Abstract
O-antigen-specific monoclonal antibodies were generated against Acinetobacter strains from international type culture collections and characterized by enzyme immunoassay and Western and colony blotting. The antibodies aid in the further completion of an O-serotyping scheme for Acinetobacter and, due to their high specificity, are especially useful to all working with these strains.
Strains pertaining to the genus Acinetobacter are ubiquitous in nature (7) but are recognized as being important opportunistic pathogens within the hospital environment as well (6). We have recently started to generate and serologically characterize monoclonal antibodies (MAb) against Acinetobacter O antigens, with the aim to develop an O-serotype-based identification scheme for this group of bacteria. To date, we have focused mainly on the generation of MAb against the clinically more important Acinetobacter species, i.e., A. baumannii (genomic species 2), unnamed genomic species 3, and unnamed genomic species 13 sensu Tjernberg and Ursing. The antibodies described so far have been generated using clinical isolates (3, 4); since we are interested in developing a serotyping scheme for the whole genus, we have expanded our studies also to those strains which are at present of less biomedical relevance. In this study, we report on O-antigen-specific MAb which were generated against Acinetobacter strains obtained from international type culture collections.
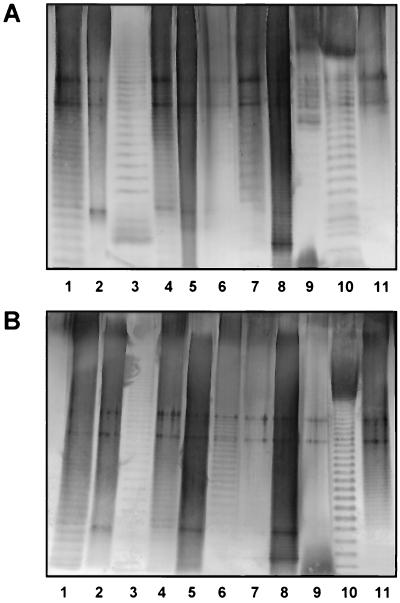
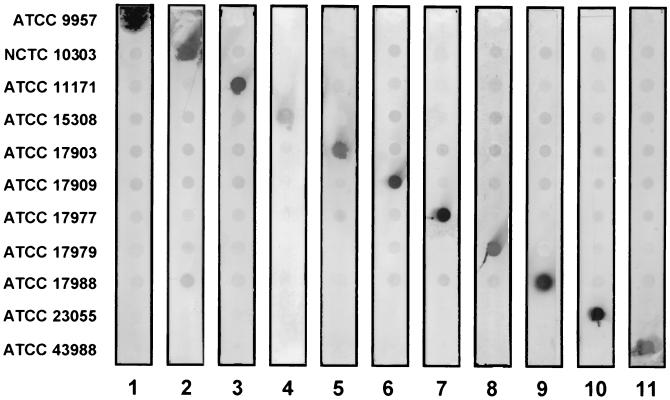
Strains (n = 11) (Table 1) belonging to various Acinetobacter genomic species were purchased from the American Type Culture Collection (Manassas, Va.) and the National Collection of Type Cultures (London, United Kingdom). Extraction of bacterial lipopolysaccharides (LPS), preparation of whole-cell lysates, proteinase K digestion, enzyme immunoassays (EIA), and Western blotting were performed as described earlier (3, 5); colony blotting was performed as described in another study (1). MAb were generated by inoculating BALB/c mice with heat-killed (1 h at 100°C) bacteria according to an immunization protocol described previously (3), except that booster injections were given intravenously. The generation of MAb S53-1 and S53-32 directed against the O-antigen of Acinetobacter strain ATCC 23055 and NCTC 10303, respectively, has been reported recently (R. Pantophlet, J. A. Severin, A. Nemec, L. Brade, L. Dijkshoorn, and H. Brade, submitted for publication). For each immunizing antigen, one hybridoma with good reactivity in EIA (Table 1) was subjected to limiting dilution (three times) to achieve monoclonality. The MAb so obtained were isotyped with a commercially available kit (Bio-Rad) and purified by affinity chromatography on protein G (Pharmacia). Three MAb were of the immunoglobulin G1 (IgG1) isotype; two MAb were of the IgG2a and IgG2b isotype, respectively; and six MAb were of the IgG3 isotype. Purity was ascertained by Coomassie staining of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (data not shown). The specificity of the antibodies was determined by EIA using isolated LPS as solid-phase antigen (5 μg/ml; 50 μl/well). They reacted (optical density at 405 nm [OD405] > 0.2) at concentrations between 2 and 250 ng/ml with the homologous antigen (Table 1). No heterologous reactivity was observed (MAb concentration yielding an OD405 of >0.2, >50,000 ng/ml). The specificity of all MAb for the homologous O antigen was verified by Western blotting with proteinase K-treated lysates as well as with isolated LPS using a 10% separating gel (Fig. 1). For 9 of the 11 MAb, the characteristic banding pattern of LPS possessing an O-polysaccharide chain could be observed (Fig. 1A). For strain ATCC 9957 (Fig. 1, lanes 6) and strain NCTC 10303 (Fig. 1, lanes 11), a better resolution of banding patterns could be achieved when isolated LPS instead of proteinase K-digested whole-cell lysates were used (Fig. 1B). Less distinct patterns were observed for Acinetobacter strains ATCC 17977 (Fig. 1, lanes 2) and ATCC 43998 (Fig. 1, lanes 5), indicating that these strains may possess an O antigen with repeating units of relatively small size. The absence of O-antigen-specific bands is not unusual and has also been observed with other Acinetobacter strains in a previous study (5). To determine whether these antibodies are also useful in simple screening experiments using crude antigen mixtures, colony blotting was performed (Fig. 2). As in the EIA, no heterologous reactivity was observed, thus confirming the high specificity of these antibodies for their respective homologous antigens.
TABLE 1.
MAb used in this study and reactivities with homologous and heterologous Acinetobacter LPS in EIA
| MAb | Immunogena (genomic species) | Isotype | Final concn of MAb (ng/ml) yielding OD405 of >0.2 with LPS of Acinetobacter strain:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 23055T | ATCC 17977 | ATCC 17903T | ATCC 15308 | ATCC 43998 | ATCC 9957T | ATCC 17909T | ATCC 17979T | ATCC 11171T | ATCC 17988T | NCTC 10303 | |||
| S53-1 | ATCC 23055T (1) | IgG3 | 63 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 |
| S53-10 | ATCC 17977 (4) | IgG2a | >50,000 | 63 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 |
| S53-11 | ATCC 17903T (13TUc) | IgG1 | >50,000 | >50,000 | 8 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 |
| S53-13 | ATCC 15308 (2) | IgG3 | >50,000 | >50,000 | >50,000 | 250 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 |
| S53-16 | ATCC 43998 (12) | IgG1 | >50,000 | >50,000 | >50,000 | >50,000 | 4 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 |
| S53-19 | ATCC 9957T (9) | IgG2b | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | 2 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 |
| S53-20 | ATCC 17909T (7) | IgG3 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | 8 | >50,000 | >50,000 | >50,000 | >50,000 |
| S53-23-3 | ATCC 17979T (6) | IgG3 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | 63 | >50,000 | >50,000 | >50,000 |
| S53-23-6 | ATCC 11171T (11) | IgG3 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | 8 | >50,000 | >50,000 |
| S53-25 | ATCC 17988T (16) | IgG1 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | 16 | >50,000 |
| S53-32 | NCTC 10303 (2) | IgG3 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | >50,000 | 125 |
BALB/c mice were immunized with heat-killed bacteria.
EIA plates were coated with purified LPS at a concentration of 5 μg/ml (50 μl/well); results of homologous reactions are boldfaced.
Unnamed genomic species 13 sensus Tjernberg and Ursing.
FIG. 1.
Reactivity of proteinase K-treated bacterial lysates (A) and isolated LPS (B) from Acinetobacter strains investigated in this study with homologous MAb on a Western blot following Sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% resolving gel and transfer onto a polyvinylidene difluoride membrane. Lanes: 1, strain ATCC 23055; 2, strain ATCC 17977; 3, strain ATCC 17903; 4, strain ATCC 15308; 5, strain ATCC 43998; 6, strain ATCC 9957; 7, strain ATCC 17909; 8, strain ATCC 17979; 9, strain ATCC 11171; 10, strain ATCC 17988; 11, strain NCTC 10303.
FIG. 2.
Reactivity of MAb with bacteria in colony blots. Bacteria were grown for 2 h on agar plates in contact with nitrocellulose which was then developed with the respective MAb. Strain numbers are indicated on the left. Lane 1, S53-19; lane 2, S53-32; lane 3, S53-23-6; lane 4, S53-13; lane 5, S53-11; lane 6, S53-20; lane 7, S53-10; lane 8, S53-23-3; lane 9, S53-25; lane 10, S53-1; lane 11, S53-16.
The increased recognition of members of the genus Acinetobacter as nosocomial pathogens (6) has led to a search for practical and reliable identification methods for Acinetobacter strains (2) that may be implemented in clinical diagnostic laboratories. The successful use of LPS, specifically of the O-antigenic moiety, as a taxonomic marker for numerous gram-negative bacteria has led us to pursue the possibility of developing a serotype-based identification scheme for Acinetobacter as well. Although our focus has been mainly on the generation of O-antigen-specific MAb against species which are currently considered to be clinically of most importance, we are also interested in the generation of antibodies against the other species. In this study, MAb were generated against the O polysaccharides of the LPS of Acinetobacter reference strains obtained from two international type culture collections. The antibodies were found to be highly specific for the homologous O antigen, independent of the assay employed: EIA with purified LPS, Western blotting with proteinase K-treated bacterial lysates, or colony blotting with whole bacteria gave comparable results. The antibodies are therefore not only useful for the further development of an O-serotyping scheme for Acinetobacter but also are useful for bacteriologists and biotechnologists working with these reference strains. These MAb will certainly also be of use to researchers who wish to study LPS biosynthesis, particularly of the O antigen, in Acinetobacter.
Acknowledgments
We thank M. Willen, A. Denzin, U. Agge, and V. Susott for technical assistance.
REFERENCES
- 1.Brade L, Grimmecke H-D, Holst O, Brabetz W, Zamojski A, Brade H. Specificity of monoclonal antibodies against Escherichia coli K-12 lipopolysaccharide. J Endotox Res. 1996;3:39–47. [Google Scholar]
- 2.Dijkshoorn L. Acinetobacter—microbiology. In: Bergogne-Bérézin E, Joly-Guillou M L, Towner K J, editors. Acinetobacter: microbiology, epidemiology, infections, management. Boca Raton, Fla: CRC Press, Inc.; 1996. pp. 37–69. [Google Scholar]
- 3.Pantophlet R, Brade L, Brade H. Identification of Acinetobacter baumannii strains with monoclonal antibodies against the O-antigen of their lipopolysacchairde. Clin Diagn Lab Immunol. 1999;6:323–329. doi: 10.1128/cdli.6.3.323-329.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantophlet R, Brade L, Brade H. Use of a murine O-antigen-specific monoclonal antibody to identify Acinetobacter strains of unnamed genomic species 13 sensu Tjernberg and Ursing. J Clin Microbiol. 1999;37:1693–1698. doi: 10.1128/jcm.37.6.1693-1698.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pantophlet R, Brade L, Dijkshoorn L, Brade H. Specificity of rabbit antisera against lipopolysaccharide of Acinetobacter. J Clin Microbiol. 1998;36:1245–1250. doi: 10.1128/jcm.36.5.1245-1250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Towner K J. Clinical importance and antibiotic resistance of Acinetobacter spp. J Med Microbiol. 1997;46:721–746. doi: 10.1099/00222615-46-9-721. [DOI] [PubMed] [Google Scholar]
- 7.Towner K J, Bergogne-Bérézin E, Fewson C A. Acinetobacter: portrait of a genus. In: Towner K J, Bergogne-Bérézin E, Fewson C A, editors. The biology of Acinetobacter: taxonomy, clinical importance, molecular biology, physiology, industrial relevance. New York, N.Y: Plenum Press; 1991. pp. 1–24. [Google Scholar]