To The Editor:
In June 2019, a new epidemic spread throughout the United States with thousands of adolescents and young adults hospitalized with lung injury following e-cigarette use. This new clinical entity of e-cigarette, or vaping, product use-associated lung injury (EVALI) has affected over 2,800 individuals as of February 2020 (last national report by the Center for Disease Control (CDC))1. In January 2020, labs affiliated with the New York State Department of Health identified vitamin E acetate (VEA) as a primary compound found in many e-cigarette/vaping cartridges from those hospitalized with EVALI. VEA was subsequently identified also in the bronchoalveolar lavage fluid (BALF) of those patients hospitalized with EVALI by a CDC working group2. Hence, VEA remains the lead chemical compound associated with EVALI.
However, a significant majority of individuals affected by EVALI do not require ICU level care3, and only a small minority of individuals hospitalized undergo flexible bronchoscopy with lavage (BAL), demonstrated by only 51 BAL samples from >2800 individuals affected at the epidemic’s peak2. Only a handful of labs nationwide have the training to complete the tedious process of isolating and identify VEA from BAL. Collectively, these factors significantly limit the use of BALF VEA levels as a diagnostic biomarker of EVALI, and for most hospitalized patients, EVALI remain a diagnosis of exclusion. Here, we identified reduced plasma phosphatidylethanolamines as a potential non-invasive biomarker for diagnosing EVALI.
In this pilot study, three groups of subject were enrolled: non-smoking controls (n=5), e-cig users without EVALI (n=5), and e-cig users hospitalized with EVALI (n=5). Written informed consent was obtained from all subjects under the University of Rochester Medical Center approved IRB (#CR00003968). Age of eligibility was between 18 and 35 years. Groups were matched for age at enrollment. Exclusion criteria included: (1) prior history of heart or lung disease, diabetes, cancer, and/or current viral respiratory tract infection identified by viral PCR or blood culture (including PCR negativity for COVID-19); (2) current medical use of daily anti-inflammatories or corticosteroids; and (3) currently pregnant or breast feeding. Subjects in the non-user group were required to have never smoked or used e-cigarette or tobacco products. Subjects in the e-cig user group were not hospitalized and recruited from the community by flyer advertisement. All subjects in the EVALI group met clinical criteria for diagnosis1,3, were hospitalized at time of enrollment and were enrolled after February 2020 (after CDC reporting of EVALI cases ceased). All EVALI subject plasma samples were also obtained prior to corticosteroid treatment.
Subject demographics, clinical presentation and management are listed in Table 1. Untargeted lipidomics was performed on human plasma via lipid extraction and subsequent LC-MS/MS identification (Methods described in greater detail within the supplement). Lipidomics was chosen considering many e-cigarette liquids, in addition to VEA, are highly viscous and lipophilic chemicals. Additionally, early data from chronic e-cig users as well as from sub-chronic e-cig exposures in mice4 support abnormal lipid metabolism in exposed plasma samples.
Table 1.
Demographics, clinical presentation and management of non-users (n=5), e-cig users (n=5), and EVALI users (n=5).
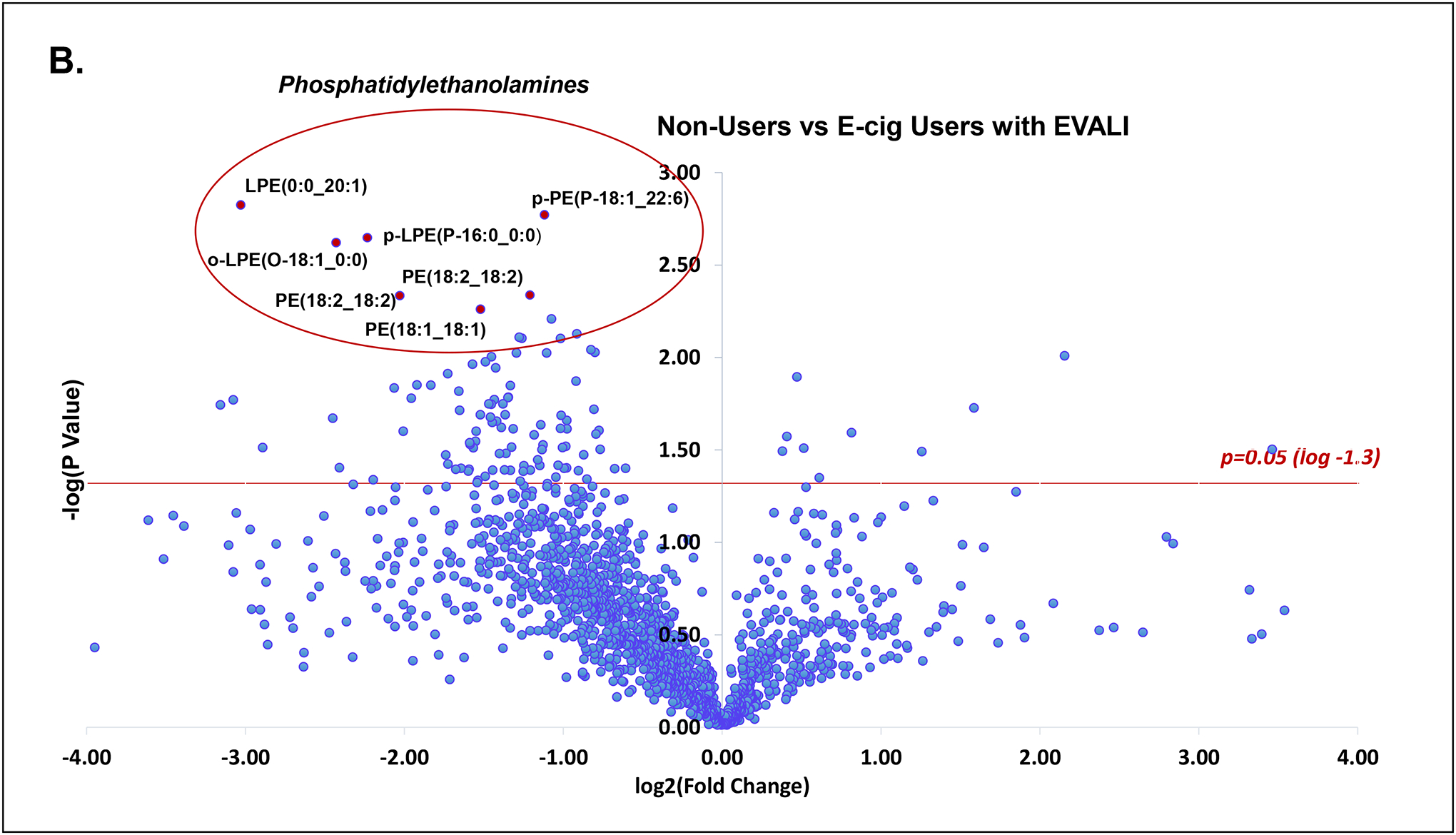
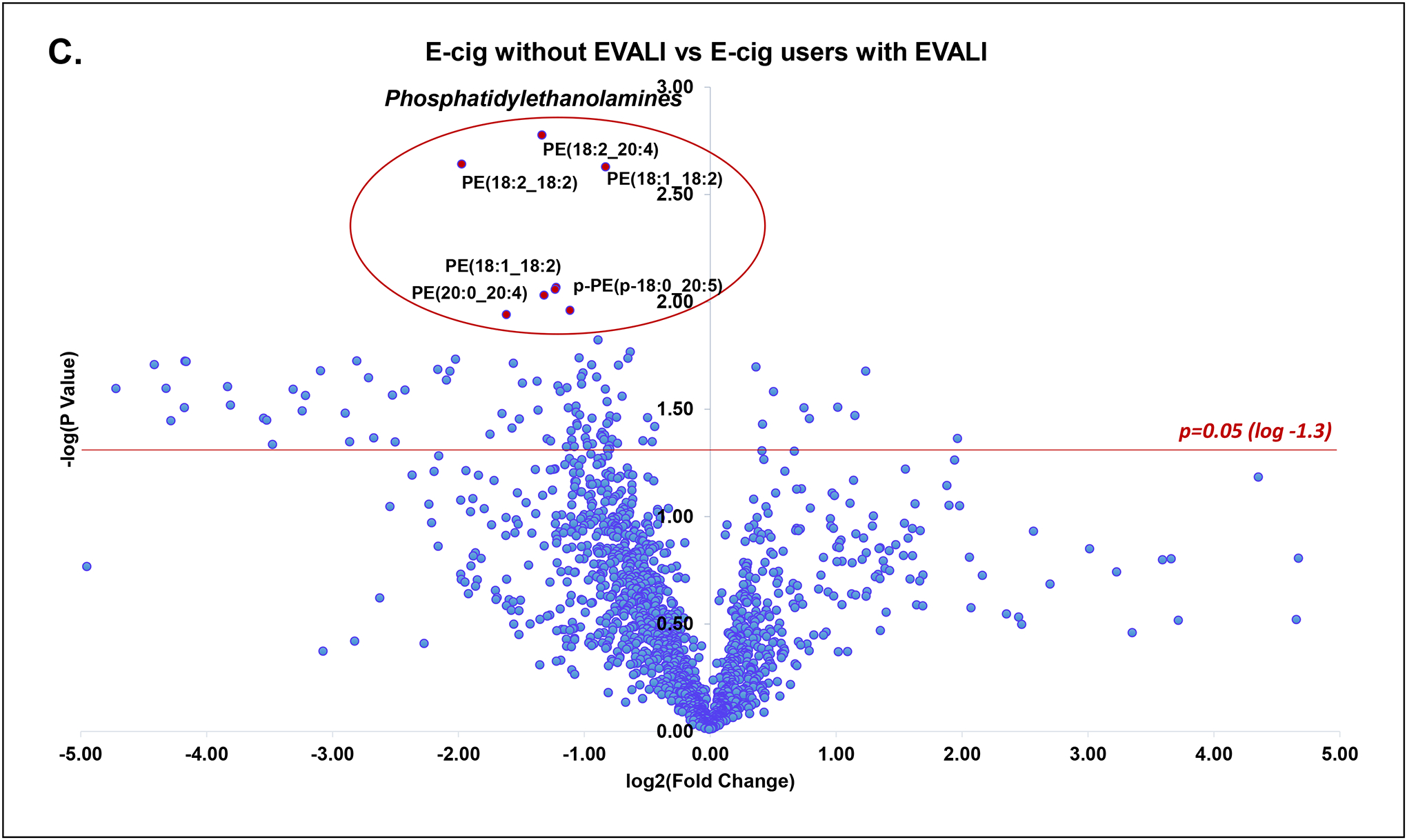
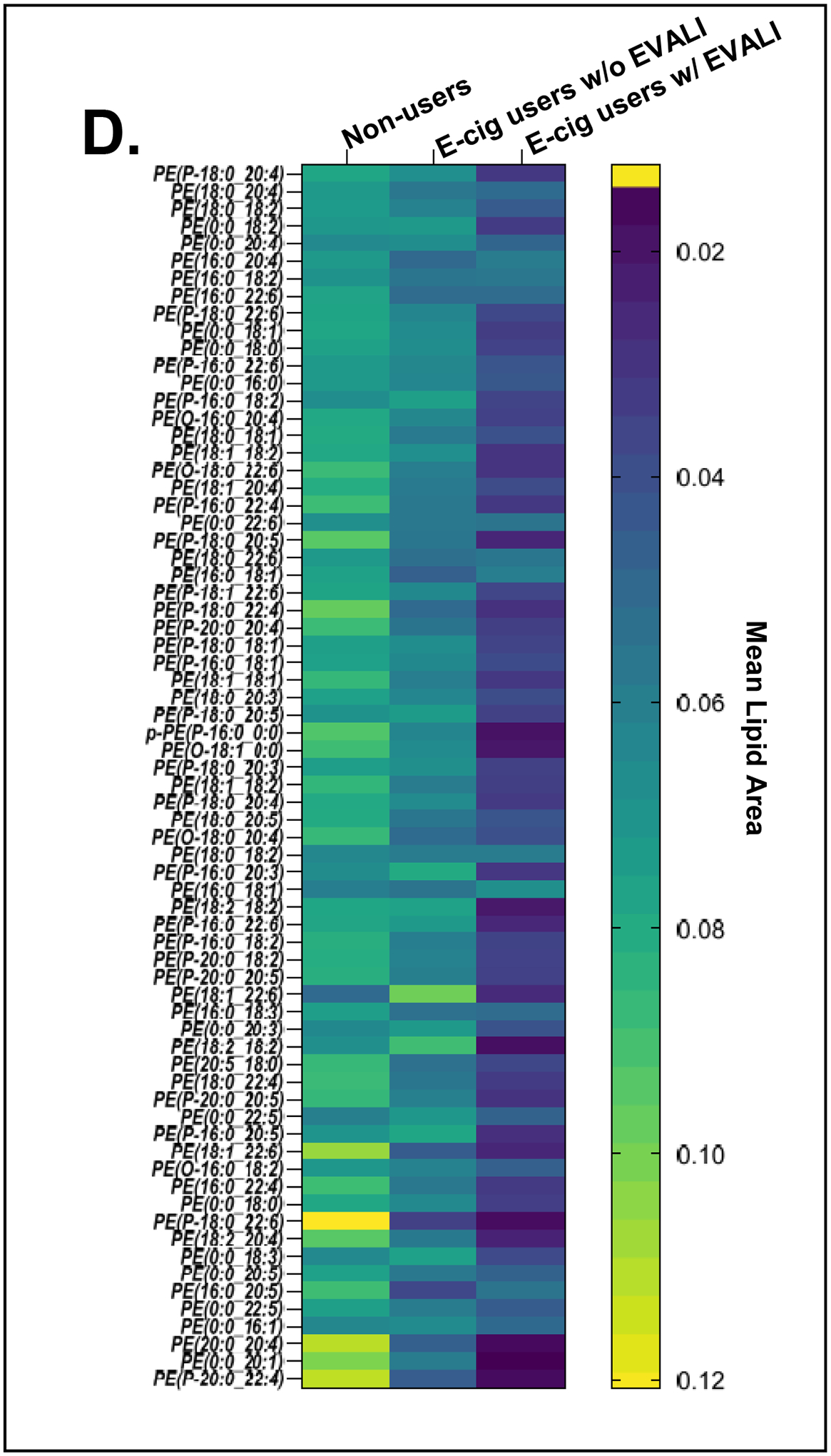
A total of 513 unique molecular species were identified from untargeted lipidomics grouped by lipid class. Principal component analysis of all lipid classes failed to delineate separation between groups. However, in lipid subclass analysis, phosphatidylethanolamines (PE’s) were significantly reduced in all EVALI subjects compared to control groups of non-users and e-cig users (Figure 1a, Kruskal-Wallis, **p=0.0029). The median plasma level of PE’s in EVALI users was 56.0 (IQR: 39.3–65.2) while 88.4 (IQR: 74.3–108.2) and 81.5 (IQR: 77.9–101.1) in non-users and e-cig users, respectively. In other words, the median plasma levels of PE’s were reduced by 36.7% and 31.3% in EVALI versus non-users and EVALI versus e-cig users, respectively. Specific plasma PE’s identified as being significantly reduced are highlighted in volcano plot comparisons between individual groups (Figure 1b and 1c) and across all groups (Figure 1d). Other structurally similar phospholipids (PL’s), such as certain phosphatidylcholines, phosphatidylserines and phosphoinositols, were also identified as reduced when comparing individual lipid components between groups, however individual sub-class analysis did not differ significantly between groups.
Figure 1.
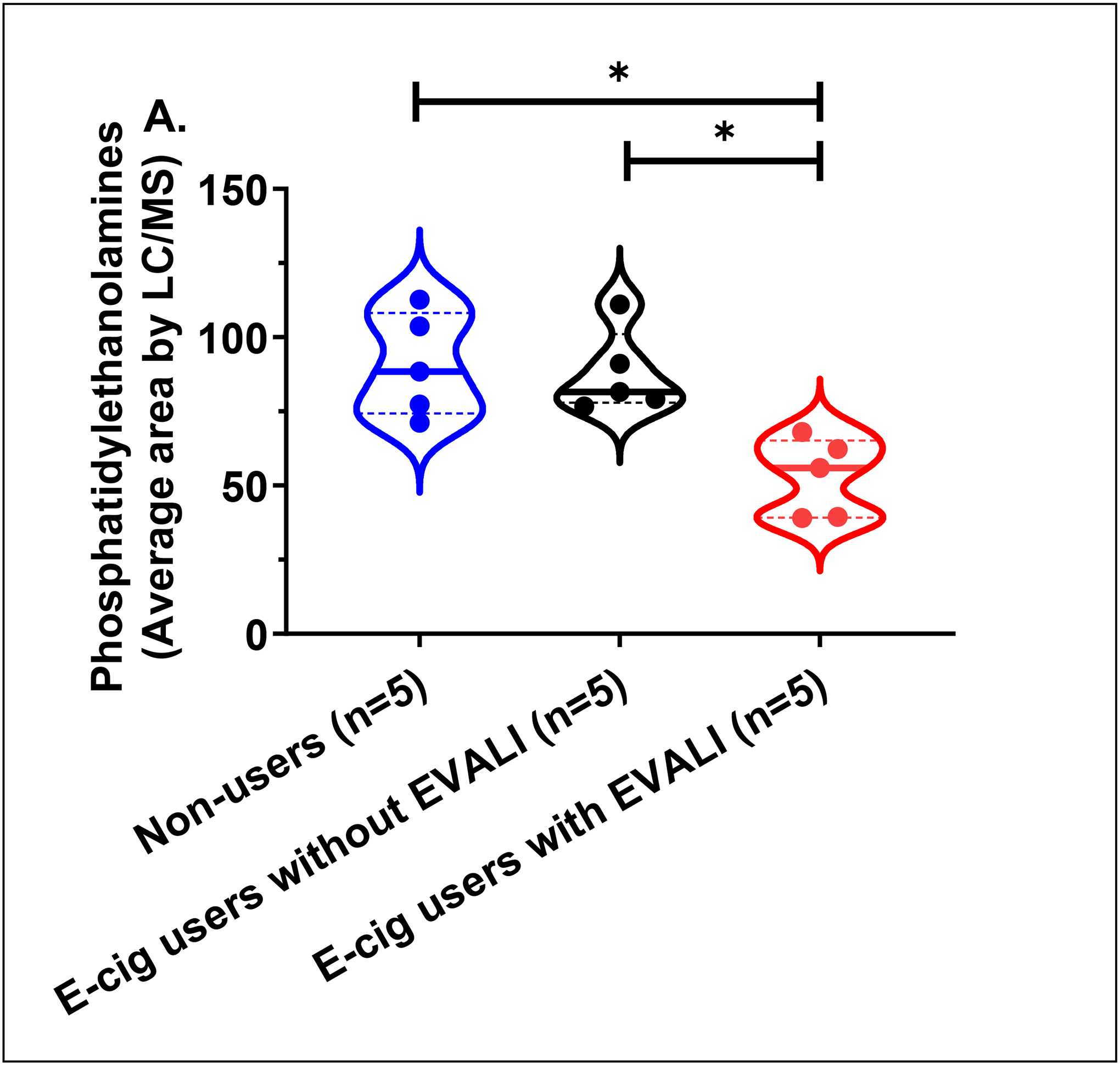
(A) Plasma phosphatidylethanolamine (PE’s) levels in non-users, e-cig users without EVALI, and e-cig users with EVALI (n=5 samples/group; median area with interquartile range).
(B) Volcano plot of lipid species comparing non-users and e-cig users with EVALI (log2 fold change vs. log2 p-value). Multiple PE’s identified as significantly reduced (p<0.05, red line).
(C) Volcano plot of lipid species comparing e-cig users without EVALI and e-cig users with EVALI (log2 fold change vs. log2 p-value). Multiple PE’s were identified as significantly reduced (p<0.05, red line).
(D) Heatmap of relative mean area for individual phosphatidylethanolamines (PE’s) in non-users, e-cig, and EVALI.
Untargeted lipidomics identified phosphatidylethanolamines (PE’s) as significantly reduced from plasma of hospitalized EVALI subjects prior to corticosteroid treatment compared to plasma PE’s levels of age-matched non-users and e-cig users without respiratory insufficiency. PE’s are a class of phospholipids found in all human cells, composing ~20% of all PL’s within the cell membrane. In conjunction with phosphatidylcholine, PE’s are classified as plasmalogens based on their lipid structure containing a vinyl ether bond at the sn-1 position and an ester bond at sn-2 position of the glycerol backbone. Plasmalogens are most abundant in the nervous, immune, and cardiovascular systems, but are also commonly seen in the kidneys, skeletal muscles, and lungs5. Three of the most common biologic functions of plasmalogens are cell membrane stability, oxidative potential, and reservoirs for second messengers5.
Specific to the lung, plasmalogens play an essential role in surfactant homeostasis. Plasmalogens help to reduce surface tension and viscosity of surfactant in combination with cholesterol. Lohner et al. (1991) first demonstrated that plasmalogens promote and stabilize the non-lamellar membrane phase structure of surfactant. Rudiger et al. (1998) showed the addition of plasmalogens to surfactant-like PL mixtures resulted in lower surface tensions, emphasizing the role of plasmalogens in surfactant’s function of preventing alveolar collapse. Additionally, in preterm infants who received surfactant supplemented with higher plasmalogen content for respiratory distress after birth, the likelihood of developing bronchopulmonary dysplasia was reduced. Related to oxidative potential, PE’s are preferentially oxidized over other diacyl phospholipids functioning also as an antioxidant when in the presence of free radicals and singlet oxygen. When mouse surfactant was exposed to ozone, plasmalogen content was reduced close to 50% and prior to any significant change in other diacyl PL concentration, further supporting the function of plasmalogens as an antioxidant for the lung.
Related to EVALI, Dipasquale et al. (2020) recently showed VEA significantly changed the mechanical properties of two surfactant mimics. Both of these surfactant mimics contained plasmalogens. Using neutron spin echo spectroscopy, increasing concentrations of VEA nonlinearly increased membrane fluidity and area compressibility. Modulation of these two properties in the presence of VEA increased surface tension, resulting in monolayer collapse. We hypothesize that the reduced concentrations of plasmalogens identified here from EVALI subjects are physiologically relevant to the respiratory insufficiency seen in those subjects hospitalized with hypoxemia and bilateral ground glass opacities. Specifically, reduced plasmalogen concentration of the lung would predispose EVALI subjects to alveolar collapse from increased surface tension of surfactant. However, we recognize that additional analyses of surfactant composition from subjects with EVALI would help strengthen this association between reduced plasma PE’s and injury specific to the lung after e-cigarette exposure.
Different from other published cohorts of EVALI, the subjects in this EVALI cohort did not require intubation, ventilation, and most (4/5) did not require admission to the ICU. Of note, none of the subjects received an arterial blood gas, limiting our ability to diagnosis acute respiratory distress syndrome (ARDS) due to the absence of a partial pressure of oxygen (PaO2). However, all of the subjects had bilateral infiltrates on chest radiograph and the lack of left-sided heart disease. Outside of neonatal respiratory distress syndrome (RDS), few clinical studies have evaluated plasmalogens in ARDS or ALI. Schmidt et al. (2004) identified reduced plasmalogens in BALF of adults with ARDS. Most of the adults included within this study were older (mean: >50 years) and were in severe respiratory failure (requiring intubation and ventilation). Hence, two additional advantages of the current study are (1) identifying reduced PE’s in a younger cohort of subjects with ALI and (2) identifying reduced plasma PE’s levels prior to the development of severe respiratory failure.
Future studies are underway to validate PE’s as a potential, non-invasive biomarker for diagnosing EVALI as well as differentiating PE plasma levels in EVALI from other forms of ALI/ARDS. Defining non-invasive biomarkers in the diagnosis of EVALI is essential considering flexible bronchoscopy remains non-essential in the routine evaluation and management of EVALI and hence VEA from BALF of EVALI subjects is not routinely performed. Additionally, the presentation of subjects with EVALI is nearly identical to other forms of ALI. One of the limitations of the current studies is sample size. This pilot study was not powered to provide accurate measurements of sensitivity and specificity for PE’s as biomarkers of diagnosing EVALI, but solely used for identification of biologically relevant plasma biomarkers using untargeted lipidomics. Unique to this study, all EVALI subjects were enrolled after CDC stopped reporting. Similar to other institutions6, our institution continues to see multiple patients hospitalized for EVALI after February 2020 (when the CDC stopped reporting and the COVID-19 pandemic began), emphasizing the continued importance and need for non-invasive biomarkers for this new disease that remains a diagnosis of exclusion and significantly different from other forms of acute lung injury.
Acknowledgements
We thank Cayman Chemicals for technical assistance in untargeted lipidomics analyses as well as Carl J. Johnston, PhD, Thivanka Muthumalage, PhD and Stephanie Podguski (University of Rochester Medical Center) for technical support in subject recruitment and sample processing.
FUNDING SOURCES:
MDM reports grant support of NIH P30 ES001247 and NIH L40 ES030909 from the National Institute of Environmental Health Sciences and 2KL2TR001999 from National Center for Advancing Translational Sciences. IR reports grant support of NIH R01 HL135613 and HL135613-S1 through the National Heart, Lung and Blood Institute.
Footnotes
DISCLAIMER: Research reported in this manuscript was supported in part by the National Institute of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or the Food and Drug Administration (FDA).
CONFLICT OF INTEREST: No conflicts of interest, financial or otherwise, are declared by the authors.
OPEN SOURCE: This data generated are published publically at: https://figshare.com/articles/dataset/Reduced_Plasma_Phosphatidylethanolamines_in_E-cigarette_or_Vaping_Product_Use-Associated_Lung_Injury/17089133?file=31600265
REFERENCES
- 1.Center for Disease Control & Office on Smoking and Health. Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. 2021.
- 2.Blount BC, Karwowski MP, Shields PG, et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N Engl J Med. 2020;382(8):697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madison MC, Landers CT, Gu BH, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest. 2019;129(10):4290–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta. 2012;1822(9):1442–1452. [DOI] [PubMed] [Google Scholar]
- 6.Kligerman SJ, Kay FU, Raptis CA, et al. CT Findings and Patterns of e-Cigarette or Vaping Product Use-Associated Lung Injury: A Multicenter Cohort of 160 Cases. Chest. 2021;160(4):1492–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]


