Abstract
Objectives:
We characterized antibiotic prescribing patterns and management practices among recurrent urinary tract infection (rUTI) patients, and we identified factors associated with lack of guideline adherence to antibiotic choice, duration of treatment, and urine cultures obtained. We hypothesized that prior resistance to nitrofurantoin or trimethoprim–sulfamethoxazole (TMP-SMX), shorter intervals between rUTIs, and more frequent rUTIs would be associated with fluoroquinolone or β-lactam prescribing, or longer duration of therapy.
Methods:
This study was a retrospective database study of adult women with International Classification of Diseases, Tenth Revision (ICD-10) cystitis codes meeting American Urological Association rUTI criteria at outpatient clinics within our academic medical center between 2016 and 2018. We excluded patients with ICD-10 codes indicative of complicated UTI or pyelonephritis. Generalized estimating equations were used for risk-factor analysis.
Results:
Among 214 patients with 566 visits, 61.5% of prescriptions comprised first-line agents of nitrofurantoin (39.7%) and TMP-SMX (21.5%), followed by second-line choices of fluoroquinolones (27.2%) and β-lactams (11%). Most fluoroquinolone prescriptions (86.7%), TMP-SMX prescriptions (72.2%), and nitrofurantoin prescriptions (60.2%) exceeded the guideline-recommended duration. Approximately half of visits lacked a urine culture. Receiving care through urology via telephone was associated with receiving a β-lactam (adjusted odds ratio [aOR], 6.34; 95% confidence interval [CI], 2.58–15.56) or fluoroquinolone (OR, 2.28; 95% CI, 1.07–4.86). Having >2 rUTIs during the study period and seeking care from a urology practice (RR, 1.28, 95% CI, 1.15–1.44) were associated with longer antibiotic duration.
Conclusions:
We found low guideline concordance for antibiotic choice, duration of therapy and cultures obtained among rUTI patients. These factors represent new targets for outpatient antibiotic stewardship interventions.
Urinary tract infections (UTIs) are a common condition in ambulatory care, accounting for an estimated 10.5 million visits to primary care annually. 1 A subset of patients experience recurrent UTIs (rUTIs). Prospective studies identified UTI recurrence in 24% of college-aged females within 6 months and in 44% of adult women (mean age, 48 years) within 1 year. 2,3 Sequalae include physical pain and negative impacts on physical and social functioning, which are further amplified in patients with rUTI. 4
Evidenced-based rUTI management recommendations in the British Medical Journal in 2013 and the 2018 American Urogynecologic Society (AUS) best-practice statement specific to rUTI management in females largely follow the 2011 Infectious Disease Society of America (IDSA) guidelines for uncomplicated cystitis in terms of antibiotic selection and duration. 5,6 After accounting for availability, allergy history, tolerance, and regional resistance, recommended agents available in the United States include nitrofurantoin, trimethoprim-sulfamethoxazole (TMP-SMX), and fosfomycin, prescribed for 5-, 3-, and 1-day periods, respectively. 7 Fluoroquinolones and β-lactams are considered second-line agents. Due to potentially serious side effects, the US Food and Drug Administration (FDA) issued an initial ‘black box’ warning in 2008 for fluoroquinolones. In 2016, the FDA recommended fluoroquinolone use only in uncomplicated UTI (uUTI) patients with no alternative treatment. 8,9 The AUS statement and others also recommend obtaining a urine culture prior to antibiotic initiation, prescribing vaginal estrogen therapy in peri- or postmenopausal females, and providing low-dose antibiotic prophylaxis. 5,6,10
Multiple studies have investigated prescribing practices for sporadic, uUTI in ambulatory settings and found substantial discordance with IDSA guidelines in terms of antibiotic choice and duration. 11–14 Durkin et al 12 found overall IDSA guideline concordance at 26% in terms of antibiotic choice, dose, and duration. 12 Among 6 family medicine practices, Cowart et al 11 found that >75% of prescriptions exceeded the recommended treatment duration. Overall, fluoroquinolone prescribing prevalence in uUTI ranged from 35.3% to 51.6% across 4 studies. 11–14
To the best of our knowledge, no US studies have examined prescribing or management practices among patients with rUTI—a population at greater risk for experiencing adverse events from repeated antibiotic exposure. 15 Therefore, our study objectives included characterizing antibiotic choice and duration, nonantibiotic UTI-related therapies, and the percentage of urine cultures obtained at visits. We also identified factors associated with guideline nonadherence in terms of antibiotic choice, duration of therapy, and lacking a visit-associated urine culture. We hypothesized that antibiotic resistance to a first-line agent, a shorter duration between visits and more frequent rUTIs may trigger providers to select a second-line agent or extend therapy duration in the latter 2 cases.
Materials and methods
Setting and study design
We conducted a retrospective database study of adult females who sought care for uncomplicated rUTI between November 1, 2016, and December 31, 2018, at family medicine, internal medicine, and urology clinics within an academic medical center in a large urban area. The patient population at these clinics generally includes privately insured patients; however, public insurance (Medicare or Medicaid) is also accepted. 14 Patients do not require a referral for primary care or internal medicine practices. However, a referral to urology may be required depending on insurance plans. In general, referrals to urology are sought when a patient presents with any of the following: elevated prostate-specific antigen test, hematuria, suspected anatomic abnormality, or noteworthy voiding difficulties. Noninfectious disease primary care physicians typically refer patients with rUTI to urology or infectious disease for care.
Data procurement occurred over 2 stages: (1) electronic extraction of patient data from the Epic Clarity Database that met our inclusion and exclusion criteria based on International Classification of Diseases Tenth Revision (ICD-10) codes; and (2) a manual chart review to identify additional ICD-10 codes and visit details indicating complicated UTI or pyelonephritis.
Inclusion and exclusion criteria
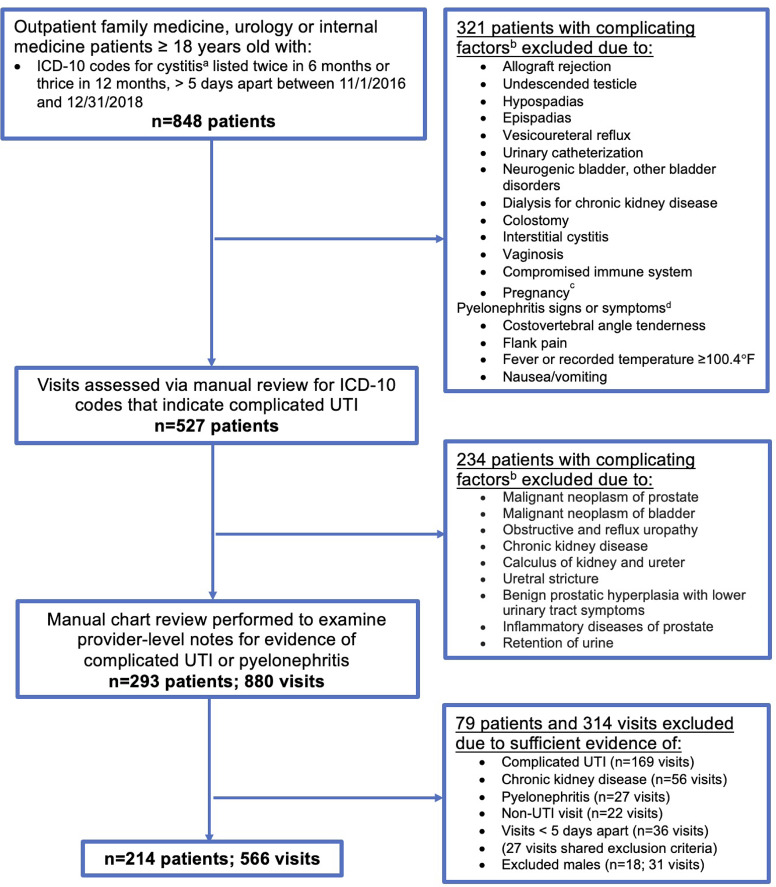
Figure 1 provides details on the selection process and criteria for study inclusion and exclusion. Females 18 and older were included if their record contained an ICD-10 code for cystitis (N30.0, N30.9, and N39.0) that occurred either in 2 visits within 6-months or 3 visits within 12 months (qualifying events), per the American Urological Association definition for rUTI. 10 Visits also had to occur >5 days apart to exclude any follow-up visits, and records had to be available 6 months following the last visit to capture recurrences within 12 months. Patients were excluded if they had ICD-10 codes listed in the prior year or at their qualifying visit indicating complicating factors that would impact the structural integrity or functional ability of the genitourinary tract, interstitial cystitis, vaginosis, impaired immune functioning, or an ICD-10 code for fever or nausea (qualifying visit only).
Fig. 1.
Flow chart depicting methods for rUTI patient and visit inclusion and exclusion process.
aInternational Classification of Diseases (ICD-10) codes N30.0, N30.9, and N39.0. bExclusionary ICD-10 codes for complicating factors were applied for all visits 12 months prior or at the qualifying visit. Exclusionary codes included T86, Q53, Q54, Q64.0, N13.7, T83, N31, N32, Z99.2, Z93.3, N30.10, N30.11, N76.0, D89.9, Z33.1, Z33.3 C61, C67, N13, N18, N20, N35, N40, N41 and R33. cExcluded if the ICD-10 code listed in the prior year or 6 months after the qualifying visit. dExclusionary criteria for pyelonephritis only applied at qualifying visit; ICD-10 codes were R50.9 and R11.
During the manual chart review, a trained clinician reviewed the patient history, review of symptoms, physical exam, and provider assessment to identify and exclude cases of pyelonephritis (Fig. 1). 16 The problem list, history of present illness, and differential diagnosis were examined to identify and exclude patients with chronic kidney disease or complicated UTI. Sufficient evidence of complicated UTI included a urologic abnormality, evidence indicating an immunocompromised state (eg, HIV, corticosteroid use, autoimmune disease), pregnancy, and nephrolithiasis.
For each eligible visit, we extracted demographic details and the following visit data: encounter date, type (office or telephone), practice type, ICD-10 codes, and allergy data. Antibiotic type, frequency, and quantity, coupled with urine culture data including organism type, concentration and antibiotic susceptibility were extracted when present. We searched and extracted urine culture results within a 3-day window before and after the visit date. Elixhauser scores were calculated for each visit by identifying ICD-10 codes matching any Elixhauser comorbidity category and assigning points based on a weighted metric validated by Moore et al. for predicting hospital readmission. 17 In addition, patients with clinical evidence of diabetes mellitus, based on HbA1c scores, diabetic medications, and a history of diabetes mellitus, were assigned the corresponding points for having ‘uncomplicated diabetes’ per Moore et al. and were also evaluated independently. The Baylor College of Medicine Ethics Committee approved the study protocol.
Statistical analysis
Descriptive statistics were calculated for demographic, health, and visit data, nonantibiotic therapies, and antibiotic type and duration. Antibiotics were classified as prophylactic if therapy exceeded 14 days or were directed to use ‘as needed.’ We used the 2011 IDSA recommended duration of therapy for uUTI of 7 days for β-lactams, 5 days for nitrofurantoin, and 3 days for fluoroquinolones and TMP-SMX when evaluating whether duration exceeded guideline recommendations. 7 When multiple antibiotics were listed for the same visit, these were counted independently in the overall antibiotic summary. Patients prescribed a second-line agent with allergies to both nitrofurantoin and TMP-SMX (5 patients, 8 visits) were excluded from the descriptive totals and risk factor analysis for antibiotic choice.
To identify factors associated with antibiotic choice for episodic therapy, we used generalized estimating equations (GEE) with a logit link (multivariate logistic regression). For antibiotic choice, we evaluated factors associated with prescription of a second-line agent (β-lactam or fluoroquinolone) compared to a first-line agent (nitrofurantoin, TMP-SMX or fosfomycin). Visits in which both first- and second-line agents were prescribed were excluded.
We used GEE Poisson regression to evaluate predictors associated with lacking a visit-associated urine culture and duration of antibiotic therapy. We included all antibiotics prescribed episodically except for ceftriaxone and doxycycline, as the former was a one-time, intravenous administration, while the latter serves as a non-traditional antibiotic for UTI. If a visit contained different antibiotics, each was included separately in the analysis.
GEE analyses were conducted in R Studio version 1.5.17 software (R Foundation for Statistical Computing, Vienna, Austria) using the geepack package specifying an exchangeable correlational structure. 18 A backward, stepwise regression process was used with a threshold P < .20 required to enter the model and 0.1 to stay in the multivariable model. We evaluated collinearity between significant predictors in the univariate analysis using GEE. A P < .05 was considered significant. We detected collinearity between practice type and visit type because telephone visits were significantly associated with urology (P < .001). Therefore, we combined both practice and visit type into a multilevel categorical variable (Table 4). This was also done for urine culture ordering, but with internal medicine separated from family medicine. More frequent rUTI visits and urology were also significantly associated (P < .001) and subcomponents were parsed out (Table 5).
Table 4.
Factors Associated With β-Lactam or Fluoroquinolone Prescribing, Using Generalized Estimating Equations Logistic Regression
| Predictors | β-Lactam Prescribing | Fluoroquinolone Prescribing | ||||
|---|---|---|---|---|---|---|
| Univariate Model | Multivariate Model | Univariate Model | ||||
| OR (95% CI) | P Value | aOR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | 1.03 (1.01–1.05) | .006 | 1.02 (1.00–1.04) | .115 e | … | .49 |
| Race | ||||||
| White | Reference | … | Reference | … | Reference | … |
| Black | 0.86 (0.22–3.42) | .83 | … | … | … | .797 |
| Other | 0.47 (0.16–1.38) | .17 | … | … | … | .225 |
| Elixhauser score | … | .830 | … | … | … | .38 |
| Diabetes mellitus | … | .84 | … | … | … | .43 |
| Practice and visit type | ||||||
| Primary care a office | Reference | … | Reference | … | Reference | … |
| Primary care telephone | 2.87 (1.01–8.15) | .047 | 2.74 (0.94–7.95) | .064 | 0.94 (0.42–2.08) | .876 |
| Urology office | 3.09 (0.71–13.47) | .13 | 2.41 (0.44–13.10) | .309 | 0.94 (0.34–2.61) | .898 |
| Urology telephone | 7.84 (3.18–19.32) | <.001 | 6.34 (2.58–15.56) | <.001 | 2.28 (1.07–4.86) | .033 |
| Interval between UTI, d b | 1 (0.99–1.00) | .100 | … | … | … | .67 |
| No. of UTI visits | 1.08 (1.01–1.14) | .014 | … | … | … | .58 |
| Antibiotic resistance in prior culturec,d | 2.63 (0.71–9.67) | .146 | … | … | … | .60 |
Note. aOR, adjusted odds ratio; CI, confidence interval.
Bold text in the univariate model indicates a P-value < .2 and inclusion in the initial model. Bold text in the multivariate model indicates a significant finding, or a finding with a P-value <0.05.
Includes patients seeking care at family medicine or internal medicine.
Different sample size for β-lactams (n=134)/fluoroquinolones (n=153) compared to overall sample size for β-lactams (n=233)/ fluoroquinolones (n=284) for this variable.
Defined as having a urine culture on the previous visit with resistance to either nitrofurantoin or TMP-SMX.
Different sample size for β-lactams(n=45)/fluoroquinolones (n=54) compared to overall sample size for β-lactams (n=233)/ fluoroquinolones (n=284) for this variable.
Age included in final β-lactam model due to its role as a confounder.
Table 5.
Factors Associated With Antibiotic a Duration in Days Using Generalized Estimating Equations Poisson Regression
| Predictors | Antibiotic Duration in Days | |
|---|---|---|
| Univariate Model | ||
| RR (95%CI) | P Value | |
| Age | 1 (1–1) | .21 |
| Race | ||
| White | Reference | … |
| Black | 0.92 (0.81–1.04) | .20 |
| Other | 1.029 (0.93–1.14) | .57 |
| Elixhauser score | … | .21 |
| Clinical evidence of DM | … | .74 |
| Practice type and number of visit | ||
| Primary careb 2 visits | Reference | … |
| Primary care > 2 visits | 1.09 (0.99–1.19) | .068 |
| Urology 2 visits | 1.11 (0.94–1.33) | .22 |
| Urology >2 visits | 1.28 (1.15–1.44) | <.001 |
| Visit type | ||
| Office visit | Reference | … |
| Telephone | … | .72 |
| Diabetes mellitus | … | .74 |
| Interval between UTI (days) c | … | .27 |
| Prior resistant cultured,e | … | .95 |
Note. RR, relative risk; CI, confidence interval; aOR, adjusted relative risk; DM, diabetes mellitus.
Bold text indicates a significant finding, or a finding with a P-value <0.05.
Includes β-lactams, fluoroquinolones, nitrofurantoin, and TMP-SMX, while excluding ceftriaxone (n=1). When visits contained duplicate entries for same antibiotic (n=10), only 1 instance was used for the analysis.
Includes patients seeking care at family medicine or internal medicine practice.
Different sample size (n=192) compared to the overall sample size (n=330), as no interval available for first visit.
Defined as having a urine culture on the previous visit with resistance to either nitrofurantoin or TMP-SMX.
Different sample size (n=66) compared to overall sample size (n=330); not each visit had a prior visit with susceptibility data.
Results
The rUTI population consisted of 214 unique patients that had 566 visits. The majority were white (63.1%) females that had a median age of 56 (IQR, 40–58) (Table 1). Approximately 19% of patients had evidence of diabetes mellitus; however, the median Elixhauser score was 0 (IQR, 0–0), indicating a relatively healthy population. Patients sought care for UTI a median of 2 times (IQR, 2–3), and two-thirds of those visits transpired in an office setting and one-third via the telephone. Slightly more than half of the rUTI population received care at internal or family medicine practices compared to urology (46%).
Table 1.
Patient Characteristics and Recurrent UTI Visit Details
| Patient Characteristics | Cohort (n=214), No. (%) |
|---|---|
| Age, median (IQR) a | 56 (40–68) |
| Race | |
| White | 135 (63.1) |
| Black | 27 (12.6) |
| Other b | 52 (24.3) |
| Marital status | |
| Single | 62 (29) |
| Married or significant other | 109 (50.9) |
| Divorced/separated/widowed | 32 (15) |
| Other/unknown | 11 (5.1) |
| Language spoken | |
| English | 205 (95.8) |
| Spanish | 8 (3.7) |
| Other | 1 (0.5) |
| Diabetes mellitus | 40 (18.7) |
| Elixhauser score, median (IQR) | 0 (0–0) |
| Visit details | (n=566) |
| Visits per patient, median (IQR) | 2 (2–3) |
| Interval between visits, median d (IQR) | 58 (22–122) |
| Visit type | |
| Office visit | 378 (66.8) |
| Telephone | 188 (33.2) |
| Practice type | |
| Family medicine | 264 (46.6) |
| General internal medicine | 41 (7.2) |
| Urology | 261 (46.1) |
| Symptoms reported c | |
| Dysuria | 196 (73.7) |
| Urgency | 164 (75.6) |
| Frequency | 178 (79.5) |
| Hematuria | 39 (26.5) |
| Incontinence | 28 (68.3) |
Note. UTI, urinary tract infection; IQR, interquartile range.
Age from first rUTI visit in study period.
Other includes Asian (n=10), American Indian (n=1), Hispanic (n=19), and unknown (n=22).
The percentage of reported symptoms was calculated based on visits that had explicit documentation of the patient’s symptoms (either as present or absent).
Antibiotic choice details
Overall, 61.5% of patients received a first-line agent and 27.2% had a fluoroquinolone prescribed for episodic rUTI (Table 2). Nitrofurantoin was the most common first-line drug prescribed (39.7%) followed by TMP-SMX (21.5%), while fosfomycin was prescribed only once. β-lactams comprised 11% of prescribed antibiotics and one patient received an intravenous ceftriaxone dose in addition to oral antibiotics. Prophylactic therapy was more aligned with rUTI treatment recommendations, with 93.2% of antibiotics falling within a recommended category.
Table 2.
Descriptive Summary of Antibiotics Prescribed to Patients With Recurrent UTI by Episodic and Prophylactic Treatment
| Antibiotics Prescribed a | Total (n=395) | Episodic (n=335) d | Prophylactic (n=44) e |
|---|---|---|---|
| β-lactams | 59 (14.9) | 37 (11) | 3 (6.8) |
| Amoxicillin | 7 (1.8) | 4 (1.2) | 3 (6.8) |
| Amoxicillin-clavulanate | 13 (3.3) c | 11 (3.3) | … |
| Ampicillin | 1 (0.3) | 1 (0.3) | … |
| Cefpodoxime | 3 (0.8) c | 1 (0.3) | … |
| Ceftriaxone | 1 (0.3) | 1 (0.3) | … |
| Cefuroxime | 11 (2.8) | 11 (3.3) | … |
| Cephalexin b | 23 (5.8) c | 8 (2.4) | NA |
| Fluroquinolones | 95 (24.1) | 91 (27.2) | … |
| Ciprofloxacin | 84 (21.3) c | 81 (24.2) | … |
| Levofloxacin | 11 (2.8) c | 10 (3) | … |
| First-line treatments | 240 (60.8) | 206 (61.5) | 41 (93.2) |
| Fosfomycin | 1 (0.3) | 1 (0.3) | … |
| Nitrofurantoin | 163 (41.3) c | 133 (39.7) | 25 (56.8) |
| TMP-SMX | 76 (19.2) c | 72 (21.5) | 2 (4.6) |
| Cephalexin b | NA | NA | 14 (31.8) |
| Other | 1 (0.3) | 1(0.3) | |
| Doxycycline | 1 (0.3) | 1 (0.3) | … |
Note. NA, not applicable.
Excluded second-line antibiotics prescribed to patients with allergies to first-line agents (nitrofurantoin and TMP-SMX).
Cephalexin is considered a first-line therapy for prophylactic treatment; therefore, in column 4, it is included as part of ‘first-line treatments’ and excluded from ‘β-lactams.’
Contains observations with an unknown length of treatment.
Episodic antibiotic therapy defined as treatment duration ≤14 d.
Prophylactic therapy defined as antibiotic duration >14 d.
Antibiotic duration details
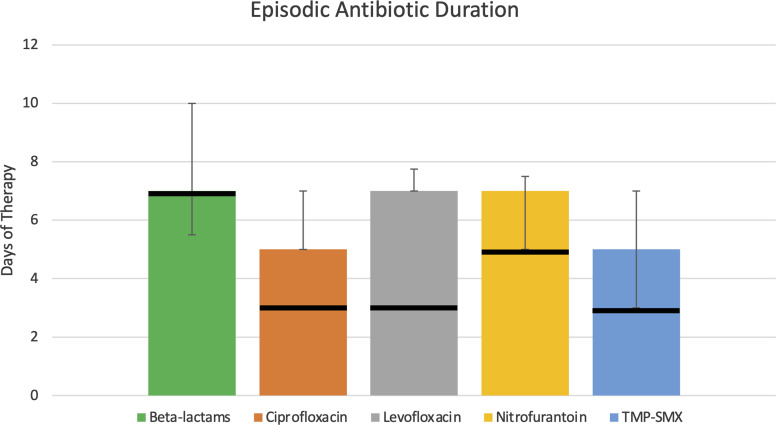
Duration of therapy also exceeded IDSA guideline recommendations, with the median duration eclipsing the 3-day cutoff points for fluoroquinolones and TMP-SMX, and 5-day threshold for nitrofurantoin (Fig. 2). Ciprofloxacin, levofloxacin, TMP-SMX, and nitrofurantoin prescriptions had inappropriate durations in 85.2%, 100%, 72.2%, and 60.2% of cases, respectively.
Fig. 2.
Episodic antibiotic duration—a bar chart depicting the median length of therapy by antibiotic class or drug for episodic rUTI treatment. Error bars represent first and third quartiles, and solid black lines represent recommended duration of therapy according to the Infectious Disease Society of America guidelines for uncomplicated cystitis. 7
Nonantibiotic therapies
Nonantibiotic therapies were prescribed to a lesser extent. Only 11.7% of eligible females had vaginal estrogen therapy prescribed. Other agents less frequently prescribed included nonopioid pain relievers (7.2%), bladder antispasmodics (3.2%), cranberry products, and probiotics (0.9%) (Supplementary Table 1).
Urine cultures obtained and predictors
Only 52% of visits had an accompanying urine culture. In a sensitivity analysis evaluating a patient’s second visit or later, only 44.8% of visits had a culture. Visit-associated cultures were highest for office visits (66.4%) but were lower among telephone visits (22.9%). Urine cultures collected also varied by practice type: internal medicine had the lowest percentage (34.1%), followed by urology (42.5%). Also, 64% of family medicine visits had a documented culture. Regression modeling found seeking care at internal medicine, regardless of visit type, increased the risk of lacking a urine culture; however, telephone visits across all practice types displayed higher relative risks compared to office encounters (Table 3). In the univariate analysis, patients within ∼2 months of their prior visit had a 26% higher risk of not having a urine culture; however, this association became nonsignificant in the multivariate model (P = .074).
Table 3.
Factors Associated With Lacking a Visit-Associated Urine Culture Using Generalized Estimating Equations Poisson Regression
| Predictors | Urine Culture Ordering | |||
|---|---|---|---|---|
| Univariate Model | Multivariate Modela | |||
| RR (95% CI) | P Value | aRR (95% CI) | P Value | |
| Age | 1 (1–1.01) | .17 | … | … |
| Elixhauser score | … | .73 | … | … |
| Race | ||||
| White | Reference | … | Reference | … |
| Black | … | .86 | … | … |
| Other | … | .64 | … | … |
| Practice and visit type | ||||
| FM office | Reference | … | Reference | … |
| FM telephone | 2.99 (2.27–3.94) | <.001 | 2.38 (1.73–3.26) | <.001 |
| IM office | 2.24 (1.45–3.45) | <.001 | 2.09 (1.35–3.24) | .001 |
| IM telephone | 2.89 (1.98–4.20) | <.001 | 2.43 (1.72–3.43) | <.001 |
| Urology office | 1.55 (1.13–2.13) | .007 | 1.43 (0.99–2.07) | .055 |
| Urology telephone | 2.95 (2.32–3.76) | <.001 | 2.58 (1.97–3.38) | <.001 |
| Interval between UTI a | ||||
| >56 d | Reference | … | Reference | … |
| ≤56 d | 1.26 (1.02–1.54) | .03 | 1.18 (0.98–1.43) | .074 |
| No. of UTI visits | … | .25 | … | … |
| Antibiotic resistance in prior cultureb,c | … | .97 | … | … |
Note. aRR, adjusted relative risk; FM, family medicine; IM, internal medicine.
Bold text indicates a significant finding, or a finding with a P-value <0.05.
Different sample size (n=362) compared to overall sample size (n=566) for this variable/model.
Defined as having a urine culture on the previous visit with resistance to either nitrofurantoin or TMP-SMX.
Different sample size (n=111) compared to overall sample size (n=566) for this variable.
Predictors of second-line therapies
Telephone visits with urology significantly increased the odds of β-lactam and fluoroquinolone prescribing by 6.34 and 2.28 times, respectively, but urology office visits did not (Table 4). In the univariate analysis, primary-care telephone visits were also associated with β-lactam prescribing but became nonsignificant in the final model. Contrary to our expectations, we did not find significant associations between prior resistance to a first-line agent, decreased intervals between UTIs or more frequent UTIs, and prescribing second-line agents.
Predictors of antibiotic duration
Having 3 or more visits with urology increased therapy days by 28% (95% CI, 1.15–1.44), but this was not the case for primary care patients with 3 or more visits (OR, 1.09; 95% CI, 0.99–1.19) or urology patients with 2 visits (OR, 1.11; 95% CI, 0.94–1.33) (Table 5). We found no evidence to support our hypothesis that a shorter interval between visits (OR, 1; 95% CI, 1–1) was associated with longer duration of therapy.
Discussion
Our study revealed moderate concordance (61.5%) with first-line agent prescribing; however, fluoroquinolones were prescribed in 27% of visits and almost 90% exceeded the 3-day recommended duration. Overall, only 21.5% of prescriptions consisted of a first-line agent prescribed for the guideline-concordant duration. Lack of concordance with choice of drug and duration of therapy increases opportunities for antibiotic resistance and adverse drug events. For example, Chalmers et al 19 found each additional day of antibiotics increased the risk of C. difficile infection by 9% and a meta-analysis found antibiotic courses >3 days significantly increased the risk of adverse drug reactions by 17%. 19,20 Meanwhile, patients with trimethoprim courses >7 days had 2.89 higher odds of developing resistance than those with regimens <7 days (95% CI, 1.44–5.78). 19–21
In terms of management, only 52% of visits had an accompanying urine culture result. The 2018 AUS best-practice statement and others advocate for urine culture ordering to establish susceptibility information and confirm rUTI diagnosis. 5,6,10 The uropathogen sensitivity data are key to ensure antibiotics with congruent susceptibility profiles are selected, especially with heightened levels of antibiotic resistance from selective pressure induced by repeated antibiotic therapy. Two recent studies detected uropathogen-antibiotic susceptibility mismatch in 31% and 40% of patients presenting at emergency departments (EDs) for a UTI. 22,23 In one of the studies, uropathogen-cephalexin susceptibility mismatch significantly increased the odds of ED readmission. 22 This finding underscores the potential for drug–pathogen susceptibility mismatches, which can delay appropriate treatment with the targeted drug, increase healthcare utilization, and associated costs.
A positive urine culture helps confirm a UTI, and a negative culture should prompt consideration of other diagnoses. In a prospective study, 22% of catheterized and mid-stream urine samples from adult females symptomatic for UTI had no uropathogen growth (0 colony forming units (CFU)/mL). 24 Tomas et al 25 analyzed urine cultures from ED patients diagnosed with UTI and found that only 48% had a positive culture (≥103 CFU/mL). In addition, 37% of those with sexually transmitted infections were misdiagnosed as UTIs. 25 Thus, a negative urine culture could facilitate treatment of the underlying cause and prevent the patient from receiving inappropriate antibiotics.
Factors associated with lacking a urine culture included seeking care at internal medicine or having telephone visits at urology or family medicine practices. Having a telephone visit compared to an office visit in each setting further increased the risk of lacking a culture by an additional 238%, 34%, and 115% in family medicine, internal medicine, and urology clinics, respectively. Ewen et al 26 examined telephone prescribing practices in ambulatory settings and found the most common indication for antibiotic prescribing was for UTI, and >75% of antibiotics were prescribed empirically without a culture, which supports our findings. Murray et al 27 found significantly lower levels of urine culture ordering for episodic UTI in an RN-led telephone treatment protocol (7%), compared to face-to-face visits (21%). However, the relationship between decreased urine cultures and practice type is not clear.
Telephone visits with urology were significantly associated with having a β-lactam or fluoroquinolone ordered. Ewen et al 26 found telephone visits compared to office visits had significantly higher levels of fluoroquinolones prescribed overall, and fluoroquinolones were most frequently prescribed for telephone based-UTI treatment. Interestingly, herein, the relationship between telephone visits and prescribing second-line agents only demonstrated significance in urology. Urology practices may attract patients that suffer from rUTI over longer periods, and providers may opt for a second-line agent if a first-line agent fails to provide long-term resolution. Furthermore, patients with a longer history of rUTI may opt to call clinicians rather than schedule an office visit.
In terms of antibiotic duration, patients with more frequent rUTIs that sought care at a urology practice had significantly longer therapy duration. Urology practices may attract patients that experience rUTIs over longer periods of time. This, coupled with more frequent rUTIs may spur providers to extend therapy duration to reverse that patient’s disease course. Thus, the confluence of these factors may prompt providers to extend therapy in this subset of patients; urology patients with 2 rUTIs or primary care patients with >2 rUTIs did not have significantly longer therapy.
Very few women received vaginal estrogen therapy, even though it can significantly lower rUTI recurrence. 5,6 Estrogen plays myriad of beneficial roles in promoting eubiotic effects that hamper colonization with uropathogenic bacteria. 28–30 Thus, incorporating vaginal estrogen therapy into practice may be a target for stewardship as an antibiotic-sparing means to prevent rUTI among perimenopausal and postmenopausal women.
Although our findings stem from a single academic medical center, we considered 4 clinics representing 3 different medical fields: family medicine, internal medicine, and urology. Use of an electronic health record database can be prone to diagnosis coding inconsistences or incomplete charting. Incomplete ICD-10 coding may have created information bias because the extent of comorbidities may have been underreported, potentially underestimating Elixhauser scores and obscuring their relationship with prescribing outcomes. We mitigated these effects by conducting a manual chart review to examine the problem list and medical history for evidence of complicated UTI or chronic kidney disease. We also captured the presence of UTI symptoms, lowering the probability of including patients with asymptomatic bacteriuria; however, explicit affirmation or denial of symptoms were not available for all visits. Our urine culture variable depends not only on a written order but also the patient fulfilling the request. Thus, this outcome may reflect shortcomings in terms of ordering and in a patient’s inability or failure to submit a specimen. Medications captured only represented orders and medications listed from that specific visit. Therefore, nonantibiotic therapies may have been underrepresented if ordered through a different provider. Lastly, we did not capture other factors that may contribute to prescribing practices, such as provider type (MD vs DO vs NP) or years in practice, due to anonymity.
In conclusion, we conducted the first examination of rUTI prescribing practices in a US outpatient setting. Fewer than 25% of prescriptions consisted of a first-line agent at the guideline-recommended duration. We detected suboptimal levels of visit-associated urine cultures, which could lead to delayed or missed treatment for other comorbidities that mimic UTI symptomology or to uropathogen antibiotic-susceptibility mismatch. Telephone encounters across all practice types were associated with decreased urine cultures obtained, and telephone visits with urology were associated with both β-lactam and fluoroquinolone prescribing. Thus, these settings and visit types are potential stewardship targets. Resistance or allergies to first-line agents did not explain second-line agent selection, indicating that these prescribing practices can change. Overall, our study has demonstrated opportunities to further antibiotic stewardship and to improve management among rUTI patients.
Acknowledgments
Rebiotix, a Ferring Company, provided input for study inclusion and exclusion criteria.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/ash.2021.224.
click here to view supplementary material
Financial support
M. Valentine-King is supported in part by the Health Resources and Services Administration, an agency of the US Department of Health and Human Services (grant no. T32 HP1003). This investigator-initiated research study was funded by Rebiotix.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
References
- 1. Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015;13:269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol 2000;151:1194–1205. [DOI] [PubMed] [Google Scholar]
- 3. Ikaheimo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis 1996;22:91–99. [DOI] [PubMed] [Google Scholar]
- 4. Ellis AK, Verma S. Quality of life in women with urinary tract infections: is benign disease a misnomer? J Am Board Fam Pract 2000;13:392–397. [DOI] [PubMed] [Google Scholar]
- 5. Gupta K, Trautner BW. Diagnosis and management of recurrent urinary tract infections in nonpregnant women. BMJ 2013;346:f3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brubaker L, Carberry C, Nardos R, Carter-Brooks C, Lowder JL. American Urogynecologic Society best-practice statement: recurrent urinary tract infection in adult women. Female Pelvic Med Reconstr Surg 2018;24:321–335. [DOI] [PubMed] [Google Scholar]
- 7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011;52:e103–e120. [DOI] [PubMed] [Google Scholar]
- 8. Tanne JH. FDA adds “black box” warning label to fluoroquinolone antibiotics. BMJ 2008;337:a816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FDA reinforces safety information about serious low blood sugar levels and mental health side effects with fluoroquinolone antibiotics; requires label changes. US Food and Drug Administration website. https://www.fda.gov/drugs/drug-safety-and-availability/fda-reinforces-safety-information-about-serious-low-blood-sugar-levels-and-mental-health-side. Published 2018. Accessed December 15, 2021.
- 10. Anger J, Lee U, Ackerman AL, et al. Recurrent uncomplicated urinary tract infections in women: AUA/CUA/SUFU guideline. J Urol 2019;202:282–289. [DOI] [PubMed] [Google Scholar]
- 11. Cowart K, Worley M, Rouby NE, Sando K. Evaluation of FDA boxed warning on prescribing patterns of fluoroquinolones for uncomplicated urinary tract infections. Ann Pharmacother 2019;53:1192–1199. [DOI] [PubMed] [Google Scholar]
- 12. Durkin MJ, Keller M, Butler AM, et al. An assessment of inappropriate antibiotic use and guideline adherence for uncomplicated urinary tract infections. Open Forum Infect Dis 2018;5:ofy198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bratsman A, Mathias K, Laubscher R, Grigoryan L, Rose S. Outpatient fluoroquinolone prescribing patterns before and after US FDA boxed warning. Pharmacoepidemiol Drug Saf 2020;29:701–707. [DOI] [PubMed] [Google Scholar]
- 14. Grigoryan L, Zoorob R, Wang H, Trautner BW. Low concordance with guidelines for treatment of acute cystitis in primary care. Open Forum Infect Dise 2015;2:ofv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hisano M, Bruschini H, Nicodemo AC, Gomes CM, Lucon M, Srougi M. The bacterial spectrum and antimicrobial susceptibility in female recurrent urinary tract infection: how different they are from sporadic single episodes? Urology 2015;86:492–497. [DOI] [PubMed] [Google Scholar]
- 16. Herness J, Buttolph A, Hammer NC. Acute pyelonephritis in adults: rapid evidence review. Am Fam Physician 2020;102:173–180. [PubMed] [Google Scholar]
- 17. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser comorbidity index. Med Care 2017;55:698–705. [DOI] [PubMed] [Google Scholar]
- 18. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Statist Softw 2005;15:11. doi: 10.18637/jss.v015.i02. [DOI] [Google Scholar]
- 19. Chalmers JD, Akram AR, Singanayagam A, Wilcox MH, Hill AT. Risk factors for Clostridium difficile infection in hospitalized patients with community-acquired pneumonia. J Infect 2016;73:45–53. [DOI] [PubMed] [Google Scholar]
- 20. Katchman EA, Milo G, Paul M, Christiaens T, Baerheim A, Leibovici L. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med 2005;118:1196–1207. [DOI] [PubMed] [Google Scholar]
- 21. Hillier S, Roberts Z, Dunstan F, Butler C, Howard A, Palmer S. Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection: a case-control study. J Antimicrob Chemother 2007;60:92–99. [DOI] [PubMed] [Google Scholar]
- 22. Jorgensen S, Zurayk M, Yeung S, et al. Emergency department urinary antibiograms differ by specific patient group. J Clin Microbiol 2017;55:2629–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Torres EL, Cantu JR, Bazan DZ, Verduzco RA, Hernández-Muñoz JJ. Travel to Mexico and uropathogen-antibiotic susceptibility mismatch in the emergency department. Am J Emerg Med 2021;46:619–624. [DOI] [PubMed] [Google Scholar]
- 24. Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med 2013;369:1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015;53:2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ewen E, Willey VJ, Kolm P, McGhan WF, Drees M. Antibiotic prescribing by telephone in primary care. Pharmacoepidemiol Drug Saf 2015;24:113–120. [DOI] [PubMed] [Google Scholar]
- 27. Murray MA, Penza KS, Myers JF, Furst JW, Pecina JL. Comparison of evisit management of urinary symptoms and urinary tract infections with standard care. Telemed J e-health 2020;26:639–644. [DOI] [PubMed] [Google Scholar]
- 28. Amabebe E, Anumba DOC. The vaginal microenvironment: the physiologic role of lactobacilli. Front Med 2018;5:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lüthje P, Brauner H, Ramos NL, Ovregaard A, et al. Estrogen supports urothelial defense mechanisms. Sci Transl Med 2013;5:190ra80. [DOI] [PubMed] [Google Scholar]
- 30. Stanton A, Mowbray CA-O, Lanz M, et al. Topical estrogen treatment augments the vaginal response to Escherichia coli flagellin. Sci Rep 2020;10:8473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/ash.2021.224.
click here to view supplementary material