Abstract
Following infection with Neospora caninum, BALB/c mice were shown to be resistant to an acute infection but developed a latent chronic infection. However, BALB/c background gamma interferon (IFN-γ)-deficient mice were sensitive to the acute infection. Since the immune response in IFN-γ-deficient mice is scantly known, we examined the function of macrophages, major histocompatibility complex (MHC) class II expression, T-cell responses, and serum cytokine levels in the mice. All IFN-γ-deficient mice died within 9 days of infection with N. caninum, whereas those treated with exogenous IFN-γ lived longer. Although N. caninum invaded various organs in both types of mice at the early stage of infection, the parasite was not detected in the brains of resistant hosts until 21 days postinfection (dpi). Peritoneal macrophages from IFN-γ-deficient mice were activated by exogenous IFN-γ associated with inhibition of parasite growth and nitric oxide production as were those from BALB/c mice. IFN-γ-deficient mice failed to increase MHC class II expression on macrophages. Moreover, BALB/c mice induced T-cell proliferation while IFN-γ-deficient mice did not. However, in vivo treatment with exogenous IFN-γ induced up-regulated MHC class II expression in IFN-γ-deficient mice. BALB/c mice treated with an antibody to CD4 showed an increase in morbidity and mortality after parasite infection. In serum, significant levels of IFN-γ and interleukin-4 (IL-4) were detected in resistant hosts, whereas IL-10 was detected in IFN-γ-deficient mice. The levels of IL-12 in IFN-γ-deficient mice were higher than those in BALB/c mice at 7 dpi. The present study indicates that early IFN-γ production has a crucial role in the activation of peritoneal macrophages for the induction of protective immune responses against N. caninum.
Neospora caninum was originally identified as a Toxoplasma gondii-like parasite causing predominantly neuromuscular disorders in dogs (3) and abortion and stillbirth in cattle (7, 14). The highly effective resistance induced by T. gondii-like protozoans is thought to be mediated by T cells (9, 13) and is associated with a highly polarized Th1 type cytokine expression pattern (10). Gamma interferon (IFN-γ) and interleukin-12 (IL-12) seem to be important in natural protection against N. caninum infection (16). Indeed, CD4+ and CD8+ T cells have a role in protective immune responses against N. caninum infection (26). For T. gondii, IFN-γ (12), tumor necrosis factor alpha (12, 30), IL-4 (23), and IL-6 (22) are critical for protective immunity. Also, IL-10 synthesis plays an important role in down-regulating IFN-γ responses to acute infection (11).
Macrophages have a crucial role in protective immunity against parasite infection. Activation of macrophages with IFN-γ exhibits a killing activity against N. caninum (27) and T. gondii (1), which is associated with increased production of nitric oxide (NO). Moreover, macrophages activate specific T lymphocytes by presenting pathogen-derived antigens in association with major histocompatibility complex (MHC) molecules. MHC class II knockout mice displayed a sensitivity to T. gondii infection consistent with the absence of CD4+ effector cells (5). On the other hand, MHC class I-deficient mice survived a T. gondii infection following vaccination with an attenuated parasite (6). Also, T. gondii interfered with the MHC class I and class II antigen presentation pathway in murine macrophages (19). In spite of the importance of MHC class II antigen presentation for host immunity, the exact role of the MHC during N. caninum infection is still unknown.
Mice have been used as laboratory models for the study of parasite infection in mammals. Although BALB/c mice are resistant to acute N. caninum infection, BALB/c background IFN-γ-deficient mice and BALB/c mice treated with an antibody to IFN-γ showed an increase in morbidity and mortality after parasite infection (2, 26). However, it is not clear how the absence of the IFN-γ gene affects host immune responses. In this study, we assessed the immune responses of BALB/c mice and BALB/c background IFN-γ-deficient mice to clarify the role of IFN-γ in host survival of N. caninum infection.
MATERIALS AND METHODS
Animals.
Female inbred BALB/c mice were purchased from a commercial supplier (Clea Japan). Female IFN-γ-deficient mice were generated as previously described (25). Male and female IFN-γ-deficient mice were backcrossed to BALB/c mice for seven generations and maintained by interbreeding heterozygous animals. Homozygous (−/−) littermates were identified by isolation of genomic tail DNA (15). The animals were 7 weeks of age at the beginning of the experiments.
Cultures and purification of parasites.
N. caninum tachyzoites of the Nc-1 isolate (8) were maintained in human foreskin fibroblasts (Hs68) grown in Dulbecco's modified Eagle's medium (Sigma, St. Louis, Mo.) supplemented with 10% heat-inactivated fetal bovine serum. For purification of tachyzoites, N. caninum-infected cells were washed with cold phosphate-buffered saline (PBS), and the cells were resuspended in cold PBS and passed through a 27-gauge needle and a 5.0-μm-pore-size filter (Millipore, Bedford, Mass.).
IFN-γ.
Recombinant mouse IFN-γ was purchased from Genzyme (Cambridge, Mass.).
DNA isolation and PCR analysis.
For DNA preparation, each tissue or organ (brain, heart, kidney, liver, lungs, and spleen) was thawed in 10× extraction buffer (10 mM Tris-HCl [pH 9.0], 0.1% sodium dodecyl sulfate, 10 mM NaCl, 0.1 mM EDTA)–1 mg of proteinase K/ml at 10 ml/g of tissue or organ at 55°C. DNA was purified by phenol-chloroform extraction and ethanol precipitation. The DNA concentration (template DNA) was adjusted to 100 μg/ml for the brain and 20 μg/ml for other organs. DNA of uninfected brains or organs and 0.2 μg of T. gondii (RH strain) tachyzoite DNA/ml were prepared as negative controls, and 0.2 μg of N. caninum tachyzoite DNA/ml was prepared as a positive control. The DNA amplified by PCR was suspended in 10 μl of a reaction mixture containing 2.5 μl of template DNA, 1 μl of 10× PCR buffer, which contained 15 mM MgCl2 (Perkin-Elmer, Foster City, Calif.), 1 μl of 10 mM deoxynucleoside triphosphate mixture (GIBCO BRL, Gaithersburg, Md.), 0.1 μl of 5-U/μl AmpliGold Taq DNA polymerase (Perkin-Elmer), and 2 pM N. caninum-specific primers Np6 and Np21 (29). Amplification was done in a thermal cycler (GeneAmp PCR System 2400; Perkin-Elmer) employing 40 cycles for denaturation (94°C, 1 min), annealing (63°C, 1 min), and primer extension (74°C, 3.5 min). At the end, a primer extension was continued for 10 min at 74°C, and then the samples were kept at 4°C. The PCR products were visualized by electrophoresis in agarose gels.
Monolayer cultures of peritoneal macrophages.
Mouse peritoneal macrophages (MPM) were harvested from the peritoneal cavities of naive mice. They were centrifuged at 800 × g for 10 min and suspended in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. The macrophage suspension was applied to 24-well tissue culture microplates containing round coverslips (15 by 15 mm) at 5 × 105 cells/well. The suspensions were incubated at 37°C for 3 h, washed thoroughly to remove nonadherent cells, and further incubated at 37°C.
In vitro N. caninum proliferation assays.
The MPM (5 × 105 cells) were infected with an equal number of parasites for 2 h. After free parasites were removed by being washed with medium, MPM were incubated for 48 h with medium alone or medium containing recombinant mouse IFN-γ. The proliferation of intracellular parasites was examined by microscopy. The parasite growth capacity is expressed as the number of tachyzoites per parasitophorous vacuole among 50 vacuoles.
Measurement of nitric oxide.
Nitrate and nitrite production in the culture medium was measured using a nitrite/nitrate assay kit (Cayman Chemical Co., Ann Arbor, Mich.) according to the manufacturer's recommendations. The amounts of nitrite and nitrate were calculated with a standard absorbance curve.
Flow cytometry analysis and antibodies.
Phycoerythrin (PE)-labeled anti-mouse CD8 monoclonal antibodies (MAbs), PE-labeled anti-mouse CD4 MAbs, PE-labeled anti-mouse CD11b (Mac-1 α chain) MAbs, fluorescein isothiocyanate (FITC)-labeled anti-mouse I-Ab (Aαb) MAbs, FITC-labeled anti-mouse CD45R/B220 MAbs, FITC-labeled anti-mouse CD3e MAbs, and purified rat anti-mouse CD16/CD32 (Fcγ III/II receptor) MAbs (FcBlock) were from Pharmingen (San Diego, Calif.).
After single cells were washed with cold PBS, 2 × 106 cells were resuspended in cold PBS containing 0.5% bovine serum albumin and 0.01% sodium azide. The cells were treated with FcBlock to avoid the nonspecific adherence of MAbs to Fc receptors. The cells were incubated with MAbs for 30 min at 4°C and washed with cold PBS. The cells (104), fixed with 0.5% paraformaldehyde in PBS, were examined with an EPICS XL flow cytometer (Coulter, Hialeah, Fla.). Within the splenocyte population, T cells were gated as CD3+ cells. Within the peritoneal cavity, macrophages were gated as CD11b+ and CD45R/B220− cells.
Effect of anti-T-cell subset MAbs.
GK1.5 and 53-6.72 MAbs were used for depletion of CD4+ and CD8+ T cells, respectively (18, 28). The peripheral blood mononuclear cells from each mouse were prepared 2 days later, and expression of CD4 and CD8 was examined by flow cytometry. After treatment of MAbs, the level of CD4+ or CD8+ T cells was always less than 1% (data not shown). The vaccinated mice were inoculated intraperitoneally (i.p.) with the MAb every 4 days after the first inoculation. BALB/c mice were challenged i.p. with 106 N. caninum tachyzoites 3 days after the first inoculation with the MAb.
Measurement of cytokine levels.
IL-2, IL-4, IL-10, IL-12 (p70), and IFN-γ levels were measured by enzyme-linked immunosorbent assay kits (Endogen, Woburn, Mass.) according to the manufacturer's recommendations. The amounts of cytokines were calculated using standard cytokine curves run on the same immunoplate.
RESULTS
Survival rates and parasite invasion in IFN-γ-deficient mice following N. caninum infection.
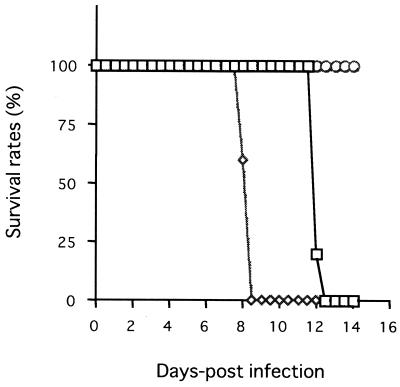
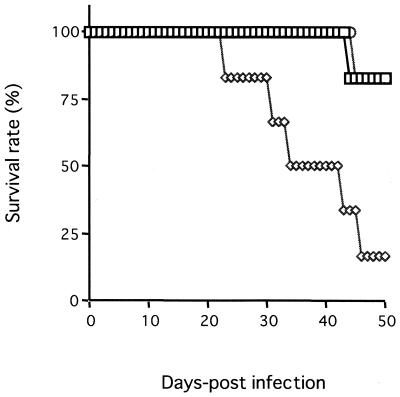
As shown in Fig. 1, all IFN-γ-deficient mice infected with N. caninum died within 9 days while all BALB/c mice survived during this time. IFN-γ-deficient mice tended to lose weight after N. caninum infection (data not shown). Administration of exogenous IFN-γ apparently extended the lives of IFN-γ-deficient mice. The presence of parasites in various organs or tissue of BALB/c and IFN-γ-deficient mice infected i.p. with 2.5 × 103 N. caninum tachyzoites was monitored by means of N. caninum-specific PCR following the infection (Table 1). In IFN-γ-deficient mice, the parasite DNA was first detected in the heart 1 day postinfection (dpi). Parasite DNA was detectable in all collected organs and tissues at 8 dpi, when all IFN-γ-deficient mice were dead. By comparison, parasite DNA in BALB/c mice was detectable at 7 dpi. Moreover, parasite DNA was detectable in the brain only at 21 dpi, while the rates of parasitized organs and tissue in BALB/c mice increased from 4 to 8 dpi. These results indicate that IFN-γ has a crucial role in protective immunity against N. caninum infection, as well as the control of parasite invasion into organs and tissues.
FIG. 1.
IFN-γ-deficient mice succumb to acute infection with N. caninum tachyzoites. BALB/c (○ and ▵) and IFN-γ-deficient (⋄ and □) mice were infected i.p. with 2.5 × 103 N. caninum tachyzoites. ▵ and □, mice treated i.p. with 500 U of recombinant IFN-γ twice a day; ○ and ⋄, untreated mice. The survival of the infected mice was monitored daily, and cumulative mortality was calculated. In the experiment shown, five mice per group were used. Comparable results were obtained in six repeat experiments. (The survival rates of both groups of BALB/c remained at 100% throughout the experiment, and hence triangles overlap with circles.)
TABLE 1.
N. caninum invasion into micea
| dpi | Mouse typeb | No. of mice positive for N. caninum by PCR/no. of mice used in:
|
|||||
|---|---|---|---|---|---|---|---|
| Brain | Heart | Lungs | Liver | Kidney | Spleen | ||
| 0 | BALB/c | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| IFN-γ KO | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | |
| 1 | BALB/c | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| IFN-γ KO | 0/3 | 1/3 | 0/3 | 0/3 | 0/3 | 0/3 | |
| 4 | BALB/c | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| IFN-γ KO | 2/3 | 1/3 | 1/3 | 2/3 | 1/3 | 1/3 | |
| 7 | BALB/c | 0/3 | 3/3 | 3/3 | 0/3 | 0/3 | 1/3 |
| IFN-γ KO | 3/3 | 3/3 | 3/3 | 3/3 | 2/3 | 3/3 | |
| 8 | BALB/c | 2/3 | 3/3 | 3/3 | 2/3 | 0/3 | 2/3 |
| IFN-γ KO | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | |
| 14 | BALB/c | 1/4 | 0/4 | 1/4 | 0/4 | 0/4 | 0/4 |
| 21 | BALB/c | 3/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
The presence of parasite DNA was examined by N. caninum-specific PCR.
KO, knockout.
N. caninum proliferation in MPM.
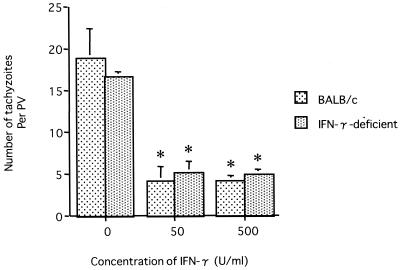
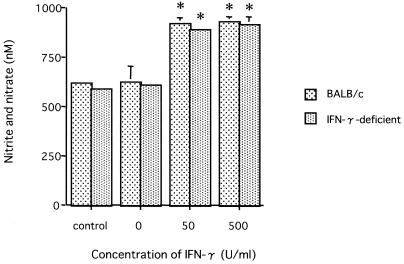
We examined whether N. caninum could proliferate in MPM of BALB/c and IFN-γ-deficient mice in the presence of IFN-γ (Fig. 2). In the absence of IFN-γ, N. caninum grew in MPM of both BALB/c and IFN-γ-deficient mice. The administration of IFN-γ inhibited N. caninum proliferation in MPM from both types of mice (P < 0.05). There was no significant difference in growth inhibition by IFN-γ between BALB/c and IFN-γ-deficient mice. Furthermore, we compared levels of NO production in MPM infected with N. caninum (Fig. 3). In MPM from both types of mice, the amounts of NO in infected MPM containing IFN-γ were significantly larger than those in the absence of IFN-γ (P < 0.01). N. caninum infection did not induce NO production in the absence of IFN-γ as was observed for MPM cultured with medium alone.
FIG. 2.
Growth of N. caninum tachyzoites in macrophages. Macrophages infected with N. caninum tachyzoites were incubated with different doses of IFN-γ for 48 h. N. caninum growth capacity is expressed as the number of tachyzoites per parasitophorous vacuole (PV) among 50 vacuoles. Each value is the mean of the number of tachyzoites ± the standard deviation of triplicate samples. According to Student's t test, the differences between the absence and the presence of IFN-γ (50 and 500 U/ml) in each mouse were significant (asterisk, P < 0.05). Data are representative of two similar experiments.
FIG. 3.
Production of nitrate and nitrite in activated macrophages. Naive macrophages cultured with medium alone for 48 h were used as the control. Production of nitrate and nitrite was measured in culture supernatants from the macrophages incubated with 0, 50, or 500 U of recombinant IFN-γ/ml for 48 h following N. caninum infection. Each value is the mean of nitrate and nitrite production ± the standard deviation of triplicate samples. According to Student's t test, the differences between the control and the presence of IFN-γ (0, 50, and 500 U/ml) in each mouse were significant (asterisk, P < 0.01). Data are representative of two similar experiments.
MHC class II expression following N. caninum infection.
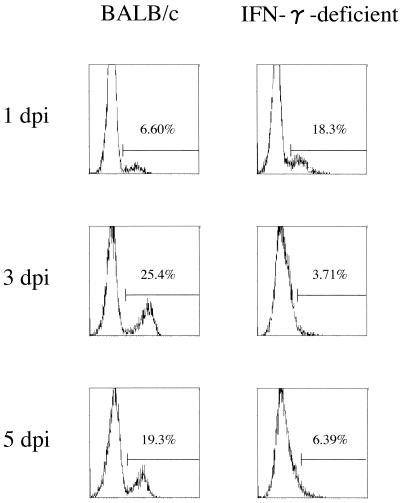
To examine MHC class II expression after N. caninum infection, MPM were prepared from BALB/c and IFN-γ-deficient mice (Fig. 4). Flow cytometry analysis indicated that MHC class II expression on MPM of BALB/c mice increased following N. caninum infection. However, significant expression of MHC class II was not observed in IFN-γ-deficient mice. We also investigated whether the administration of exogenous IFN-γ in vivo recovered MHC class II expression on MPM of IFN-γ-deficient mice (Fig. 5). The exogenous IFN-γ induced MHC class II expression on MPM of IFN-γ-deficient mice in a dose-dependent manner, suggesting that IFN-γ-deficient mice could not induce sufficient expression of MHC class II due to lack of the IFN-γ gene.
FIG. 4.
MHC class II expression in BALB/c and IFN-γ-deficient mice following N. caninum infection. The MHC class II expression on Mac-1 α chain+/CD45R/B220− cells was examined in mice infected i.p. with 5 × 105 N. caninum tachyzoites at 1, 3, and 5 dpi. Data are representative of three similar experiments.
FIG. 5.
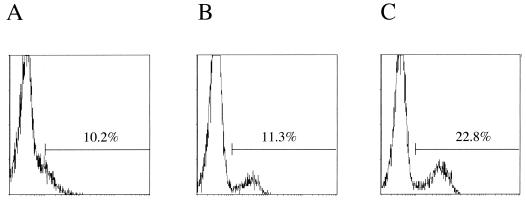
Effect of exogenous IFN-γ on MHC class II expression in IFN-γ-deficient mice. The mice infected i.p. with 5 × 105 N. caninum tachyzoites were treated with 0 (A), 500 (B), and 5,000 (C) U of recombinant IFN-γ every day. The MHC class II expression on Mac-1 α chain+/CD45R/B220− cells was examined at 3 dpi.
In vivo T-cell proliferation following N. caninum infection.
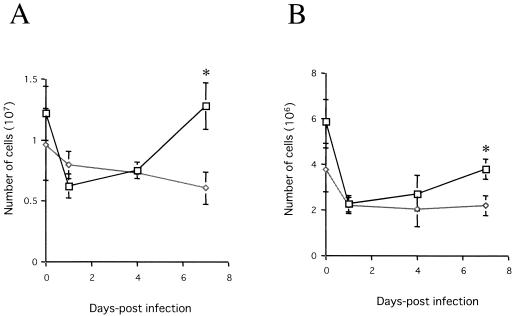
MHC class II molecules were displayed as MHC class II-peptide complexes on the surfaces of antigen-presenting cells for recognition by CD4+ T cells. Therefore, we examined the proliferation of CD4+ T cells in vivo (Fig. 6A). The number of CD4+ T cells gradually increased after N. caninum infection in BALB/c mice but not in IFN-γ-deficient mice (P < 0.01). A similar pattern in the proliferation of CD8+ T cells was observed (P < 0.01; Fig. 6B).
FIG. 6.
The number of CD4+ (A) and CD8+ (B) cells following N. caninum infection in BALB/c (□) and IFN-γ-deficient (⋄) mice. The splenocytes were collected from each mouse infected i.p. with 2.5 × 103 N. caninum tachyzoites at 0, 1, 4, and 7 dpi. Each value is the mean of the cell numbers ± the standard deviation of triplicate samples. According to Student's t test, the differences between BALB/c and IFN-γ-deficient mice were significant (asterisk, P < 0.01). Data are representative of two similar experiments.
Effect of anti-T-cell subset MAbs on N. caninum infection.
The role of anti-T-cell subset MAbs in BALB/c mice against N. caninum infection was examined (Fig. 7). The in vivo administration of an anti-CD8 MAb had no effect against N. caninum infection compared to results for control mice for the duration of the experiment. However, mice inoculated with an anti-CD4 MAb were highly susceptible to the infection. This result suggests that the role of CD4+ T cells may be crucial for protective immunity against N. caninum infection.
FIG. 7.
Effect of anti-T-cell subset MAbs on N. caninum infection. BALB/c mice treated with anti-CD4 MAbs (⋄), anti-CD8 MAbs (○), or PBS (□) were infected i.p. with 106 N. caninum tachyzoites. The survival of the infected mice was monitored daily, and cumulative mortality was calculated. In the experiment shown, six mice per group were used. Comparative results were obtained in two repeat experiments.
Levels of serum cytokines following N. caninum infection.
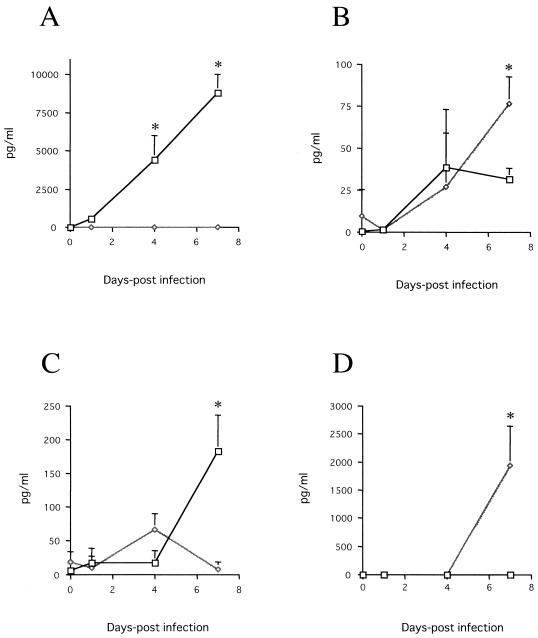
We compared levels of serum cytokines (IFN-γ, IL-2, IL-4, IL-10, and IL-12 [p70]) after N. caninum infection for BALB/c and IFN-γ-deficient mice (Fig. 8). The parasite infection induced IFN-γ, IL-12 (p70), and IL-4 production in BALB/c mice (P < 0.05). IFN-γ and IL-12 (p70) production was earlier than IL-4 production. The levels of IL-12 (p70) in IFN-γ-deficient mice were higher than those in BALB/c mice at 7 dpi (P < 0.05). On the other hand, IL-10 was produced in IFN-γ-deficient mice (P < 0.05). IL-2 production was not observed in either type of mouse (data not shown).
FIG. 8.
Serum cytokine synthesis in response to acute N. caninum infection in BALB/c (□) and IFN-γ-deficient (⋄) mice. The levels of IFN-γ (A), IL-12 (p70) (B), IL-4 (C), and IL-10 (D) were measured in each mouse infected with 2.5 × 103 N. caninum tachyzoites at 0, 1, 4, and 7 dpi by enzyme-linked immunosorbent assay. Each value is the mean of the amount of cytokine produced ± the standard deviation of triplicate samples. According to Student's t test, the differences between BALB/c and IFN-γ-deficient mice were significant (asterisk, P < 0.05).
DISCUSSION
The aim of the present study was to assess immune responses in genetically IFN-γ-deficient mice against N. caninum infection. Whereas wild-type BALB/c mice showed no clinical signs of neosporosis, all IFN-γ-deficient mice died within 9 dpi. IFN-γ-deficient mice infected with N. caninum and treated with exogenous mouse IFN-γ lived longer, suggesting that IFN-γ may have a crucial role during the parasite infection. It might be difficult to design successful experiments to protect IFN-γ-deficient mice completely by treatment with exogenous IFN-γ because it is known that overproduction of IFN-γ induces toxic effects in the host (11). In the early stage of infection (8 dpi), the spread of parasites in various organs and the brain in IFN-γ-deficient mice was measured by PCR. The death of IFN-γ-deficient mice following N. caninum infection was associated with parasite burden. Although the parasite spread in wild-type BALB/c mice was slow compared with that in IFN-γ-deficient mice, N. caninum invaded various organs (except for the kidney) and the brain in wild-type BALB/c mice. However, N. caninum DNA was detectable only in the brains of wild-type BALB/c mice at 21 dpi, indicating that IFN-γ may control parasite proliferation in the hosts.
Since macrophages play an initial role in the immune response against pathogens, we compared their function in BALB/c and IFN-γ-deficient mice. One of the functions of macrophages is their killing activity against intracellular parasites associated with increased production of NO (1, 27). Parasite growth was inhibited by treatment with IFN-γ in macrophages from BALB/c and IFN-γ-deficient mice. In addition, increased NO production was detected in macrophages from both mice groups infected with N. caninum in the presence of IFN-γ while the increased levels of NO production were the same in both types of mice. This result shows that macrophages from IFN-γ-deficient mice can be activated by treatment with IFN-γ. In the absence of IFN-γ, the level of NO production did not increase following N. caninum infection compared with control levels, suggesting that this phenomenon may be observed in IFN-γ-deficient mice in vivo.
Another major function of macrophages is antigen presentation. Wild-type BALB/c mice treated with an anti-CD4 MAb became sensitive to N. caninum infection compared with those treated with an anti-CD8 MAb. MHC class II knockout mice were sensitive to infection with T. gondii (5). Therefore, we assessed the levels of MHC class II expression in IFN-γ-deficient mice. The levels of MHC class II expression in wild-type BALB/c mice, but not in IFN-γ-deficient mice, increased following N. caninum infection. However, exogenous treatment with IFN-γ increased MHC class II expression in IFN-γ-deficient mice infected with parasites. Our finding suggests that macrophages from IFN-γ-deficient mice failed to activate specific T lymphocytes by presenting N. caninum antigens. In contrast to wild-type BALB/c mice, IFN-γ-deficient mice did not experience an increase in the number of splenic T cells (CD4+ and CD8+ cells) after N. caninum infection. This finding suggests that macrophages of IFN-γ-deficient mice could not efficiently present N. caninum antigens as MHC class II-antigen complexes, resulting in low levels of T-cell proliferation compared to those in wild-type BALB/c mice. The present study indicates that early IFN-γ production has a crucial role in the induction of protective immune responses to N. caninum infection associated with the activation of peritoneal macrophages.
The analysis of serum cytokines showed that, following N. caninum infection, the production of IFN-γ and IL-4 was induced in wild-type BALB/c mice whereas IL-10 production was induced in IFN-γ-deficient mice. Since macrophages appear to be the major source of IL-10 in acutely infected mice (17), MPM exposed to a large number of the tachyzoites might produce high levels of IL-10 in IFN-γ-deficient mice. However, the role of IL-10 produced in IFN-γ-deficient mice is unclear. Although IL-12 production was observed in both types of mice, the levels in IFN-γ-deficient mice were higher than those in BALB/c mice at 7 dpi. The high levels of IL-12 were determined in the absence of endogenous IFN-γ, suggesting that IL-12 production might be regulated by sufficient synthesis of IFN-γ for protection against the infection. A comparison of the IL-4 production pattern to that of IFN-γ and IL-12 showed a significant delay in BALB/c mice. Three of the cytokines (IFN-γ, IL-12, and IL-4) were shown to be essential for resistance to N. caninum (2, 16) and T. gondii (12, 23, 34) infection in the mouse model. Moreover, the simultaneous induction of IL-10 and IFN-γ is a common feature of the immune response to intracellular parasites. IL-10 stimulation allows the pathogen to evade IFN-γ-dependent mechanisms of host resistance (4, 17, 20, 21). In addition, overproduction of IFN-γ increased the sensitivity of the hosts to the pathogen (11). In agreement with these observations, our finding suggests that the balance of cytokine production is controlled in resistant hosts. We have shown in the present study that cytokine synthesis in resistant hosts in response to N. caninum infection could be controlled.
In conclusion, this study supports the crucial roles that macrophages and CD4+ cells play in the IFN-γ-mediated host immune responses to N. caninum infection. Moreover, the up-regulation of MHC class II expression on macrophages during N. caninum infection is associated with host survival of parasite infection.
ACKNOWLEDGMENTS
We thank J. P. Dubey (United States Department of Agriculture, Agriculture Research Service, Livestock and Poultry Sciences Institute, and Parasite Biology and Epidemiology Laboratory) for the gift of N. caninum and the Nc-1 isolate and Y. Iwakura (Institute of Medical Sciences, The University of Tokyo) for the gift of IFN-γ-deficient mice.
This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan. Y.N. was supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
REFERENCES
- 1.Adams L B, Hibbs J B, Jr, Taintor R R, Krahenbuhl J L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- 2.Baszler T V, Long M T, McElwain T F, Mathison B A. Interferon-gamma and interleukin-12 mediate protection to acute Neospora caninum infection in BALB/c mice. Int J Parasitol. 1999;29:1635–1646. doi: 10.1016/s0020-7519(99)00141-1. [DOI] [PubMed] [Google Scholar]
- 3.Bjerkas I, Presthus J. The neuropathology in toxoplasmosis-like infection caused by a newly recognized cyst-forming sporozoon in dogs. APMIS. 1989;97:459–468. doi: 10.1111/j.1699-0463.1989.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 4.Denis M, Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425–5430. [PubMed] [Google Scholar]
- 5.Denkers E Y, Scharton-Kersten T, Barbieri S, Caspar P, Sher A. A role for CD4+ NK1.1+ T lymphocytes as major histocompatibility complex class II independent helper cells in the generation of CD8+ effector function against intracellular infection. J Exp Med. 1996;184:131–139. doi: 10.1084/jem.184.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denkers E Y, Gazzinelli R T, Martin D, Sher A. Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J Exp Med. 1993;178:1465–1472. doi: 10.1084/jem.178.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubey J P, Lindsay D S. Neosporosis. Parasitol Today. 1993;9:452–458. doi: 10.1016/0169-4758(93)90099-2. [DOI] [PubMed] [Google Scholar]
- 8.Dubey J P, Carpenter J L, Speer C A, Topper M J, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc. 1988;192:1269–1285. [PubMed] [Google Scholar]
- 9.Frenkel J K. Adoptive immunity to intracellular infection. J Immunol. 1967;98:1309–1319. [PubMed] [Google Scholar]
- 10.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 11.Gazzinelli R T, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- 12.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;15:3672–3681. [PubMed] [Google Scholar]
- 13.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 14.Gottstein B, Hentrich B, Wyss R, Thur B, Busato A, Stark K D, Muller N. Molecular and immunodiagnostic investigations on bovine neosporosis in Switzerland. Int J Parasitol. 1998;28:679–691. doi: 10.1016/S0020-7519(98)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igarashi I, Suzuki R, Waki S, Tagawa Y, Seng S, Tum S, Omata Y, Saito A, Nagasawa H, Iwakura Y, Suzuki N, Mikami T, Toyoda Y. Roles of CD4+ T cells and gamma interferon in protective immunity against Babesia microti infection in mice. Infect Immun. 1999;67:4143–4148. doi: 10.1128/iai.67.8.4143-4148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan I A, Schwartzman J D, Fonseka S, Kasper L H. Neospora caninum: role for immune cytokines in host immunity. Exp Parasitol. 1997;85:24–34. doi: 10.1006/expr.1996.4110. [DOI] [PubMed] [Google Scholar]
- 17.Khan I A, Matsuura T, Kasper L H. IL-10 mediates immunosuppression following primary infection with Toxoplasma gondii in mice. Parasite Immunol. 1995;17:185–195. doi: 10.1111/j.1365-3024.1995.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 18.Ledbetter J A, Herzenberg L A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 19.Luder C G, Lang T, Beuerle B, Gross U. Down-regulation of MHC class II molecules and inability to up-regulate class I molecules in murine macrophages after infection with Toxoplasma gondii. Clin Exp Immunol. 1998;112:308–316. doi: 10.1046/j.1365-2249.1998.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sher A, Fiorentino D, Caspar P, Pearce E, Mosmann T. Production of IL-10 by CD4+ T lymphocytes correlates with down-regulation of Th1 cytokine synthesis in helminth infection. J Immunol. 1991;147:2713–2716. [PubMed] [Google Scholar]
- 21.Silva J S, Morrissey P J, Grabstein K H, Mohler K M, Anderson D, Reed S G. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki Y, Rani S, Liesenfeld O, Kojima T, Lim S, Nguyen T A, Dalrymple S A, Murray R, Remington J S. Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infect Immun. 1997;65:2339–2345. doi: 10.1128/iai.65.6.2339-2345.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki Y, Yang Q, Yang S, Nguyen N, Lim S, Liesenfeld O, Kojima T, Remington J S. IL-4 is protective against development of toxoplasmic encephalitis. J Immunol. 1996;157:2564–2569. [PubMed] [Google Scholar]
- 24.Suzuki Y, Joh K. Effect of the strain of Toxoplasma gondii on the development of toxoplasmic encephalitis in mice treated with antibody to interferon-gamma. Parasitol Res. 1994;80:125–130. doi: 10.1007/BF00933779. [DOI] [PubMed] [Google Scholar]
- 25.Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-gamma(−/−) mice, but not in TNF-alpha(−/−) mice: role for IFN-gamma in activating apoptosis of hepatocytes. J Immunol. 1997;159:1418–1428. [PubMed] [Google Scholar]
- 26.Tanaka T, Hamada T, Inoue N, Nagasawa H, Fujisaki K, Suzuki N, Mikami T. The role of CD4(+) or CD8(+) T cells in the protective immune response of BALB/c mice to Neospora caninum infection. Vet Parasitol. 2000;90:183–191. doi: 10.1016/s0304-4017(00)00238-7. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka T, Nagasawa H, Fujisaki K, Suzuki N, Mikami T. Growth-inhibitory effects of interferon-gamma on Neospora caninum in murine macrophages by a nitric oxide mechanism. Parasitol Res. 2000;86:768–771. doi: 10.1007/s004360000242. [DOI] [PubMed] [Google Scholar]
- 28.Wilde D B, Marrack P, Kappler J, Dialynas D P, Fitch F W. Evidence implicating L3T4 in class II MHC antigen reactivity monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte line. J Immunol. 1983;131:2178–2183. [PubMed] [Google Scholar]
- 29.Yamage M, Flechtner O, Gottstein B. Neospora caninum: specific oligonucleotide primers for the detection of brain “cyst” DNA of experimentally infected nude mice by the polymerase chain reaction (PCR) J Parasitol. 1996;82:272–279. [PubMed] [Google Scholar]
- 30.Yap G S, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J Immunol. 1998;160:1340–1345. [PubMed] [Google Scholar]