F M, Sanders M C, King M, Szabolcs P, Friedman D, Gomez F. Effect of endogenous and synthetic sex steroids on the clearance of antibody-coated cells. J Immunol. 1988;141:2959–2966. [PubMed] [Google Scholar]
- 20.Schreiber A D, Parson J, McDermott P, Cooper R A. Effect of corticosteroids on the human monocyte IgG and complement receptors. J Clin Investig. 1975;56:1189–1197. doi: 10.1172/JCI108196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiner G F, Unanue E R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin alteration. Adv Immunol. 1976;31:67. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]
- 22.Shimamura T, Nakamura T, Koyama J. Demonstration of the evidence of two distinct Fc receptors for IgG isotypes on guinea pig macrophages by the use of monoclonal antibodies. Mol Immunol. 1987;24:67–74. doi: 10.1016/0161-5890(87)90112-x. [DOI] [PubMed] [Google Scholar]
- 23.Sisk D. The endocrine system. In: Wagner J E, Manning P J, editors. The biology of the guinea pig. New York, N.Y: Academic Press, Inc.; 1976. pp. 79–103. [Google Scholar]
- 24.Vernon-Roberts B. The effects of steroid hormones on macrophage activity. Int Rev Cytol. 1969;25:131–159. doi: 10.1016/s0074-7696(08)60202-8. [DOI] [PubMed] [Google Scholar]
- 25.Werb Z, Foley R, Nunck A. Interaction of glucocorticoids with macrophages. Identification of glucocorticoid receptors in monocytes and macrophages. J Exp Med. 1978;147:1684–1694. doi: 10.1084/jem.147.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]