Abstract
Introduction:
Alterations in blood glucose levels are common and an important determinant of a patient’s admission outcomes, point-of-care glucometers, which are affected by a variety of factors, are increasingly used in clinical care. In this study we compared blood glucose levels determined by two commonly used glucometers (One Touch® and Accu-check®) with those of a standard laboratory method and determined the effect of haematocrit on glucose readings
Methods:
Blood glucose levels were measured with One Touch® and Accu-Check® glucometers and the glucose oxidase method at the same time in 295 children aged 0 to 15 years over a 6-month period. Bland-Altman and correlation analysis were used to explore biases among the three methods. For all statistical tests, a p-value of less than 0.05 was considered statistically significant.
Results:
Most were males (51.2%) and the median (range) age was 1 year (1 day, 12 years). There was a significant correlation between each of the glucometer methods and laboratory blood sugar, and the correlation between the two glucometers was strong and significant. This correlation remained statistically significant even after controlling for haematocrit values. There was an acceptable level of bias (3.9 mg/dL) between the One Touch® and Accu-check® glucometers, but each had a remarkably large bias compared with the glucose oxidase method.
Conclusion:
The use of a tested glucometer in clinical settings can aid in rapid decision-making, but there is a need to periodically cross-check with the glucose oxidase method in the laboratory to optimise treatment outcomes for children with dysglycaemia.
Keywords: Comparison, glucometers, glucose, laboratory-based glucose oxidase test
Introduction
Alteration in glucose homeostasis is a common metabolic emergency especially in the neonatal unit and the Children’s emergency room and is an important determinant of patient outcomes.1–6 In Nigeria, the prevalence of hypoglycaemia among children admitted into a typical paediatric emergency ward could be as high as 6.2% and it is commonly associated with severe malaria, septicaemia, pneumonia, and protein energy malnutrition.7 To prevent severe complications and improve patient outcomes, it is necessary to reliably determine the blood glucose levels as quickly as possible and institute intervention when needed. Point of care testing using the glucose meters have become increasingly available in most clinical settings and it plays significant role in patient management. The use of glucose meter requires very little blood sample and give immediate results expediting the decision-making process.
In most developing countries glucose meters are increasingly available and becoming a part of standard of care in paediatric emergency rooms and neonatology unit. These devices are fraught with errors which can lead to wrong clinical judgment and interventions.8 In a study to evaluate accuracy of 27 glucose meters Freckmann et al8 found 40% of them did not meet the required standards. Similar findings have been reported by other investigators and some inaccuracies have been associated with dire consequences on patient health and indeed some fatalities.9 In addition, glucose meter readings are affected by factors like haematocrit, hypoxia, ambient temperature, and hypotension amongst others.1,10,11
With the increasing availability of glucose meters in developing countries we evaluated the accuracy of two commonly used glucose meters in hospitals in Nigeria, Accu-Check® and One-Touch®, compared to standard laboratory-based glucose oxidase method. We also investigated the effect of haematocrit on the glucose readings
Methods
Study Design and Subjects
In a cross-sectional design, we enrolled neonates and children less than 15 years old over a 6-month period, presenting to the emergency room and outpatient clinics after parental consent. The settings of this study were the children’s emergency and outpatient clinics of two large hospitals, University College Hospital and Adeoyo Maternity Teaching Hospital, in Ibadan, Southwest Nigeria.
Glucose and Haematocrit Measurements
For each study participant, 1–1.5 mls of blood was collected into bottles with fluoride oxalate and blood glucose readings were obtained using two glucose meters, One-Touch® (OT) and Accu-check® (AC) glucometers. Two readings were obtained from each glucometer, and the average was calculated. The remaining blood sample was used to obtain reference blood glucose in the laboratory using the standard glucose oxidase method. The glucose oxidase (GO) based random plasma glucose (RPG) results were available for 173 children and 295 for both Accu-Check® and OT. Venous blood was collected into two capillary tubes for haematocrit assessment and the average of the two samples was taken. The packed cell volume (PCV) results were available for 265 patients.
Data analysis
The blood sugar values obtained from the glucose oxidase method laboratory were regarded as the gold standard against which the results of the two glucometers were compared. Bland-Altman plots were used to graphically display the bias and 95% confidence interval (CI) of the results of the two glucometers from the laboratory method (gold standard). In addition, a Pearson correlation analysis was carried out to examine relationships among the three methods, while a partial correlation was carried out to explore the effect of haematocrit levels on the relationships. The statistical package SPSS for Windows version 16.0 (SPSS Inc, Chicago, IL, USA) was used to analyse the data. All statistical tests were two-tailed, and a p-value of less than 0.05 was considered statistically significant.
Ethical Consideration
The study was approved by the University of Ibadan/University College Hospital, Ibadan joint Ethics Review Committee and done in line with the Helsinki Declaration of 1975, as updated in 2000.
Results
Of the 295 children who participated in the study, 144 (48.8%) were females; median age was 1 year (range = 1 day to 12 years). The RPG ranges for glucose oxidase method were 33 mg/dl to 169 mg/dl, Accu-Check® was 15 mg/dl to 337 mg/dl, and OT was 43 mg/dl to 356 mg/dl. The mean glucose oxidase method, Accu-Check®, and One-Touch® RPG were respectively 87.7 mg/dl (20.3), 94.1 mg/dl (28.2), and 98.4 mg/dl (30.4). The packed cell volume (PCV) results ranged from 19%–69% with a mean (SD) of 38.7% (9.3).
There was a significant correlation between RPG levels obtained from each of the glucometer methods and the glucose oxidase method, while the correlation between Accu-Check® and One-Touch® values was strong and significant (Table 1). Even when haematocrit values were considered, the correlation between each glucometer method and lab blood sugar remained statistically significant.
Table 1:
Correlation between blood sugar levels obtained by laboratory method and glucometers.
| Method | Pearson Correlations | Partial Correlations | ||||||
|---|---|---|---|---|---|---|---|---|
| Laboratory | One-touch | Laboratory | One-touch | |||||
| r | p | r | p | r | P | r | p | |
| Accu-check | 0.513 | <0.001 | 0.885 | <0.001 | 0.502 | <0.001 | 0.892 | <0.001 |
| One-touch | 0.546 | <0.001 | 0.537 | <0.001 | ||||
Pair-wise assessment of agreements between methods
Bland Altman plots of difference versus average of RPG levels from laboratory and Accu-check® shows the mean of differences (bias) between Accu-check® and laboratory values readings was 14.3 mg/dl (95% limits of agreement = −17.0 to 45.6) as shown in Figure 1. There were 5.8% (10 out 173) of the readings outside the limit of agreement. The average lies between 43.0 mg/dl and 174.0 mg/dl.
Fig 1:
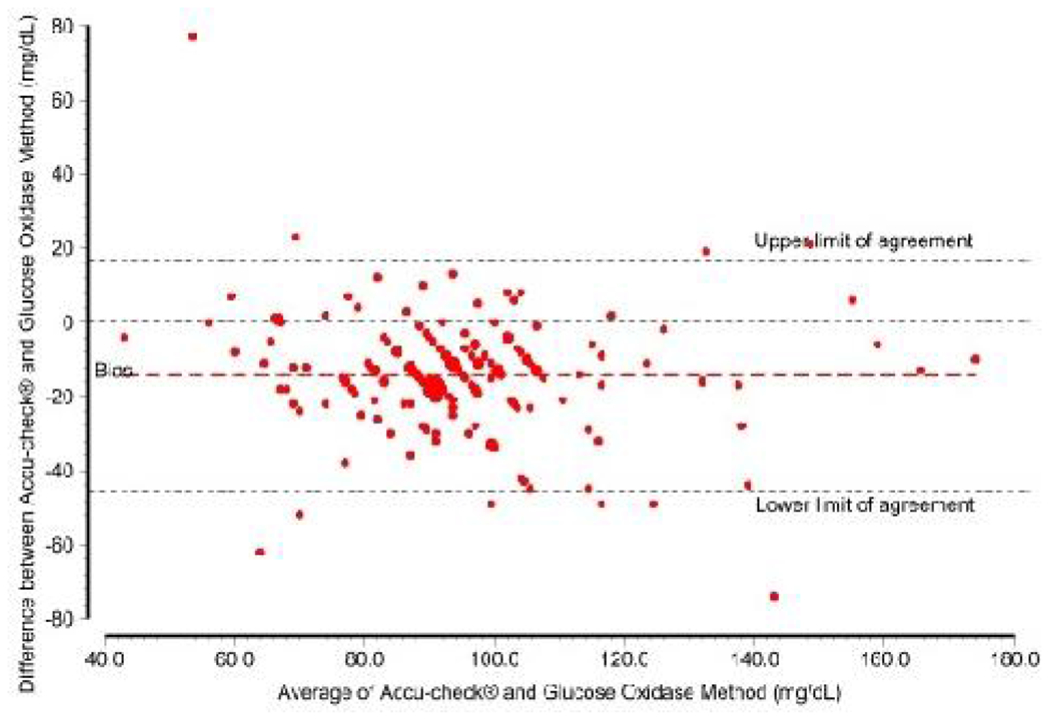
Blend-Altman plot of agreement between Accu-Check and glucose oxidase method
Similarly, the mean of differences (bias) between One Touch® and laboratory values readings was −19.7 mg/dl (95% limits of agreement = −10.4 to 49.9) as shown in Figure 2. There were 4.1% (7 out 173) of the readings outside the limit of agreement. The average lies between 44.5 mg/dl and 174 mg/dl.
Fig 2:

Blend-Altman plot of agreement between One-Touch and glucose oxidase method
Also, the mean of differences (bias) between Accu-check® and One Touch® was −3.9 mg/dl (95% limits of agreement = −31.1 to 23.2) (Figure 3). There were 4.1% (12 out 294) of the readings outside the limit of agreement. The average lies between 41.5 mg/dl and 179.0 mg/dl.
Fig 3:
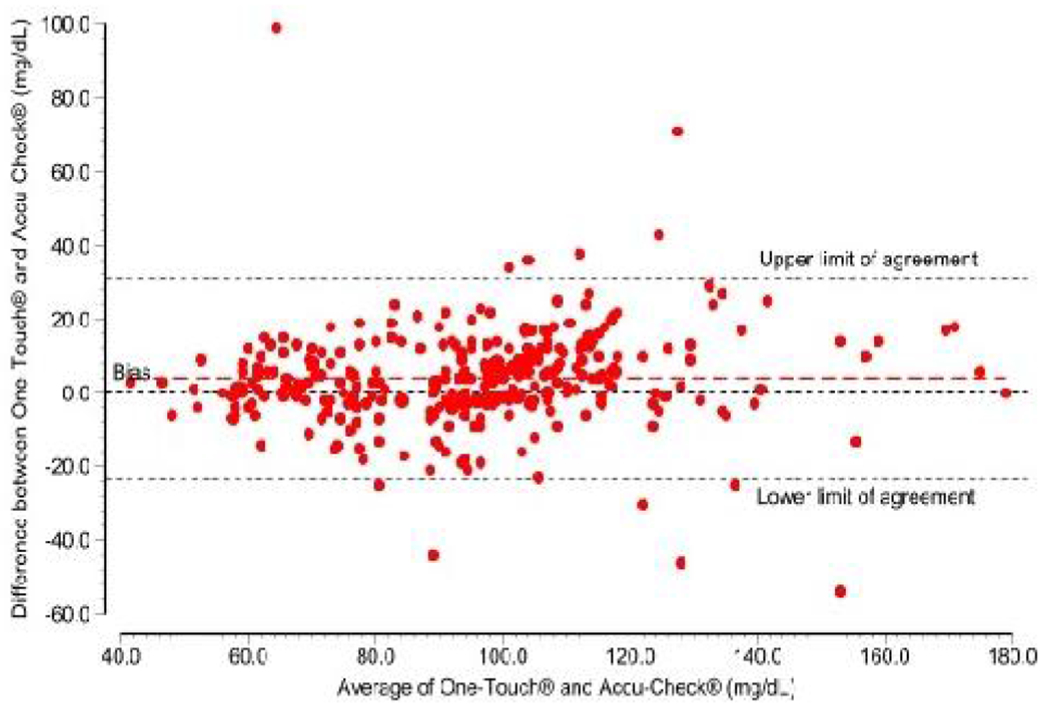
Blend-Altman plot of agreement between Accu-Check and One-Touch
Discussion
This study showed that among children presenting to emergency or outpatient clinic settings in Nigeria, there was a significant correlation between RPG values measured by standard laboratory and point-of-care glucose meters. The observed correlation also did not vary across a wide range of age at presentation and pack cell volumes. The observed significant correlation was greater between the two glucometers tested than between glucose oxidase and any of the glucometer-based RPG in this study. This is not unexpected as glucose is unstable in whole blood and glycolysis continues in red blood cells after sample collection. This is increased in sepsis and leucocytosis. Inhibitors of glucolysis, e.g. fluoride, have a 1-2 hour lag before crossing the cell membrane and reaching optimal activity12. In the study, efforts were made to transport the samples to the laboratory within 30 minutes to 1 hour of collection, but it cannot be ascertained how soon after reaching the laboratory the samples were processed.
This study supports the use of glucometers in paediatric in-patient and out-patient settings in the face of challenging laboratory services, and the two glucometers evaluated in this study had good correlation and can be used in our setting. This is important because as many as 41% of glucometers may not meet the specified standards8 and a recent review found a significant proportion did not meet the current ISO standards.13 Glucose metabolism disorders are a recognised risk factor for mortality in children, and the availability of point-of-care glucometers, which provide reliable blood glucose measurements for interventions, will significantly improve outcomes in these children.1,5,6 Disturbances of glucose metabolism are also common accompanying presentations in neonates and children with severe malaria, sepsis, and diabetes mellitus in developing countries.6 Prompt recognition of this and early intervention can be lifesaving. Unfortunately, in most resource-limited settings, laboratory backup for prompt and adequate patient care is either lacking or results are often returned too late to have an impact on patient care. The provision of point-of-care diagnostics like glucose metres can thus be lifesaving.
In the clinical setting, there are various pre-analytical, analytical, and post-analytical factors that could affect the accuracy of point-of-care glucometers13,14. Pre-analytical factors include operator or strip related factors like particles on the test finger, wet or too dry fingers, wrong sample site selection, expired or strip exposed to humidity. Analytical factors include test performance of the glucometer, environmental factors like temperature, altitude, humidity, and altered patient physiologic states. Temperature changes, especially hypothermia, increase the glucometer blood glucose reading on glucometers, while high altitude and humidity cause a reduction in reading.15 Also, a number of drugs, such as mannitol, acetaminophen, ascorbic acid, dopamine, and maltose, cause a fictitious increase in glucose reading.13,14,16,17 In post analysis, errors may arise in the display, reading, or recording of results.17 The results from our study showed good correlation across a wide range of PCV, 16–69%, in the study population. Extremely low PCV has been associated with increased readings and increased PCV with reduced readings12. In neonates, the haematocrit has been found to be the most important factor responsible for the poor performance of glucometers, especially at extremely low glucose levels.11 Therefore, this should be borne in mind when using glucometers in this population, and there might be a need for follow-up testing in the laboratory. However, the observed good correlation in this study was not affected across the PCV range in the study population. The minimum observed PCV of 16% approximates the 15% cut off for WHO defined severe malaria anaemia, a common feature in our setting. Also, hematocrit above the maximum observed hematocrit in the study population of 69%, often seen in patients with cyanotic cardiac disease and some neonates, is seen in only a fraction of patients in our clinical setting.
Although this study was conducted in children, the findings may be applicable to adults, as previously reported.18 An exception to this is in the intensive care unit setting, where various physiologic states may influence the clinical accuracy of the glucometer, e.g., oedema and shock. In addition, various intraoperative patient physiologic dynamics that change, at times rapidly, can affect the accuracy, especially temperature, perfusion, blood volume, PCV, sympathetic discharge, and peripheral vasoconstriction.19 Hence, glucometer use is not recommended in these settings by the Food and Drug Administration.13
A limitation of our study is the inability to evaluate the effects of extremely low or high PCV on test performance in our clinical setting, especially in specific populations like neonates. However, the study was conducted among a randomly selected group of patients in the emergency and outpatient settings, as patients who presented were enrolled irrespective of their clinical state. This might very well represent the general population mix of patients in these settings, and so the results of the study remain relevant in such settings. Another limitation of this study was the inability to analyse the effects of altered patient physiologic states and medications that might affect the performance of the glucometers. In our emergency room, hypoxaemia is seen in about 28.6% of patients20, and hypoxaemia may lead to a falsely low glucose reading by glocosemeters. Glucose requires oxygen to complete a chemical reaction that generates a proportional electric current reported by the glucometer. In hypoxic states, the amount of chemical reaction generated and thus the glucose reading reported is reduced.13
In spite of the favourable performance of most meters, over 90% of inaccuracies are due to operator error.21 This stems from mistakes with sample site selection, wiping the site with alcohol or using a wet finger, bubbles in the sample, inadequate blood samples, etc. Also, as most glucometers are shared amongst patients in most clinical settings like ours, the risk of transmission of bloodborne infections remains a threat.[22] It is therefore important to educate operators on its use to ensure maximum utility is derived from the test procedure while minimising risk to the patients. This is particularly important in emergency room settings, where stress levels can be rather high and the pace of activities quite fast.
Footnotes
Conflict of Interest: None
Contributor Information
Babatunde Oluwatosin Ogunbosi, Department of Paediatrics, Faculty of Clinical Sciences, College of Medicine, University of Ibadan, Nigeria.
Olatokunbo Olumide Jarrett, Department of Paediatrics, Faculty of Clinical Sciences, College of Medicine, University of Ibadan, Nigeria.
Adebola Emmanuel Orimadegun, Institute of Child Health, College of Medicine, University of Ibadan, Nigeria.
Omolola Oluwakemi Ayoola, Paediatric Endocrinology, Royal Manchester Children’s Hospital, University of Manchester.
Kike Osinusi, Department of Paediatrics, Faculty of Clinical Sciences, College of Medicine, University of Ibadan, Nigeria.
References
- 1.Deshpande S, Ward Platt M The investigation and management of neonatal hypoglycaemia. Semin Fetal Neonatal Med. 2005. Aug;10(4):351–61. Epub 2005/06/01. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15922680 [DOI] [PubMed] [Google Scholar]
- 2.Smith RL, Lin JC, Adelson PD, Kochanek PM, Fink EL, Wisniewski SR, et al. Relationship between hyperglycemia and outcome in children with severe traumatic brain injury. Pediatr Crit Care Med. 2012. Jan;13(1):85–91. Epub 2011/04/19. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21499170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asilioglu N, Turna F, Paksu MS Admission hyperglycemia is a reliable outcome predictor in children with severe traumatic brain injury. J Pediatr (Rio J). 2011. Jul-Aug;87 (4):325–8. Epub 2011/05/21. engpor.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21597650 [DOI] [PubMed] [Google Scholar]
- 4.Marik PE, Zaloga GP Adrenal insufficiency in the critically ill: a new look at an old problem. Chest. 2002. Nov;122 (5):1784–96. Epub 2002/11/12. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12426284 [DOI] [PubMed] [Google Scholar]
- 5.Lodha R, Bhutia TD, Kabra SK, Thukral A Day 1 blood glucose and outcome in critically ill children. Indian Pediatr. 2009. Sep;46(9):809–10. Epub 2009/10/09. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19812427 [PubMed] [Google Scholar]
- 6.Osier FH, Berkley JA, Ross A, Sanderson F, Mohammed S, Newton CR Abnormal blood glucose concentrations on admission to a rural Kenyan district hospital: prevalence and outcome. Arch Dis Child. 2003. Jul;88(7):621–5. Epub 2003/06/24. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12818911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elusiyan JBE, Adejuyigbe EA, Adeodu OO. Hypoglycaemia in a Nigerian paediatric emergency ward. J tropical pediatrics. 2005;52(2):96–102. [DOI] [PubMed] [Google Scholar]
- 8.A multicenter study of the accuracy of the One Touch Ultra home glucose meter in children with type 1 diabetes. Diabetes Technol Ther. 2003;5(6):933–41. Epub 2004/01/08. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14709195 [DOI] [PubMed] [Google Scholar]
- 9.Freckmann G, Baumstark A, Jendrike N, Zschornack E, Kocher S, Tshiananga J, et al. System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technol Ther. 2010. Mar;12(3):221–31. Epub 2010/02/16. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20151773 [DOI] [PubMed] [Google Scholar]
- 10.Hellman R Glucose meter inaccuracy and the impact on the care of patients. Diabetes Metab Res Rev. 2012. Mar;28 (3):207–9. Epub 2012/01/05. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22215509 [DOI] [PubMed] [Google Scholar]
- 11.Balion C, Grey V, Ismaila A, Blatz S, Seidlitz W Screening for hypoglycemia at the bedside in the neonatal intensive care unit (NICU) with the Abbott PCx glucose meter. BMC Pediatr. 2006;6:28. Epub 2006/11/07. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17083737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman DG Evaluation of a new blood glucose meter. Lancet. 1987. May 23;1 (8543):1205–6. Epub 1987/05/23. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2883516 [DOI] [PubMed] [Google Scholar]
- 13.Klonoff David C, Prahalad Priya. Performance of cleared blood glucose monitors. J diabetes science and technology. 2015;9(4):895–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ksenia Tonyushkina, Nichols James H. Glucose meters: a review of technical challenges to obtaining accurate results. J diabetes science and technology. 2009;3 (4):971–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fink KS, Christensen DB, Ellsworth A Effect of high altitude on blood glucose meter performance. Diabetes Technol Ther. 2002;4(5):627–35. Epub 2002/11/27. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12450444 [DOI] [PubMed] [Google Scholar]
- 16.Ho KM, Liang J Toxic levels of paracetamol falsely elevate blood glucose readings by handheld glucose meter (Glucocard II). Anaesth Intensive Care. 2003. Jun;31(3):333–4. Epub 2003/07/26. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12879685 [PubMed] [Google Scholar]
- 17.Klonoff David C Point-of-care blood glucose meter accuracy in the hospital setting. Diabetes Spectrum. 2014;27(3):174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warner JV, Wu JY, Buckingham N, McLeod DS, Mottram B, Carter AC Can one point-of-care glucose meter be used for all pediatric and adult hospital patients? Evaluation of three meters, including recently modified test strips. Diabetes Technol Ther. 2011. Jan;13 (1):55–62. Epub 2010/12/24. eng.http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21175272 [DOI] [PubMed] [Google Scholar]
- 19.Boris Mraovic, Schwenk Eric S, Epstein Richard H. Intraoperative accuracy of a point-of-care glucose meter compared with simultaneous central laboratory measurements. J Diabetes Science and Technology. 2012;6(3):541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orimadegun AE, Ogunbosi BO, Carson SS Prevalence and predictors of hypoxaemia in respiratory and non-respiratory primary diagnoses among emergently ill children at a tertiary hospital in south western Nigeria. Trans R Soc Trop Med Hyg. 2013. Nov;107 (11):699–705. https://www.ncbi.nlm.nih.gov/pubmed/24062524 http://trstmh.oxfordjournals.org/content/107/11/699.full.pdf [DOI] [PubMed] [Google Scholar]
- 21.Dungan Kathleen, Chapman John, Braithwaite Susan S, Buse John. Glucose measurement: confounding issues in setting targets for inpatient management. Diabetes care. 2007;30(2):403–9. [DOI] [PubMed] [Google Scholar]
- 22.Kadi Z, Saint-Laurent P, Cadranel JF, Joly C, Dumouchel P, Jeanne S, et al. Retrospective investigation of patients exposed to possible transmission of hepatitis C virus by a capillary blood glucose meter. J Hosp Infect. 2006. May;63 (1):65–9. Epub 2006/03/07. eng.http://www.ncbi.nlm.nih.gov/ [DOI] [PubMed] [Google Scholar]


